SUMMARY
Blood glucose levels are tightly controlled, a process thought to be orchestrated primarily by peripheral mechanisms (insulin secretion by β-cells, and insulin action on muscle, fat and liver). The brain also plays an important, albeit less well-defined role. Subsets of neurons in the brain are excited by glucose; in many cases this involves ATP-mediated closure of KATP channels. To understand the relevance of this, we are manipulating glucose sensing within glucose-excited neurons. In the present study, we demonstrate that glucose excitation of MCH-expressing neurons in the lateral hypothalamus is mediated by KATP channels, is negatively regulated by UCP2 (a mitochondrial protein that reduces ATP production), and that glucose sensing by MCH neurons plays an important role in regulating glucose homeostasis. Combined, the glucose-excited neurons are likely to play key, previously unexpected roles in regulating blood glucose.
Keywords: MCH neurons, KATP channel, UCP2, glucose sensing
INTRODUCTION
Numerous studies have demonstrated that the brain can affect blood glucose levels (Rother et al., 2008; Sandoval et al., 2009; Schwartz and Porte, 2005), raising the possibility that the CNS contributes to maintenance of the euglycemic state (and by inference, to the pathogenesis of type 2 diabetes). With this in mind, it is of interest that subsets of neurons in the brain are capable of sensing glucose (Levin et al., 2004). By acting in conjunction with downstream autonomic efferents, these glucose-sensing neurons could provide a basis for central regulation of blood glucose levels. While there has been much speculation about the existence and importance of such neural mechanisms, complexities of the brain combined with difficulties in probing its function, have left this issue unresolved.
One approach is to genetically perturb glucose sensing in select subsets of neurons, and then assess effects on blood glucose levels. Glucose inhibits certain neurons (for example, AgRP neurons in the arcuate nucleus and orexin neurons in the lateral hypothalamus) and stimulates others (for example, POMC neurons in the arcuate nucleus, MCH neurons in the lateral hypothalamus, and chemically undefined neurons in the ventral medial hypothalamus and in the hindbrain) (Balfour et al., 2006; Burdakov et al., 2005; Claret et al., 2007; Ibrahim et al., 2003; Levin et al., 2004; Mountjoy et al., 2007; Parton et al., 2007; Routh, 2002). The mechanism responsible for glucose inhibition of neurons is incompletely understood (Burdakov and Lesage, 2009). In contrast, a well-defined mechanism accounts for most cases of glucose excitation of neurons (Ashford et al., 1990; Ibrahim et al., 2003; Kang et al., 2004; Miki et al., 2001; Parton et al., 2007). This pathway is similar to that which occurs in glucose-excited pancreatic β-cells (Ashcroft, 2005) (i.e. ↑ glucose → ↑ ATP production → ATP-mediated closure of KATP channels ↑ depolarization). Using an approach originally developed for pancreatic β-cells (Koster et al., 2000; Remedi et al., 2009), we are perturbing glucose sensing by genetically expressing mutant Kir6.2 subunits in specific groups of glucose-excited neurons. The mutation (Kir6.2[K185Q,ΔN30]) being employed renders KATP channels resistant to closure by ATP, hence abrogating excitation by glucose (Koster et al., 2000; Parton et al., 2007; Remedi et al., 2009).
We previously used a related approach to disturb glucose sensing in POMC neurons (Parton et al., 2007). Of interest, this led to impairment in peripheral glucose homeostasis. It is important to note, however, that POMC neurons represent only a small percentage of all glucose-excited neurons. Left unresolved is the role of these other glucose-excited neurons. This question is significant because if these other glucose-excited neurons are also playing an important role, then their combined effects on glucose homeostasis are expected to be large.
The present study focuses on MCH neurons, which are of interest because their anatomy is distinctly different from that of POMC neurons (Bittencourt et al., 1992). MCH neurons are found exclusively in the lateral hypothalamus and zona incerta while POMC neurons are found in the arcuate nucleus and the nucleus of the solitary tract. Two approaches have been used to perturb glucose sensing in MCH neurons. To decrease glucose sensing, we expressed a mutant Kir6.2 subunit (Kir6.2[K185Q,DN30]) in MCH neurons, rendering KATP channels resistant to closure by ATP. To increase glucose sensing, we deleted UCP2 selectively from MCH neurons. UCP2 is a mitochondrial protein that reduces ATP production (Krauss et al., 2005). In its absence, MCH neurons are expected to have increased glucose-stimulated ATP levels, thus augmenting glucose-stimulated closure of KATP channels (just as UCP2 deficiency does in pancreatic β-cells (Krauss et al., 2003; Zhang et al., 2001; Zhang et al., 2006) and in POMC neurons (Parton et al., 2007)).
RESULTS
Generation and Characterization of Mch-Cre Transgenic Mice
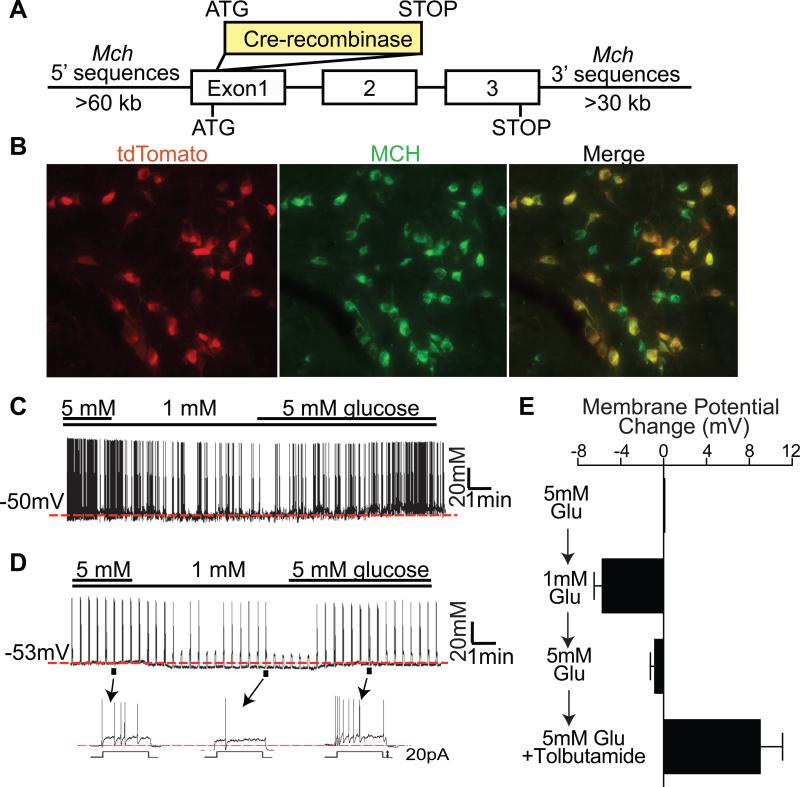
A BAC genomic clone containing the mouse Mch gene was used to drive expression of Cre recombinase (Cre) (Figure 1A). Eight lines of Mch-Cre mice were generated, and then crossed with lox-GFP reporter mice (Z/EG) (Novak et al., 2000) (referred to as Mch-Cre/Z/EG mice). Two lines were found to express GFP in the lateral hypothalamus and zona incerta (known sites of MCH expression) (Bittencourt et al., 1992; Elias et al., 1998), but not in other sites in the brain. To characterize Cre expression in greater detail, one of the two lines was then crossed with the more sensitive lox-tdTomato reporter mouse, Gt(ROSA)26Sortm14(CAG-tdTomato)Hze (Madisen et al., 2010). Mch-Cre/lox-tdTomato mice expressed tdTomato in neurons in the lateral hypothalamus (LH) and in the zona incerta (ZI), and as expected, not in other sites in the brain. Double immunohistochemical detection of MCH peptide and tdTomato protein (Figure 1B) revealed that all tdTomato-positive cell bodies expressed MCH, and that the majority (~80%) of MCH peptide-positive cell bodies expressed tdTomato.
Figure 1. MCH neurons are glucose excited and express Sur1-containing KATP channels.
(A) Structure of Mch-Cre BAC transgene. (B) Double immunohistochemistry for tdTomato (red) and MCH peptide (green) in the lateral hypothalamus of Mch-Cre/lox-tdTomato mice. (C-D) Representative traces recorded in the whole-cell patch clamp mode from GFP-positive neurons of Mch-Cre/Z/EG mice. (C) Spontaneously firing MCH neuron that responded to glucose. (D) Silent MCH neuron that responded to glucose with depolarizing current injection (20pA for 3 sec with 20sec interval). (E) Effects of glucose (5mM → 1mM → 5mM) on membrane potential of “tolbutamide-responding” GFP-positive neurons of Mch-Cre/Z/EG mice. The averaged membrane potential of the last three minutes of the indicated condition from each recording was used for calculation (n=9, mean ± SEM).
MCH Neurons Are Excited By Glucose and Express KATP Channels
Whole-cell electrophysiological recordings were performed on GFP-positive neurons (i.e. MCH neurons) of Mch-Cre/Z/EG mice, and membrane potentials and firing rates were assessed (Figure 1C-E). Consistent with previous findings (Burdakov et al., 2005; van den Pol et al., 2004), we observed that ~30% (11/39) of MCH neurons were spontaneously firing while ~70% (28/39) were electrically silent, requiring injection of depolarizing current for induction of action potentials. Regardless of their firing properties, ~70% of all MCH neurons were excited by glucose. Specifically, 8/11 of the spontaneously firing neurons (example shown in Figure 1C) and 20/28 of the silent neurons (example shown in Figure 1D) were reversibly hyperpolarized when extracellular glucose was lowered from 5 to 1mM. Of note, these glucose concentrations are consistent with those reported, in vivo, in the lateral hypothalamus (Silver and Erecinska, 1998). In addition, in response to 1 mM glucose, the spontaneously firing MCH neurons showed a reduction in firing rates (Figure 1C) and, likewise, the “silent” MCH neurons showed a decrease in firing rates induced by injection of current (Figure 1D). These findings demonstrate that the majority (~ 70%) of MCH neurons are excited by glucose, which is in agreement with an earlier study (Burdakov et al., 2005).
Glucose-excited POMC neurons and pancreatic β-cells express Sur1-containing KATP channels (Ibrahim et al., 2003; Inagaki et al., 1995; Parton et al., 2007; Sakura et al., 1995). To determine if MCH neurons are similar in this regard, a Sur1 subunit-specific KATP channel blocker, tolbutamide, was added at the end of the recordings (Figure S1A and S1B). In total, ~70% of all MCH neurons were excited by tolbutamide. For the glucose-excited subset of MCH neurons (which represents ~70% of all MCH neurons as noted above), nearly all were excited by tolbutamide (17 of 19 neurons). For the subset that was not excited by glucose (which represents ~30% of all MCH neurons, of which 5 were tested for effects of tolbutamide), none were excited by tolbutamide. In a separate set of studies, summarized in Figure 1E, we examined membrane potential as glucose was changed from 5 mM → 1 mM → 5 mM, and then again as tolbutamide was added. As expected, the “tolbutamide-responding” MCH neurons were reversibly hyperpolarized by 1 mM glucose. In contrast, the “tolbutamide-resistant” MCH neurons (Figure S1C), and also randomly selected thalamic neurons (Figure S1D), were unaffected by 1 mM glucose. Finally, an immunohistochemical approach was used to assess Sur1 expression (Figure S1E). Consistent with the observed electrophysiologic responses to tolbutamide, ~75% (335 out of 435) of all MCH-positive neurons were immunopositive for Sur1. Thus, as assessed using tolbutamide, nearly all glucose sensing MCH neurons have Sur1-containing KATP channels, and this trait distinguishes the “glucose-sensing” from the “non-glucose-sensing” MCH neurons.
Expression of Mutant Kir6.2 in MCH Neurons
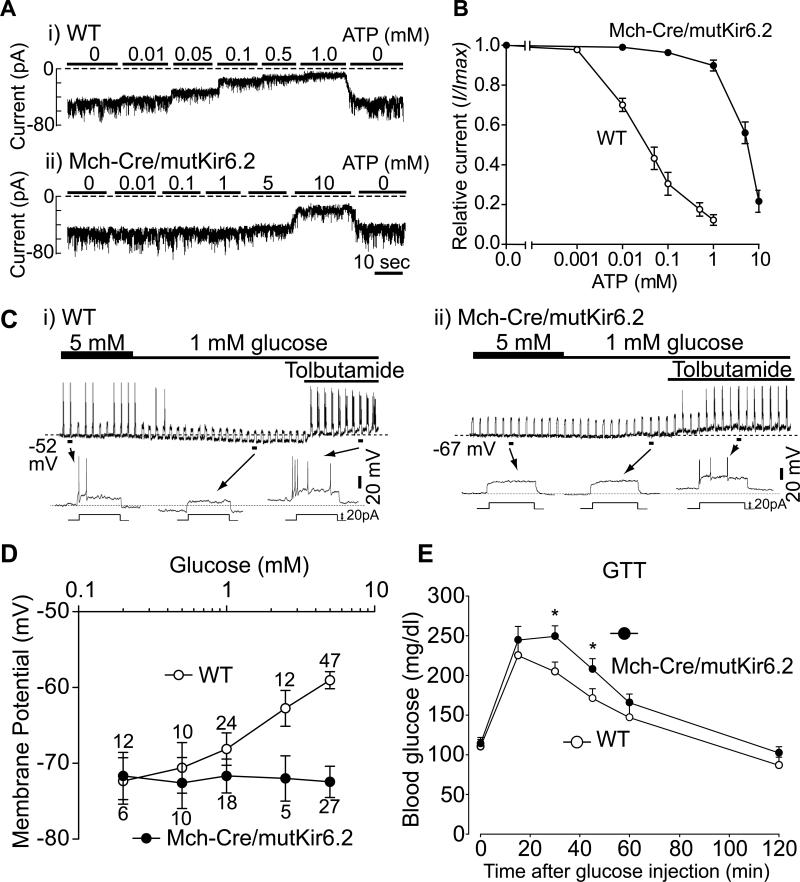
To impair glucose sensing in MCH neurons, we utilized Rosa26 knockin mice in which, following Cre-mediated deletion of a STOP cassette, CMV/Actin promoter elements drive expression of Kir6.2[K185Q,ΔN30] (Remedi et al., 2009). This renders KATP channels resistant to closure by ATP, abrogating excitation by glucose (Koster et al., 2000; Remedi et al., 2009). To confirm that ATP sensitivity of KATP channels was indeed altered in MCH neurons of Mch-Cre/mutKir6.2 mice, we performed recordings on inside-out excised patches derived from MCH neurons of Mch-Cre/Z/EG (control) and Mch-Cre/mutKir6.2/Z/EG mice (expressing mutant Kir6.2). Currents were measured as ATP was applied, in increasing concentrations, to the cytosolic surface of the patches. As can be seen in Figure 2A and 2B, KATP channels from control MCH neurons closed as ATP concentrations were increased with a Ki ([ATP] that causes half-maximal inhibition) of ~30 μM. As expected, expression of mutant Kir6.2 in MCH neurons greatly reduced sensitivity to closure by ATP (by ~200 fold), with the Ki for ATP increasing to ~5.8 mM.
Figure 2. Expression of mutant Kir6.2 in MCH neurons blocks glucose sensing.
(A-B) Effects of ATP on KATP channel current. Recordings were performed on inside-out patches derived from GFP-positive (i.e. MCH) neurons. (A) Representative macroscopic current traces recorded at -60 mV from patches derived from i) WT (Mch-Cre/Z/EG) and ii) Mch-Cre/mutKir6.2/Z/EG mice. Concentrations of ATP are indicated above the corresponding traces. (B) Summarized effects of ATP on KATP channel current. Data was normalized to the maximal current recorded in the absence of ATP (mean ± SEM, n=5-7). (C) Effects of glucose on MCH neurons within brain slices. Representative traces recorded in the whole-cell patch clamp mode from GFP-positive neurons derived from i) WT (Mch-Cre/Z/EG) and ii)Mch-Cre/mutKir6.2/Z/EG mice. (D) Effects of glucose on membrane potential (mean ± SEM, values above each point represent the number of neurons assessed). (E) Effects of mutant Kir6.2 on glucose tolerance in intact mice. Representative glucose tolerance curves of 8-week-old male mice (mean ± SEM, n=7-10 per genotype, 2 g/Kg glucose i.p.). Asterisk, P<0.05 (unpaired t Tests), compared with wildtype littermates at a given time point.
Using brain slice preparations, we next determined if expression of ATP-insensitive KATP channels disrupted glucose sensing in MCH neurons. First, we observed that only ~14% of MCH neurons (3/22) from Mch-Cre/mutKir6.2 mice (compare with ~30% of MCH neurons from control mice) had spontaneous action potentials at 5mM glucose. Membrane resistance of MCH neurons was calculated from the change in membrane potential in response to a hyperpolarizing current pulse (-40 pA, 1sec in duration). At 5mM glucose, membrane resistance of MCH neurons in Mch-Cre/mutKir6.2 mice was significantly reduced (WT: 1081 ± 65 MΩ, n=7; mutKir6.2: 540 ± 47 MΩ, n=7; mean ± SEM), consistent with KATP channels being more open. Second, nearly all MCH neurons from Mch-Cre/mutKir6.2 mice (18/20) were no longer hyperpolarized as extracellular glucose was lowered from 5 to 1 mM (example shown in Figure 2C). This is in striking contrast to MCH neurons from control mice, as noted above, in which ~70% were hyperpolarized by a similar fall in glucose. Although MCH neurons with ATP-insensitive KATP channels are no longer excited by glucose, they are still responsive to tolbutamide, as application of the KATP channel blocker resulted in robust membrane depolarization and stimulation of action potentials (Figure 2C) in 14 of 16 tolbutamide-treated neurons. Third, as shown in Figure 2D, glucose-induced depolarization of MCH neurons, while readily observed in MCH neurons from control mice, was completely abrogated in MCH neurons from Mch-Cre/mutKir6.2 mice. Thus, expression of mutant KATP channels in MCH neurons disrupts glucose sensing. To assess for effects of mutant KATP channels on signaling processes thought to be independent of KATP channels, we evaluated the effects of orexin and glutamate on MCH neurons. As previously reported (van den Pol et al., 2004), orexin depolarized MCH neurons from control mice, and this effect was unaltered in MCH neurons from Mch-Cre/mutKir6.2 mice (Figure S2). Likewise, glutamate similarly excited MCH neurons from both control and Mch-Cre/mutKir6.2 mice (Data not shown). Taken together, the above findings provide direct evidence that MCH neurons sense glucose via a KATP channel-dependant pathway.
Glucose Sensing by MCH Neurons Regulates Peripheral Glucose Homeostasis
We next investigated the effect of defective glucose sensing in MCH neurons on whole body glucose homeostasis. By comparing Mch-Cre/mutKir6.2 mice (mutKir6.2) with their wildtype littermates (WT) (8-week-old males), we observed no difference in body weight (WT: 26.94 ± 0.47 g, n=15; mutKir6.2: 26.46 ± 0.61g, n=16; mean ± SEM), daily food intake (WT: 6.17 ± 0.29 g chow/day, n=12; mutKir6.2 mice: 6.95 ± 1.23 g chow/day, n=8; mean ± SEM), ad lib fed blood glucose levels (WT: 193.3 ± 9.1 mg/dl, n=11; mutKir6.2: 196.1 ± 8.1 mg/ml, n=11; mean ± SEM), or overnight fasted blood glucose levels (WT: 110.0 ± 7.6 mg/dl, n=7; mutKir6.2 mice: 113.9 ± 7.7 mg/dl, n=8; mean ± SEM). To examine the dynamic response of Mch-Cre/mutKir6.2 mice to an acute increase in blood glucose concentration, glucose tolerance tests were performed on 8-week-old wildtype and Mch-Cre/mutKir6.2 mice (Figure 2E). Following the exogenous glucose load, Mch-Cre/mutKir6.2 mice displayed increased excursions in blood glucose (i.e. impaired glucose tolerance). These effects were not due to unexpected expression of mutant Kir6.2 in pancreatic β-cells (see Figure S3 for details), and were not due to nonspecific effects of the Mch-Cre transgene as no effect was seen in Mch-Cre mice lacking the mutKir6.2 allele (data not shown). Thus, glucose sensing by MCH neurons plays an important role in maintaining peripheral glucose homeostasis.
MCH Neurons Express UCP2
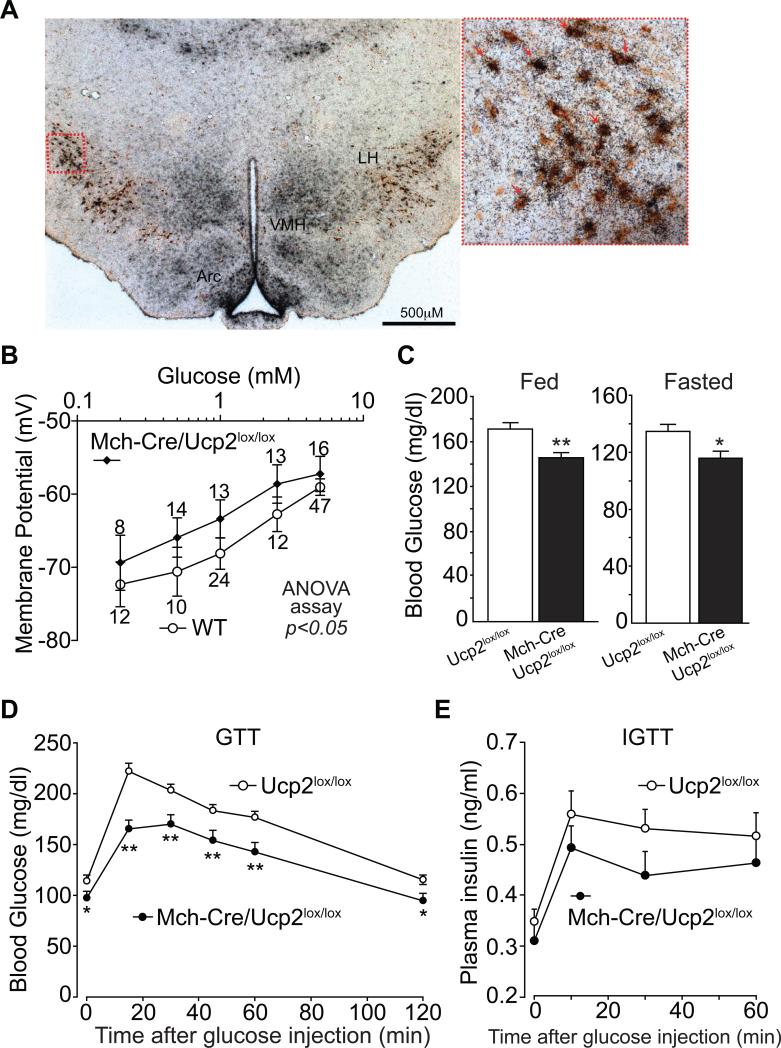
In pancreatic β-cells and POMC neurons, glucose sensing is negatively regulated by UCP2 (Parton et al., 2007; Zhang et al., 2001). As Ucp2 mRNA is abundantly expressed in the LH (Parton et al., 2007; Richard et al., 1999), we hypothesized that UCP2 might negatively regulate glucose sensing in MCH neurons as well. Double in situ hybridization/immunohistochemistry analyses for Ucp2 mRNA and MCH peptide demonstrate that ~95% of MCH neurons in LH and ZI express abundant Ucp2 mRNA (Figure 3A). Thus, like pancreatic β-cells (Zhang et al., 2001) and POMC neurons (Parton et al., 2007), glucose-excited MCH neurons co-express Sur1-containing KATP channels (Figure S1E) and Ucp2 (Figure 3A).
Figure 3. Deleting UCP2 in MCH neurons augments glucose sensing.
(A) Double immunohistochemistry / in situ hybridization for MCH peptide (DAB, brown stain) and Ucp2 mRNA (35S-labelled cRNA probe, silver grains) in wildtype mice. (B) Effects of UCP2 deletion on glucose sensing by MCH neurons. Dose response curve of glucose-stimulated depolarization of GFP-positive neurons from WT (Mch-Cre/Z/EG) and Mch-Cre/Ucp2lox/lox/Z/EG mice (mean ± SEM values above each point represent the number of neurons assessed, two-way ANOVA test, pinteraction=0.95, pcolume=0.02, prow=0.001). (C) Blood glucose levels of ad lib fed and overnight fasted, 8-week old male mice (mean ± SEM, n=8-10 per genotype). Asterisk, P<0.05; Two asterisks, P<0.01, compared with wildtype littermates (unpaired t Tests). (D) Representative glucose tolerance curves of 8-week-old male mice (mean ± SEM, n=8-10 per genotype, 2 g/Kg glucose i.p.). Asterisk, P<0.05; Two asterisks, P<0.01, compared with wildtype at a given time point (unpaired t Tests). (E) Insulin levels during glucose tolerance test. (mean ± SEM, n=8-10 per genotype, 2 g/Kg glucose i.p.).
Glucose Sensing in MCH Neurons Is Regulated by UCP2
Mice were generated with lox sites flanking exons 2 (start codon) and 3 of the Ucp2 gene (see Figure S4A-D for details and validation of the Ucp2lox allele). Ucp2lox/lox mice were then crossed with Mch-Cre mice to generate Mch-Cre/Ucp2lox/lox animals. The Z/EG GFP reporter transgene was also present for visualization of MCH neurons. Electrophysiologic studies revealed that, in the case of MCH neurons lacking UCP2, ~44% of MCH neurons (7/16) fired spontaneously at 5mM glucose. This is an increase over the 30% of control neurons (11/39) that fired spontaneously (as previously noted). When membrane potential was examined in response to different concentrations of glucose (Figure 3B), we found that MCH neurons from Mch-Cre/Ucp2lox/lox mice exhibited a parallel but significantly left-shifted dose-response curve to glucose, indicating that disruption of UCP2 in MCH neurons enhances glucose-induced depolarization.
We next assessed the physiologic consequences of deleting UCP2 from MCH neurons. Although Mch-Cre/Ucp2lox/lox mice showed no difference from control littermates (8-week-old males) in either body weight (Ucp2lox/lox: 28.10 ± 0.69 g, n=12; Mch-Cre/Ucp2lox/lox: 28.79 ± 1.04 g, n=8; mean ± SEM) or food intake (Ucp2lox/lox: 4.40 ± 0.11 g chow/day, n=10; Mch-Cre/Ucp2lox/lox: 4.61 ± 0.15 g chow/day, n=7; mean ± SEM), ad lib fed and overnight fasted blood glucose levels were significantly reduced (Figure 3C). In glucose tolerance tests, Mch-Cre/Ucp2lox/lox mice had a markedly reduced rise in blood glucose levels (Figure 3D). This effect is not due to deletion of UCP2 from pancreatic β-cells because Mch-Cre mice do not express Cre activity in pancreatic islets (as noted earlier), and in agreement with this, Ucp2 mRNA levels were unaltered in islets of Mch-Cre/Ucp2lox/lox mice (Figure S4E). Finally, we assessed insulin levels during the glucose tolerance test (Figure 3E) and found that they were not increased (but instead, tended to be decreased) in Mch-Cre/Ucp2lox/lox mice. This indicates that alterations in insulin secretion by β-cells, mediated by either direct (i.e. via unexpected consequences in the β-cell) or indirect pathways (i.e. via the brain), are not driving the fall in glucose levels – instead β-cells are responding secondarily to the lower glucose levels. This suggests that MCH neurons affect blood glucose levels by controlling glucose production by the liver, and/or by increasing glucose uptake into muscle and/or fat.
Mutant KATP Channels Block the Effects of UCP2-deficiency
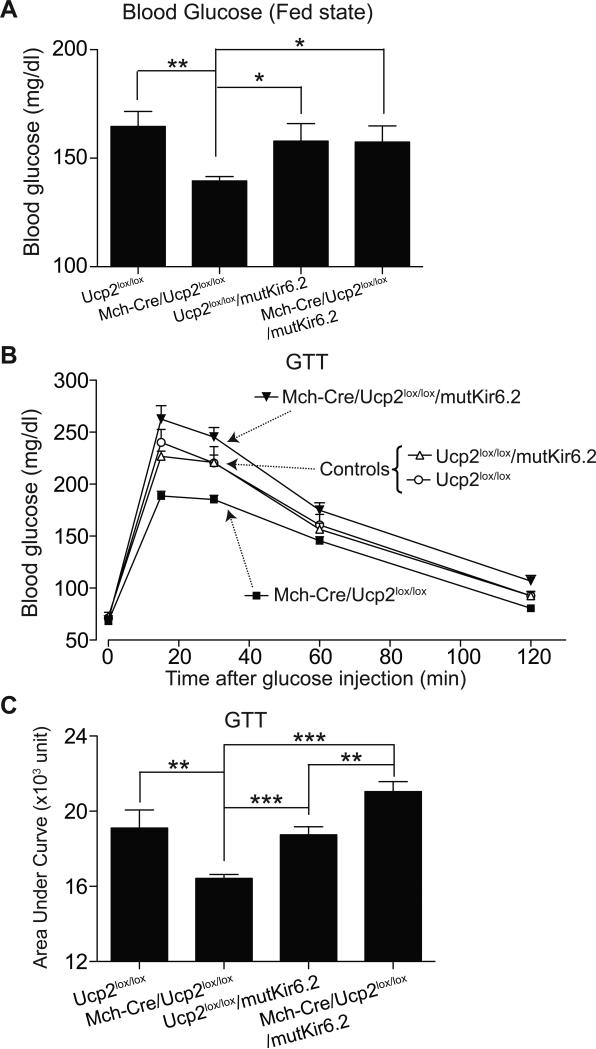
We hypothesize that UCP2 deficiency increases glucose-mediated excitation of MCH neurons (with the subsequent effects on peripheral glucose homeostasis) by increasing ATP within MCH neurons, which then closes KATP channels. However, it is also possible that UCP2 deficiency could produce effects via other signaling pathways. If ATP-mediated closure of KATP channels is involved (as we hypothesize), then expression of mutant Kir6.2 (which renders KATP channels resistant to closure by ATP) should completely suppress the UCP2-deficient phenotype. To evaluate this, we generated Mch-Cre/Ucp2lox/lox/mutKir6.2 mice. This was accomplished by crossing Mch-Cre/Ucp2lox/lox mice with Ucp2lox/lox/mutKir6.2 mice to produce the four groups of littermate study subjects shown in Figure 4. As observed before, Mch-Cre/Ucp2lox/lox mice (lacking UCP2 in MCH neurons) had reduced blood glucose levels in the fed state (Figure 4A) and a reduced rise in blood glucose during the glucose tolerance test (Figure 4B and 4C). Of note, in Mch-Cre/Ucp2lox/lox/mutKir6.2 mice, expression of mutant Kir6.2 completely prevented the glucose-lowering effects of UCP2 deficiency. In fact, blood glucose levels during the glucose tolerance test were increased above that seen in control mice, which is what we observed in Mch-Cre/mutKir6.2 mice (Figure 2E). In the presence of mutant Kir6.2, UCP2 deficiency was without effect. These results are consistent with the view that ATP-mediated regulation of KATP channels in MCH neurons is downstream, and hence the mechanism for, UCP2-mediated effects on glucose homeostasis.
Figure 4. Mutant KATP Channels Block the Effects of UCP2-deficiency in MCH Neurons.
(A) Blood glucose levels of ad lib fed 8-week-old male mice (mean ± SEM, n=7-8 per genotype). Asterisk, P<0.05; Two asterisks, P<0.01, compared with littermates (one-way ANOVA assay). (B) Representative glucose tolerance curves of 8-week-old male mice (mean ± SEM, n=7-8 per genotype, 2 g/kg glucose i.p.). (C) GTT data from (B) expressed as Area Under the Curve. Asterisk, P<0.05; Two asterisks, P<0.01, Three asterisks, P<0.001, compared with littermates (one-way ANOVA assay).
DISCUSSION
Diverse subsets of neurons in the brain, including POMC neurons in the arcuate nucleus, MCH neurons in the lateral hypothalamus, and chemically undefined neurons in the VMH and hindbrain, are excited by glucose (Balfour et al., 2006; Burdakov et al., 2005; Ibrahim et al., 2003; Levin et al., 2004; Parton et al., 2007; Ritter et al., 1981; Song and Routh, 2005). This is of interest because it provides a means by which the brain can sense and subsequently respond to increased blood glucose levels. In support of an important role for this process, we have previously disrupted glucose sensing in POMC neurons and found that this impaired peripheral glucose homeostasis (Parton et al., 2007). Left unresolved, however, is whether POMC neurons uniquely perform this function, or, if other glucose-excited neurons, one example being MCH neurons, along with POMC neurons comprise a network of glucose-sensing, peripheral blood glucose-regulating neurons.
In the present study, we genetically altered glucose excitation of MCH neurons - we decreased it by expressing ATP-resistant KATP channels and increased it by deleting UCP2 (which is expected to increase glucose-stimulated ATP production). These studies show 1) that glucose sensing in MCH neurons operates via ATP-mediated closure of Sur1-containing KATP channels, 2) that this process is negatively regulated by UCP2 (likely via an ATP-KATP channel-based mechanism), and 3) that glucose sensing in MCH neurons (either decreased or increased) impacts meaningfully on peripheral glucose homeostasis. Thus, MCH neurons, like POMC neurons, regulate glucose homeostasis. Combined, glucose-excited neurons (MCH neurons, POMC neurons, and possibly other glucose-excited neurons) are likely to play key roles in maintaining the euglycemic state.
EXPERIMENTAL PROCEDURES
Animal Care
Care of all animals and procedures were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Mice were housed at 220C-240C using a 12 hour light/12 hour dark cycle with standard mouse chow (Teklad F6 Rodent Diet 8664, 4.05 kcal/g, 3.3 kcal/g metabolizable energy, 12.5% kcal from fat, Harlan Teklad, Madison, WI) and water provided ad libitum.
Generation of Mch-Cre BAC Transgenic Mice
A mouse BAC genomic clone containing the Mch gene along with ~64 kb of 5’ and ~34 kb of 3’ flanking sequence was used to generate MCH-Cre mice as previously reported (Dhillon et al., 2006). Please see Supplemental Experimental Procedures for greater details.
Generation of Mch-Cre/mutKir6.2 Mice
Mch-Cre mice were mated with Rosa26-Kir6.2[K185Q,ΔN30] mice (Remedi et al., 2009) and only littermates were used as study subjects. Please see Supplemental Experimental Procedures for genotyping information.
Generation of Mch-Cre/Ucp2lox/lox Mice
Mch-Cre transgenic mice were mated with Ucp2lox/+ mice (129, C57Bl/6 mixed background) and a breeding colony was maintained by mating Mch-Cre/Ucp2lox/lox mice with Ucp2lox/lox mice. Littermates were used as study subjects to avoid any potential confounding effects due to differences in genetic backgrounds. Please see Supplemental Experimental Procedures for greater details in the generation of lox-Ucp2 allele and genotyping information.
Double Immunohistochemistry/in situ hybridization for MCH peptide/Ucp2
Ucp2 in situ hybridization was performed as described previously (Parton et al., 2007). For double Ucp2 in situ hybridization and MCH immunohistochemistry, sections processed for in situ hybridization were incubated with chicken anti-MCH primary antiserum (Santa Cruz), followed by a 2-h incubation in biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories; 1:1,000). Sections were incubated with avidin–biotin complex (Vectastain Elite ABC Kit, Vector Laboratories; 1:500) and then incubated in 0.04% diaminobenzidine tetrahydrochloride (DAB; Sigma) and 0.01% hydrogen peroxide in PBS. The DAB reaction was quenched by two washes with PBS.
Glucose Homeostasis Studies
ad lib fed-state blood glucose levels were measured between 9 am and 10 am. Fasted blood glucose levels were measured after an overnight fast (food removed at 5 pm, blood sampled between 9 am and 10 am the next day). For glucose tolerance studies, animals fasted overnight (~16 hours) were administered i.p. with 2 g/kg of glucose. Blood was sampled from the tail vein and glucose levels were measured with a glucometer (Roche Diagnostics) at 15, 30, 60, and 120 minutes. For assessment of insulin levels during the GTT, blood samples were collected at above time points and serum insulin levels were assayed using a commercially available kit (Rat Insulin ELISA kit, Crystal Chem Inc. Chicago, IL).
Electrophysiology
Primary hypothalamic cultures and inside-out excised patch recordings
Lateral hypothalamic nuclei were microdissected, minced, and mechanically dissociated using the Papain Dissociation System (Worthington, Lakewood, NJ). Cells were isolated and seeded onto Thermanox glass coverslips (Nalge Nunc International, Rochester, NY) in Neurobasal A medium (Invitrogen) supplemented with 1X B27 (Invitrogen), 0.4 mM L-glutamine, and 1% fetal calf serum. For the initial plating, 25 uM glutamic acid was added. The media was changed every 3-5 days and excised patch recordings were made on GFP-expressing neurons after 7-10 days of culture. For the KATP channel recordings, both the pipette and internal bath solution contained 140 mM K+. Bath solutions with varying Mg++-ATP concentrations were perfused onto the cytoplasmic side of the membrane patches during recording. All macroscopic currents were recorded at a membrane potential of -60 mV.
Slice preparation and whole-cell recordings
Brain slices were prepared from young adult mice (5-7 weeks old) as described previously (Burdakov et al., 2005; Parton et al., 2007). GFP-positive neurons were visualized using epifluorescence imaging and patched under infrared differential interference contrast (IR-DIC) optics. Recordings were made using a MultiClamp 700B amplifier using pClamp 9.0 software (Axon Instruments). To further confirm if a recorded neuron was indeed a MCH neuron, we examined the following electrophysiological properties of MCH neurons: exhibition of spike rate adaptation and lack of a H-current mediated voltage change as previously described (van den Pol et al., 2004).
Bath perfusions were used for changing glucose concentrations and for the addition of 250uM tolbutamide during recording. For aCSF solution containing varying glucose level, the osmolarity was adjusted to the same level by adding sucrose. Recording electrodes had resistances of 2.5-4 MΩ when filled with the internal solution (128 mM K-gluconate, 10 mM HEPES, 1 mM EGTA, 10 mM KCL, 1 mM MgCl2, 0.3 mM CaCl2, 2 mM Na-ATP, 0.3 mM Na-GTP (pH 7.35 with KOH)). Since the majority of MCH neurons are electrically silent, under current clamp, depolarizing current pulses (20-30 pA, for 3 sec, with 20 sec interval) were used to elicit action potentials for those silent neurons.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge members of Lowell lab and Dr. Hu Huang for helpful discussions. This work was supported by the National Institute of Health / the National Institute of Diabetes and Digestive and Kidney Diseases (DK053477 to BBL; DK078478 to LV), and American Diabetes Association (7-07-MN-38 to BBL/DK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental information includes four figures and Supplemental Experimental Procedures.
REFERENCES
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–483. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Lesage F. Glucose-induced inhibition: how many ionic mechanisms? Acta Physiol (Oxf) 2009;195:71–78. doi: 10.1111/j.1748-1716.2009.02005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Elias CF, Saper CB, Maratos-Flier E, Tritos NA, Lee C, Kelly J, Tatro JB, Hoffman GE, Ollmann MM, Barsh GS, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement J.P.t., Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Kang L, Routh VH, Kuzhikandathil EV, Gaspers LD, Levin BE. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes. 2004;53:549–559. doi: 10.2337/diabetes.53.3.549. [DOI] [PubMed] [Google Scholar]
- Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell. 2000;100:645–654. doi: 10.1016/s0092-8674(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–512. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50:168–177. doi: 10.1007/s00125-006-0473-3. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, Tong A, Bruning JC, Koster JC, Nichols CG. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard D, Huang Q, Sanchis D, Ricquier D. Brain distribution of UCP2 mRNA: in situ hybridization histochemistry studies. Int J Obes Relat Metab Disord. 1999;23(Suppl 6):S53–55. doi: 10.1038/sj.ijo.0800947. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Rother E, Konner AC, Bruning JC. Neurocircuits integrating hormone and nutrient signaling in control of glucose metabolism. Am J Physiol Endocrinol Metab. 2008;294:E810–816. doi: 10.1152/ajpendo.00685.2007. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov. 2009;8:386–398. doi: 10.1038/nrd2874. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. J Neurophysiol. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- Song Z, Routh VH. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Acuna-Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42:635–652. doi: 10.1016/s0896-6273(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–755. doi: 10.1016/s0092-8674(01)00378-6. [DOI] [PubMed] [Google Scholar]
- Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, Porco JA, Jr., Lowell BB. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.