Abstract
Vitamin D deficiency continues to attract considerable attention because of claims that an adequate status can reduce the risk of a wide range of diseases. The facts are that this hormone modulates the expression of a very large number of genes, possibly some 5 to 10% of the genome; that it has been subject to very strong evolutionary pressures; and that its biological activities are exerted across a wide range of tissues, and these all contribute to the plausibility that such claims may eventually be found to be valid. While the endocrine action of the active metabolite, 1,25-dihydroxyvitamin D, has been well-characterised to contribute to maintaining plasma calcium and phosphate homeostasis through regulation of intestinal absorption, recent research has focused on its autocrine and/or paracrine activities. Such activities of vitamin D have been best characterised in skin tissues and the immune system where it regulates cell differentiation and maturation as well as the innate immune system. Recent data are now available to implicate autocrine/paracrine activities in each of the major bone cell types where it also regulates cell proliferation and differentiation. In rodent models, adequate levels of serum 25-hydroxyvitamin D have been found to be critical to optimise bone health and to protect against osteoporosis. These findings are consistent with clinical data that such activity is present in humans. The introduction of an autocrine/paracrine paradigm for vitamin D has significant implications for critical levels of serum 25-hydroxyvitamin D for optimal health.
Introduction
The beneficial actions of vitamin D continue to achieve considerable publicity in the lay and scientific press alike, as the number of disease states associated with a low vitamin D status increases. The wide variety of diseases associated with a low vitamin D status has tested the credulity of many biological scientists, but the claims keep being made, and evidence, much of it only demonstrating significant associations, keeps being published. Thus, it is worthwhile to assess the actions and factors influencing vitamin D levels in mammals in the wider perspective to see if it is at all probable that such an agent could play an important role in such a variety of organ systems. A second issue is to review its mode of action. Vitamin D3 was identified in 1919 as the critical factor in resolving the childhood bone disease of rickets.1 It was not until 50 years later, with identification of the metabolic pathway of two sequential hydroxylations to finally form the biologically active metabolite 1,25-dihydroxyvitamin D3 (1,25D)2 and a specific nuclear receptor3 that it was realised that it acts as a steroid hormone in an endocrine manner. Thus, the paradigm of vitamin D3 as a precursor of an endocrine agent has dominated thinking in this area until the end of the 20th century. However the 21st century has seen a blossoming of data obtained from a number of organ systems indicating that vitamin D3 has an autocrine action (that is, the activity of vitamin D3 arises from 1,25D synthesised within those cells), and/or a paracrine action (that is 1,25D is synthesised in one cell type and acts within adjacent cells). Some of these data have ruled out an endocrine action of 1,25D in some tissues.
This new paradigm for vitamin D activity provides an explanation of many new activities for vitamin D and certainly has implications for the required serum levels of the pro-hormone 25-hydroxyvitamin D3 (25D) as a marker of vitamin D status for optimal health. The synthesis of adequate 1,25D by any tissue is dependent on the level of the enzyme 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1), which catalyses the conversion of 25D to 1,25D, and the level of the substrate, (25D). The kidney is a suitable example to discuss the importance of the level of CYP27B1 because it is finely regulated to contribute to the homeostasis of extracellular fluid (ECF) levels of calcium and phosphate.4 As will be discussed in more detail, when plasma 25D levels fall, there is a rise in parathyroid hormone (PTH) levels which itself up-regulates the expression of renal CYP27B1, the site of synthesis for circulating 1,25D. The renal synthesis of 1,25D continues even when 25D levels fall to levels below 40 nmol/L, levels considered to be inadequate.5 This circulating 1,25D continues, or even increases the stimulation of the intestinal absorption of calcium and phosphate to maintain normal ECF levels under these conditions. At this time there is limited knowledge of the regulation of CYP27B1 expression in other tissues, although, as discussed below, the control of 1,25D synthesis in bone as well as other tissues is clearly different from that in kidney. Non-renal CYP27B1 may be unable to increase during low levels of 25D causing 1,25D levels to fall earlier in these tissues than in the plasma.
Our previous reviews over some eight years in this journal,4,5 have described the regulation of the vitamin D endocrine actions. This current article briefly addresses some of the wider aspects of the actions of vitamin D and factors influencing vitamin D status in all animal life. It focuses on recent developments supporting the concept of autocrine/paracrine actions of vitamin D and implications for desirable levels of vitamin D status for optimal health in bone.
Is it Plausible for Vitamin D to Have the Wide Range of Biological Activities That Have Been Ascribed to it?
Over recent years a low vitamin D status has been reported to increase the risk of numerous diseases apart from its well-characterised role in the aetiology of rickets in children and osteomalacia in adults. This list includes cancer, cardiovascular disease, skeletal muscular disorders and autoimmune diseases such as Type I diabetes, rheumatoid arthritis and multiple sclerosis, thus contributing to increased risk of falls, infections, reduced fertility and finally and perhaps most importantly, mortality.6 If a low vitamin D status increased the risk of each and every one of these states, then vitamin D would have to meet a number of criteria including those discussed below:
Modulate the Expression of a Large Number of Genes
A number of studies, particularly arising from cancer research, have reported that the vitamin D receptor (VDR), acting through the binding of its ligand 1,25D, can modulate the expression of a large number of genes. The key tumour suppressor gene TP53 codes for a protein, p53, which plays a key role in regulating cell proliferation and is mutated in many human cancers. Vitamin D shares many pathways with p53 that can be regulated either directly by p53 or indirectly through vitamin D.7 Wang and colleagues8 studied the ability of 1,25D to increase the expression of genes within a squamous cell carcinoma cell line in culture and identified that the expression of a total of 913 genes were affected with 734 (∼80%) being increased. They also conducted an in silico analysis for DNA sequences that bind the liganded vitamin D receptor (vitamin D receptor response elements, VDREs) shared between human and mouse genomes and identified 3065 genes which are potentially modulated by vitamin D. A recent major review on vitamin D activity acknowledged that ‘a wide-range of studies agree that 1,25D regulates …. a very large number of genes (0.8–5% of the total genome) … involved in … growth regulation, DNA repair, differentiation, apoptosis, membrane transport, metabolism, cell adhesion and oxidative stress.’9 Thus it appears that vitamin D does modulate a very large number of genes, possibly up to 10 per cent of the human genome, a very large number indeed.
Subject to Powerful Evolutionary Pressure
The great natural events of our planet are largely determined by its orientation to the sun, changes of which are responsible for the changing of the seasons and of vast ecosystems including the melting of the polar icecaps, flooding of the deserts or generating the monsoons at the tropics. Any forms of life that can adapt their metabolism to such changes are likely to outperform others by increasing their energy intake and reproductive capacity. Vitamin D3 is synthesised in response to UV-B (wavelength 295–310 nm) irradiation of the skin. As the orientation of the earth to the sun changes and therefore the seasons change, the intensity of visible light and UV-A irradiance on the surface of the earth change to a lesser extent than does UV-B irradiance. For example at 35º latitude, the intensity of UV-B irradiance is three times greater at the summer solstice compared to the winter solstice.10 Thus, the synthesis of vitamin D3 is the only known biological marker of the changing of the seasons. Thus vitamin D3 has the capacity to act as a signal for the body to optimise metabolism and other functions such that it can adapt to take greatest advantage of these great changes to the various ecosystems on our planet.
Expression of VDR and 25-hydroxyvitamin D 1α-hydroxylase (CYP27B1) Genes is Widespread Throughout Tissues
It is well recognised that the VDR gene is expressed in ‘virtually every tissue of the body’9 and therefore it meets the criterion for having the capacity to influence activities within a very large proportion of the body’s tissues. However, it is not so well known that the enzyme responsible for the synthesis of 1,25D from 25D, CYP27B1, is also expressed in a very large number of tissues. Immunohistochemical techniques have been used to identify the presence of the enzyme in a wide range of human tissues including kidney, skin, lymph nodes, tonsil, colon, pancreas, adrenal gland, placenta and brain cells.11 This is not a complete list but simply the range of tissues utilised by these researchers.
An alternative approach to study the distribution of tissues which express the CYP27B1 gene was to prepare a transgenic mouse model in which the −1501 base pairs of the human CYP27B1 gene, that is, the promoter region regulating expression of this gene, was linked to the luciferase reporter gene.12 Confocal microscopy and immunofluorescence demonstrated that the luciferase enzyme and the endogenous CYP27B1 enzyme were expressed in the identical cells in the kidney, testis and brain organs.13 A non-exhaustive survey of tissues found that human CYP27B1 gene promoter activity was also identified in skin, bone, bone marrow, spleen, skeletal muscle, heart, distal small intestine, lung and liver (Figure 1). However, it must be emphasised that circulating 1,25D, in most cases, is accounted for, only by metabolism of 25D by the kidney and there is no evidence that extra-renal production of 1,25D contributes to the circulating levels other than in disease or following placentation.14
Figure 1.
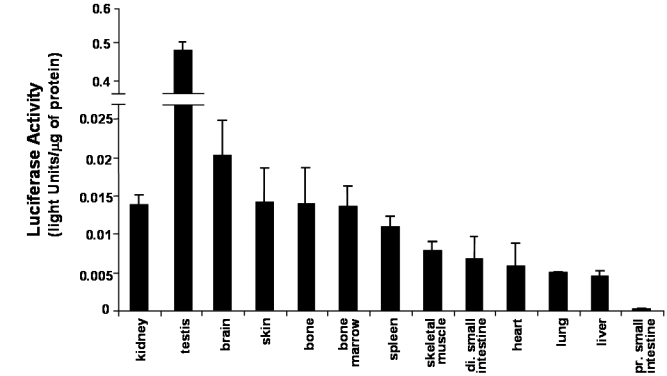
Human CYP27B1 promoter activity, expressed as luciferase reporter gene activity in various tissues isolated from male 12-week old transgenic mice (n=3) containing the −1501 base pairs human CYP27B1 gene linked to the luciferase gene fed a laboratory chow diet. (Reprinted from Reference 12 with permission of the publishers).
The quantity of promoter activity is also of interest. In 12 week-old male mice fed a normal chow diet containing high levels of calcium (normally 0.8% to 1% calcium), the major tissue of expression was the testis followed by the brain (Figure 1). The kidney exhibited comparable levels of CYP27B1 promoter activity as skin, bone and bone marrow.
Thus, under conditions of adequate dietary calcium intake, the kidney is not the site of the highest level of CYP27B1 activity. However, under conditions of low dietary calcium intake and low vitamin D status marked induction of renal enzyme activity takes place.14,15
Thus, it is quite feasible for a low vitamin D status to increase the risk of a wide range of disease states. The hormone is capable of modulating the expression of a very large number of genes, possibly up to 10% of the genome. It is the biological indicator of the changing of the seasons, a time when ecosystems markedly change and therefore has been subjected to the strongest of evolutionary pressures. Finally, vitamin D is capable of exerting activities in a very wide range of tissues since expression of the VDR occurs in almost all tissues and the expression of the CYP27B1 enzyme which is responsible for synthesising 1,25D from 25D, is also very widely expressed.
Well-established Autocrine Activities of Vitamin D in Skin and Immune Cells
Vitamin D Metabolism and Activities in Skin Tissue
Skin is a unique tissue with regard to vitamin D synthesis and activity as it is the only tissue in which UV-B irradiation can directly synthesise pre-vitamin D3 from 7-dehydrocholesterol which is then converted into vitamin D3 at 37°C.
Skin cells also express each of the enzymes required for the sequential hydroxylation of vitamin D3 to generate the active 1,25D metabolite as well as expressing the 25-hydroxyvitamin D 24-hydroxylase (CYP24) enzyme that inactivates vitamin D metabolites providing a detoxification of cells with regard to 1,25D activity.16 This tissue also expresses the vitamin D receptor which allows the modulation of gene expression responsible for cell proliferation and differentiation (Figure 2). Secondly, the differentiation of keratinocytes to form the various levels of the epidermis, including the superficial cornified layer, is particularly sensitive to extracellular calcium concentrations.17 Extracellular calcium stimulates the expression of genes for cross-linking proteins necessary for the formation of the cornified envelope of skin and there is a gradient of calcium across the epidermis with the lowest level in the basal layer and the highest level in the granular layer just below the cornified layer.18
Figure 2.
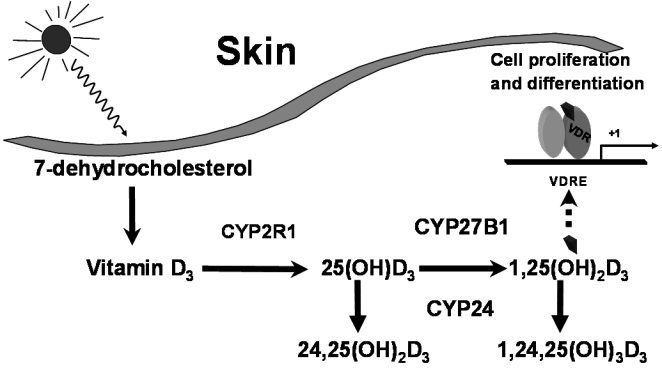
The synthesis and metabolism of vitamin D within keratinocytes including metabolism by the vitamin D-25-hydroxylase enzyme (CYP2R1), the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) and the 25-hydroxyvitamin D-24-hydroxylase (CYP24). (© Howard A Morris)
The critical role of vitamin D3 in skin development was immediately evident with the production of a mouse line in which the VDR gene was ablated, the VDR null mice. These mice lose their hair irrespective of whether they are hypo- or normocalcaemic or the status of their skeleton.19 The VDR null mice also demonstrate a defect in epidermal differentiation with reduced levels of the cross-linking proteins necessary for development of the cornified layer. However, this state is corrected when plasma calcium is returned to normal. Interestingly the CYP27B1 null mouse, which shares most of the features of calcium, phosphate and bone phenotypes with the VDR null mouse, has a different phenotype with regard to the epidermis. CYP27B1 null mice also show a reduction in the levels of the cross-linking proteins but normocalcaemia does not rescue this phenotype. Furthermore, the order of their epidermal calcium gradient is destroyed and when the permeability barrier of skin is disrupted, their ability to recover is delayed.20
These in vivo findings clearly indicate the critical role of vitamin D receptor and local synthesis of 1,25D in skin tissue with subtle differences of action between the VDR and the 1,25D-liganded VDR. Cellular-based studies demonstrate that 1,25D as an autocrine factor promotes keratinocyte differentiation via many of the same pathways as calcium, although in the absence of calcium, this differentiation is not optimised.17 The level of VDR and CYP27B1 vary with differentiation of keratinocytes with the lowest levels occurring in the later stages suggesting feedback regulation of this pathway. 1,25D increases the expression of the cross-linking proteins producing increased cornified envelope production, an effect that can be obtained with 25D because of its conversion to 1,25D by the CYP27B1 enzyme present in these cells. Most interesting is the interaction between the calcium signalling system and the actions of 1,25D. The expression of the calcium-sensing receptor is increased by 1,25D. The activities of intracellular signalling pathways are modulated by both 1,25D and calcium. 1,25D and calcium also interact in their ability to increase expression of the cross-linking proteins in keratinocytes. One explanation is that the regulatory region of the gene, the promoter, for at least one of these proteins, involucrin, contains both a VDRE and a calcium response element. Furthermore, calcium, at least indirectly, regulates coactivator proteins that complex with the liganded-VDR when it is bound to the VDRE to enhance gene expression.17 These molecular interactions between the vitamin D pathway and extracellular calcium are most interesting and clearly are likely to have implications for other tissues including bone cell metabolism.
Vitamin D Activities and the Innate Immune System
It should be of no surprise that vitamin D acts to optimise the immune system as a Nobel Prize in Physiology and Medicine in 1903 was awarded to Niels Ryberg Finsen ‘in recognition of his contribution to the treatment of diseases, especially lupus vulgaris, with concentrated light radiation, whereby he has opened a new avenue for medical science.’21 Of course clinical practice for the treatment of tuberculosis for many years had been to encourage patients to move to lower latitudes or spend more time in the sunshine, each of which would likely increase vitamin D status. However, the molecular basis of this activity was not elucidated until the 21st century.
The innate immune system provides a rapid host mechanism for defence against microbial pathogens which in mammals is mediated in part by the toll-like receptors which recognise a variety of microbial-derived ligands including bacterial lipoproteins.22 A microarray study of gene expression stimulated by exposure of human monocytes to a synthetic 19,000 D Mycobacterium tuberculosis-derived lipopeptide, identified that the VDR and a calcium-binding pro-inflammatory molecule, S100A12, were two genes that were markedly upregulated compared to incubation in culture medium alone.23 The identification of the VDR prompted the researchers to investigate further the expression of VDR-related genes and the next to be identified was the increased expression of the CYP27B1 gene at 12 to 24 hours after exposure. When 1,25D or 25D were included in the incubation medium of primary human monocytes, a dose-dependent upregulation of expression of the gene for the antimicrobial peptide cathlecidin was detected.
This ground-breaking study eventually identified that activation of the toll-like receptors of human monocytes and macrophages by microorganisms such as M. tuberculosis, induces expression of VDR and CYP27B1 genes, as well as engulfment of the microorganism into phagosomes. When the plasma 25D level is approximately 80 nmol/L, but certainly greater than 20 nmol/L, CYP27B1 enzyme activity synthesises adequate 1,25D to activate the VDR and increases induction of the antimicrobial peptide cathlecidin. Cathlecidin is translocated to the phagosomes where it kills the intracellular M. tuberculosis.23 This work provides an explanation for the higher susceptibility of African-Americans to tuberculosis because of their lower vitamin D status compared to Caucasian Americans and suggests that different vitamin D levels of various human populations may contribute to their susceptibility to microbial infection.
Vitamin D Actions on Bone - Endocrine and Autocrine/Paracrine Activities
Osteomalacia or rickets in children, is the index disease of vitamin D deficiency, therefore when a low vitamin D status was found to be associated with elderly patients suffering a hip fracture at both high and medium latitudes,24,25 there was a view that this fracture may be the result of osteomalacia rather than osteoporosis, a view that is even present today.26 Confusion as to the cellular and structural impact of vitamin D deficiency of bone arises from the necessity to examine the status of mineralisation using invasive bone biopsy techniques.
Thus, the use of rodent models to investigate the interaction between vitamin D status and dietary calcium intake with respect to their effects on bone architecture and bone cell activity has been most helpful. In one such study adult female rats were allocated to either vitamin D deficient or replete diets and then further allocated to 0.1% or 1% calcium diets for 3 months. Rats fed the vitamin D deplete diet achieved mean serum 25D levels of 14 nmol/L while animals fed vitamin D replete diets achieved 25D levels of 97 to 135 nmol/L.27 Osteoid maturation time (OMT), a measurement reflecting the rate of mineralisation, was significantly higher (12.6 days) in the vitamin D deplete/0.1% calcium group, a result which is diagnostic for osteomalacia since it signifies a mineralisation defect. In the remaining groups, OMT was reduced to 6 days, a value excluding osteomalacia. However metaphyseal trabecular bone volume and cortical width were reduced in both vitamin D replete/0.1% calcium and vitamin D deplete/1% calcium groups compared with rats fed the vitamin D replete/1% calcium diet (Figure 3).28 These data indicate that in the context of vitamin D and dietary calcium depletion, osteomalacia occurs only when both vitamin D and dietary calcium levels are markedly reduced and osteoporosis occurs with either a low calcium diet with replete vitamin D levels or when vitamin D status is low but dietary calcium is adequate. Thus, osteoporosis, which is defined as a reduced quantity of normal bone within the bone tissue, can result from vitamin D depletion when dietary calcium is adequate. These results provide an explanation for the osteoporosis and increased risk of hip fracture observed when elderly subjects are depleted for vitamin D.
Figure 3.
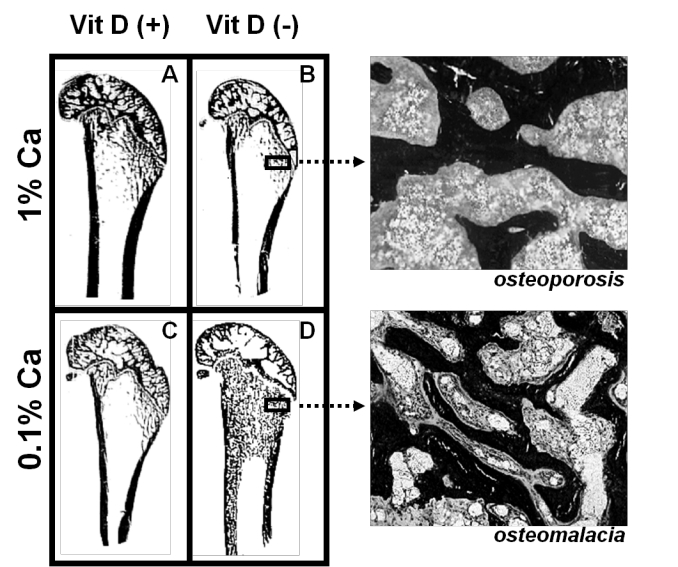
Longitudinal sections (Von Kossa stained) of 9-month old Sprague-Dawley rat distal femora following 3 months feeding either A. 1% calcium/20 IU vitamin D3/day; B. 1% calcium/0 IU vitamin D3/day; C. 0.1% calcium/20 IU vitamin D3/day; D. 0.1% calcium/0 IU vitamin D3/day. Highly trabecularised bone with osteomalacia (D) is evident in contrast to reduced trabecular bone volume (B &C) compared to A. (© Howard A Morris)
Further information on the aetiology of osteomalacia has been derived from studies on animal models which are vitamin D deficient or in which vitamin D activity has been destroyed through ablation of genes either for the VDR or the CYP27B1 enzyme. These animals, when fed a 1% calcium, 0.6% phosphorus diet, develop severe osteomalacia with hypocalcaemia, hypophosphataemia and severe secondary hyperparathyroidism. However, if they are fed a 2% calcium, 1.25% phosphorus, 20% lactose diet from weaning until 10 weeks of age, they maintain normocalcaemia and normophosphataemia, do not demonstrate a bone mineralisation defect and apparently achieve normal trabecular bone volume status.29 Thus, vitamin D deficiency gives rise to osteomalacia as a result of the loss of the 1,25D endocrine action on the intestinal absorption of calcium and phosphate since it can be resolved by overcoming this requirement for vitamin D by feeding high levels of these nutrients sufficient to maintain normal plasma levels. Based on these results, some researchers have concluded that the action of vitamin D to maintain a healthy skeleton is limited to regulation of intestinal calcium and phosphate absorption.
Similar experimentation was conducted by Panda and colleagues,30 however, they maintained their feeding experiments until mice were aged 16 weeks of age. They confirmed the resolution of the osteomalacia as shown previously but in these older animals the trabecular bone volume was actually only about 50% of the level achieved by wild type mice. These data now suggest that vitamin D has an extra activity on bone tissue in adult animals to maintain bone volume and to protect against osteoporosis. Such data are consistent with observational clinical data from hip fracture patients where bone histomorphometry has indicated osteoporosis is evident in most cases and osteomalacia in only a small minority of cases.31
Clinical studies indicate that plasma levels of 1,25D and intestinal calcium absorption do not decrease until plasma 25D levels fall below 20 nmol/L.32 However, the mean value for serum 25D levels in hip fracture cases is significantly above that value at 40 nmol/L.25 Interestingly, the risk of nonvertebral fractures including the hip does not reduce until serum 25D levels of 80 nmol/L or greater are achieved.33
The effects of serum 25D levels between 20 and 80 nmol/L on bone cell activities are ill-defined and rodent studies have again been most useful for the investigation of this issue. Young adult rats (3 months of age) fed a 0.4% calcium, vitamin D deficient diet for 4 months achieved a mean serum 25D level of 12 nmol/L with an OMT indicative of osteomalacia.34 When serum 25D was raised to 22 nmol/L, osteomalacia was resolved while osteoporosis was evident in the groups with serum 25D levels between 22 and 80 nmol/L. This loss of trabecular bone volume was due to increased bone resorption as a result of increased expression of the RANKL gene in bone and increased osteoclastogenesis. No relationship was found between bone volume and either serum 1,25D or parathyroid hormone.34 Further studies have been conducted with 15 month old rats comparing the effects of varying vitamin D status on 0.1% or 1% calcium diets (Lee MC, Anderson PH, O’Loughlin PD unpublished results). The positive relationship between trabecular bone volume and serum 25D levels was confirmed and with these older animals it extended to a similar relationship between cortical bone volume and 25D levels with a maximum cortical bone volume being achieved at 25D levels of 100 nmol/L or greater. Furthermore, these studies indicate that only in animals achieving a positive calcium balance did increasing 25D levels to greater than 80 nmol/L increase cortical bone volume. Consistent with the increased cortical bone volume, increased bone strength was only achieved in animals fed 1% calcium and serum 25D levels greater than 80–100 nmo/L.
Therefore, for rodent models, osteomalacia occurs when serum 25D levels are below 20 nmol/L when 1,25D levels are low and when the dietary calcium intake is low. Serum 25D levels above 20 nmol/L, largely normalises the serum 1,25D level, which restores the endocrine action at the intestine with active calcium and phosphate transport at the intestine and normalisation of plasma calcium and phosphate levels. Under these conditions the bone mineralisation is also normalised, resolving osteomalacia. However 25D levels between 20 and 80 nmol/L result in the loss of normally mineralised bone at both the trabecular and cortical compartments. This activity is not related to plasma 1,25D suggesting an autocrine or paracrine action within the bone tissue itself. Maximum bone architecture and strength is only achieved when an adequate vitamin D status as represented by 25D levels greater than 80–100 nmol/L combined with sufficient dietary calcium to provide calcium intakes greater than the losses of calcium in urine and faeces. It is interesting to note that these critical values for serum 25D in rodents are comparable to suggested critical values in humans.
Vitamin D Metabolism and Activities in Bone Cells
The observations that serum 25D correlate with trabecular or cortical bone volumes have led some to suggest that 25D may have a direct biological action in bone cells, however, only at 25D levels of 500 nmol/L or more, can this pro-hormone activate the VDR.35 What is important here however, is that each of the major bone cell types, osteoblasts, osteoclasts and osteocytes, is capable of metabolising 25D to 1,25D to elicit biological activities. Human and rodent osteoblasts strongly express the CYP27B1 enzyme which is essential to convert 25D to 1,25D and to increase expression of key genes associated with maturation and mineralization when 25D is included in the culture media.36 25D in the media also reduces cell proliferation and stimulates osteoblast maturation and mineralisation in vitro.
In pre-osteoclasts, such as in human peripheral blood mononuclear cell preparations, CYP27B1 expression is also necessary for 25D to optimise osteoclastogenesis in the presence of RANKL and M-CSF in vitro.37 Most interestingly, the osteoclasts formed in the presence of 25D, demonstrated reduced resorbing activity compared with cells matured in the absence of vitamin D metabolites or cells in which the VDR gene has been ablated.38 Such data are consistent with the in vivo finding described above that osteoclastogenesis was reduced when serum 25D levels were above 80 nmol/L in rat models.
Osteocytes also express the CYP27B1 gene and mRNA levels increase with differentiation and are associated with the acquisition of mature osteocyte genes including MEPE (Matrix Extracellular PhosphoglycoprotEin), DMP1 (Dentin Matrix Protein 1), PHEX (Phosphate-regulating gene with Homologies to Endopeptidase on the X chromosome) (Atkins GJ, unpublished data) and FGF23 (Fibroblast Growth Factor 23).39 Thus, in vitro data provide strong evidence that the prohormone 25D is capable of metabolism by bone cells to the active hormone 1,25D to elicit various activities including the reduction of bone resorption by osteoclasts and to enhance maturation and mineralisation by osteoblasts and osteocytes. Each of these activities is consistent with the actions of adequate circulating levels of 25D observed in vivo.
The anabolic effects of circulating 25D on the skeleton are dependent on a dietary calcium intake which provides a greater amount of calcium than that excreted from the body. The question arising from such findings is whether calcium exerts any biological activities within the skeletal system itself rather than simply providing a raw material, in conjunction with phosphate, for bone mineralisation. In vitro studies demonstrate that the addition of calcium to culture media enhances the ability of osteoblast-like cells isolated from long bones to mineralise in vitro (Yang D, Atkins GJ, Morris HA unpublished results) although such effects may depend on the source of the cells.40 Interestingly, a recent report describes that when rats are fed a dietary calcium level sufficient to ensure a positive calcium balance, the levels of CYP27B1 mRNA are 3-fold higher in bone tissue compared with animals fed a low calcium diet.27 Messenger RNA levels for CYP24, which codes for the vitamin D catabolic enzyme 25-hydroxyvitamin D 24-hydroxylase and are highly regulated by 1,25D, were also elevated in bone from these animals suggesting that 1,25D levels are higher in the bone tissue. Thus, a contributing effect of adequate dietary calcium intake to their positive effects on bone mineral homeostasis may be, at least in part, through increasing the synthesis of 1,25D within bone tissue.
Implications of Autocrine/Paracrine Activities of Vitamin D in Bone Tissue for Vitamin D Requirements
As discussed above, the renal metabolism of 25D to 1,25D, which is the source of the circulating vitamin D activity, is exquisitely regulated to contribute to plasma calcium and phosphate homeostasis. Firstly, it is important to revise a principle of enzymology used in daily practice by clinical biochemists. The level of a product formed by any enzyme reaction is dependent on the level of substrate or the level of enzyme - usually described as Michaelis-Menten kinetics.41 Thus, when the level of enzyme remains constant, the amount of product formed will be dependent on the level of the substrate in the reaction. Conversely, if the level of the substrate remains constant, then the amount of product produced is dependent on the level of enzyme in the reaction. Thus, in biological systems, the level of the CYP27B1 enzyme in any tissue and its binding properties for 25D will determine the critical level of the substrate, serum 25D, required to produce the product, 1,25D. If the circulating 25D level falls below this critical level for a particular tissue, then that tissue will become 1,25D deficient. It is likely that different tissues have different levels of CYP27B1 under different conditions although there are limited data available (Figure 1). Hypocalcaemia, low serum 1,25D and increased PTH levels markedly increase renal CYP27B114,15 and high dietary calcium intakes increase bone cell CYP27B1.27
The contribution of the endocrine action of 1,25D to calcium homeostasis includes interaction with PTH secretion, regulation of intestinal calcium absorption and renal tubular reabsorption of calcium.4 The endocrine activity of 1,25D is solely dependent on the renal level of CYP27B1 in health or in non-pregnant women. The regulation of PTH secretion by the 25D may involve an autocrine activity within parathyroid cells. CYP27B1 is expressed in bovine parathyroid cells and converts 25D to 1,25D, while 25D and 1,25D suppress PTH mRNA and secretion of PTH protein.42 Further studies including the effects of ablating CYP27B1 activity in parathyroid cells are required to unequivocally identify that an autocrine action of 25D in the parathyroid gland regulates PTH secretion. However we can speculate from the current data on the contribution of endocrine and autocrine actions of 1,25D to regulate calcium and phosphate homeostasis. As vitamin D status declines, the level of serum 25D falls below an as yet unknown critical level for an autocrine action in parathyroid cells whereupon the inhibition of locally synthesised 1,25D on PTH synthesis and secretion is relieved. The consequent increase in circulating PTH levels acts to conserve plasma calcium by various mechanisms including increasing the level of CYP27B1 enzyme in renal cells, which in turn increases serum 1,25D levels. It is the increased renal CYP27B1 enzyme level that is key to the continued production of renal 1,25D at lower levels of circulating 25D. The increased serum 1,25D, acting in an endocrine manner, increases the efficiency of intestinal absorption of calcium and phosphate which attempts to normalise their levels in plasma.
In extra-renal tissues the existence of such feedback mechanisms for 1,25D synthesis is largely unknown. Unlike the kidney, in bone tissue low levels of serum 1,25D and high PTH do not up-regulate CYP27B1 levels.15 No mechanisms have been identified in other tissues.14 Therefore, as circulating 25D levels fall, it is likely that the synthesis of 1,25D will cease in non-renal tissues before it ceases in the kidney. In bone tissue, the fall of serum 25D levels results, at least in rodent models, in an increase in bone resorption which would contribute to maintaining calcium and phosphate homeostasis.34 As discussed above, clinical data strongly suggest that in humans a serum 25D level of approximately 20 nmol/L is a critical value for the kidney.32 Data from rodent and clinical studies suggest that optimal bone health requires serum 25D levels of 80 nmol/L or more. Thus, this value could represent a critical substrate level for CYP27B1 present in bone cells but this will need to be confirmed by biochemical studies of this enzyme activity in these cells.
Conclusions
The endocrine paradigm for vitamin D has been most useful for understanding its contribution to maintaining plasma calcium and phosphate homeostasis. However, once the association of a low vitamin D status with increased risk of hip fracture in the elderly was identified, it was evident that this paradigm was incomplete with regard to vitamin D biology in bone tissue. It was evident that in hip fracture patients, although serum 25D levels were low at approximately 40 nmol/L, serum 1,25D levels were not significantly reduced.43 What was interesting was the report that bone levels of 1,25D were lowered in patients with hip fracture.43 It has been known since the early 1980s that osteoblast-like cells had the capacity to convert 25D to 1,25D44 but such data were ignored at that time largely because the molecular tools were unavailable to investigate the cell physiology of this activity. During the latter years of the 1990s these tools became available providing the means for the generation of data on the extra-renal synthesis of 1,25D and provide evidence for a new, extra paradigm for vitamin D action, involving the autocrine/paracrine activity of 1,25D. This new paradigm now provides a plausible explanation for the observational data that optimal health outcomes are associated with higher levels of serum 25D than previously considered. Further basic research in this area will likely continue elucidating this most fascinating story.
Acknowledgments
The authors gratefully acknowledge the valuable and insightful discussions with Professor BEC Nordin. Experimental work produced within the laboratory of the authors was supported by grants from the NHMRC, ARC and Cancer Council SA.
Footnotes
Competing Interests: None declared.
References
- 1.Mellanby E. An experimental investigation on rickets. Lancet. 1919;1:407–12. doi: 10.1111/j.1753-4887.1976.tb05815.x. [DOI] [PubMed] [Google Scholar]
- 2.Fraser DR, Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970;228:764–6. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- 3.Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969;62:155–62. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PH, May BK, Morris HA. Vitamin D metabolism: new concepts and clinical implications. Clin Biochem Rev. 2003;24:13–26. [PMC free article] [PubMed] [Google Scholar]
- 5.Morris HA. Vitamin D: a hormone for all seasons—how much is enough? Clin Biochem Rev. 2005;26:21–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Grant WB, Cross HS, Garland CF, Gorham ED, Moan J, Peterlik M, et al. Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog Biophys Mol Biol. 2009;99:104–13. doi: 10.1016/j.pbiomolbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Maruyama R, Aoki F, Toyota M, Sasaki Y, Akashi H, Mita H, et al. Comparative genome analysis identifies the vitamin D receptor gene as a direct target of p53-mediated transcriptional activation. Cancer Res. 2006;66:4574–83. doi: 10.1158/0008-5472.CAN-05-2562. [DOI] [PubMed] [Google Scholar]
- 8.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19:2685–95. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nozawa H, Yamamoto H, Makita K, Schuch NJ, Pinheiro DK, Carbonne S, et al. Ground-based observations of solar UV radiation in Japan, Brazil and Chile. Revista Brasileira de Geofısica. 2007;5(Suppl. 2):S17–25. [Google Scholar]
- 11.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin D(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 12.Hendrix I, Anderson P, May B, Morris H. Regulation of gene expression by the CYP27B1 promoter-study of a transgenic mouse model. J Steroid Biochem Mol Biol. 2004;89–90:139–42. doi: 10.1016/j.jsbmb.2004.03.093. [DOI] [PubMed] [Google Scholar]
- 13.Anderson PH, Hendrix I, Sawyer RK, Zarrinkalam R, Manavis J, Sarvestani GT, et al. Co-expression of CYP27B1 enzyme with the 1.5kb CYP27B1 promoter-luciferase transgene in the mouse. Mol Cell Endocrinol. 2008;285:1–9. doi: 10.1016/j.mce.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix I, Anderson PH, Omdahl JL, May BK, Morris HA. Response of the 5′-flanking region of the human 25-hydroxyvitamin D 1α-hydroxylase gene to physiological stimuli using a transgenic mouse model. J Mol Endocrinol. 2005;34:237–45. [Google Scholar]
- 15.Anderson PH, O’Loughlin PD, May BK, Morris HA. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone. 2005;36:654–662. doi: 10.1016/j.bone.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann B, Genehr T, Knuschke P, Pietzsch J, Meurer M. UVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol. 2001;117:1179–85. doi: 10.1046/j.0022-202x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- 17.Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol. 1990;143:294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD, Oda Y, Xie Z. Calcium and 1,25(OH)2D: interacting drivers of epidermal differentiation. J Steroid Biochem Mol Biol. 2004;89–90:355–60. doi: 10.1016/j.jsbmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 20.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–92. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 21.The Nobel Prize in Physiology or Medicine. 1903. http://nobelprize.org/nobel_prizes/medicine/laureates/1903/ (Accessed 5 July 2010)
- 22.Modlin RL, Cheng G. From plankton to pathogen recognition. Nat Med. 2004;10:1173–74. doi: 10.1038/nm1104-1173. [DOI] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Aaron JE, Gallagher JC, Anderson J, Stasiak L, Longton ED, Nordin BE, et al. Frequency of osteomalacia and osteoporosis in fractures of the proximal femur. Lancet. 1974;1:229–33. doi: 10.1016/s0140-6736(74)92545-8. [DOI] [PubMed] [Google Scholar]
- 25.Morris HA, Morrison GW, Burr M, Thomas DW, Nordin BE. Vitamin D and femoral neck fractures in elderly South Australian women. Med J Aust. 1984;140:519–21. doi: 10.5694/j.1326-5377.1984.tb108222.x. [DOI] [PubMed] [Google Scholar]
- 26.Priemel M, von Domarus C, Klatte TO, Kessler S, Schilie J, Meier S, et al. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–12. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 27.Anderson PH, Iida S, Tyson JH, Turner AG, Morris HA. Bone CYP27B1 gene expression is increased with high dietary calcium in mineralising osteoblasts. J Steroid Biochem Molec Biol. 2010;121:71–5. doi: 10.1016/j.jsbmb.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 28.Morris HA, O’Loughlin PD, Anderson PH. Experimental evidence for the effects of calcium and vitamin D on bone: a review. Nutrients. 2010;2:1026–35. doi: 10.3390/nu2091026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, et al. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology. 1999;140:4982–7. doi: 10.1210/endo.140.11.7110. [DOI] [PubMed] [Google Scholar]
- 30.Panda DK, Miao D, Bolivar I, Li J, Huo R, Hendy GN, et al. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–66. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 31.Arnala I, Kyrölä K, Kröger H, Alhava EM. Analysis of 245 consecutive hip fracture patients with special reference to bone metabolism. Ann Chir Gynaecol. 1997;86:343–7. [PubMed] [Google Scholar]
- 32.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Bone Res. 2008;23:1859–63. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 34.Anderson PH, Sawyer RK, Moore AJ, May BK, O’Loughlin PD, Morris HA. Vitamin D depletion induces RANKL-mediated osteoclastogenesis and bone loss in a rodent model. J Bone Miner Res. 2008;23:1789–97. doi: 10.1359/jbmr.080616. [DOI] [PubMed] [Google Scholar]
- 35.Rowling MJ, Gliniak C, Welsh J, Fleet JC. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–15. doi: 10.1093/jn/137.12.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, et al. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–28. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Kogawa M, Anderson PH, Findlay DM, Morris HA, Atkins GJ. The metabolism of 25-(OH)vitamin D3 by osteoclasts and their precursors regulates the differentiation of osteoclasts. J Steroid Biochem Mol Biol. 2010;121:277–80. doi: 10.1016/j.jsbmb.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 38.Kogawa M, Findlay DM, Anderson PH, Ormsby R, Vincent C, Morris HA, et al. Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology. 2010;151:4613–25. doi: 10.1210/en.2010-0334. [DOI] [PubMed] [Google Scholar]
- 39.Tang W-J, Wang L-F, Xu X-Y, Zhou Y, Jin WF, Wang HF, et al. Autocrine/paracrine action of vitamin D on FGF23 expression in cultured rat osteoblasts. Calcif Tissue Int. 2010;86:404–10. doi: 10.1007/s00223-010-9355-2. [DOI] [PubMed] [Google Scholar]
- 40.Liu YK, Lu QZ, Pei R, Ji HJ, Zhou GS, Zhao XL, et al. The effect of extracellular calcium and inorganic phosphate on the growth and osteogenic differentiation of mesenchymal stem cells in vitro: implication for bone tissue engineering. Biomed Mater. 2009;4:025004. doi: 10.1088/1748-6041/4/2/025004. [DOI] [PubMed] [Google Scholar]
- 41.Bais R, Panteghini M. Principles of clinical enzymology. In: Burtis CA, Ashwood ER, Bruns DE, editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. 4th ed. St Louis: Elsevier Saunders; 2006. pp. 191–218. [Google Scholar]
- 42.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–9. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 43.Lidor C, Sagiv P, Amdur B, Gepstein R, Otremski I, Hallel T, et al. Decrease in bone levels of 1,25-dihydroxyvitamin D in women with subcapital fracture of the femur. Calcif Tissue Int. 1993;52:146–8. doi: 10.1007/BF00308324. [DOI] [PubMed] [Google Scholar]
- 44.Turner RT, Puzas JE, Forte MD, Lester GE, Gray TK, Howard GA, et al. In vitro synthesis of 1 alpha, 25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci USA. 1980;77:5720–4. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]


