Abstract
A host of animal studies have been used to model the effects of exposure to a low protein diet in utero on adult blood pressure. Collection of systolic blood pressure data by the indirect tail-cuff plethysmography method consistently shows increased pressures in low protein exposed rodent offspring compared to controls, but this technique has been criticised as the associated stress artefacts may confound the observed effects. Conversely, radiotelemetry systems allow unrestrained and continuous monitoring of blood pressure through the awake and sleep phases of the diurnal cycle. In this novel study, we directly compared blood pressure parameters in male offspring from low protein and control-fed dams measured simultaneously using tail-cuff and radiotelemetry systems. Control rats showed a good correlation between tail-cuff and radiotelemetry derived blood pressure data. Conversely, low protein males were relatively hypertensive at 8 weeks of age when measured by tail-cuff, but had significantly lower blood pressure than controls at 12 weeks of age when measured by telemetry. Heart rate and length of systole did not differ between the two groups. Individual stress protocols mimicking those imposed by tail-cuff plethysmography (novel environment, heat, restraint, inflation), caused similar increases in blood pressure and heart rate in control and low protein animals, ruling out an effect of enhanced pressor response to stress following prenatal protein restriction. Instead, an increase in peripheral vascular resistance in these animals is considered possible. Such a disparity between central and peripheral blood pressure measurements could have important clinical implications regarding cardiovascular risk assessment and treatment.
Introduction
The developmental origins of adult health and disease hypothesis proposes that maternal undernutrition causes permanent changes in structure and function of tissues and organs, resulting in cardiovascular dysfunction and hypertension in the offspring (Barker, 1997; Alexander, 2006). Human studies are strongly supported by animal models showing that prenatal malnutrition retards fetal growth and induces adult hypertension (Langley-Evans et al. 1994; Langley-Evans, 2000; Lewis et al. 2002). Rat dams fed low protein (9% casein) (Langley-Evans et al. 1994; Langley-Evans & Jackson, 1995; Langley-Evans, 2000) or low iron (Gambling et al. 2003) diets before mating and throughout pregnancy bear offspring with systolic blood pressure (SBP) elevated by up to 28 mmHg throughout adulthood. A severe protein-calorie restricted diet throughout pregnancy (30% control diet) similarly led to a modest elevation of SBP (5 to 8 mmHg) (Woodall et al. 1996). However, these data were obtained by indirect tail-cuff blood pressure measurements while the conscious rat is under restraint, and rely on a temperature-induced increase in blood flow in the caudal artery. Both of these conditions are a source of stress which could introduce a measurement artefact across subjects. It has been suggested that this may occur differentially across dietary treatment groups, and so the effect of dietary restriction on SBP which is observed using the tail-cuff technique could represent a difference in stress response rather than a difference in basal peripheral blood pressure.
The development of radiotelemetric implants offers the opportunity to measure blood pressure directly and continuously without animal handling, restraint, or modifications to the environment. Simultaneous SBP, diastolic blood pressure (DBP), heart rate (HR), mean arterial pressure (MAP) and activity data are collected from free-living animals in their home cages and stored for later analysis. Several studies have used this method rather than tail-cuff and provide evidence to suggest that tail-cuff measurements of blood pressure produce higher readings of heart rate and blood pressure compared to telemetry, and that stress artefacts are involved (Bazil et al. 1993; Irvine et al. 1997; Tonkiss et al. 1998). For example, telemetered spontaneously hypertensive rats (SHRs) and Wistar/Kyoto controls showed a significant hypertensive response to minor handling (an elevated SBP of 40 mmHg) and restraint in the manner used for tail-cuff readings (30 mmHg) (Irvine et al. 1997). Importantly, prenatally malnourished rats (6% casein diet) exhibited a small but significant rise in DBP and HR but not SBP in comparison to well-nourished controls during a baseline period, but a greater increase in DBP and SBP than controls during first exposure to an olfactory stress (Tonkiss et al. 1998). This evidence supports the theory that differences observed between treatment groups using the tail-cuff technique could reflect a differential response to stress rather than a difference in basal blood pressure. Other studies have shown differences in response to antihypertensive treatments when blood pressure is measured by the two techniques. The acute antihypertensive effects of captopril in SHRs as measured by tail-cuff and direct femoral cannula methods were not evident when blood pressure was measured by telemetry implants in the aorta (Bazil et al. 1993). The authors suggest that the apparent insensitivity to the hypotensive effects of angiotensin-converting enzyme (ACE) inhibition in telemetered animals indicates a lower level of stress associated with this technique. In contrast, another study has shown captopril and losartan (an angiotensin receptor blocker, ARB), to have identical hypotensive effects in restrained and unrestrained animals (Irvine et al. 1997). However, hydralazine, a vasodilator acting directly at the level of vascular smooth muscle cells, had a significantly greater effect in restrained animals. In addition to the different levels of stress associated with each technique, it is important to note that the discrepancies in the data measured could also reflect differential regulation of vascular resistance at different sites of measurement. For example, it has been shown previously that vascular reactivity to angiotensin II and the mechanisms of vasoconstriction differ between vascular beds (Chen et al. 1995; Jackson & Herzer, 2001). In addition, it has been shown that there is an amplification of blood pressure between central and peripheral sites of measurement and that the extent of the amplification differs depending on a range of cardiovascular functional indices (Tsoucaris et al. 1995; Nichols & O’Rourke, 1998; Safar et al. 2000).
Together these observations demonstrate that the method of blood pressure determination is critical to the observed outcome, and suggest that the apparent differences in response to treatment noted in animals whose blood pressure is measured in different ways may give important information about the mediators of hypertension. In this study we measured blood pressure in the offspring of rats fed a control or low protein diet during pregnancy using both tail-cuff and radiotelemetry methods simultaneously. The objective was to establish whether the commonly found hypertension in offspring exposed to low protein diets in utero is due to a differential stress response or some other reason. This is important because an extensive body of research into the mechanisms which underpin programming of cardiovascular function by maternal undernutrition has been based upon tail-cuff observations. As expected we noted discrepancies between the two methods, but these were not wholly attributable to stress-related artefacts.
Methods
Ethical approval
All experiments were performed under license from the United Kingdom Home Office in accordance with the Animals (Scientific Procedures) Act 1986 and complied with the policy and regulations of The Journal of Physiology (Drummond, 2009).
Animals
Female virgin Wistar rats (Harlan, UK) were subjected to a 12 h light (08.00–20.00 h)–dark (20.00–08.00 h) cycle at a temperature of 20–22°C with ad libitum access to food and water. At a weight of approximately 180–200 g, females were mated with Wistar stud males. After conception, determined by the presence of a vaginal plug on the cage floor, females were single-housed and fed either a control 18% (w/w) casein (CON; n= 4) or a 9% (w/w) casein (MLP; n= 4) diet throughout gestation, as described previously (Langley-Evans et al. 1996). During pregnancy the animals were weighed and food intake was recorded daily. At birth (day 21) the animals were transferred to a standard laboratory chow diet (Harlan). Litters were sexed and weighed and culled to eight pups to ensure a standard level of nutrition during the suckling period. As males have been shown in many experiments to be more vulnerable to developmental programming effects (Woods, 2004), measurements were collected in male offspring only and selection of pups at birth was biased towards males.
Determination of blood pressure by tail-cuff
At 4 and 8 weeks of age, tail-cuff blood pressure was determined in all of the male offspring (CON, n= 19; MLP, n= 19) using the IITC Life Sciences Model 229 Blood Pressure amplifier/pump (IITC Inc., Woodlands, CA, USA), which uses a photoelectric sensor to detect changes in blood flow in response to manipulation of cuff occlusion pressure. All rats were housed at 27°C in the room where the measurements were made, for at least 2 h before testing, and all animals were handled and measured by the same technique and operator. The cuff was inflated twice before measurement collection to acclimatise the animal to the procedure. At 12 weeks of age, blood pressure was determined using the same technique in the smaller cohort of male offspring (CON, n= 8; MLP, n= 7) which had been implanted with telemetry devices. The tail cuff procedure was performed whilst telemetric data were being collected, in order to obtain directly comparable data. Measurements were taken in triplicate and an average value was derived for each animal.
Determination of blood pressure by radiotelemetry
Surgical procedure
The Data Science International (DSI, St Paul, MN, USA) telemetric system was used to monitor continuous blood pressure. The implants (model TA11PA-C40) are for use in small animals with a minimum weight of 175 g, but their use in young animals undergoing rapid linear growth poses surgical problems and can result in loss of cannula patency. Therefore, at 10 weeks of age, 2 male offspring per litter underwent surgery for implantation of a radiotelemetric pressure transducer. This was considered an ample representation of each litter, without skewing the statistical analysis. Rats were anaesthetised with isoflorane and supplemental oxygen. Buprenorphine (0.016 units (100 g)−1) and enrofloxacin (0.02 units (100 g)−1) were administered subcutaneously to the unconscious animal, and the abdominal area shaved and cleaned. Rats were placed on a heat mat for the duration of the surgery and aseptic techniques were adopted throughout. The catheter of a newly calibrated implant was inserted into the abdominal aorta below the level of the renal arteries and secured in place with a cellulose patch and tissue adhesive. The transmitter was sutured to the abdominal wall. Rats were given 0.004 units (100 g)−1 meloxicam subcutaneously and housed in single cages on heat mats to recover in the telemetry suite, which was free from noise and disturbance. A warm sugar and chow mash was provided and animals were weighed daily following surgery to ensure a return to pre-surgery body weight within 7 days. Of the 16 telemetry probes implanted, one failed to work and the host animal (MLP) was excluded from data collection.
Baseline measurements
Eight to eleven days after surgery, implanted rats were placed on telemetry receivers, in the same room to that in which they recovered, in three batches to adjust to their environment. The mean age at recording did not differ between the two groups and the range was tight (CON: 79.5 ± 0.87 days; MLP: 78.8 ± 1.39 days). Animals were unrestrained and free to move within their home cages. Room temperature and humidity were as standard for the animal unit. Blood pressure and heart rate data were then simultaneously and continuously collected every 10 s for 5 days.
Stress measurements
In order to establish the effect of stressors associated with the collection of tail-cuff data, each factor associated with the tail-cuff technique was exactly recreated in a step-wise fashion while continuous telemetry data were collected. Initially, animals were individually placed into a heat box over a telemetry receiver at room temperature for 1 h to establish the effect of being placed in a novel environment, as is routine for tail-cuff measurements. They were removed to their home cage for a minimum of 24 h (untelemetered) and then reintroduced to the heat box over the telemetry receiver at 27°C for a period of 30 min to simulate the heating used during tail-cuff measurements. While still inside the heat box, the rats were then restrained in the same Plexiglas tube as was used for tail-cuff plethysmography. The tail-cuff was positioned without inflation and telemetry data collected for a further 3 min. After this time the tail-cuff was inflated to 300 mmHg to both establish the effect of inflation stress on telemetric blood pressure and obtain directly comparable, simultaneously collected tail-cuff and telemetry blood pressure measurements. All stress measurements were collected between 09.00 and 16.00 h, in the same room as the collection of basal blood pressure data, and collection intervals of telemetric data were increased to 2 s.
Statistical analysis
Tail-cuff data for each animal at each time point was averaged. Telemetry data were extracted from the Dataquest ART Analysis program and hourly averages for the baseline period calculated and condensed to a 24 h interval (e.g. all measurements between 01.00 and 01.59 h on each of days 1–5 were averaged and represented as 01.00 in the 24 h scale). As well as repeated measures over a 24 h period, day, night and total means were calculated for each parameter. For the stress effects, data were averaged over the period of the stressor task. Periods of peak systolic pressure at the same time over alternate 24 h periods (Day 1, 3 and 5 and Night 1, 3 and 5) of the baseline period were used as representative data to calculate average day and night length of systole. SBP values obtained by synchronised tail-cuff and telemetry were correlated against each other and differences calculated by subtracting the radiotelemetry SBP from the tail-cuff SBP in each animal. The effect of diet was robustly analysed using the linear mixed model in SPSS 16.0 (SPSS Inc., Chicago, IL, USA). This model adjusts for the fact that multiple offspring from each litter were measured, and for body weight on the day of blood pressure measurement. Therefore, the effect of maternal diet on each blood pressure parameter is of primary interest while simultaneously taking account of litter in the model.
Results
There was no difference in body weight between CON and MLP males at birth or at the time of blood pressure measurements (4, 8 and 12 weeks of age; Table 1). Systolic blood pressure measured by tail-cuff plethysmography was comparable between CON and MLP males at 4 weeks of age, but MLP rats had higher blood pressure than controls (18.5 mmHg higher SBP) by 8 weeks of age (P < 0.02; Fig. 1). Technical difficulties during the 4 week measurements prevented all animals from being tail-cuffed at this age, reducing the statistical power. Although the same magnitude of difference was noted at 12 weeks of age, it no longer achieved statistical significance (CON: 143 ± 4.7, MLP: 161 ± 6.6; P < 0.08; Fig. 1). This was due to the reduced statistical power of the smaller cohort that underwent telemetry surgery (n= 7–8 per group versus n= 19 per group in full cohort measured at 8 weeks). Without the most robust statistical controls for body weight and litter of origin, pressures at this age were significantly higher at P < 0.02 (independent samples t test). SBP increased significantly from 4 to 12 weeks in CON (P < 0.003) and earlier, from 4 to 8 weeks, in MLP rats (P < 0.03).
Table 1.
Body weights in grams of male offspring of control (CON) and low protein (MLP) dams at birth (n= 19 per group), 4 (n= 13), 8 (n= 19), 10 (n= 7–8) and 12 (n= 7–8) weeks of age
| Diet | Birth | 4 weeks | 8 weeks | 10 weeks (pre-surgery) | 12 weeks (telemetry) |
|---|---|---|---|---|---|
| CON | 5.26 ± 0.09 | 78.8 ± 2.04 | 268.5 ± 4.79 | 332.6 ± 12.4 | 344.9 ± 10.6 |
| MLP | 5.35 ± 0.13 | 76.8 ± 2.00 | 255.5 ± 3.91 | 315.3 ± 8.55 | 333.8 ± 9.49 |
| P | ns | ns | ns | ns | ns |
Data are presented as means ±s.e.m., ns = not significantly different.
Figure 1. Systolic blood pressure (SBP) measurements collected in male offspring of control (CON) and low protein (MLP) dams at 4, 8 and 12 weeks of age by tail-cuff plethysmography.
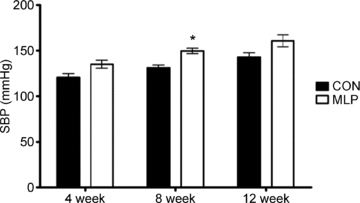
Data are plotted as means ±s.e.m.; *P < 0.02 between CON and MLP at 8 weeks, n= 13 (4 weeks), 19 (8 weeks) and 7–8 (12 weeks) per group.
On the basis of telemetric recordings, MLP rats demonstrated significantly lower SBP (mean, day and night; Fig. 2), DBP (mean and day; Fig. 3) and MAP (mean, day and night; data not shown) measurements than CON rats during the 5 day baseline period. Pulse pressure (the difference between SBP and DBP; data not shown) and HR (Fig. 4) did not differ between the groups. Novel environment, heat, restraint and inflation disturbances raised all parameters except for pulse pressure, but to the same degree in CON and MLP animals (Figs 2B–4B and 5).
Figure 2. Systolic blood pressure (SBP) measurements collected in male offspring of control (CON) and low protein (MLP) dams at 12 weeks of age by radiotelemetry.
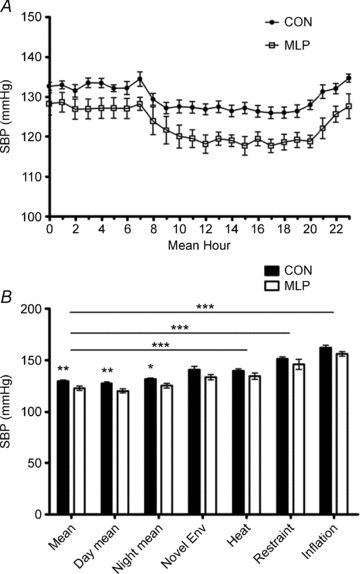
A, baseline data collected for 5 days and presented over a 24 h period, P < 0.01; B, mean values for 24 h period; day phase (08.00–20.00 h); night phase (20.00–08.00 h); introduction of novel environment (env); heat stress; restraint stress and inflation stress. Data are plotted as means ±s.e.m.*Statistical difference between CON and MLP groups at each condition. Horizontal bars indicate differences between conditions for both CON and MLP groups. ***P < 0.001, **P < 0.01, *P < 0.05, n= 8 (CON) or 7 (MLP).
Figure 3. Diastolic blood pressure (DBP) measurements collected in male control (CON) and low protein (MLP) offspring at 12 weeks of age by radiotelemetry.
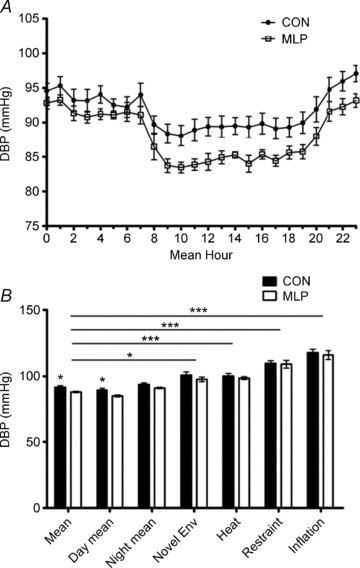
A, baseline data collected for 5 days and presented over a 24 h period, P < 0.03; B, mean values for 24 h period; day phase (08.00–20.00 h); night phase (20.00–08.00 h); introduction of novel environment (env); heat stress; restraint stress and inflation stress. Data are plotted as means ±s.e.m. *Statistical difference between CON and MLP groups at each condition. Horizontal bars indicate differences between conditions for both CON and MLP groups. ***P < 0.001, *P < 0.05, n= 8 (CON) or 7 (MLP).
Figure 4. Heart rate (HR) measurements collected in male control (CON) and low protein (MLP) offspring at 12 weeks of age by radiotelemetry.
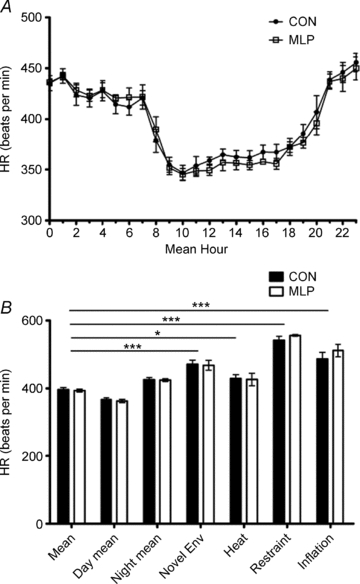
A, baseline data collected for 5 days and presented over a 24 h period; B, mean values for 24 h period; day phase (08.00–20.00); night phase (20.00–08.00 h); introduction of novel environment (env); heat stress; restraint stress and inflation stress. Data are plotted as means ±s.e.m. *Statistical difference between CON and MLP groups at each condition. Horizontal bars indicate differences between conditions for both CON and MLP groups. ***P < 0.001, *P < 0.05, n= 8 (CON) or 7 (MLP).
Figure 5. Systolic blood pressure (SBP) and heart rate (HR) data collected over ‘stress’ period by radiotelemetry in male offspring of control (CON) and low protein (MLP) dams at 12 weeks of age: introduction of novel environment – 60 min; heat stress – 30 min; restraint stress – 3 min, and inflation stress – 4 min.
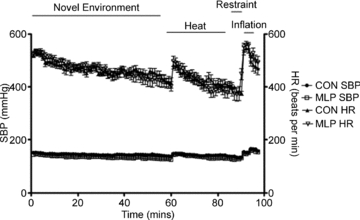
Data are plotted as means ±s.e.m., n= 8 (CON) or 7 (MLP).
Simultaneous tail-cuff and telemetry measurements resulted in a strong and significant correlation of SBP values in control animals (R= 0.92, P < 0.01). In contrast, the correlation was much weaker in MLP animals and did not reach statistical significance (R= 0.75, NS). In CON rats, tail-cuff SBP values ranged from 8.0 mmHg lower to 8.2 mmHg higher than telemetry readings and a paired t test showed no significant difference between SBPs obtained by tail cuff versus telemetry (Fig. 6). In MLP rats, tail-cuff data were always higher than telemetry readings in the range of 6.9 to 43.2 mmHg, and this difference was statistically significant (Fig. 6, P < 0.01). There was no difference in systole length between CON and MLP animals over the baseline period, or in response to stress (Fig. 7).
Figure 6. Difference in systolic blood pressure (SBP) measurements collected in male control (CON) and low protein (MLP) offspring at 12 weeks of age at the same time by tail-cuff and radiotelemetry methods.
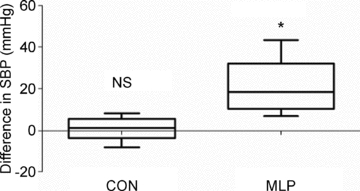
Data presented as tail-cuff SBP minus radiotelemetry SBP, showing median (central line), minimum and maximum values (whiskers) and range of remaining data (length of box), *P < 0.01, NS – non-significant, n= 8 (CON) or 7 (MLP).
Figure 7. Systole length in male control (CON; n= 8) and low protein (MLP; n= 7) offspring at 12 weeks of age during 5 day baseline collection and ‘stress’ periods (introduction of novel environment (env); heat stress; restraint stress and inflation stress).
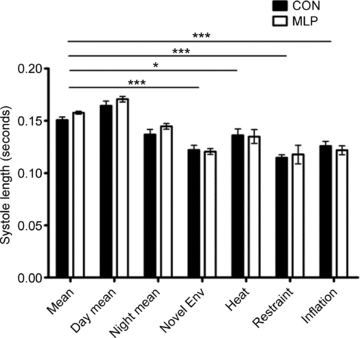
Data are plotted as means ±s.e.m. *Statistical difference between CON and MLP groups at each condition. Horizontal bars indicate differences between conditions for both CON and MLP groups. ***P < 0.001, *P < 0.05.
Discussion
A range of scientific techniques have been developed to study hypertension and blood pressure (Kurtz et al. 2005). This study has used the simultaneous collection of blood pressure data by radiotelemetry and tail-cuff plethysmography, under baseline conditions and in response to various stressors, to directly compare the data produced by these two techniques in an established model of hypertension programmed by manipulation of the maternal diet. To the authors’ knowledge, this is the first time that the stress response associated with both prenatal protein restriction and tail-cuff plethysmography restraint has been teased apart to resolve the possible mechanism behind the well-established programming of hypertension. Importantly, the results demonstrate that the lack of agreement in data obtained by the two methods is isolated to animals which were exposed to a prenatal low protein diet, and that the differences observed between control and low protein offspring cannot be explained by differences in response to stress.
The feeding of a low protein diet during rat gestation has been consistently shown to produce a systolic blood pressure rise of between 7 and 30 mmHg (Langley-Evans et al. 1994) by 4 weeks of age. This increase in blood pressure appears permanent, remaining elevated well into adult life (Langley-Evans & Jackson, 1995). In this study, the low protein exposed offspring were found to have elevated blood pressure measured by tail-cuff by 8 weeks of age, compared to the control group. This observation has been widely reported by many research groups using a variety of species, strain, timing and severity of protein restriction and age of examination with tail-cuff or cannulation blood pressure collection (Langley-Evans, 2000; Vehaskari et al. 2001; Brawley et al. 2004; Woods et al. 2004; Watkins et al. 2008; Sathishkumar et al. 2009). It was decided to delay implantation of radiotelemetry devices to allow linear growth of the animals to be completed and to allow tail-cuff hypertension to become firmly established. However, at 12 weeks of age the same animals had significantly lower blood pressure than controls according to radiotelemetry measurements, and no difference in tail-cuff pressures (a non-significant increase of 18 mmHg). The use of radiotelemetry for blood pressure monitoring eliminates the need to restrain or to raise the body temperature of the animals, so the higher pressures recorded using the tail-cuff technique could be interpreted as the product of an enhanced pressor response to stress in MLP offspring.
This was previously suggested by Tonkiss et al. (1998), who reported no differences in blood pressure between control and low protein offspring using radiotelemetry, but an enhanced pressor response to an olfactory stress in the low protein group. The earlier study was not, however, a fair test of whether the raised blood pressure of offspring exposed to a 9% casein maternal diet (Langley-Evans, 2000; Langley-Evans et al. 1994; Langley-Evans et al. 1996) was attributable to enhanced stress responses. In the study of Tonkiss et al. the level of protein restriction was greater and the olfactory stress (exposure to ammonia odour) was not directly equivalent to the processes experienced by an animal undergoing the tail-cuff procedure. Porter et al. (2007) used restraint in a Plexiglas tube to measure the stress response in male and female Sprague–Dawley rats by telemetry following a prenatal high-salt diet. Exposure to a high-salt diet in utero only was insufficient to programme baseline hypertension in male or female adult offspring. Alternatively, Contreras et al. (2000) induced persisting high blood pressure in adult offspring following a high-salt diet during pregnancy, lactation and for 10 days after weaning. Restraint stress resulted in an enhanced pressor and tachycardic response in female high-salt rats, but not males, suggesting an oestrogen-mediated mechanism (Porter et al. 2007). O’Regan et al. (2008) found a decrease in blood pressure in male and female offspring following prenatal dexamethasone treatment when measured by telemetry, contrary to previous reports using tail-cuff and carotid cannulation methods (Levitt et al. 1996). The expected hypertensive phenotype was only seen in male offspring, following handling and restraint stress in a Plexiglas tube. To establish if low protein offspring exhibit an enhanced pressor response to the stressors experienced during the tail-cuff procedure, both groups of animals in the present study were subjected to a novel environment, heat stress, restraint and occlusion of the tail-cuff while under continuous telemetry recording. All of these stressors did significantly increase SBP, DBP and HR compared to baseline measurements, but the pressor response to stress was identical in CON and MLP rats. This therefore eliminates an increased stress response in MLP rats as a driver of their apparent hypertension when subjected to the tail-cuff procedure. Therefore, it appears that while some models of fetal programming of hypertension lead to increased sensitivity to environmental stress, prenatal protein restriction may not.
An alternative explanation for the disparity between the two techniques is a difference in regulation of aortic and peripheral pressures. It is possible that MLP rats demonstrate an increase in peripheral vascular resistance which does not impact upon blood pressure in the aorta. This would be consistent with some of the mechanistic studies in the literature. Modified vascular reactivity (Whitworth et al. 1995) reduced nephron endowment and renal insufficiency (Nwagwu et al. 2000; Vehaskari et al. 2001; Sahajpal & Ashton, 2003), greater activity of the renin–angiotensin system (Langley-Evans & Jackson, 1995), stress-induced stimulation of the hypothalamic–pituitary–adrenal axis (Hadoke et al. 2006) and a decreased nitric oxide (NO)-mediated vascular response (Sathishkumar et al. 2009) have been reproducibly demonstrated in the offspring of rats fed a low protein diet, and are independent of any controversy around methods of blood pressure determination. Each of these programmed effects would impact upon peripheral vascular resistance, which would be detected as a change in pressure using the tail-cuff approach. In the current study, telemetry transducers were implanted in the abdominal aorta, which is not a resistance vessel, and therefore the impact on pressure at this site may not be comparable. Importantly, no studies have indicated changes in heart size or function that would be consistent with greater stroke volume or cardiac output, which are more likely to impact upon aortic pressure (Nichols & O’Rourke, 1998). It has generally been assumed that SBP at the tail reflects central SBP, but our findings suggest that it is not valid to extrapolate the data.
Studies in Wistar/Kyoto rats, both conscious and under anaesthesia, have shown significant amplifications in blood pressure between the carotid artery and terminal aorta, but no amplification was seen in spontaneously hypertensive rats (SHRs) (Tsoucaris-Kupfer et al. 1993; Tsoucaris et al. 1995). Studies in normotensive and hypertensive humans have shown that the amplification between aortic and brachial pulse pressures averaged 18–31% (Nichols & O’Rourke, 1998). The extent of amplification, and thus the difference between central and peripheral blood pressure, differs depending upon a range of factors, including arterial stiffness (Marque et al. 1999; Safar et al. 2000), length of the arterial tree (Nichols & O’Rourke, 1998) and length of the systole (Nichols & O’Rourke, 1998). The differences observed between data collected by the tail-cuff and telemetry techniques could therefore relate to a range of other cardiovascular factors, rather than a differential stress artefact. We were able to assess length of the systole in this study and observed no difference in CON and MLP offspring, either under baseline conditions or in response to stress. More detailed analysis of longitudinal growth data and arterial stiffness are required to investigate further the potential for differences in amplification between the two groups. The data from this study demonstrate quite clearly that the relationship between the tail-cuff and telemetry readings differs between control and low protein offspring in a manner not modified by various stressors. We therefore suggest that other aspects of cardiovascular structure or function are mediating the effects observed. Study of the factors relating to the extent of blood pressure amplification between central and peripheral sites could give important insight into the mechanisms underlying the increase in peripheral blood pressure observed.
Conclusion
Although it is well-established that tail-cuff determination of blood pressure is prone to artefacts, this study is the first to demonstrate that, once the impact of stress has been discounted, discrepancies between blood pressure data collected by the simultaneous use of tail-cuff and telemetry techniques are isolated to offspring exposed to a prenatal low protein diet. Having systematically eliminated the contribution of an enhanced pressor response to the stresses involved in the tail-cuff technique, the data suggest that the increase in systolic blood pressure observed in MLP offspring reflects an increase in peripheral vascular resistance and a change in the degree of amplification of blood pressure between central and peripheral regions. Investigation of the mechanisms which mediate the increased peripheral vascular resistance will be a high priority for further investigation as the current study was not designed or powered to consider such questions. This finding nevertheless highlights the importance of understanding the impact of prenatal diet on the mechanisms of regional vascular function and the differences in central and peripheral blood pressure control, whilst using the most appropriate site and method of blood pressure data collection. The dissociation between central and peripheral blood pressure observed in this and previous studies has clinical implications for the determination of cardiovascular risk in humans, where blood pressure recordings are conventionally taken from the brachial artery. Increasing evidence from invasive and non-invasive studies shows that central, more than peripheral, blood pressure is associated with target organ damage and potentially with cardiovascular risk (Williams et al. 2006; Roman et al. 2007; Jankowski et al. 2008). Commonly prescribed antihypertensive drugs mainly reduce blood pressure by decreasing total peripheral resistance and cardiac output (Harvey et al. 2008). Further investigation of the impact of such drugs on regional blood pressure and cardiovascular risk is warranted.
Acknowledgments
This work was supported by BBSRC and BHF. The authors wish to thank Mr Richard Plant for technical assistance with surgical techniques and Mrs Carol Armett and Miss Sarah Kirkland for their care of the animals.
Glossary
Abbreviations
- ACE
angiotensin converting enzyme
- ARB
angiotensin receptor blocker
- CON
control protein
- DBP
diastolic blood pressure
- HR
heart rate
- MAP
mean arterial pressure
- MLP
maternal low protein
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
Author contributions
A.S., S.M. and S.L.E. were all involved with conception and design of experiments, collection, analysis and interpretation of data, and drafting and revision of manuscript. All authors approved the final version for publication. Experiments were carried out within the School of Biosciences, University of Nottingham.
References
- Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br Med Bull. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- Bazil MK, Krulan C, Webb RL. Telemetric monitoring of cardiovascular parameters in conscious spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1993;22:897–905. doi: 10.1097/00005344-199312000-00019. [DOI] [PubMed] [Google Scholar]
- Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, Clough GF, Poston L, Hanson MA. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, McNeill JR, Wilson TW, Gopalakrishnan V. Heterogeneity in vascular smooth-muscle responsiveness to angiotensin-II – role of endothelin. Hypertension. 1995;26:83–88. doi: 10.1161/01.hyp.26.1.83. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Wong DL, Henderson R, Curtis KS, Smith JC. High dietary NaCl early in development enhances mean arterial pressure of adult rats. Physiol Behav. 2000;71:173–181. doi: 10.1016/s0031-9384(00)00331-0. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambling L, Dunford S, Wallace DI, Zuur G, Solanky N, Srai SK, McArdle HJ. Iron deficiency during pregnancy affects postnatal blood pressure in the rat. J Physiol. 2003;552:603–610. doi: 10.1113/jphysiol.2003.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188:435–442. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- Irvine RJ, White J, Chan R. The influence of restraint on blood pressure in the rat. J Pharmacol Toxicol Methods. 1997;38:157–162. doi: 10.1016/s1056-8719(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Herzer WA. Regional vascular selectivity of angiotensin II. J Pharmacol Exp Ther. 2001;297:736–745. [PubMed] [Google Scholar]
- Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45:299–310. doi: 10.1161/01.HYP.0000150857.39919.cb. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Critical differences between two low protein diet protocols in the programming of hypertension in the rat. Int J Food Sci Nutr. 2000;51:11–17. doi: 10.1080/096374800100859. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clin Nutr. 1994;13:319–324. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Lewis RM, Forhead AJ, Petry CJ, Ozanne SE, Hales CN. Long-term programming of blood pressure by maternal dietary iron restriction in the rat. Br J Nutr. 2002;88:283–290. doi: 10.1079/BJN2002656. [DOI] [PubMed] [Google Scholar]
- Marque V, Kieffer P, Atkinson J, Lartaud-Idjouadiene I. Elastic properties and composition of the aortic wall in old spontaneously hypertensive rats. Hypertension. 1999;34:415–422. doi: 10.1161/01.hyp.34.3.415. [DOI] [PubMed] [Google Scholar]
- Nichols WW, O’Rourke M. Theoretical, Experimental and Clinical Principles. 4th edn. London, Sydney, Aukland: Arnold; 1998. McDonald's blood flow in arteries; pp. 54–113.pp. 201–222.pp. 284–292.pp. 347–401. [Google Scholar]
- Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr. 2000;83:79–85. [PubMed] [Google Scholar]
- O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol. 2008;196:343–352. doi: 10.1677/JOE-07-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JP, King SH, Honeycutt AD. Prenatal high-salt diet in the Sprague-Dawley rat programs blood pressure and heart rate hyperresponsiveness to stress in adult female offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R334–342. doi: 10.1152/ajpregu.00887.2006. [DOI] [PubMed] [Google Scholar]
- Harvey RA, Champe PC, Clark MA, Cubeddu LX. Pharmacology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- Safar ME, Blacher J, Mourad JJ, London GM. Stiffness of carotid artery wall material and blood pressure in humans: application to antihypertensive therapy and stroke prevention. Stroke. 2000;31:782–790. doi: 10.1161/01.str.31.3.782. [DOI] [PubMed] [Google Scholar]
- Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 2003;104:607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J Vasc Res. 2009;46:229–239. doi: 10.1159/000166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- Tsoucaris-Kupfer D, Benetos A, Legrand M, Safar ME. Pulse pressure gradient along the aortic tree in normotensive Wistar-Kyoto and spontaneously hypertensive rats: effect of nicardipine. J Hypertens. 1993;11:135–139. doi: 10.1097/00004872-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Tsoucaris D, Benetos A, Legrand M, London GM, Safar ME. Proximal and distal pulse pressure after acute antihypertensive vasodilating drugs in Wistar-Kyoto and spontaneously hypertensive rats. J Hypertens. 1995;13:243–249. [PubMed] [Google Scholar]
- Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, Fleming TP. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008;586:2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth JA, Brown MA, Kelly JJ, Williamson PM. Mechanisms of cortisol-induced hypertension in humans. Steroids. 1995;60:76–80. doi: 10.1016/0039-128x(94)00033-9. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr Res. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Woods LL. Renal disease and fetal undernutrition. In: Langley-Evans SC, editor. Fetal Nutrition and Adult Disease: Programming of Chronic Disease Through Fetal Exposure to Undernutrition. Wallingford: 2004. pp. 235–258. [Google Scholar]
- Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]


