Abstract
Background:
Formal family meetings have been recommended as a useful approach to assist in goal setting, facilitate decision making, and reduce use of ineffective resources in the ICU. We examined patient outcomes before and after implementation of an intensive communication system (ICS) to test the effect of regular, structured formal family meetings on patient outcomes among long-stay ICU patients.
Methods:
One hundred thirty-five patients receiving usual care and communication were enrolled as the control group, followed by enrollment of intervention patients (n = 346), from five ICUs. The ICS included a family meeting within 5 days of ICU admission and weekly thereafter. Each meeting discussed medical update, values and preferences, and goals of care; treatment plan; and milestones for judging effectiveness of treatment.
Results:
Using multivariate analysis, there were no significant differences between control and intervention patients in length of stay (LOS), the primary end point. Similarly, there were no significant differences in indicators of aggressiveness of care or treatment limitation decisions (ICU mortality, LOS, duration of ventilation, treatment limitation orders, or use of tracheostomy or percutaneous gastrostomy). Exploratory analysis suggested that in the medical ICUs, the intervention was associated with a lower prevalence of tracheostomy among patients who died or had do-not-attempt-resuscitation orders in place.
Conclusions:
The negative findings of the main analysis, in combination with preliminary evidence of differences among types of unit, suggest that further examination of the influence of patient, family, and unit characteristics on the effects of a system of regular family meetings may be warranted. Despite the lack of influence on patient outcomes, structured family meetings may be an effective approach to meeting information and support needs.
Trial registry:
ClinicalTrials.gov; No.: NCT01057238 ; URL: www.clinicaltrials.gov
Treatment decision making for patients requiring prolonged mechanical ventilation is a challenge for both critical care professionals and family surrogate decision makers.1-3 Zilberberg et al4 have projected that the number of patients undergoing prolonged mechanical ventilation will double from the year 2000 to reach > 600,000 by 2020. The majority of these prolonged-stay ICU patients, often referred to as “chronically critically ill,” are cognitively impaired and thus family surrogates are faced with the burden of decision making.5,6
Effective, consistent communication with the families of critically ill patients has been identified as one of the most important needs of families and the strongest predictor of satisfaction with care.7-12 Interventions designed to enhance communication and decision making in critical care units have included daily phone calls to families by nurses, ethics consultation, and proactive palliative care consultation.13-16 Recently, formal, scheduled family meetings, involving multiple disciplines, and occurring away from the bedside, have been recommended as a more effective approach to talking with families and encouraging dialogue about goals of care.17-19
Although evidence is clear that adequate attention to the needs of families is associated with greater satisfaction and perhaps reduced adverse psychologic outcomes among families, there has been less exploration of the influence of family communication on patient outcomes. Lautrette et al17 tested the effect of using a structured format for a single end-of-life conference, supplemented with a bereavement brochure, for families of patients expected to die within the next few days. They demonstrated a lower prevalence of posttraumatic stress symptoms, anxiety, and depressive symptoms among families in the intervention group. As expected, given the enrollment criteria, there was no difference in patient length of stay (LOS). In contrast, Lilly et al20 did find a reduction in LOS among patients with the highest acuity scores following use of a system of regular, structured family meetings. However, their intervention occurred only in the medical ICU (MICU) in which the investigators were attending physicians.
To more fully evaluate the feasibility and effectiveness of regular, structured family meetings, we implemented a controlled trial of an “intensive communication system” (ICS) for family decision makers of long-stay ICU patients in five ICUs. The primary end point of the trial was ICU LOS. We also wanted to measure the effect on decisions about prolongation of life-support interventions, and thus, use of tracheostomy was a secondary end point. Additional exploratory analyses were performed to examine possible differences in effectiveness among various ICU specialties. In this report, we focus only on patient outcomes, testing the hypothesis that implementing a system of regular family meetings for long-stay patients would be associated with a reduction in length of ICU stay.
Materials and Methods
A pre-post (before-after) design was used, and all patients meeting the eligibility criteria were enrolled consecutively. The control group (usual care) consisted of 135 patients and corresponding families enrolled from November 2005 to April 2006. We then implemented the intervention from May 2006 through February 2008, and enrolled 354 patients and family members. Institutional review board approvals from the two hospitals were obtained prior to study initiation.
Participants
Eligibility criteria for patients were (1) 72 h of mechanical ventilation and no expectation by the attending physician of extubation or discharge from the ICU within the next 48 h; (2) lack of decisional capacity as judged by the ICU attending physician or by Glasgow Coma Scale below 6; (3) not on mechanical ventilation prior to admission; and (4) having an identified family surrogate decision maker. Family surrogates were eligible if they were (1) identified as the appointed surrogate and (2) available for participation in family meetings.
Power analysis for the test statistic of multiple linear regression for the outcome variable ICU LOS incorporated the following assumptions: α = 0.05, directional hypotheses, and medium effect size. A medium effect size was incorporated into the a priori power analysis based on prior intervention studies with this population and on Lilly’s work.11,12,20 Given these assumptions, a total sample (intervention + control) of 480 patients was needed to achieve a power of 0.80.21
Settings
Subjects were enrolled from five ICUs at two academic medical centers: a surgical ICU (SICU), MICU, and neuroscience ICU at a university-affiliated, not-for-profit medical center, and a MICU and SICU at a university-affiliated public medical center in the same city. The two medical units were closed-model ICUs where a single intensivist and medical team were primarily responsible for all care. The surgical and neuroscience units had a collaborative care model with active comanagement by the intensivist and surgeon.
Intervention
During the usual care (control) phase, informed consent was obtained from families as soon as the patient met eligibility criteria. Families were told that the study was evaluating family satisfaction with care and communication in the ICU. Formal family meetings were held if and when the ICU staff believed they were needed, with no attempt by investigators to influence frequency or content. Formal family meetings were defined as meetings that involved a physician, with at least one family member, held away from the patient’s bedside.
In the second phase, the ICS was implemented as the standard of care for all patients who met the enrollment criteria, regardless of whether their family members agreed to participate in the study data collection. Two dedicated advanced practice nurses (APNs) who had worked in and were familiar with the ICUs in each hospital were employed for the study. They were responsible for scheduling and participating in the family meetings to ensure that the communication structure was implemented consistently. Formal training sessions, consisting of an explanation of the rationale for the study and a review of the study protocol, were held for the APNs, each ICU physician group, ICU staff nurses, and social workers prior to the intervention. The APNs attended most of the sessions for the ICU physicians, as well as a 2-h session specifically for them. In addition, weekly debriefings for the APNs were held for most of the 2-year intervention period.
The ICS structure included a family meeting, held away from the bedside, within 5 days of ICU admission and at least weekly thereafter. Each meeting addressed medical update, values and preferences of the patient, and goals of care; treatment plan; and milestones for determining if the treatment plan was effective. There was no attempt to alter the communication style of the participants or to direct decisions. Following Lilly’s protocol, the aim was to include the ICU attending physician, the social worker/case manager, and the APN at every meeting, in addition to appropriate consulting physicians and bedside nurses. Thirty percent of the meetings were taped, allowing the investigators to monitor adherence to the protocol. The intervention ended when the patient left the ICU.
Measurements
Demographic and clinical information about each patient was obtained from medical records. This included age, gender, race, Acute Physiology and Chronic Health Evaluation (APACHE) III,22 major diagnostic category, and use of interventions that reflected resource use or goals of care such as placement of tracheostomy and percutaneous endoscopic gastrostomy (PEG) tubes. Treatment limitation decisions, hospital and ICU lengths of stay, discharge disposition, and ICU and hospital survival were also recorded.
Interrater reliability for medical record abstraction was assessed every 4 months throughout the data collection period. Pearson correlations ranged from 0.63 to 1.0 for continuous variables and κs ranged from 0.55 to 1.0 for categorical variables.
Analysis
One variable, LOS, exhibited significant deviation from a normal distribution, with seven cases having standardized scores > 3 SD above the mean. These seven cases (all with LOS > 80 days) were dropped from all analyses. Univariate comparisons of demographic and clinical characteristics of the sample were performed using independent sample t tests for continuous variables and χ2 analysis for categorical variables. Multivariate analyses included linear regression to address the primary end point (ICU stay) and logistic regression to address the secondary end point (tracheostomy placement). χ2 Goodness-of-fit was used to examine whether the distribution of the experimental group was similar to the distribution of the control group for key treatment limitation variables.
Results
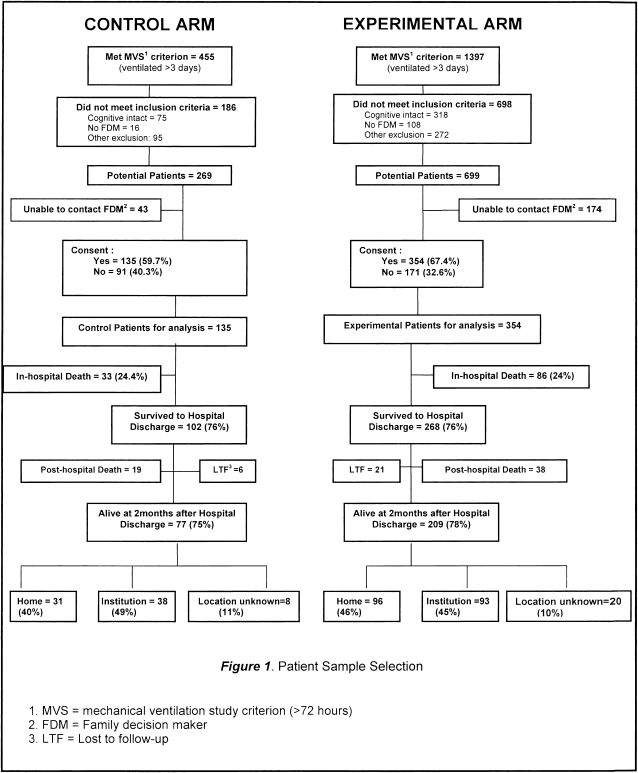
Figure 1 displays the sample enrollment, refusals, and dropouts, and Table 1 compares the experimental and control group on key clinical and demographic variables for patients as well as caregivers. There were significant differences between control and intervention groups (Table 1) on univariate analysis. Patients in the control group were older, more likely to have received care in the neuroscience ICU, less likely to have had treatment limitations in addition to a do-not-attempt-resuscitation (DNAR) order, and less likely to have a parent as a caregiver than those in the experimental group. Postdischarge mortality was higher in the control group. Contrary to our expectation, there were no significant differences between the control and experimental group in length of ICU stay or hospital stay.
Figure 1.
Patient sample selection. FDM = family decision maker; LTF = lost to follow-up; MVS = mechanical ventilation study criterion (> 72 h).
Table 1.
—Univariate Comparisons of Demographic and Clinical Variables Between Experimental and Control Patients (n = 481) and Caregivers (n = 475)
| Variable | Control (n = 135) | Experimental (n = 346) | t | χ2 | P Value |
| Age of patient, y, mean (SD) | 59.39 (16.90) | 55.88 (17.87) | 1.96 | … | .05 |
| 95% CI | 56.51-62.26 | 53.99-57.77 | … | ||
| Acute physiologic score, mean (SD) | 57.72 (30.40) | 60.91 (29.36) | -1.06 | … | .29 |
| 95% CI | 52.52-62.91 | 57.79-64.02 | … | ||
| Charlson comorbidity, mean (SD) | 1.65 (2.10) | 1.54 (1.88) | 0.54 | … | .59 |
| 95% CI | 1.29-2.01 | 1.34-1.74 | … | ||
| Mechanical ventilation, d, mean (SD) | 10.41 (9.23) | 10.12 (8.31) | 0.34 | … | .74 |
| 95% CI | 8.84-11.98 | 9.24-11.00 | … | ||
| ICU stay, d, mean (SD) | 13.44 (9.18) | 14.41 (9.85) | -0.99 | … | .16a |
| 95% CI | 11.88-15.01 | 13.37-15.46 | … | ||
| Length of hospital stay, d, mean (SD) | 22.84 (13.36) | 24.86 (13.04) | -1.51 | … | .07a |
| 95% CI | 20.57-25.12 | 23.48-26.23 | … | ||
| ICU stay in patients in fourth quartile APACHE, mean (SD) | 13.72 (10.67) | 13.66 (10.36) | 0.03 | … | .49a |
| 95% CI | 9.87-17.57 | 11.40-15.92 | … | ||
| Age of caregiver, y, mean (SD) | 52.59 (14.99) | 53.36 (14.18) | -0.52 | … | .60 |
| 95% CI | 50.04-55.14 | 51.84-54.87 | … | … | |
| Male, No. (%) | 67 (49.6) | 196 (56.6) | … | 1.93 | .17 |
| White, No. (%) | 102 (75.6) | 232 (67.1) | … | 3.31 | .07 |
| ICU service, No. (%) | … | 6.23 | .04 | ||
| MICU | 41 (30.4) | 100 (28.9) | … | ||
| SICU | 61 (45.2) | 192 (55.5) | … | ||
| NSU | 33 (24.4) | 54 (15.6) | … | ||
| Living will: yes, No. (%) | 30 (22.2) | 53 (15.3) | … | 3.24 | .07 |
| DNAR order, No. (%) | 46 (34.1) | 107 (30.9) | … | 0.44 | .51 |
| Added treatment limits (of DNAR patients),b No. (%) | 27 (54.0) | 80 (76.9) | … | 8.37 | .004 |
| PEG, No. (%) | 53 (40.2) | 127 (37.6) | … | 0.27 | .61 |
| Tracheostomy, No. (%) | 74 (55.6) | 169 (49.3) | … | 1.56 | .21 |
| Discharge ventilation status, No. (%) | … | 3.71 | .16 | ||
| None | 78 (71.6) | 223 (80.5) | … | ||
| Partial | 4 (3.7) | 8 (2.9) | … | ||
| Full (24 h) | 27 (24.8) | 46 (16.6) | … | ||
| APACHE fourth quartile, No. (%) | 32 (23.9) | 84 (24.3) | … | 0.03 | .86 |
| Mortality, No. (%) | … | ||||
| ICU | 26 (19.3) | 67 (19.4) | … | 0.001 | .98 |
| Hospital | 33 (24.4) | 86 (24.9) | … | 0.009 | .93 |
| Postdischargec | 19 (21.6) | 38 (15.9) | … | 4.57 | .03 |
| Cumulative 2-mod | 52 (40.3) | 124 (38.2) | … | 0.18 | .67 |
| Caregiver, No. (%) | … | ||||
| Female | 97 (71.9) | 265 (76.6) | … | 1.17 | .28 |
| White | 100 (74.1) | 233 (67.3) | … | 2.07 | .15 |
| Relationship | … | 9.06 | .03 | ||
| Spouse | 58 (43.0) | 121 (35.0) | … | … | … |
| Child | 45 (33.3) | 93 (26.9) | … | … | … |
| Parent | 17 (12.6) | 73 (21.1) | … | … | … |
| Other relative | 15 (11.1) | 59 (17.1) | … | … | … |
APACHE = Acute Physiology and Chronic Health Evaluation; DNAR = do not attempt resuscitation; MICU = medical ICU; NSU = neuroscience ICU; PEG = percutaneous endoscopic gastrostomy; SICU = surgical ICU.
The P values reported are for directional hypotheses stated a priori.
This number represents patients who had a DNAR order in place and had additional treatment limitations in place, such as no vasopressors or no dialysis.
Postdischarge mortality rates reflect the percentage of patients who were discharged alive and for whom 2-mo outcomes were known (control = 88, experimental = 239).
χ2 goodness-of-fit performed.
As seen in Table 2, 80% of control patients (109/135) and 25.7% of experimental patients (89/346) did not have any formal family meetings (P < .0001). For those who did have formal meetings, the average (SD) number of meetings was 1.27 (0.67) for those in the control group and 1.82 (1.18) for those in the experimental group (P = .82). The average length of formal meetings was 20.76 (11.31) min for the control group and 31.19 (14.77) min for the experimental group [t (261) = −2.51, P = .01]. At least two of the three specified professionals were present at all meetings in both groups. There were no differences between the control and experimental groups in the number of questions asked by either family members or physicians; however, the number of meetings held in the control period was quite small (see Table 2 for meeting characteristics).
Table 2.
—Characteristics of Family Meetings
| Variable | Control | Experimental | P Value |
| Had ≥ 1 formal family meeting (control: n = 135; experimental: n = 346), No. (%) | 26/135 (19.3) | 257/346 (74.3) | .0001 |
| Attendance at first family meeting (control: n = 26; experimental: n = 257), No. (%) | |||
| Physician | 15 (100.0) | 238 (94.1) | .64 |
| Nurse | 6 (40.0) | 243a (96.0) | .0001 |
| Social worker/case manager | 4 (26.7) | 91 (36.0) | .001 |
| For those with formal family meetings (control: n = 26; experimental: n = 257), mean (SD) | |||
| Total number of meetings | 1.27 (0.67) | 1.82 (1.18) | .001 |
| Length of meetings (min) | 20.76 (11.31) | 31.19 (14.77) | .013 |
| Number of questions by MD | 1.45 (1.26) | 1.36 (1.47) | .85 |
| Number of questions by family | 12.85 (13.78) | 7.05 (5.15) | .22 |
For the experimental group, this variable reflects the presence of the intervention nurse.
To evaluate the primary outcome, ICU LOS, we conducted a multiple linear regression analysis. Controlling for variables that differed between control and experimental groups (patient age, caregiver relationship to patient), as well as key clinical variables that had been shown to relate to ICU LOS in other research,23-25 we then added the intervention variable. As can been seen in Table 3, the model explained only 3% of the variance in ICU LOS. Addition of study group (control vs experimental) made little contribution (R2 change = 0.001), and admitting unit was the only variable with significant influence on our outcome variable.
Table 3.
—Standardized Estimates From Ordinary Least-Squares Regression of ICU Length of Stay on Patient and Family Caregiver Variables (N = 466)a
| Variables | β (B) | SE B | β | t | P Value |
| Patient | |||||
| Age, y | -0.02 | 0.03 | -0.03 | -0.51 | .61 |
| Living will (0 = no, 1 = yes) | -0.89 | 1.24 | -0.35 | -0.72 | .47 |
| ICU admitting unit (0 = MICU, 1 = non-MICU) | 3.96 | 0.96 | 0.19 | 4.14 | .0001 |
| Study group (0 = control, = experimental) | 0.81 | 0.98 | 0.04 | 0.82 | .41 |
| Caregiver | |||||
| Race (0 = white, 1 = nonwhite) | -0.17 | 0.99 | -0.01 | -0.17 | .87 |
| Relationship to patientb | |||||
| Child | -1.16 | 1.16 | -0.05 | -0.10 | .32 |
| Parent | -1.66 | 1.47 | -0.07 | -1.13 | .26 |
| Other | 1.07 | 1.33 | 0.04 | 0.80 | .42 |
Model: R2 (adj) = 0.031, F8,471 = 2.92, P = .003. B = slope; SE B = standard error of B. See Table 1 for expansion of other abbreviation.
Original sample size was reduced for analyses because of missing data for some variables.
Reference group for dummy coding was Spouse.
Influence of Diagnosis and Unit
In the second stage of analysis, we explored through logistic regression the influence of key demographic and clinical variables on the secondary end point of tracheostomy. We selected tracheostomy as the outcome variable of interest because it represents a decision point, indicated if life-sustaining therapies are to be continued in the presence of prolonged mechanical ventilation. As with the previous analysis, tracheostomy placement (yes/no) was regressed on patient and caregiver variables previously shown to affect decision making (Acute Physiologic Score, Charlson comorbidity, presence of a living will, race and age of the family decision maker), as well as ICU admitting unit. The two MICUs were grouped together because their organizational structure was the same and patient characteristics were almost identical. As the final step, the intervention variable was added to evaluate its contribution to the model.
As seen in Table 4, with study group added, the overall R2 was 0.06, with an overall correct classification of 59.7%. Adding the intervention variable into the model made no significant difference to the R2. Odds of receiving a tracheostomy were significantly related to admitting unit (medical < nonmedical).
Table 4.
—Logistic Regression of Tracheostomy Placement on Patient and Caregiver Variables (N = 466)a
| Variables | β | OR | 95% CI | P Value |
| Patient | ||||
| APACHE | -0.01 | 0.99 | 0.985-0.999 | .03 |
| Total Charlson | 0.03 | 1.03 | 0.92-1.14 | .64 |
| Living will (0 = no, 1 = yes) | -0.05 | 0.95 | 0.57-1.59 | .84 |
| ICU admitting unit (0 = MICU, 1 = non-MICU) | 0.69 | 1.99 | 1.25-3.19 | .004 |
| Study group (0 = control, 1 = experimental) | -0.30 | 0.74 | 0.48-1.12 | .74 |
| Caregiver | ||||
| FDM race (0 = white, 1 = nonwhite) | -0.01 | 0.99 | 0.65-1.51 | .97 |
| FDM age | -0.001 | 0.99 | 0.99-1.01 | .86 |
Nagelkerke R2 = 0.07. Model χ2 (7) = 26.38, P = .0001. Overall correct classification 60.5%. FDM = Family Decision Maker. See Table 1 for expansion of other abbreviations.
Tracheostomy placement in ICU (0 = no, 1 = yes).
Finally, we examined the effect of the intervention on the subgroup of patients for whom continued aggressive interventions were most likely to be ineffective (DNAR order or tracheostomy for patients who died, and tracheostomy in patients who had DNAR orders in place). Using the control group as the “expected” outcome, we used χ2 goodness of fit to examine whether the intervention yielded results that were significantly different from expected. Although the numbers in these subgroup analyses were small, the prevalence of tracheostomy among patients who had a DNAR order in place or among patients who died was significantly lower in the experimental group compared with the control group in the MICUs, but not in the non-MICUs (Table 5).
Table 5.
—Treatment Limitation Patterns by Study Group and Unit
| Control | Experimental | χ2 | P Value | |
| DNAR order in place among patients who died | ||||
| Medical ICUs | 10/12 (83.3) | 37/38 (97.4) | 3.19 | .07 |
| Nonmedical ICUs | 17/21 (81.0) | 39/48 (81.3) | 0.001 | .98 |
| Tracheostomy performed among patients who died | ||||
| Medical ICUs | 6/11 (54.5) | 5/38 (13.2) | 8.39 | .004a |
| Nonmedical ICUs | 9/21 (42.9) | 18/48 (37.5) | 0.18 | .68 |
| Tracheostomy performed among patients with DNAR | ||||
| Medical ICUs | 10/19 (52.6) | 13/51 (25.5) | 4.62 | .03b |
| Nonmedical ICUs | 13/27 (48.1) | 27/56 (48.2) | 0.00 | .99 |
Data are presented as No./Total (%). See Table 1 for expansion of abbreviations.
ɸ = 0.41.
ɸ = 0.26.
Discussion
There is widespread agreement about the importance of skilled and sensitive communication with families of critically ill patients. Previous studies have focused on specific communicative techniques, such as active listening, use of emotionally supportive statements, and facilitation of shared decision making.26-28 The hypothesized mechanism of our intervention was that early and consistent communication that included explicit discussion of patient preferences, values, and goals would provide more effective and efficient support to families who were considering treatment limitations, thus reducing LOS and use of ineffective resources.
Although previous research suggested that structured communication interventions were efficacious, our project was designed as an effectiveness trial for the specific purpose of evaluating whether such a structured system would be effective under “real world” conditions in a variety of clinical settings. In contrast to the results found in Lilly’s earlier study, which was conducted in a single MICU, we were unable to demonstrate a significant reduction in LOS among those patients with the highest APACHE scores.
There are at least two possible explanations for the lack of association of the intervention with a reduced LOS. First, it is possible that the support of a more deliberative decision-making process encouraged family decision makers to take more time to consider decisions to continue or limit continuation of aggressive interventions. The opportunity to explore values and preferences may have delayed decisions to proceed with tracheostomy, for example, which, in turn, could delay transfer to extended-care facilities. Future research would be needed to explore this and, if confirmed, to evaluate the possible impact on decision satisfaction or regret.
Similarly, the practical difficulties of arranging a formal meeting, compared with the relative efficiency of a brief unscheduled discussion at the bedside with an individual family member, may also have contributed to delays in decision making. It often required planning several days ahead to allow the family decision maker to schedule time away from work when the physician could be present or to assemble other family members who wished to participate in a formal meeting.
Additional factors that may have contributed to the ineffectiveness of the intervention include differences among the clinicians in their comfort and confidence in using the study protocol. We made no attempt to alter the personal style of each clinician because the purpose was to determine if imposing a structure would be sufficiently powerful to have an effect, without requiring that individuals learn new communication skills. The personal styles of the clinician participants may have been a stronger influence than the imposed structure of the communication system.
We also observed considerable variation in the response of family members to the system of formal meetings. In some cases, families seemed uncomfortable with the formality of meeting away from the bedside or saw it as simply unnecessary. This suggests that factors such as the trust families feel in those caring for their loved one, the baseline (ie, preintervention) skills of the doctors and nurses in talking with families on an ongoing basis, and personal characteristics of families, including medical literacy and preferences for shared decision making, all likely act as significant mediators of intervention effectiveness.29
Although caution must be used in interpreting exploratory analyses, differences in the apparent effectiveness of the intervention between medical units and surgical units may suggest that both patient characteristics and unit culture may have influenced the effectiveness of the intervention. The potential benefits of continued aggressive interventions in a young auto accident victim being cared for in a SICU are predictably of a different magnitude than the potential for benefit in an older adult with exacerbation of advanced chronic illness being cared for in a MICU. In the former situation, formal family conferences are likely to be an effective support to family decision makers and improve continuity of and satisfaction with care, but they are unlikely to alter the timing or direction of decisions, nor should they.
It is not possible to determine whether differences in culture among the units, distinct from patient characteristics, affected the power of the intervention. Cassell et al30 have written of the unique covenant between surgeons and their patients, entailing an implicit promise to do all that can be done to “save” the patient. The differences in the effect of the intervention between medical and surgical units among patients who died or who had DNAR orders in place suggests that there may be subtle and not-so-subtle influences against early or explicit consideration of treatment limitation.
Limitations
A number of limitations should be considered in interpreting our results. First, we elected to use liberal enrollment criteria because we knew that it could sometimes take several days to locate and approach family members for consent, and we did not want to delay beginning the intervention beyond the first week; this required identifying eligible patients early. The resulting heterogeneity of the sample, combined with the significant differences between control and intervention groups, contributed to the limited overall effect size.
Second, it may be the case that the family members who refused participation and inclusion of data from their loved one were those who were most distressed and who might have responded most strongly to the intervention. Conversely, it could also be that the families who refused were relatively comfortable with the communication about care and simply had no need to participate, or interest in participating, in a study of communication.
We recognize that most of our patients had multiple professionals, including consulting physicians, social workers, therapists, and numerous nurses, caring for them and interacting with family decision makers. Communication from these other sources undoubtedly influenced treatment decisions to some extent and may have contributed to, or detracted from, the effect of the intervention.
Last, the pre-post design has inherent limitations. Although we observed no major changes in ICU operation or personnel during the study period, we also were unable to control for the threat of history (the possibility that relevant conditions in the study site [eg, norms, practices, clinicians, etc] might change over time and influence study results) and subtle changes in clinician behavior and norms. Stratified random sampling might have avoided this threat and yielded more equivalent control and intervention groups but it would have raised the possibility of diffusion of the intervention.
Conclusions
The lack of the hypothesized effect of the intervention on the sample as a whole is strong evidence that the dynamics of decision making surrounding goals of care and aggressiveness of intervention are sufficiently complex that no single communication intervention is likely to have equivalent effects with all family members, in all environments. Directions for future research suggested by our findings include testing approaches that are tailored to family decision-making preferences and further investigation of the influence of unit characteristics on decision making.
It is clear that there will be continued growth in the numbers of patients who are able to be initially supported through a critical illness or injury but who are ultimately unable to recover and will suffer prolonged exposure to ineffective interventions. The results reported here focus only on the effect of the communication intervention on resource use. Even if the use of regular formal family meetings does not alter resource use in all settings, the literature is replete with evidence of other beneficial effects of providing families with time to sit in a quiet location and talk at some length about the patient’s goals and preferences and to explore issues related to quality of life, and providing families with consistent support as they face difficult decisions.10,17,18,29,31 Critical care clinicians have a strong obligation to continue to identify and test specific approaches in their own units that can be effective aids to families in decision making.
Acknowledgments
Author contributions: Dr Daly had full access to all data in the study and takes full responsibility for the integrity of all data and the accuracy of the data analysis.
Dr Daly: contributed to conceptualization of the project, all aspects of the conduct of the research, manuscript preparation, and approval of the final manuscript.
Dr Douglas: contributed to conceptualization of the project, all aspects of the conduct of the research, statistical analysis, manuscript preparation, and approval of the final manuscript.
Dr O’Toole: contributed to conceptualization of the project, all aspects of the conduct of the research, manuscript preparation, and approval of the final manuscript.
Dr Gordon: contributed to statistical analysis and approval of the final manuscript
Dr Hejal: contributed to implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Dr Peerless: contributed to implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Dr Rowbottom: contributed to implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Dr Garland: contributed to implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Dr Lilly: contributed to conceptualization of the design, ongoing evaluation of the project, and approval of the final manuscript.
Dr Wiencek: contributed to implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Dr Hickman: contributed implementation and ongoing evaluation of the intervention and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- APN
advanced practice nurse
- DNAR
do not attempt resuscitation
- ICS
intensive communication system
- LOS
length of stay
- MICU
medical ICU
- PEG
percutaneous endoscopic gastrostomy
- SICU
surgical ICU
Funding/Support: This research was funded by the National Institute of Nursing Research, National Institutes of Health [Grant RO1NR008941].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Curtis JR. The long-term outcomes of mechanical ventilation: what are they and how should they be used? Respir Care. 2002;47(4):496–505. [PubMed] [Google Scholar]
- 2.Nelson JE. Palliative care of the chronically critically ill patient. Crit Care Clin. 2002;18(3):659–681. doi: 10.1016/s0749-0704(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 3.Gries CJ, Curtis JR, Wall RJ, Engelberg RA. Family member satisfaction with end-of-life decision making in the ICU. Chest. 2008;133(3):704–712. doi: 10.1378/chest.07-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zilberberg MD, de Wit M, Pirone JR, Shorr AF. Growth in adult prolonged acute mechanical ventilation: implications for healthcare delivery. Crit Care Med. 2008;36(5):1451–1455. doi: 10.1097/CCM.0b013e3181691a49. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166(18):1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 6.Daly BJ, Douglas SL, Kelley CG, O’Toole E, Montenegro H. Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest. 2005;128(2):507–517. doi: 10.1378/chest.128.2.507. [DOI] [PubMed] [Google Scholar]
- 7.Hickey M. What are the needs of families of critically ill patients? A review of the literature since 1976. Heart Lung. 1990;19(4):401–415. [PubMed] [Google Scholar]
- 8.Johnson D, Wilson M, Cavanaugh B, Bryden C, Gudmundson D, Moodley O. Measuring the ability to meet family needs in an intensive care unit. Crit Care Med. 1998;26(2):266–271. doi: 10.1097/00003246-199802000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Heyland DK, Rocker GM, Dodek PM, et al. Family satisfaction with care in the intensive care unit: results of a multiple center study. Crit Care Med. 2002;30(7):1413–1418. doi: 10.1097/00003246-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Fumis RRL, Nishimoto IN, Deheinzelin D. Families’ interactions with physicians in the intensive care unit: the impact on family’s satisfaction. J Crit Care. 2008;23(3):281–286. doi: 10.1016/j.jcrc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Douglas SL, Daly BJ, Gordon NH, Brennan PF. Survival and quality of life: short-term versus long-term ventilator patients. Crit Care Med. 2002;30(12):2655–2662. doi: 10.1097/00003246-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Daly BJ, Douglas SL, Gordon NH, et al. Composite outcomes in the chronically critically ill: survival, location, and cognitive status at four months. Am J Crit Care. 2009;18(5):456–465. doi: 10.4037/ajcc2009580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medland JJ, Ferrans CE. Effectiveness of a structured communication program for family members of patients in an ICU. Am J Crit Care. 1998;7(1):24–29. [PubMed] [Google Scholar]
- 14.Schneiderman LJ, Gilmer T, Teetzel HD, et al. Effect of ethics consultations on nonbeneficial life-sustaining treatments in the intensive care setting: a randomized controlled trial. JAMA. 2003;290(9):1166–1172. doi: 10.1001/jama.290.9.1166. [DOI] [PubMed] [Google Scholar]
- 15.Dowdy MD, Robertson C, Bander JA. A study of proactive ethics consultation for critically and terminally ill patients with extended lengths of stay. Crit Care Med. 1998;26(2):252–259. doi: 10.1097/00003246-199802000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123(1):266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 17.Lautrette A, Darmon M, Megarbane B, et al. A communication strategy and brochure for relatives of patients dying in the ICU. N Engl J Med. 2007;356(5):469–478. doi: 10.1056/NEJMoa063446. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JR, Engelberg RA, Wenrich MD, Shannon SE, Treece PD, Rubenfeld GD. Missed opportunities during family conferences about end-of-life care in the intensive care unit. Am J Respir Crit Care Med. 2005;171(8):844–849. doi: 10.1164/rccm.200409-1267OC. [DOI] [PubMed] [Google Scholar]
- 19.Gay EB, Pronovost PJ, Bassett RD, Nelson JE. The intensive care unit family meeting: making it happen. J Crit Care. 2009;24(4):629. doi: 10.1016/j.jcrc.2008.10.003. e1-e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lilly CM, De Meo DL, Sonna LA, et al. An intensive communication intervention for the critically ill. Am J Med. 2000;109(6):469–475. doi: 10.1016/s0002-9343(00)00524-6. [DOI] [PubMed] [Google Scholar]
- 21.Elashoff J. nQuery Advisor. Boston, MA: Statistical Solutions; 2002. [Google Scholar]
- 22.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 23.Degenholtz HB, Thomas SB, Miller MJ. Race and the intensive care unit: disparities and preferences for end-of-life care. Crit Care Med. 2003;31(5) Suppl:S373–S378. doi: 10.1097/01.CCM.0000065121.62144.0D. [DOI] [PubMed] [Google Scholar]
- 24.Kwak J, Haley WE. Current research findings on end-of-life decision making among racially or ethnically diverse groups. Gerontologist. 2005;45(5):634–641. doi: 10.1093/geront/45.5.634. [DOI] [PubMed] [Google Scholar]
- 25.Reignier J, Dumont R, Katsahian S, et al. Patient-related factors and circumstances surrounding decisions to forego life-sustaining treatment, including intensive care unit admission refusal. Crit Care Med. 2008;36(7):2076–2083. doi: 10.1097/CCM.0b013e31817c0ea7. [DOI] [PubMed] [Google Scholar]
- 26.McDonagh JR, Elliott TB, Engelberg RA, et al. Family satisfaction with family conferences about end-of-life care in the intensive care unit: increased proportion of family speech is associated with increased satisfaction. Crit Care Med. 2004;32(7):1484–1488. doi: 10.1097/01.ccm.0000127262.16690.65. [DOI] [PubMed] [Google Scholar]
- 27.White DB, Braddock CH, III, Bereknyei S, Curtis JR. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med. 2007;167(5):461–467. doi: 10.1001/archinte.167.5.461. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton RD, Engelberg RA, Wenrich MD, Goss CH, Curtis JR. Clinician statements and family satisfaction with family conferences in the intensive care unit. Crit Care Med. 2006;34(6):1679–1685. doi: 10.1097/01.CCM.0000218409.58256.AA. [DOI] [PubMed] [Google Scholar]
- 29.Azoulay E, Pochard F, Chevret S, et al. FAMIREA Study Group Half the family members of intensive care unit patients do not want to share in the decision-making process: a study in 78 French intensive care units. Crit Care Med. 2004;32(9):1832–1838. doi: 10.1097/01.ccm.0000139693.88931.59. [DOI] [PubMed] [Google Scholar]
- 30.Cassell J, Buchman TG, Streat S, Stewart RM. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life—Updated. Crit Care Med. 2003;31(5):1551–1557. [PubMed] [Google Scholar]
- 31.Azoulay E, Pochard F, Chevret S, et al. French FAMIREA Study Group Meeting the needs of intensive care unit patients: a multicenter study. Am J Respir Crit Care Med. 2001;163(1):135–139. doi: 10.1164/ajrccm.163.1.2005117. [DOI] [PubMed] [Google Scholar]