Abstract
The authors examined the extent to which socioeconomic position, behavior-related factors, cardiovascular risk factors, inflammatory markers, and chronic diseases explain the association between poor lung function and mortality in 4,817 participants (68.9% men) from the Whitehall II Study aged 60.8 years (standard deviation, 5.9), on average. Forced expiratory volume in 1 second (FEV1) was used to measure lung function in 2002–2004. A total of 139 participants died during a mean follow-up period of 6.4 years (standard deviation, 0.8). In a model adjusted for age and sex, being in the lowest tertile of FEV1/height2 was associated with a 1.92-fold (95% confidence interval: 1.35, 2.73) increased risk of mortality compared with being in the top 2 tertiles. Once age, sex, and smoking history were taken into account, the most important explanatory factors for this association were inflammatory markers (21.3% reduction in the FEV1/height2-mortality association), coronary heart disease, stroke, and diabetes (11.7% reduction), and alcohol consumption, diet, physical activity, and body mass index (9.8% reduction). The contribution of socioeconomic position and cardiovascular risk factors was small (≤3.5% reduction). Taken together, these factors explained 32.5% of the association. Multiple pathways link lung function to mortality; these results show inflammatory markers to be particularly important.
Keywords: forced expiratory volume, inflammation, middle aged, mortality, respiratory function tests
Poor lung function, often characterized by low forced expiratory volume in 1 second (FEV1), is a well-established predictor of mortality, with early work in this domain dating from 1970 (1). This association is seen across the continuum of the FEV1 distribution (2–5) and over short and long follow-up periods (2–7), with the longest duration of follow-up being over 40 years (2, 8). Several mechanisms have been proposed to explain these associations (5, 9–11), including smoking, age-related chronic conditions, and inflammation. However, the relative importance of different mechanisms remains unclear, as few investigators have been able to use a comprehensive range of explanatory risk factors within a single methodological setup. Therefore, our objective in the present study was to examine the extent to which socioeconomic factors, health behaviors, cardiovascular risk factors and diseases, and inflammatory markers explain the association between lung function and all-cause mortality.
MATERIALS AND METHODS
Study population
Data were drawn from the Whitehall II Study, which was established in 1985 as a longitudinal study of 10,308 United Kingdom civil servants (6,895 men and 3,413 women) (12). All civil servants aged 35–55 years in 20 London-based departments were invited to participate by letter; 73% agreed. The baseline examination (phase 1) took place during 1985–1988 and involved a clinical examination and a self-administered questionnaire including sections on lifestyle factors. Subsequent phases of data collection have alternated between a postal questionnaire alone (phases 2 (1988–1990), 4 (1995–1996), 6 (2001), and 8 (2006)) and a postal questionnaire accompanied by a clinical examination (phases 3 (1991–1993), 5 (1997–1999), 7 (2002–2004), and 9 (2007–2009)).
Participants gave written consent to participate in the study, and the University College London ethics committee approved the protocol. Measures of lung function were introduced to the study in 2002–2004 (phase 7), when the mean age of participants was 60.8 years (standard deviation, 5.9; range, 50.5–73.7).
Lung function
Lung function was measured using a portable flow spirometer (MicroPlus Spirometer; Micro Medical Ltd., Kent, United Kingdom) administered by a trained nurse. Participants with the following conditions were allowed to opt out of lung function tests: persons who had recently been coughing up blood of unknown origin, had ever had a pneumothorax, had experienced severe angina requiring hospitalization in the previous 6 months, had ever had a heart attack or stroke, had ever had a pulmonary embolism or an aneurysm, had recently undergone ear or eye surgery, had recently undergone stomach or chest surgery, had ever suffered a perforated eardrum or hernia, or had blood pressure higher than 180/96 mm Hg on the test day. Thus, 4,832 (74.5%) of the 6,483 participants who came in for the clinical examination between 2002 and 2004 underwent spirometry. We assessed forced vital capacity (FVC) and FEV1. FVC measures the volume of air that can forcibly be blown out after full inspiration, measured in liters. FEV1 measures the volume of air expelled in the first second during the FVC maneuver, again measured in liters (13). The largest FVC and FEV1 values from 3 maneuvers (pairwise correlations were 0.91–0.93 for the 3 measures of FEV1 and 0.94–0.96 for FVC) were used in the analysis.
Mortality
A total of 10,297 respondents (99.9%) were successfully traced for mortality through the national mortality register kept by the National Health Service Central Register using the National Health Service identification number assigned to each British citizen. In our analysis, mortality follow-up began at the measurement of lung function during the medical examination (2002–2004, phase 7) and ended on January 31, 2010.
Covariates
Sociodemographic variables used were age, sex, and socioeconomic position (6-level civil service employment grade). Employment grade in the Whitehall II Study is a comprehensive marker of socioeconomic circumstances and is related to salary, social status, and level of responsibility (12).
Data on health behaviors were drawn from questionnaires in phases 1, 3, 5, and 7. Smoking history was assessed using questions on smoking status and current amount of tobacco smoked and was categorized as “current smoker in phase 7,” “recent ex-smoker” (cessation of smoking between phases 1 and 7), “long-term ex-smoker” (cessation of smoking before phase 1), and “never smoker.” The “current smoker” category was further divided into 3 groups according to the number of cigarettes smoked per day (<10, 10–20, or >20). Alcohol consumption was assessed via questions on the number of alcoholic drinks (“measures” of spirits, “glasses” of wine, and “pints” of beer) consumed in the last 7 days. This was converted to number of units (1 unit = 8 g) of alcohol. Frequency of fruit and vegetable consumption was assessed on an 8-point scale, ranging from “seldom or never” to “2 or more times a day.” Hours of moderate and vigorous physical activity per week were calculated from a question on the number of hours spent in physical activity at different levels (asked in phases 1 and 3) and from a 20-item questionnaire on the frequency and duration of participation in walking, cycling, sports, gardening, housework, and home maintenance (administered in phases 5 and 7) (14). Summary measures of these health behaviors over the 4 study phases were used in the present analysis. Smoking history was used as defined above, and for the other health behavior measurements, mean values over the 4 study phases were used.
Body mass index in phase 7 was calculated as weight (kg) divided by height squared (m2). Weight was measured in underwear to the nearest 0.1 kg using an electronic Soehnle scale with a digital readout (Leifheit AS, Nassau, Germany). Height was measured in bare feet to the nearest millimeter using a stadiometer, while the participant stood completely erect with the head in the Frankfort plane. Body mass index was categorized as <20, 20–24.9, 25–29.9, and ≥30.
Cardiovascular risk factors included in the analysis were serum cholesterol, systolic blood pressure, and diastolic blood pressure and were measured during the clinical examination in phases 1, 3, 5, and 7. Blood pressure was measured twice with the participant sitting after a 5-minute rest, using a Hawksley random-zero sphygmomanometer (Hawksley & Sons Ltd., Lancing, United Kingdom). The average of 2 readings was taken to be the measured blood pressure. Serum cholesterol was measured within 72 hours in serum stored at 4°C, using enzymatic colorimetric methods. As for the health behaviors, the mean of these measures over the 4 phases of data collection (phases 1, 3, 5, and 7) was used to reflect history of these risk factors in the analysis.
Chronic conditions included the prevalence of coronary heart disease, stroke, and diabetes. Coronary heart disease prevalence was based on clinically verified events and included myocardial infarction and definite angina (15). Stroke was assessed using a self-reported measure of physician diagnosis. Diabetes was defined by a fasting glucose concentration ≥7.0 mmol/L, a 2-hour postload glucose concentration ≥11.1 mmol/L, reported doctor-diagnosed diabetes, or use of diabetes medication (16).
Inflammatory markers included interleukin-6 and C-reactive protein, assessed in phases 3 and 7. Fasting serum was collected during the clinical examination and was stored at −70°C until analysis. Samples from both phases were analyzed at the same time. C-reactive protein was measured using a high-sensitivity immunonephelometric assay in a BN ProSpec nephelometer (Dade Behring, Milton Keynes, United Kingdom). Interleukin-6 was measured using a high-sensitivity enzyme-linked immunosorbent assay (R & D Systems, Oxford, United Kingdom) (17). Because the distributions of C-reactive protein and interleukin-6 were skewed, these data were log-transformed for the analysis. The mean of the phase 3 and phase 7 measures was used in the analysis.
Statistical analysis
FEV1 divided by height squared was used in the analysis because it adjusts the lung function measure for body size and has been shown to be a good predictor of mortality (4, 6). We also examined the associations with FVC/height2 and FEV1 percentage of the predicted value (FEV1% pred) and report these results briefly. Because the distribution of lung function values differed by sex, we used sex-specific tertiles of the lung function measures in the analyses.
We first assessed the differences in all covariates between persons who were alive and persons who had died by the end of the follow-up period, using t tests for continuous variables and chi-square tests for categorical variables. We also investigated the association between sex-specific tertiles of FEV1/height2 and covariates, using linear regression for continuous variables and logistic regression for dichotomous variables.
In order to examine the relation between lung function and mortality graphically over the follow-up period, we plotted unadjusted and fully adjusted survival curves for tertiles of FEV1/height2 (18). Subsequently, we used Cox regression with age as the time scale to estimate hazard ratios and 95% confidence intervals for the association between tertiles of lung function and mortality. The proportional hazards assumption for the Cox model was confirmed formally by means of Shoenfeld's tests.
Because no difference in risk was found between the 2 upper tertiles of FEV1/height2, the results show the hazard ratio for mortality associated with being in the lowest FEV1/height2 tertile as compared with the upper 2 tertiles of FEV1/height2. We first examined the extent to which this association was explained by smoking history. In order to do this, we added smoking history to the model including age and sex (model 1). The reduction in model 1 attributed to smoking history was calculated using the formula 100 × (βmodel 1 − βmodel 1+smoking history)/(βmodel 1). Subsequently, the extent to which this association was attenuated was examined by sequentially adding socioeconomic position, other health behaviors, body mass index, cardiovascular risk factors, chronic diseases, and inflammatory markers and then simultaneously adding all covariates to the model adjusted for age, sex, and smoking history (model 2). The reduction in model 2 attributed to the group of covariates under consideration was calculated using the formula 100 × (βmodel 2 − βmodel 2+covariates)/(βmodel 2). All analyses were repeated using FVC/height2 instead of FEV1/height2 as the measure of lung function. We also undertook analysis using FEV1% pred, computed on the basis of reference values obtained from Quanjer et al. (19).
The main analysis was performed using STATA 10 statistical software (StataCorp LP, College Station, Texas). Adjusted survival curves were calculated using SAS 9 software and the macro proposed by Cole et al. (18).
RESULTS
Sample description
A total of 4,817 participants with complete data on lung function and all covariates were included in the analysis; 139 died during a mean follow-up period of 6.4 years (standard deviation, 0.8). Compared with the 1,666 participants who came to the clinical examination in phase 7 (2002–2004) but did not participate in the spirometry test, the 4,817 persons on whom the analysis was based had a lower rate of all-cause mortality (2.9% vs. 6.1%; P < 0.001), particularly mortality from respiratory diseases (0.2% vs. 0.3%; P < 0.001), cardiovascular diseases (0.6% vs. 1.5%; P < 0.001), and cancer (1.4% vs. 2.1%; P < 0.001), but not lung cancer (3 cases in study participants vs. no cases in others). This group was also younger (60.8 vs. 61.9 years; P < 0.001) and had fewer current smokers (7.3% vs. 9.2%; P < 0.001) but showed no difference in self-reported history of asthma (9.9% vs. 10.0%; P = 0.96).
Table 1 presents characteristics of the study participants as a function of their vital status at the end of follow-up. Table 2 shows that there was a linear association between all of the covariates and the sex-specific tertiles of FEV1/height2 (P ≤ 0.005). Of the 4,817 participants in the present study, 721 (15.0%) had an FEV1:FVC ratio lower than 70%; their inclusion in the analysis did not change the results much, so all analyses were based on all 4,817 persons. There was no sex difference in the association between FEV1/height2 and mortality (P for interaction = 0.79), leading us to combine men and women in the analysis.
Table 1.
Characteristics of the Study Population as a Function of Vital Status at the End of Follow-Upa (n = 4,817), Whitehall II Study, United Kingdom, 2002–2010
| Alive at the End of Follow-Up |
Deceased at the End of Follow-Up |
P Value | |||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| No. and % of participants | 4,678 | 97.1 | 139 | 2.9 | |||
| Sociodemographic factors | |||||||
| Age, years | 60.7 (5.9) | 63.7 (6.1) | <0.001 | ||||
| Female sex | 1,448 | 30.9 | 51 | 36.7 | 0.15 | ||
| Low employment grade | 500 | 10.7 | 23 | 16.6 | 0.03 | ||
| Health behaviors | |||||||
| Current smoker | 354 | 7.6 | 19 | 13.7 | 0.008 | ||
| Units of alcohol per week | 11.4 (11.4) | 13.1 (16.0) | 0.10 | ||||
| Frequency of fruit and vegetable consumption per week | 8.1 (3.3) | 7.8 (3.5) | 0.24 | ||||
| Hours of moderate and vigorous physical activity per week | 3.7 (2.7) | 3.0 (2.6) | 0.003 | ||||
| Body mass indexb | 26.5 (4.3) | 28.3 (5.3) | <0.001 | ||||
| Cardiovascular risk factors | |||||||
| Systolic blood pressure, mm Hg | 122.6 (12.0) | 125.6 (12.3) | 0.004 | ||||
| Diastolic blood pressure, mm Hg | 76.3 (7.7) | 77.5 (8.2) | 0.07 | ||||
| Serum total cholesterol, mmol/L | 6.0 (0.9) | 6.0 (0.9) | 0.94 | ||||
| Chronic diseases | |||||||
| Diabetes mellitus | 406 | 8.7 | 26 | 18.7 | <0.001 | ||
| Coronary heart disease | 298 | 6.4 | 19 | 13.7 | 0.001 | ||
| Stroke | 61 | 1.3 | 6 | 4.3 | 0.003 | ||
| Inflammatory markers | |||||||
| C-reactive protein (log-transformed) | 0.04 (1.00) | 0.49 (1.08) | <0.001 | ||||
| Interleukin-6 (log-transformed) | 0.50 (0.54) | 0.80 (0.61) | <0.001 | ||||
| Lung function | |||||||
| FEV1, L | 2.9 (0.8) | 2.5 (0.8) | <0.001 | ||||
| FEV1/height2, L/m2 | 1.0 (0.2) | 0.9 (0.2) | <0.001 | ||||
| FVC, L | 3.9 (1.0) | 3.4 (0.9) | <0.001 | ||||
| FVC/height2, L/m2 | 1.3 (0.3) | 1.2 (0.3) | <0.001 | ||||
Abbreviations: FEV1, forced expiratory volume; FVC, forced vital capacity; SD, standard deviation.
The end of follow-up was defined as January 31, 2010, or the date of censoring, whichever occurred first.
Weight (kg)/height (m)2.
Table 2.
Characteristics of the Study Population by Tertile of FEV1/Height2 (n = 4,817), Whitehall II Study, United Kingdom, 2002–2010
| Sex-Specific Tertile of FEV1/Height2 |
P for Trenda |
|||||||||
| Highest |
Intermediate |
Lowest |
||||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| No. and % of participants | 1,605 | 33.3 | 1,606 | 33.3 | 1,606 | 33.3 | ||||
| Sociodemographic factors | ||||||||||
| Age, years | 58.2 (4.9) | 60.8 (5.7) | 63.3 (5.9) | <0.001 | ||||||
| Female sex | 498 | 31.0 | 479 | 29.8 | 522 | 32.5 | 0.37 | |||
| Low employment grade | 107 | 6.7 | 155 | 9.7 | 261 | 16.3 | <0.001 | |||
| Health behaviors | ||||||||||
| Smoking historyb | <0.001 | |||||||||
| Current smoker | 80 | 5.0 | 128 | 8.0 | 165 | 10.3 | ||||
| Recent ex-smoker | 171 | 10.7 | 184 | 11.5 | 180 | 11.2 | ||||
| Long-term ex-smoker | 521 | 32.5 | 510 | 31.8 | 456 | 28.4 | ||||
| Never smoker | 833 | 51.9 | 784 | 48.8 | 805 | 50.1 | ||||
| Units of alcohol per week | 12.0 (11.4) | 11.8 (11.6) | 10.6 (11.8) | <0.001 | ||||||
| Frequency of fruit and vegetable consumption per week | 8.4 (3.3) | 8.2 (3.3) | 7.7 (3.2) | <0.001 | ||||||
| Hours of moderate and vigorous physical activity per week | 3.8 (2.7) | 3.7 (2.6) | 3.4 (2.7) | <0.001 | ||||||
| Body mass indexc | <0.001 | |||||||||
| <20 | 52 | 3.2 | 45 | 2.8 | 46 | 2.9 | ||||
| 20.0–24.9 | 614 | 38.3 | 539 | 33.6 | 523 | 32.6 | ||||
| 25–29.9 | 715 | 44.6 | 715 | 44.5 | 727 | 45.3 | ||||
| ≥30 | 224 | 14.0 | 307 | 19.1 | 310 | 19.3 | ||||
| Cardiovascular risk factors, mm Hg | ||||||||||
| Systolic blood pressure | 120.6 (11.2) | 122.8 (12.0) | 124.7 (12.4) | <0.001 | ||||||
| Diastolic blood pressure | 75.3 (7.7) | 76.5 (7.7) | 77.2 (7.6) | <0.001 | ||||||
| Serum total cholesterol, mmol/L | 5.9 (0.9) | 6.0 (0.9) | 6.0 (0.9) | <0.001 | ||||||
| Chronic diseases | ||||||||||
| Diabetes mellitus | 88 | 5.5 | 129 | 8.0 | 215 | 13.4 | <0.001 | |||
| Coronary heart disease | 50 | 3.1 | 97 | 6.0 | 170 | 10.6 | <0.001 | |||
| Stroke | 12 | 0.8 | 24 | 1.5 | 31 | 1.9 | 0.005 | |||
| Inflammatory markers | ||||||||||
| C-reactive protein (log-transformed) | −0.21 (0.99) | 0.06 (0.96) | 0.30 (1.00) | <0.001 | ||||||
| Interleukin-6 (log-transformed) | 0.36 (0.48) | 0.51 (0.52) | 0.67 (0.57) | <0.001 | ||||||
| No. and % of deaths | 28 | 1.7 | 33 | 2.1 | 78 | 4.9 | <0.001 | |||
Abbreviations: FEV1, forced expiratory volume in 1 second; SD, standard deviation.
Calculated from linear regression analysis for continuous variables and from logistic regression analysis for dichotomous variables.
“Recent ex-smokers” were defined participants who had stopped smoking between phases 1 (1988–1990) and 7 (2002–2004) and “long-term ex-smokers” as participants who had stopped smoking before phase 1 (1988–1990).
Weight (kg)/height (m)2.
Association between lung function and mortality
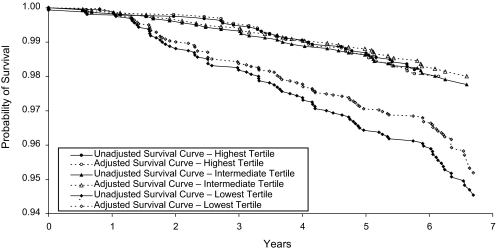
In analysis adjusted for age and sex, compared with the top tertile of FEV1/height2, there was no higher risk of mortality among persons in the second tertile (hazard ratio (HR) = 0.96, 95% confidence interval (CI): 0.57, 1.60), whereas participants in the lowest tertile showed higher risk (HR = 1.88, 95% CI: 1.19, 2.97). As a result, we combined the top 2 tertiles in subsequent analyses. Figure 1 shows unadjusted and fully adjusted survival curves using sex-specific tertiles of FEV1/height2. Table 3 shows the numbers of participants at risk of mortality during the follow-up period. As is clear from the figure, a difference in the probability of survival between these 2 groups started to appear after approximately 18 months of follow-up, and it persisted over the rest of the follow-up period. The dotted line in the figure shows the attenuation in the association with the addition of covariates to the model.
Figure 1.
Unadjusted and adjusted survival by tertile of lung function (n = 4,817), Whitehall II Study, United Kingdom, 2002–2010. See Table 3 for numbers of participants at risk of mortality.
Table 3.
Numbers of Participants at Risk of Mortality During the Follow-Up Period (n = 4,817), Whitehall II Study, United Kingdom, 2002–2010
| Sex-Specific Tertile of FEV1/Height2 | Follow-up Year |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Highest | 1,605 | 1,596 | 1,591 | 1,586 | 1,579 | 1,571 | 1,166 |
| Intermediate | 1,606 | 1,603 | 1,598 | 1,593 | 1,586 | 1,581 | 1,199 |
| Lowest | 1,606 | 1,602 | 1,585 | 1,570 | 1,557 | 1,538 | 1,209 |
Abbreviation: FEV1, forced expiratory volume in 1 second.
Table 4 presents results of the adjustment for covariates in greater detail. In model 1, adjusted for age and sex, being in the lowest tertile of FEV1/height2 was associated with a 1.92-fold (95% CI: 1.35, 2.73) increased risk of mortality compared with being the top 2 tertiles. When smoking history was entered into the model (model 2), this association was reduced by 4.9% (HR = 1.86, 95% CI: 1.31, 2.66). The inclusion of socioeconomic position (model 3) and cardiovascular risk factors (model 5) in this model did not substantially change the association (percentage reduction ≤3.5%). When physical activity, diet, alcohol consumption, and body mass index were added to model 2 (model 4), the association was reduced by 9.8%. When these behaviors were introduced separately, physical activity produced the greatest attenuation (8.0% for physical activity and less than 4% for the other health behaviors and body mass index). The percentage reduction related to chronic diseases was 11.7%. Adding inflammatory markers to model 2 reduced the association between FEV1/height2 and mortality by 21.3%. All of these covariates taken together explained 32.5% of the association, in addition to the 4.9% explained by smoking history.
Table 4.
Hazard Ratio for Mortality According to Lung Function (n = 4,817), Whitehall II Study, United Kingdom, 2002–2010
| Model | Adjustment Factors | Hazard Ratioa | 95% Confidence Interval | P Value | % Reductionb |
| 1 | Age and sex | 1.92 | 1.35, 2.73 | <0.001 | |
| 2 | Model 1 factors + smoking historyc | 1.86 | 1.31, 2.66 | 0.001 | 4.9 |
| 3 | Model 2 factors + socioeconomic position | 1.84 | 1.29, 2.63 | 0.001 | 1.7 |
| 4 | Model 2 factors + other health behaviorsd + body mass index | 1.75 | 1.23, 2.50 | 0.002 | 9.8 |
| 5 | Model 2 factors + serum cholesterol and systolic and diastolic blood pressure | 1.82 | 1.27, 2.59 | 0.001 | 3.5 |
| 6 | Model 2 factors + chronic diseasese | 1.73 | 1.21, 2.48 | 0.003 | 11.7 |
| 7 | Model 2 factors + inflammatory markersf | 1.63 | 1.14, 2.33 | 0.008 | 21.3 |
| 8 | Fully adjusted | 1.52 | 1.05, 2.19 | 0.03 | 32.5 |
Abbreviation: FEV1, forced expiratory volume in 1 second.
Hazard ratio comparing participants in the lowest sex-specific tertile of FEV1/height2 with participants in the top 2 tertiles.
Percent reduction observed when comparing model 2 with model 1 and then comparing models 3–8 with model 2.
The variable for smoking history used smoking status measured from 1985/1988 to 2002/2004 and current amount of tobacco smoked in 2002/2004.
Alcohol consumption, physical activity, and fruit and vegetable consumption.
Diabetes, coronary heart disease, and self-reported stroke.
Interleukin-6 and C-reactive protein (log-transformed).
When we replaced FEV1/height2 by FVC/height2 as the measure of lung function, the hazard ratio for mortality for the lowest tertile as compared with the top 2 tertiles was 1.83 (95% CI: 1.29, 2.58) in the model adjusted for age and sex. All of the covariates taken together explained 52.3% of this association, in addition to the 3.7% explained by smoking history, with inflammatory markers leading to the greatest attenuation in the association (28.0%). The analysis using FEV1% pred (see Web Table 1, which is available on the Journal’s Web site (http://aje.oxfordjournals.org/)) showed being in the lowest tertile to be associated with a 1.55-fold (95% CI: 1.11, 2.17) higher risk of mortality than being in the top 2 tertiles. Here again, inflammation markers explained the greater part of the association (33.9%).
We also undertook analyses using cause-specific mortality, but because of small numbers of deaths, these analyses were restricted to cardiovascular (n = 35) and cancer (n = 68) mortality. Persons in the lowest FEV1/height2 tertile had greater risk of cardiovascular (HR = 1.90, 95% CI: 0.94, 3.85) and cancer (HR = 2.08, 95% CI: 1.25, 3.43) mortality, although the first association did not reach statistical significance at the conventional level. Inflammatory markers explained the greater part of the associations with cardiovascular mortality (33.5%, in addition to the 5.0% explained by smoking history) and cancer mortality (12.5%, in addition to the 10.9% explained by smoking history). All of the covariates together explained 70.1% of the association with cardiovascular mortality and 13.3% of the association with cancer mortality, in addition to smoking history.
Sensitivity analysis
We performed further analyses in several subgroups in order to ascertain the robustness of our findings. We repeated the analyses among never smokers (n = 2,422; Web Table 2), persons without a self-reported history of asthma (n = 4,336; Web Table 3), and persons with FEV1:FVC ratios of <70% (n = 721; Web Table 4) and ≥70% (n = 4,096; Web Table 4). In addition, analyses excluding participants in the lowest tertile of the fat-free-mass distribution (n = 2,842; Web Table 5) and separate analyses in persons with body mass indices of <30 (n = 3,976; Web Table 6) and ≥30 (n = 841; Web Table 6) yielded results largely similar to those of the main analysis. Further analyses excluding deaths from respiratory diseases (n = 2) and lung cancer (n = 1) in the first 2 years of follow-up (Web Table 7) also supported our main findings. Finally, we examined the role of the explanatory variables by excluding participants with a C-reactive protein level greater than 10 mg/L (Web Table 8), to rule out the influence of infection on the day of the examination (20). Here again, the results were similar to those reported for the full sample.
DISCUSSION
Data from this large British cohort showed that poor lung function, characterized by low FEV1/height2, was associated with higher risk of all-cause mortality in late midlife. Similar results were found for FVC/height2 and FEV1% pred. Socioeconomic position, health behaviors, body mass index, cardiovascular risk factors, cardiovascular diseases, and inflammatory markers were used to explain this association. Our results showed inflammatory markers, and to a lesser extent behavior-related factors and chronic diseases, to explain the greater part of the association between lung function and mortality. Taken together, these factors explained one-third (FEV1/height2) to one-half (FVC/height2 and FEV1% pred) of the associations.
It is well established that lung function predicts mortality (2, 4, 5, 21), even though the mechanisms underlying this association remain unclear (5, 9–11). Researchers have examined changes in this association after adjusting for the effects of smoking, cardiovascular risk factors, or diseases (4, 5, 11), but to our knowledge no study has attempted to quantify the impact of a wide range of different explanatory factors on the association. Lung function was once thought to reflect only smoking status, which was hypothesized to explain its association with mortality. However, several studies, including our study, have shown lung function to also be related to mortality in never smokers (3, 6, 11, 22, 23). Clearly, smoking is not the only explanation for this association. Physical activity (24–26) and a healthier diet (27–29) have also been found to be associated with better lung function and obesity with reduced lung function (30). Our results showed health behaviors and body mass index to explain approximately 10% of the association between lung function and mortality, with the effect being driven mainly by smoking and physical activity.
Other plausible explanatory factors include chronic comorbidity. This hypothesis is supported by the fact that patients with chronic obstructive pulmonary disease die mainly from causes other than respiratory disease (31), arguing for the “common cause” hypothesis. Aging is accompanied by an increase in multiple chronic diseases, resulting in high comorbidity at older ages, including chronic obstructive pulmonary disease (10). Thus, lung function might simply be a proxy for general health, explaining its association with mortality (5, 32). Another possibility is that impaired lung function allows excessive quantities of inhaled deleterious environmental agents to enter the body, which then leads to disease and death (33). In the present study, chronic diseases such as coronary heart disease, stroke, and diabetes explained 12% of the association between FEV1/height2 and mortality. However, our data did not allow us to distinguish between the 2 proposed pathways—1) lung function as a proxy for general health and 2) chronic diseases as mediating the association between lung function and mortality.
More recently, the inflammatory hypothesis has been proposed as a potential explanatory factor for the relation between lung function and other comorbid conditions leading to death (9, 10). Indeed, higher levels of inflammatory markers have been found among persons with impaired lung function (34, 35). However, it is still debated whether it is inflammation that leads to lung function deterioration or the inverse (10, 34, 36, 37). Inflammation is associated with several chronic conditions (10) that are risk factors for mortality, such as osteoporosis, diabetes, and atherosclerosis. Thus, there are some arguments in favor of a possible role of inflammatory markers in the association between lung function and mortality, and our results support this hypothesis. Nevertheless, the importance of inflammatory markers in this association may also be due to residual confounding by smoking, since higher levels of inflammation are found in smokers. Furthermore, it has been suggested that inflammation leads to muscle wasting (10, 38), which is itself linked to lower lung functioning (36, 39). Our analysis based on never smokers and excluding persons in the lowest tertile of the fat-free-mass distribution showed inflammation to continue to play a role in mediating the association between lung function and mortality.
Strengths and weaknesses
The primary strength of this study was the large number of factors investigated to explain the association between lung function and mortality. Indeed, data on health behaviors and cardiovascular risk factors were available over a 15-year period preceding the assessment of lung function. Moreover, to our knowledge, this was the first study to assess the role of inflammatory markers. A further strength of the study was the use of adjusted survival curves that allowed us to summarize the data by avoiding potential weaknesses of the multiplicative model (40).
Three limitations of this study must also be noted. First, although the sample covered a wide socioeconomic status range, with annual full-time salaries ranging from £4,995 (∼$7,900) to £150,000 (∼$237,400), data were obtained from white-collar civil servants and cannot be assumed to be representative of the general population. Second, the inflammatory markers considered in our study are general markers of inflammation, included in the Whitehall II Study to assess outcomes such as cardiovascular disease, and do not constitute ideal “lung function” markers. Third, our results must be interpreted with caution, since the study design did not allow us to rule out the possibility of reverse causation between lung function and other covariates. Finally, these results were based on the healthier Whitehall II participants—that is, those who were still alive and took part in the phase 7 screening and who reported no contraindicative conditions against participation in the lung function tests. Nevertheless, lower lung function still predicted mortality in this selected population, and our results are likely to have underestimated the true association.
Conclusions
To our knowledge, this study was the first to investigate several potential explanations underlying the well-established association between lung function and mortality. The main finding emphasizes the importance of inflammatory markers for this association. The other important explanatory factors were health behaviors and chronic diseases, such as coronary heart disease, stroke, and diabetes. These results show that multiple processes are likely to link lung function and mortality.
Supplementary Material
Acknowledgments
Author affiliations: Centre for Research in Epidemiology and Population Health, Unité 1018, National Institute of Health and Medical Research (INSERM), Villejuif, France (Séverine Sabia, Francine Kauffmann, Archana Singh-Manoux); Department of Epidemiology and Public Health, Faculty of Biomedical Sciences, University College London, London, United Kingdom (Martin Shipley, Mika Kivimaki, Archana Singh-Manoux); Unité 708, INSERM, Paris, France (Alexis Elbaz); Unité Mixte de Recherche en Santé 708, Université Pierre et Marie Curie–Paris 6, Paris, France (Alexis Elbaz); Unité Mixte de Recherche en Santé 1018, Université Paris-Sud 11, Villejuif, France (Francine Kauffmann); and Centre de Gérontologie, Hôpital Sainte-Périne, Paris, France (Archana Singh-Manoux).
Dr. Archana Singh-Manoux was supported by a European Young Investigator Award from the European Science Foundation; Dr. Mika Kivimaki was supported by the BUPA Foundation and the Academy of Finland; and Martin Shipley was supported by the British Heart Foundation. The Whitehall II Study has been supported by grants from the Medical Research Council, the British Heart Foundation, the United Kingdom Health and Safety Executive, the United Kingdom Department of Health, the US National Heart, Lung, and Blood Institute (grant R01HL036310), and the US National Institute on Aging (grants R01AG013196 and R01AG034454).
The authors thank all of the participating civil service departments and their welfare, personnel, and establishment officers; the United Kingdom Health and Safety Executive; the British Council of Civil Service Unions; and all members of the Whitehall II study team.
The funding bodies did not participate in the study design, analysis or interpretation of data, or manuscript preparation.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- HR
hazard ratio
References
- 1.Higgins MW, Keller JB. Predictors of mortality in the adult population of Tecumseh. Arch Environ Health. 1970;21(3):418–424. doi: 10.1080/00039896.1970.10667260. [DOI] [PubMed] [Google Scholar]
- 2.Gray L, Hart CL, Smith GD, et al. What is the predictive value of established risk factors for total and cardiovascular disease mortality when measured before middle age? Pooled analyses of 2 prospective cohort studies from Scotland. Eur J Cardiovasc Prev Rehabil. 2010;17(1):106–112. doi: 10.1097/HJR.0b013e3283348ed9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hole DJ, Watt GC, Davey-Smith G, et al. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi: 10.1136/bmj.313.7059.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MR, Pedersen OF, Lange P, et al. Improved survival prediction from lung function data in a large population sample. Respir Med. 2009;103(3):442–448. doi: 10.1016/j.rmed.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Schünemann HJ, Dorn J, Grant BJ, et al. Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest. 2000;118(3):656–664. doi: 10.1378/chest.118.3.656. [DOI] [PubMed] [Google Scholar]
- 6.Chinn S, Gislason T, Aspelund T, et al. Optimum expression of adult lung function based on all-cause mortality: results from the Reykjavik Study. Respir Med. 2007;101(3):601–609. doi: 10.1016/j.rmed.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Mannino DM, Buist AS, Petty TL, et al. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax. 2003;58(5):388–393. doi: 10.1136/thorax.58.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrie JE, Singh-Manoux A, Kivimäki M, et al. Cardiorespiratory risk factors as predictors of 40-year mortality in women and men. Heart. 2009;95(15):1250–1257. doi: 10.1136/hrt.2008.164251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fabbri LM, Rabe KF. From COPD to chronic systemic inflammatory syndrome? Lancet. 2007;370(9589):797–799. doi: 10.1016/S0140-6736(07)61383-X. [DOI] [PubMed] [Google Scholar]
- 10.Fabbri LM, Luppi F, Beghé B, et al. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31(1):204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 11.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 12.Marmot MG, Smith GD, Stansfeld S, et al. Health inequalities among British civil servants: the Whitehall II Study. Lancet. 1991;337(8754):1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 13.Hayes D, Jr, Kraman SS. The physiologic basis of spirometry. Respir Care. 2009;54(12):1717–1726. [PubMed] [Google Scholar]
- 14.Sabia S, Nabi H, Kivimaki M, et al. Health behaviors from early to late midlife as predictors of cognitive function: the Whitehall II Study. Am J Epidemiol. 2009;170(4):428–437. doi: 10.1093/aje/kwp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrie JE, Langenberg C, Shipley MJ, et al. Birth weight, components of height and coronary heart disease: evidence from the Whitehall II Study. Int J Epidemiol. 2006;35(6):1532–1542. doi: 10.1093/ije/dyl184. [DOI] [PubMed] [Google Scholar]
- 16.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 17.Elovainio M, Ferrie JE, Singh-Manoux A, et al. Organisational justice and markers of inflammation: the Whitehall II Study. Occup Environ Med. 2010;67(2):78–83. doi: 10.1136/oem.2008.044917. [DOI] [PubMed] [Google Scholar]
- 18.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- 20.Myers GL, Rifai N, Tracy RP, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110(25):e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 21.Beaty TH, Cohen BH, Newill CA, et al. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol. 1982;116(1):102–113. doi: 10.1093/oxfordjournals.aje.a113385. [DOI] [PubMed] [Google Scholar]
- 22.Bang KM, Gergen PJ, Kramer R, et al. The effect of pulmonary impairment on all-cause mortality in a national cohort. Chest. 1993;103(2):536–540. doi: 10.1378/chest.103.2.536. [DOI] [PubMed] [Google Scholar]
- 23.Batty GD, Gunnell D, Langenberg C, et al. Adult height and lung function as markers of life course exposures: associations with risk factors and cause-specific mortality. Eur J Epidemiol. 2006;21(11):795–801. doi: 10.1007/s10654-006-9057-2. [DOI] [PubMed] [Google Scholar]
- 24.Jakes RW, Day NE, Patel B, et al. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation into Cancer-Norfolk prospective population study. Am J Epidemiol. 2002;156(2):139–147. doi: 10.1093/aje/kwf021. [DOI] [PubMed] [Google Scholar]
- 25.Pelkonen M, Notkola IL, Lakka T, et al. Delaying decline in pulmonary function with physical activity: a 25-year follow-up. Am J Respir Crit Care Med. 2003;168(4):494–499. doi: 10.1164/rccm.200208-954OC. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Aymerich J, Lange P, Serra I, et al. Time-dependent confounding in the study of the effects of regular physical activity in chronic obstructive pulmonary disease: an application of the marginal structural model. Ann Epidemiol. 2008;18(10):775–783. doi: 10.1016/j.annepidem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Shaheen SO, Jameson KA, Syddall HE, et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36(2):277–284. doi: 10.1183/09031936.00114709. [DOI] [PubMed] [Google Scholar]
- 28.Varraso R, Fung TT, Hu FB, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–791. doi: 10.1136/thx.2006.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varraso R, Fung TT, Barr RG, et al. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am J Clin Nutr. 2007;86(2):488–495. doi: 10.1093/ajcn/86.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ. 2006;174(9):1293–1299. doi: 10.1503/cmaj.051299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGarvey LP, John M, Anderson JA, et al. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss ST, Segal MR, Sparrow D, et al. Relation of FEV1 and peripheral blood leukocyte count to total mortality. The Normative Aging Study. Am J Epidemiol. 1995;142(5):493–498. doi: 10.1093/oxfordjournals.aje.a117665. [DOI] [PubMed] [Google Scholar]
- 33.Cohen BH. Chronic obstructive pulmonary disease: a challenge in genetic epidemiology. Am J Epidemiol. 1980;112(2):274–288. doi: 10.1093/oxfordjournals.aje.a112994. [DOI] [PubMed] [Google Scholar]
- 34.Fogarty AW, Jones S, Britton JR, et al. Systemic inflammation and decline in lung function in a general population: a prospective study. Thorax. 2007;62(6):515–520. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61(1):10–16. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135(1):173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 37.Olafsdóttir IS, Gíslason T, Thjódleifsson B, et al. Gender differences in the association between C-reactive protein, lung function impairment, and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2(4):635–642. [PMC free article] [PubMed] [Google Scholar]
- 38.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 39.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13(1):197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 40.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21(1):13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.