Abstract
This study tested the hypothesis that passive leg heating attenuates α-adrenergic vasoconstriction within that limb. Femoral blood flow (FBF, femoral artery ultrasound Doppler) and femoral vascular conductance (FVC, FBF/mean arterial blood pressure), as well as calf muscle blood flow (CalfBF, 133xenon) and calf vascular conductance (CalfVC) were measured during intra-arterial infusion of an α1-adrenoreceptor agonist, phenylephrine (PE, 0.025 to 0.8 μg kg−1 min−1) and an α2-adrenoreceptor agonist, BHT-933 (1.0 to 10 μg kg−1 min−1) during normothermia and passive leg heating (water-perfused pant leg). Passive leg heating (∼46°C water temperature) increased FVC from 4.5 ± 0.5 to 11.9 ± 1.3 ml min−1 mmHg−1 (P < 0.001). Interestingly, CalfBF (1.8 ± 0.2 vs. 2.8 ± 0.3 ml min−1 (100 g)−1) and CalfVC (2.0 ± 0.3 vs. 3.9 ± 0.5 ml min−1 (100 g)−1 mmHg−1× 100) were also increased by this perturbation (P < 0.05 for both). Infusion of PE and BHT-933 resulted in greater absolute decreases in FVC during leg heating compared to normothermic conditions (maximal decreases in FVC during heating vs. normothermia: PE: 7.8 ± 1.1 vs. 2.8 ± 0.5 ml min−1 mmHg−1; BHT-933: 8.6 ± 1.7 vs. 2.1 ± 0.4 ml min−1 mmHg−1; P < 0.01 for both). However, the nadir FVC during drug infusion was higher during passive leg heating compared to normothermic conditions (FVC at highest dose of respective drugs during heating vs. normothermic conditions: PE: 3.7 ± 0.4 vs. 2.0 ± 0.3 ml min−1 mmHg−1; BHT-933: 3.8 ± 0.2 vs. 2.1 ± 0.3 ml min−1 mmHg−1; P < 0.001 for both). Leg heating did not alter the responsiveness of CalfBF or CalfVC to either PE or BHT-933. Taken together, these observations suggest that local heating does not decrease α-adrenergic responsiveness. However, heat-induced vasodilatation opposes α-adrenergic vasoconstriction. Furthermore, passive heating of a limb causes not only an increase in skin blood flow but also in muscle blood flow.
Introduction
Heat stress in humans causes a hyperadrenergic state (Rowell, 1990) associated with increased cardiac output (Rowell et al. 1969; Minson et al. 1998, 1999; Wilson et al. 2007, 2009), muscle and skin sympathetic nerve activity (Niimi et al. 1997; Crandall et al. 1999; Yamazaki et al. 2003; Cui et al. 2004a; Keller et al. 2006) and circulating noradrenaline (norepinephrine) concentrations (Kim et al. 1979; Escourrou et al. 1982). The result of these neural and cardiovascular adjustments is, at least in part, a preferential distribution of cardiac output to the cutaneous circulation aiding heat dissipation and thus body temperature regulation. The cost of this redistribution of blood during heat stress is a markedly reduced orthostatic tolerance (Lind et al. 1968; Allan & Crossley, 1972; Johnson et al. 1973; Wilson et al. 2002b, 2006; Cui et al. 2004b; Keller et al. 2009) accompanied with impairments in baroreflex control of arterial blood (Crandall, 2000). Therefore, an understanding of the effects of heat stress on sympathetic-mediated vasoconstriction is important towards identifying the mechanism(s) by which heat stress compromises the control of blood pressure.
Passive heating attenuates α-adrenergic-mediated vasoconstrction in isolated dog veins (Vanhoutte & Lorenz, 1970; Cooke et al. 1984), as well as in vivo and in vitro rat preparations (Kregel & Gisolfi, 1990; Massett et al. 1998; Massett et al. 2000). Additionally, increased temperature of femoral artery sections of the rat blunted P2X receptor-meditated vasoconstriction (Kluess et al. 2005), while increased skeletal muscle temperature associated with exercise has also been considered as a possible mechanism responsible for functional sympatholysis (Buckwalter & Clifford, 2001). In humans, cutaneous vascular responses to local administration of noradrenaline was blunted in locally heated skin (Wilson et al. 2002a). However, the findings of Wilson et al. may not adequately reflect the responses of an entire limb, which include both cutaneous and skeletal muscle vasculature. Cui et al. (2002) reported diminished increases in both mean arterial pressure and total peripheral resistance in response to a systemic infusion of the α1-adrenoreceptor agonist, phenylephrine, in the heat-stressed human. Together, those data support the notion that tissue heating may attenuate α-adrenergic receptor responsiveness. Such an attenuation may play an important role in the balance between temperature control and blood pressure regulation during a range of conditions (e.g. exercise, passive heating, etc.).
Relatively few investigations have examined the effects of increased tissue temperature in whole limbs, or in isolated skeletal muscle, on adrenergic control of the vasculature in humans. Considering the importance of understanding sympathetic control of peripheral vasculature during heat stress as it relates to blood flow and blood pressure regulation, the aim of this study was to test the hypothesis that passive leg heating attenuates α-adrenergic-mediated vasoconstriction in that limb compared to normothermic conditions.
Methods
Subjects
Ten healthy young men (age: 27 ± 6 years, height: 182 ± 8 cm, weight: 78 ± 14 kg) participated in the study. Written informed consent was obtained from all participants. Studies were performed at the Aviation Medicine unit, Department of Cardiology, National Hospital, Denmark. The informed consent and procedures were approved by the local ethics committee of Copenhagen and Frederiksberg (KF-01082/00) and conformed to the Declaration of Helsinki. Subjects were healthy, non-smokers, free of known cardiovascular and respiratory diseases, and were not using prescription or over-the-counter medications. Subjects were asked to refrain from caffeinated and alcoholic beverages for the 24 h before scheduled experiments.
Instrumentation
Subjects were studied while supine. Their left leg was dressed in a tube-lined perfusion pant leg connected to a water perfusion pump, enabling the control of leg temperature. Core temperature (Tcore) was measured using a telemetry temperature pill swallowed by subjects at least 1 h before data collection. Leg skin and muscle temperatures (Tsk and Tm) were measured from two thermocouples fixed to the skin with porous adhesive tape and another inserted ∼2 cm into the muscle belly, respectively. The insertion of the thermocouple into the muscle belly was accomplished by using an intravenous catheter to puncture the skin and muscle fascia, retracting the needle and then advancing the thermocouple through the sheath of the catheter. This was followed by the removal of the sheath leaving the thermocouple in place. Heart rate was collected from an electrocardiogram (ECG) signal.
Catheterization
The femoral artery of the experimental leg was cannulated under local anaesthesia (lidocaine, 5 ml, 20 mg ml−1). The catheter (Arrow, 20 gauge) was introduced in the proximal direction ∼5 cm below the inguinal ligament. This catheter was used for both arterial blood pressure measurements and the infusions of drugs. Mean arterial pressure (MAP) was determined as the mathematical mean of recorded pressure over a period of several cardiac cycles. The arterial blood pressures were adjusted for pump pressure during infusions as previously described (Wray et al. 2004).
Doppler ultrasound
The ultrasound machine (Vivid-FiVe, GE Medical) was equipped with a phased array transducer (paediatric echo transducer, 10 MHz). The femoral artery was insonated distal to the inguinal ligament. Vessel diameter was determined using a long-axis scan and a perpendicular insonation angle. The maximum diameter during the systolic phase was used for calculation of blood flow because this is the phase in which the vast majority of flow occurs in limb conductive arteries during rest. The blood velocity profile was obtained using the same transducer (pulsed wave Doppler, 5 MHz), with a sample volume depth of 5 mm. For each subject the lowest feasible insonation angle was chosen and kept constant throughout the study (in all subjects insonation angle was ≤50 deg). At all sample points, both femoral artery diameter and an angle-corrected, intensity-weighted, and time-averaged blood velocity (Vmean) were obtained (Echopac Software, GE Medical). Femoral artery blood flow was calculated as: (femoral artery diameter/2)2πVmean; femoral vascular conductance was calculated as: femoral blood flow/MAP.
133Xenon washout
For each thermal condition, calf muscle blood flow was measured via the 133xenon washout technique. This technique has been used in numerous investigations to measure skeletal muscle blood flow (Grimby et al. 1967; Henriksen & Sejrsen, 1977; Saito et al. 1997; Sorensen et al. 1999), as well as flow in other vascular beds (Stallknecht et al. 1995; Kruuse et al. 2003; Langberg et al. 2003; Simonsen et al. 2003). Gaseous 133xenon (0.1 ml; I.D.B. Holland B.V., Baarle-Nassau, The Netherlands) (Simonsen et al. 2003) was slowly injected with a 21-gauge needle into the medial portion of the belly of the gastrocnemius muscle. The injection was placed at a depth ∼3 cm below the skin into the skeletal muscle. 133Xenon in gaseous and aqueous form has been reported as virtually identical (Simonsen et al. 2003). A scintillation crystal detector was positioned directly over the injection site around the perfusion suit pant leg with a velcro strap. As 133xenon was injected two times (see Experimental protocol section), care was taken to ensure the injection site was duplicated. Washout of 133xenon was registered by a Mediscint system (Oakfield Instruments, Oxford, UK). Regional calf blood flow was determined using the following formula: Blood flow =−kλ× 100 (ml−1 (100 g)−1 min−1), where k is the rate constant of the washout curve and λ is the tissue/blood partition coefficient for 133xenon at equilibrium. λ has been determined to be 0.7 ml g−1 after correction for the specific gravity of muscle (Conn, 1961).
Drugs
Phenylephrine (PE; Danish county pharmaceutical corporation, SAD) was used as a specific α1-adrenergic agonist, while BHT-933 (BHT; Sigma-Aldrich, Denmark) was used as a specific α2-adrenergic agonist. Drugs were dissolved and diluted as appropriate with normal saline. Intra-arterial infusion rates ranged from 0.2 to 6 ml min−1 at the doses indicated below.
Experimental protocol
During the normothermic condition, 33°C water was perfused through the tube-lined pant leg. After ∼30 min, the experimental protocol illustrated in Fig. 1 was performed. Leg heating was initiated by perfusing 46°C water through the pant leg. Under both thermal conditions, dose response periods for PE (7 doses from 0.025 to 0.8 μg kg−1 min−1) and BHT-933 (6 doses from 1 to 10 μg kg−1 min−1) were ∼20 min for PE and ∼18 min for BHT-933 (i.e. 2 min per dose except for 5 min at two of the doses for each drug, termed ‘low’ and ‘high’ dose, needed for proper 133xenon measures of calf muscle blood flow). Low and high doses for PE were 0.05 and 0.2 μg kg−1 min−1, respectively, and for BHT-933 were 2.5 and 10.0 μg kg−1 min−1, respectively. Drug infusions were normalized to body weight. The order of α-agonist administration was random but equally weighted between subjects and delivered in similar order within subjects between thermal conditions.
Figure 1. Experimental protocol timeline.

Dose responses for the α1-adrenoreceptor agonist phenylephrine (0.025, 0.05, 0.1, 0.15, 0.2, 0.4, 0.8 μg kg−1 min−1) and the α2-adrenoreceptor agonist BHT-933 (1, 2, 2.5, 5, 7.5 10 μg kg−1 min−1) were determined during normothermia and leg heating conditions. The drug order (i.e. phenylephrine or BHT-933) was randomized and equally weighted between subjects. Drugs were delivered in similar order within subjects between thermal conditions.
Data and statistical analysis
Steady-state haemodynamic and thermal variables, as well as dose response data, were determined during the final 30 s of each administered dose, while 133xenon measures were determined over 300 s data collection periods. Comparisons of steady-state physiological variables between thermal conditions were made using paired t tests. Comparisons of dose response data were made using 2-way repeated measures ANOVA (thermal condition and drug dose). When necessary, post hoc analyses of multiple comparisons were performed using Bonferroni t tests. Statistical significance was set at P < 0.05.
Results
Cardiovascular and thermal variables during normothermia and leg heating
Steady-state cardiovascular and thermal data during normothermia and leg heating are presented in Table 1. Leg heating increased skin and muscle temperatures, resulting in a slight but significant increase in intestinal temperature. This was accompanied by a significant reduction in mean arterial blood pressure along with a small increase in heart rate.
Table 1.
Pre-drug infusion cardiovascular and thermal variables
| Normothermia | Leg heating | |
|---|---|---|
| Heart rate (beats min−1) | 54 ± 2 | 59 ± 3* |
| Arterial pressure (mmHg) | 88 ± 2 | 73 ± 2* |
| Core temperature (°C) | 37.0 ± 0.1 | 37.3 ± 0.1* |
| Calf muscle temperature (°C) | 33.8 ± 0.2 | 37.5 ± 0.2* |
| Leg skin temperature (°C) | 32.1 ± 0.2 | 39.4 ± 0.6* |
Significantly different from Normothermia (P < 0.05).
α-Adrenergic vasoconstriction during normothermia and leg heating
Whole-leg data using Doppler ultrasound
Figure 2A and B depict changes in diameter of the common femoral artery throughout the drug infusion protocol for PE (α1) and BHT-933 (α2), respectively. As expected, BHT-933 infusion did not change femoral artery diameter in either thermal condition, while PE infusion decreased femoral artery diameter throughout the drug infusion protocol for both thermal conditions. There was no significant thermal effect on vessel diameter during either drug infusion protocol.
Figure 2. Femoral artery diameter, femoral blood flow and femoral vascular conductance in response to PE and BHT-933.

Femoral artery diameter (A and B), femoral blood flow (FBF; C and D) and femoral vascular conductance (FVC; E and F) in response to PE (selective α1-adrenoreceptor agonist at 0.025, 0.05, 0.1, 0.15, 0.2, 0.4, 0.8 μg kg−1 min−1; left panels) and BHT-933 (selective α2-adrenoreceptor agonist at 1, 2, 2.5, 5, 7.5 10 μg kg−1 min−1; right panels) during normothermia (○) and leg heating (•). *P < 0.05 vs. baseline (BL); †P < 0.05 vs. normothermia (NT).
Figure 2C and D depict femoral blood flow and Fig. 2E and F depict femoral vascular conductance responses throughout the drug infusion protocol for PE (α1) and BHT-933 (α2), respectively. During both thermal conditions, femoral vascular conductance was reduced throughout the drug infusion protocol in a dose-dependent manner. The absolute changes in femoral vascular conductance were greater during leg heating conditions for both PE and BHT-933 (P < 0.01). The nadir femoral vascular conductance during the highest doses of the drugs for both PE and BHT-933 was higher during passive leg heating compared to normothermic conditions (value at highest dose of respective drugs during heating vs. normothermic conditions: PE: 3.7 ± 0.4 vs. 2.0 ± 0.3 ml min−1 mmHg−1; BHT-933: 3.8 ± 0.2 vs. 2.1 ± 0.3 ml min−1 mmHg−1; P < 0.001 for both). Across all doses, the percentage decrease in femoral vascular conductance was not significantly different for PE between thermal conditions (see Fig. 3A), while for BHT-933 the percentage change in femoral vascular conductance was greater during leg heating, particularly at the higher doses of BHT-933 (see Fig. 3B).
Figure 3. Relative changes in femoral vascular conductance.

Relative change in femoral vascular conductance (FVC) in response to PE (A) and BHT-933 (B) administration, expressed as percentage change from pre-drug baseline, during normothermia (○) and leg heating (•). †P < 0.05 vs. normothermia (NT)
Isolated skeletal muscle data using 133xenon washout
Figure 4 depicts representative 133xenon washout data from one subject during both normothermic and leg heating conditions. Figure 5A and B depict mean calf muscle blood flow and calf muscle vascular conductance responses to leg heating, respectively. Leg heating was accompanied by increases in both calf muscle blood flow (P= 0.029) and vascular conductance (P= 0.015). Figure 6 depicts calf muscle blood flow and conductance responses throughout rest, low and high dose drug infusions and 20 min recovery for PE (α1) and BHT-933 (α2), respectively. Leg heating did not alter the responsiveness of calf muscle blood flow or conductance to either PE or BHT-933.
Figure 4. 133Xenon clearance relative to time from one subject during pre-drug normothermia (○) and leg heating (•) conditions.

The calculated muscle tissue blood flow for this subject is representative of the group average. The faster the decline in counts per minute, the faster the washout of 133xenon due to higher muscle blood flow. Data on the y-axis are normalized to initial log value to account for differences in initial 133xenon counts during each condition.
Figure 5. Baseline calf muscle blood flow (A) and calf muscle vascular conductance (VC) (B) during normothermia (NT, ○) and leg heating (•) from each subject.
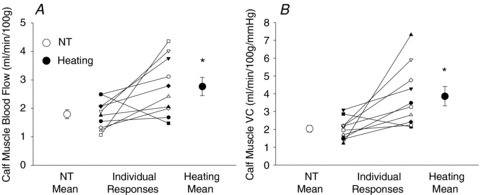
*P < 0.05 vs. NT.
Figure 6. Calf muscle blood flow and vascular conductance in response to phenylephrine and BHT-933.

Calf muscle blood flow (A and B) and vascular conductance (VC) (C and D) in response to phenylephrine (PE; left panels) and BHT-933 (right panels) during normothermia (NT) and leg heating (H). For either evaluation, there was no main effect of leg heating, yet there was a significant effect of the drug dose. Low and high doses for PE were 0.05 and 0.2 μg kg−1 min−1, respectively. Low and high doses for BHT-933 were 2.5 and 10.0 μg kg−1 min−1, respectively; †P < 0.05 vs. Baseline; ‡P < 0.05 vs. High Dose.
Discussion
The primary finding of this investigation did not support the initial hypothesis, given that α1- and α2-adrenergic vasoconstrictor responsiveness was preserved in the passively heated human leg when compared to normothermic conditions. This preservation in adrenergic responsiveness was apparent in both the whole leg, as shown by femoral artery Doppler measures, and in calf skeletal muscle vasculature, as shown by 133xenon clearance measures. Interestingly, despite profound α-adrenergic vasoconstriction at the highest doses of both agonists during leg heating, absolute femoral vascular conductance remained elevated compared to responses at the highest doses of each drug during normothermic conditions. Another interesting observation was that passive leg heating increased calf muscle blood flow and conductance.
The coordinated cardiovascular response to heat stress, which includes increases in cardiac output (Rowell et al. 1969; Minson et al. 1998, 1999; Wilson et al. 2007, 2009), muscle and skin sympathetic nerve activity (Niimi et al. 1997; Crandall et al. 1999; Yamazaki et al. 2003; Cui et al. 2004a; Keller et al. 2006) and circulating noradrenaline concentrations (Kim et al. 1979; Escourrou et al. 1982), has been referred to as a hyperadrenergic state (Rowell, 1990). Despite this hyperadrenergic state, heat stress markedly impairs blood pressure control during a hypotensive challenge as evidenced by orthostatic intolerance (Lind et al. 1968; Allan & Crossley, 1972; Johnson et al. 1973; Wilson et al. 2002b, 2006; Cui et al. 2004b; Keller et al. 2009). Possible mechanisms responsible for this reduction in orthostatic tolerance include reduced baroreflex control of arterial blood pressure (Crandall, 2000) and impaired α-adrenergic vasoconstriction, particularly in the cutaneous vasculature (Wilson et al. 2002a). However, no studies have investigated the effects of heating on limb α-adrenoreceptor responsiveness in humans.
Passive heating has been shown to attenuate α-adrenergic vasoconstriction in isolated dog veins (Vanhoutte & Lorenz, 1970; Cooke et al. 1984) as well as in vivo and in vitro rat preparations (Kregel & Gisolfi, 1990; Massett et al. 1998, 2000). Furthermore, the cutaneous vascular response to local administration of noradrenaline is blunted in locally heated human skin (Wilson et al. 2002a). Cui et al. (2002), demonstrated that whole-body heat stress reduced mean arterial pressure and total peripheral resistance responses to systemic (i.e. venous) infusion of phenylephrine leading to speculation that heat-induced attenuation of α-adrenergic vasoconstriction may contribute to accompanying orthostatic intolerance. In the current study, whole-limb vascular responses (i.e. the change in femoral vascular conductance) in the heated leg were preserved to both α1 (phenylephrine) and α2-adrenergic (BHT-933) administrations (Figs 2 and 3). In fact, during leg heating, the percentage decrease in femoral vascular conductance was greater in response to higher doses of BHT-933 (Fig. 3). However, it is notable that even at the highest doses of both drugs, absolute femoral blood flow and femoral vascular conductance remained elevated compared to the respective normothermic condition (see Fig. 2). This finding indicates that the vasodilator effect of passive heating of the whole limb is not completely countered by α-adrenergic vasoconstriction (at least at the doses used in this study). This difference in the lowest limb blood flow at the highest dose of these drugs between thermal conditions is probably the result of increased cutaneous blood flow during heating as measures of calf muscle blood flow at the highest doses of these drugs were similar between thermal conditions.
The attenuation of α-adrenergic-mediated vasoconstriction during skeletal muscle contraction, a phenomenon referred to as ‘functional sympatholysis,’ has been demonstrated in numerous animal and human models (Thomas et al. 1994; Hansen et al. 1996, 1999; Sander et al. 2000; Ruble et al. 2002; Tschakovsky et al. 2002; Rosenmeier et al. 2003; Keller et al. 2004; Wray et al. 2004). Increased temperature of skeletal muscle occurs concomitantly with skeletal muscle contraction during exercise, is dependent on exercise intensity (Saltin & Hermansen, 1966), and can be influenced by environmental temperature (Saltin et al. 1968; Morris et al. 2005). While the possible role of increased muscle temperature associated with exercise has been discussed as a potential mechanism for functional sympatholysis (Buckwalter & Clifford, 2001), relatively little attention has been given to the effect of tissue temperature on α-adrenoceptor responsiveness in humans. Findings from the current study do not support a direct effect of moderate increases in skeletal muscle temperature on α-adrenoreceptor responsiveness. However, the mechanism(s) by which α-adrenergic vasoconstriction is attenuated during exercise (i.e. active heat stress, as opposed to passive heat stress) remain incompletely understood. It is possible the combination of increased metabolite production (e.g. that which occurs during muscle contraction) and increased muscle temperature may play a role in functional sympatholysis. Future studies are required to answer these remaining questions.
Using a clearance technique (125I-labelled antipyrine), Johnson et al. (1976) demonstrated that local heating of a forearm did not increase forearm muscle blood flow, indicating an absence of a direct effect of temperature on skeletal muscle blood flow. Detry et al. (1972) also proposed that skeletal muscle blood flow was unchanged during whole-body heat stress. Taken together, those findings support what has become a tenant of thermoregulatory physiology, that is, local limb and whole-body heat stress do not increase skeletal muscle blood flow in humans. Contrary to those prior findings, in the current study leg heating resulted in a marked increase in calf muscle blood flow and vascular conductance in 9 of 10 subjects (see Fig. 5), with mean responses being an approximate doubling of the respective variables. Although these responses are relatively small compared to conditions such as exercise, the heat-induced skeletal muscle vasodilatation may be of particular physiological importance given that any potential increase in muscle blood flow in response to heating would be in direct competition for blood pressure regulation. Future studies investigating the interaction between muscle temperature and vascular responses during conditions such as exercise, or in combination with blood pressure challenge may be warranted.
One possible explanation for the discrepant findings may be inherent differences in the skeletal muscle investigated, with calf muscle blood flow examined in the current study and forearm muscle blood flow examined by Johnson et al. (1976). Subjectively, it is expected that calf skeletal muscle is exposed to much greater and more frequent increases in tissue temperature compared to forearm muscle, due to the bipedal nature of man. This ‘lifelong’ exposure may alter vascular responses of the leg in a way that favours dilatation in response to increased temperature. Another difference between the present and cited studies is the radio-labelled isotope used to investigate skeletal muscle blood flow. 133Xenon, used in the current study, has a greater affinity for adipose tissue, compared to 125I-labelled antipyrine used by Johnson et al. (1976). However, the affinity of 133xenon to adipose tissue is unlikely to explain the observed increase in flow in the present study for two reasons: (1) great care was taken to inject the tracer well below subcutaneous adipose tissue in the lean subjects, and (2) the degree of adiposity in the muscle would be unchanged between normothermic and heat-stressed conditions. Future studies examining the effects of heating on different skeletal muscle vascular beds (e.g. forearm vs. leg) may be warranted.
Leg heating was accompanied by a decrease in mean arterial pressure, along with relatively minor, but significant, increases (∼9%) in heart rate (see Table 1). This increase in heart rate is possibly due to arterial baroreceptor unloading, although the increase in core temperature of ∼0.3°C may also have contributed to this response. The mechanism by which arterial blood pressure was decreased is most probably related to an increase in vascular conductance of the heated leg that was not completely countered by a reflex increase in heart rate, cardiac output, or via decreases in vascular conductance in non-heated tissue. Another possible mechanism for this response may be via central inhibition of sympathetic outflow following BHT-933 (selective α2-adrenergic agonist) infusion. While α2-adrenergic receptor agonists, such as clonidine, can centrally inhibit sympathetic outflow, it is unlikely this played a role in the current study for two reasons: (1) arterial blood pressure was not different between pre-infusion, BHT-933 infusion and recovery periods (data not shown), indicating that the BHT-933 did not have a significant systemic effect and (2) the period of time between the drug infusions during normothermia and the baseline data collection during leg heating (when arterial blood pressure was reduced) was separated by ∼1.5 to 2 h.
It is worth noting that while skeletal muscle blood flow is reported to be unchanged during whole-body heat stress (Detry et al. 1972), muscle sympathetic nerve activity is increased during this thermal exposure (Niimi et al. 1997; Crandall et al. 1999; Yamazaki et al. 2003; Cui et al. 2004a; Keller et al. 2006). This is interesting, considering increased muscle sympathetic nerve activity would be expected to cause vasoconstriction and, according to findings in the current study, α-adrenergic responses are preserved in heated skeletal muscle. It may be that increased tissue temperature associated with whole-body heating directly dilates skeletal muscle vasculature, as observed in the present study (see Fig. 5), but this dilatory effect is countered by increased muscle sympathetic nerve activity and associated vasoconstriction during the whole-body heat stress. This would, theoretically, prevent/attenuate increases in skeletal muscle blood flow during whole-body heating and facilitate distribution of cardiac output to the skin necessary for thermoregulation.
Limitations
One limitation of the current study is related to the nature of the α-adrenoceptor activation used in this study (i.e. intra-arterial drug infusion). Unlike sympathetic neural release of vasoconstrictor substances, such as noradrenaline, which probably activates primarily abluminal adrenoceptors, intra-arterial drug infusion probably activates both abluminal and luminal adrenoceptors, the latter of which are located on the endothelium. To that end, previous studies have shown a similar muscle contraction-induced impairment between vasoconstriction elicited by α-adrenoceptor activation via phenylephrine (α1-adrenoceptor agonist) and clonidine (α2-adrenoceptor agonist) compared to the release of endogenous noradrenaline from sympathetic nerve terminals (via tyramine) (Tschakovsky et al. 2002; Rosenmeier et al. 2003). However, the location of the α-adrenoceptors activated, that is, luminal and abluminal, and the associated consequence of tissue heating on these separate receptor populations, was not accounted for in the current study. Another limitation of the present study design is that the effect of heating on endogenous neurotransmitter release was not investigated. Therefore, while post-junctional α-adrenoceptor responsiveness was preserved during leg heating, it remains possible that tissue heating impairs the release of vasoconstrictor substances, such as noradrenaline, from sympathetic nerve terminals.
In summary, α1- and α2-adrenergic receptor responsiveness was preserved in both the whole-leg and isolated calf muscle vasculature in a passively heated human leg compared to normothermic conditions. Despite these observations, absolute flow and vascular conductance in the whole leg remained elevated at the highest drug doses during leg heating. Together, these findings demonstrate that local heating does not decrease α-adrenergic responsiveness in skeletal muscle.
Acknowledgments
This work was supported by NIH NHLBI 61388 and 84072 to C.G.C. and NIH NHLBI 082426 to D.M.K.. M.S.'s research is supported by the Danish Heart Association, The Danish National Research Foundation, The Novo Nordisk Foundation, The Michaelsen Foundation, and The Danish Physicians Research Foundation.
Glossary
Abbreviations
- CalfBF
calf muscle blood flow
- CalfVC
calf vascular conductance
- FBF
femoral blood flow
- FVC
femoral vascular conductance
- MAP
mean arterial pressure
- PE
phenylephrine
Author contributions
The studies were performed in the lab of M.S. in Copenhagen, Denmark. D.M.K., M.S. and C.G.C. contributed to the conception and design of the experiments, and collection, analysis and interpretation of data; B.S. contributed to the collection, analysis and interpretation of data; all authors were responsible for drafting the article or revising it critically for important intellectual content, and all approved the final version.
References
- Allan JR, Crossley RJ. Effect of controlled elevation of body temperature on human tolerance to +G z acceleration. J Appl Physiol. 1972;33:418–420. doi: 10.1152/jappl.1972.33.4.418. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Conn HL., Jr Equilibrium distribution of radioxenon in tissue: xenon-hemoglobin association curve. J Appl Physiol. 1961;16:1065–1070. doi: 10.1152/jappl.1961.16.6.1065. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Shepherd JT, Vanhoutte PM. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ Res. 1984;54:547–553. doi: 10.1161/01.res.54.5.547. [DOI] [PubMed] [Google Scholar]
- Crandall CG. Carotid baroreflex responsiveness in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2000;279:H1955–H1962. doi: 10.1152/ajpheart.2000.279.4.H1955. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 1999;277:H2348–H2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Phenylephrine-induced elevations in arterial blood pressure are attenuated in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1221–R1226. doi: 10.1152/ajpregu.00195.2002. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Muscle sympathetic nerve activity during lower body negative pressure is accentuated in heat-stressed humans. J Appl Physiol. 2004a;96:2103–2108. doi: 10.1152/japplphysiol.00717.2003. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Orthostatic challenge does not alter skin sympathetic nerve activity in heat-stressed humans. Auton Neurosci. 2004b;116:54–61. doi: 10.1016/j.autneu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Detry JM, Brengelmann GL, Rowell LB, Wyss C. Skin and muscle components of forearm blood flow in directly heated resting man. J Appl Physiol. 1972;32:506–511. doi: 10.1152/jappl.1972.32.4.506. [DOI] [PubMed] [Google Scholar]
- Escourrou P, Freund PR, Rowell LB, Johnson DG. Splanchnic vasoconstriction in heat-stressed men: role of renin-angiotensin system. J Appl Physiol. 1982;52:1438–1443. doi: 10.1152/jappl.1982.52.6.1438. [DOI] [PubMed] [Google Scholar]
- Grimby G, Haggendal E, Saltin B. Local xenon 133 clearance from the quadriceps muscle during exercise in man. J Appl Physiol. 1967;22:305–310. doi: 10.1152/jappl.1967.22.2.305. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sayad D, Thomas GD, Clarke GD, Peshock RM, Victor RG. Exercise-induced attenuation ofα-adrenoceptor mediated vasoconstriction in humans: evidence from phase-contrast MRI. Cardiovasc Res. 1999;41:220–228. doi: 10.1016/s0008-6363(98)00226-0. [DOI] [PubMed] [Google Scholar]
- Hansen J, Thomas GD, Harris SA, Parsons WJ, Victor RG. Differential sympathetic neural control of oxygenation in resting and exercising human skeletal muscle. J Clin Invest. 1996;98:584–596. doi: 10.1172/JCI118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen O, Sejrsen P. Local reflex in microcirculation in human skeletal muscle. Acta Physiol Scand. 1977;99:19–26. doi: 10.1111/j.1748-1716.1977.tb10347.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Brengelmann GL, Rowell LB. Interactions between local and reflex influences on human forearm skin blood flow. J Appl Physiol. 1976;41:826–831. doi: 10.1152/jappl.1976.41.6.826. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Niederberger M, Rowell LB, Eisman MM, Brengelmann GL. Competition between cutaneous vasodilator and vasoconstrictor reflexes in man. J Appl Physiol. 1973;35:798–803. doi: 10.1152/jappl.1973.35.6.798. [DOI] [PubMed] [Google Scholar]
- Keller DM, Cui J, Davis SL, Low DA, Crandall CG. Heat stress enhances arterial baroreflex control of muscle sympathetic nerve activity via increased sensitivity of burst gating, not burst area, in humans. J Physiol. 2006;573:445–451. doi: 10.1113/jphysiol.2006.108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Low DA, Wingo JE, Brothers RM, Hastings J, Davis SL, Crandall CG. Acute volume expansion preserves orthostatic tolerance during whole-body heat stress in humans. J Physiol. 2009;587:1131–1139. doi: 10.1113/jphysiol.2008.165118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YD, Lake CR, Lees DE, Schuette WH, Bull JM, Weise V, Kopin IJ. Hemodynamic and plasma catecholamine responses to hyperthermic cancer therapy in humans. Am J Physiol Heart Circ Physiol. 1979;237:H570–H574. doi: 10.1152/ajpheart.1979.237.5.H570. [DOI] [PubMed] [Google Scholar]
- Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Elevated temperature decreases sensitivity of P2X purinergic receptors in skeletal muscle arteries. J Appl Physiol. 2005;99:995–998. doi: 10.1152/japplphysiol.00319.2005. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Gisolfi CV. Circulatory responses to vasoconstrictor agents during passive heating in the rat. J Appl Physiol. 1990;68:1220–1227. doi: 10.1152/jappl.1990.68.3.1220. [DOI] [PubMed] [Google Scholar]
- Kruuse C, Thomsen LL, Birk S, Olesen J. Migraine can be induced by sildenafil without changes in middle cerebral artery diameter. Brain. 2003;126:241–247. doi: 10.1093/brain/awg009. [DOI] [PubMed] [Google Scholar]
- Langberg H, Boushel R, Skovgaard D, Risum N, Kjaer M. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol. 2003;551:683–689. doi: 10.1113/jphysiol.2003.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AR, Leithead CS, McNicol GW. Cardiovascular changes during syncope induced by tilting men in the heat. J Appl Physiol. 1968;25:268–276. doi: 10.1152/jappl.1968.25.3.268. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Kregel KC. Effect of heating on the hemodynamic responses to vasoactive agents. Am J Physiol Regul Integr Comp Physiol. 1998;275:R844–R853. doi: 10.1152/ajpregu.1998.275.3.R844. [DOI] [PubMed] [Google Scholar]
- Massett MP, Lewis SJ, Stauss HM, Kregel KC. Vascular reactivity and baroreflex function during hyperthermia in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1282–R1289. doi: 10.1152/ajpregu.2000.279.4.R1282. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Pawelczyk JA, Kenney WL. Age, splanchnic vasoconstriction, and heat stress during tilting. Am J Physiol Regul Integr Comp Physiol. 1999;276:R203–R212. doi: 10.1152/ajpregu.1999.276.1.r203. [DOI] [PubMed] [Google Scholar]
- Morris JG, Nevill ME, Boobis LH, Macdonald IA, Williams C. Muscle metabolism, temperature, and function during prolonged, intermittent, high-intensity running in air temperatures of 33 degrees and 17 degrees C. Int J Sports Med. 2005;26:805–814. doi: 10.1055/s-2005-837448. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann GL, Murray JA. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27:673–680. doi: 10.1152/jappl.1969.27.5.673. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Foldager N, Mano T, Iwase S, Sugiyama Y, Oshima M. Sympathetic control of hemodynamics during moderate head-up tilt in human subjects. Environ Med. 1997;41:151–155. [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Stolwijk JA. Muscle temperature during submaximal exercise in man. J Appl Physiol. 1968;25:679–688. doi: 10.1152/jappl.1968.25.6.679. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21:1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen L, Enevoldsen LH, Bulow J. Determination of adipose tissue blood flow with local 133Xe clearance. Evaluation of a new labelling technique. Clin Physiol Funct Imaging. 2003;23:320–323. doi: 10.1046/j.1475-0961.2003.00509.x. [DOI] [PubMed] [Google Scholar]
- Sorensen VB, Wroblewski H, Galatius S, Haunso S, Kastrup J. Exercise skeletal muscle blood flow is related to peripheral microvascular stiffness in idiopathic dilated cardiomyopathy. Microvasc Res. 1999;58:268–280. doi: 10.1006/mvre.1999.2176. [DOI] [PubMed] [Google Scholar]
- Stallknecht B, Simonsen L, Bulow J, Vinten J, Galbo H. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Am J Physiol Endocrinol Metab. 1995;269:E1059–E1066. doi: 10.1152/ajpendo.1995.269.6.E1059. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition ofα2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte PM, Lorenz RR. Effect of temperature on reactivity of saphenous, mesenteric, and femoral veins of the dog. Am J Physiol. 1970;218:1746–1750. doi: 10.1152/ajplegacy.1970.218.6.1746. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Brothers RM, Tollund C, Dawson EA, Nissen P, Yoshiga CC, Jons C, Secher NH, Crandall CG. Effect of thermal stress on Frank–Starling relations in humans. J Physiol. 2009;587:3383–3392. doi: 10.1113/jphysiol.2009.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Auton Neurosci. 2002a;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Crandall CG. Heat stress reduces cerebral blood velocity and markedly impairs orthostatic tolerance in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:1443–1448. doi: 10.1152/ajpregu.00712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Zhang R, Witkowski S, Crandall CG. Skin cooling maintains cerebral blood flow velocity and orthostatic tolerance during tilting in heated humans. J Appl Physiol. 2002b;93:85–91. doi: 10.1152/japplphysiol.01043.2001. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Tollund C, Yoshiga CC, Dawson EA, Nissen P, Secher NH, Crandall CG. Effects of heat and cold stress on central vascular pressure relationships during orthostasis in humans. J Physiol. 2007;585:279–285. doi: 10.1113/jphysiol.2007.137901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Fadel PJ, Smith ML, Raven P, Sander M. Inhibition ofα-adrenergic vasoconstriction in exercising human thigh muscles. J Physiol. 2004;555:545–563. doi: 10.1113/jphysiol.2003.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki F, Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Shiraki K. Whole body heating reduces the baroreflex response of sympathetic nerve activity during Valsalva straining. Auton Neurosci. 2003;103:93–99. doi: 10.1016/s1566-0702(02)00140-6. [DOI] [PubMed] [Google Scholar]


