Abstract
Hormone secretion by thyrocytes occurs by fluid phase uptake and lysosomal degradation of the prohormone thyroglobulin (Tg). However, some Tg internalized by megalin bypasses lysosomes and is transcytosed across cells and released into the bloodstream. Because the hormone content of Tg is variable, we investigated whether this affects transcytosis. We found that rat Tg with a low hormone content [low-hormonogenic rat Tg (low-horm-rTg)] is transcytosed by megalin across thyroid FRTL-5 cells to a greater extent than rat Tg with a high hormone content [hormonogenic rat Tg (horm-rTg)]. In immunoprecipitation experiments, the Tg sequence Arg-2489-Lys-2503 (required for binding to megalin and heparan sulfate proteoglycans) was found to be more exposed in low-horm-rTg, which accounted for its preferential transcytosis. Thus, removal of surface heparan sulfate proteoglycans from FRTL-5 cells or blocking of 2489–2503 reduced transcytosis of low-horm-rTg to a greater extent than that of horm-rTg. Preferential transcytosis of low-horm-rTg affected hormone release. Thus, the increase in hormone release from horm-rTg in FRTL-5 cells determined by megalin blocking (due to reduced transcytosis and enhanced Tg degradation) was rescued by low-horm-rTg, suggesting that megalin is required for effective hormone release. This finding was confirmed in a small number of megalin-deficient mice, which had serological features resembling mild hypothyroidism. Reduced hormone formation within Tg in vivo, due to treatment of rats with aminotriazole or of patients with Graves' disease with methimazole, resulted in increased Tg transcytosis via megalin, in confirmation of results with FRTL-5 cells. Our study points to a major role of megalin in thyroid homeostasis with possible implications in thyroid diseases.
The thyroid plays a central role in the maintenance of body homeostasis through the action of thyroid hormones [thyroxine (T4) and triiodothyronine (T3)]. Excessive (hyperthyroidism) or defective (hypothyroidism) function is avoided by a negative-feedback control pathway involving the thyroid-stimulating hormone (TSH), which up-regulates hormone synthesis and is down-regulated by thyroid hormones themselves (1).
The follicle, the thyroid functional unit, is composed of a single layer of epithelial cells (thyrocytes) surrounding a lumen containing a material named colloid (2). The prohormone thyroglobulin (Tg) is the major protein component of colloid, into which it is secreted after synthesis by thyrocytes, which is up-regulated by TSH. At the cell–colloid interface, Tg undergoes iodination of tyrosyl residues, a process catalyzed by thyroperoxidase, resulting in the formation of thyroid hormones (3). If thyroid iodide uptake is effective, Tg hormone content depends on dietary iodine intake, which varies based on iodine availability in the environment (3). To face conditions of low iodine availability, large amounts of iodine are stored within colloidal Tg that, however, is not iodinated to the same extent and has a variable hormone content (2, 3).
Secretion of hormones by thyrocytes occurs by fluid-phase pinocytosis of Tg from the colloid followed by degradation in lysosomes (2, 4). Newly synthesized Tg is soluble and readily available for endocytosis and hormone release (5), whereas a proportion of Tg in storage is insoluble and undergoes solubilization by extracellular proteases before endocytosis (6). In addition to fluid-phase uptake, Tg is internalized by endocytic receptors, after which Tg is not degraded in lysosomes but is either recycled into the colloid or transported across cells by transcytosis (4). The multiligand endocytic receptor megalin is responsible for transcytosis (4, 7, 8).
Megalin was first identified by Kerjaschki and Farquhar (9) and later found to be a member of the low-density lipoprotein receptor family, composed of a single-transmembrane domain, a large ectodomain, and a short cytoplasmic tail (10–12). Megalin mediates tubular uptake of low-molecular-weight proteins in the kidney and is also involved in the development of the central nervous system, which is severely impaired in ≈98% of megalindeficient (Meg–/–) mice (13). In the thyroid, megalin is expressed in a TSH-dependent manner on the apical surface of thyrocytes, where it binds and internalizes Tg, after which Tg is transported across cells by transcytosis (7). Tg is then released from basolateral membranes into the bloodstream, being in part complexed with secretory components of megalin ectodomain (8). Transcytosis also occurs in the kidney for retinol-binding protein (14), another megalin ligand, but this is not the usual function of the receptor; instead, for unknown reasons, the majority of megalin ligands undergo lysosomal degradation (13).
If the structure of thyroid follicles is intact, transcytosis is the major route by which Tg reaches the circulation (4). However, the physiological role of this pathway is unclear. An early interpretation was that transcytosis reduces hormone release by diverting hormonogenic Tg from lysosomes (4). We have, however, considered that transcytosis may rather serve to dispose Tg molecules with a low hormone content. In support of this hypothesis, here we show that transcytosis is preferential for low-hormonogenic Tg, which plays an important role in the control of thyroid function.
Methods
Tg Preparations. Hormonogenic rat Tg (horm-rTg) was purified from frozen rat thyroid glands as described (7). Low-hormonogenic rat Tg (low-horm-rTg) was obtained from media of FRTL-5 cells (American Type Culture Collection, Rockville, MD), as described (15). Thus, FRTL-5 cells, a differentiated rat thyroid cell line (16), are unable to form hormone residues within endogenously synthesized Tg (17). By SDS/PAGE and Western blotting (WB), Tgs resolved as 660- and 330-kDa bands. The T4 content of Tg was determined as described (15). Radiolabeled Tgs were prepared with 125I-Na (NEN Life Science) by using IODO beads (Pierce) (specific activity: 1,500–7,000 cpm/ng). Tgs were labeled with biotin by using EZ-Link Sulfo-NHS-LC-Biotin (Pierce).
Antibodies. Rabbit anti-Tg was from Axell (Westbury, NY). Mouse anti-Tg (unlabeled or horseradish peroxidase conjugated) was from DAKO. A rabbit antibody against the rat Tg sequence 2489–2503 (anti-rTgP) was described previously (18). Rabbit anti-T4 was from Cappel. Two rabbit (A55 and antimegalin-GST) and one mouse (1H2) antimegalin antibodies were described previously (8). None of these reacted with Tg (not shown). A fluorescein isothiocyanate-conjugated mouse antibody against heparan sulfate proteoglycans (HSPGs) was from Seikagaku Kogyo (Tokyo).
Transcytosis and Uptake Experiments. For transcytosis assays, we used confluent FRTL-5 cells cultured on 3-μm filters in culture inserts (Becton Dickinson) placed in 24-well plates, as described (7). Paracellular transport of 3H-mannitol was measured as described (7). In experiments with unlabeled or biotin-labeled Tgs, 2 μg of Tg was applied to the upper chamber in 200 μl of binding buffer (Coon's F12 medium, 5 mM CaCl2/0.5 mM MgCl2/0.5% ovalbumin), and the lower chamber was rinsed with 200 μl of Tg-free buffer. After 1 h at 37°C, Tg was measured in media from the lower chamber by WB or ELISA. In experiments with 125I-Tg, 5 μg of Tg was applied to the upper chamber in 500 μl of culture medium (containing 5 mM CaCl2), and the lower chamber was rinsed with 1,000 μl of Tg-free medium. After 1 h at 37°C, radioactivity was measured in 100 μl of aliquots of the medium from the lower chamber. In certain experiments, Tgs were applied with 200 μg/ml 1H2, anti-rTgP, mouse (mIgG), or rabbit IgG (RIgG). In other experiments, cells were preincubated for 2 h at 37°C with heparitinase (Seikagaku, 5 milliunits/ml) or chondroitinase ABC (Sigma, 10 units/ml). Expression of cell surface HSPGs on FRTL-5 cells was determined by flow cytometry, as described (19). Uptake experiments were performed by using a technique modified from Goldstein et al. (20), as described (21). ELISAs for Tg were performed as described (7).
Analysis of Transcytosed and Serum Tg. Unlabeled purified Tgs or Tgs collected from the upper or lower chamber in transcytosis assays were analyzed by WB, either directly or after precipitation, with rabbit anti-Tg, which was performed by incubating samples overnight at 4°C with protein A beads (Pharmacia Biotec) coupled with anti-Tg. Similar experiments were performed with extracts of four thyroid tissue and serum samples collected simultaneously at surgery, in accordance with institutional guidelines, from four patients with Graves' disease (GD).
Immunoprecipitation Experiments with Purified Tgs. Tg (5 μg) was incubated overnight at 4°C with protein A beads coupled with RIgG or anti-T4. Beads were spun and supernatants subjected to WB for Tg, either directly or after precipitation with anti-rTgP, which was performed by incubating samples overnight at 4°C with protein A beads coupled with anti-rTgP or RIgG.
T3 Release Experiments. One-hundred micrograms of horm-rTg, low-horm-rTg, or both, alone or with 200 μg/ml 1H2 or mIgG, were applied to the upper chamber of FRTL-5 cells on filters in 500 μl of binding buffer. The lower chamber was rinsed with 1,000 μl of Tg-free buffer. After 6 h at 37°C, free-T3 was measured in media from the upper and lower chamber with a kit from Lysophase (Sesto S. Giovanni, Milan). T3 was undetectable in media from cells incubated with Tg-free buffer.
In Vivo Studies. (i) Mice. Sera from three homozygous Meg–/– mice generated by targeted gene disruption as reported (22) and three control littermates (Meg+/–) were collected at the Max Delbrueck Center according to institutional guidelines. Free T4 (FT4) was measured by equilibrium dialysis immunoassay (Nichols Institute, San Juan Capistrano, CA). TSH was measured as described (23). Tg was measured by ELISA.
(ii) Rats. Six female Lewis rats weighing 100–120 g (Charles River Breeding Laboratories) received 0.04% aminotriazole (3-amino-1,2,4-triazole, Sigma) in drinking water and l-thyroxine4 (L-I4) (Sigma, 20 μg/day) by i.p. injection, for 6 days. Six untreated rats were used as controls. Animal care and death were in accordance with institutional guidelines. At death, serum samples were collected and thyroid glands harvested and frozen for megalin immunofluorescence studies, which were performed as described (7). TSH (Amersham Pharmacia), total T3 (Diagnostic Products, Los Angeles), and Tg (by ELISA) were measured in all sera. To detect megalin secretory components complexed with Tg by immunoprecipitation, sera were incubated overnight at 4°C with protein A beads coupled with rabbit anti-Tg, antimegalin-GST, or RIgG and then subjected to WB for Tg. (iii) Patients with GD. Serum samples from 13 consecutive patients with GD (3 male, 10 female, age: 38.3 ± 11.4 yr), selected based on the absence of serum autoantibodies to Tg, were collected at the University of Pisa according to institutional guidelines. Patients were seen when untreated and 3 mo after beginning methimazole, which was given orally as follows: daily dose, 30 mg for 2 wk, 15 mg for 2 wk, 10 mg for 2 wk, and 5 mg up to 3 mo. The following serum tests were performed: free-T3 (Lysophase); TSH (Delfia Wallac, Gaithersburg, MD); anti-TSH receptor (TSH-R) autoantibodies (Brahms, Berlin); and Tg (Sorin Biomedica, Saluggia, Italy). To estimate megalin secretory components complexed with Tg, Tg was measured in serum samples from treated or untreated patients after overnight preadsorption at 4°C with protein A beads coupled with antimegalin A55 or RIgG. Serum antimegalin autoantibodies, measured as described (24), were undetectable (not shown). Archival formalin-fixed paraffin-embedded thyroid specimens from three GD patients and normal thyroid tissue from the contralateral normal lobe of two patients with thyroid cancer were used for megalin immunohistochemistry, performed as described (8).
Statistical Analysis. Data are presented as mean ± SD or median values, as appropriate, or are representative of similar results obtained in at least three experiments. Unpaired or paired t, Mann—Whitney, and Wilcoxon Signed Rank tests were performed when appropriate by using stat-view (SAS, Cary, NC).
Additional details are in Supporting Methods, which is published as supporting information on the PNAS web site.
Results
Transcytosis of Tgs Across FRTL-5 Cells. We used two preparations of rat Tg, horm-rTg and low-horm-rTg, which contained 2.6 and 0.3 molecules of T4/molecule, respectively. As reported (7), we used FRTL-5 cells on filters that displayed features of polarity, including apical megalin expression (not shown) and negligible paracellular leakage. Thus, transport of 3H-mannitol (≈1 kDa) was 0.78% in 1 h, compared with 47.07% in filters without cells.
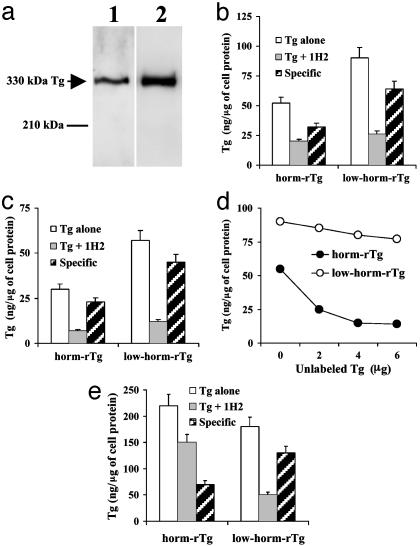
After addition of unlabeled (Fig. 1 a and b), 125I-labeled (Fig. 1c) or biotin-labeled (Fig. 1d) Tgs to the upper chamber, 15–22% of Tgs applied were found in the lower chamber after1hat37°C, compared with 39.9% in filters without cells (not shown). Based on results with 3H-mannitol, we assumed that ≈1% of Tg in the lower chamber had leaked and concluded that ≈99% had been transcytosed. Regardless of the labeling, low-horm-rTg was transcytosed to a greater extent than horm-rTg (Fig. 1 a–d). Transcytosed Tg resolved as a single band of ≈330 kDa by WB (Fig. 1a), with a pixel density 2.7-fold higher for low-horm-rTg (163.1 pixels/cm2) than for horm-rTg (58.5 pixels/cm2). By ELISA (Fig. 1b), we estimated that unlabeled low-horm-rTg was transcytosed to a 1.7-fold greater extent than horm-rTg. 125I-labeled (Fig. 1c) and biotin-labeled (Fig. 1d) low-horm-rTg were transcytosed, respectively, to a 1.9- and 1.6-fold greater extent than horm-rTg. As in previous studies (7), when Tgs were applied to FRTL-5 cells with antimegalin 1H2, transcytosis was reduced (Fig. 1 b and c), whereas mIgG had no effect (not shown). Transcytosis via megalin (total transcytosis minus transcytosis in the presence of 1H2) was greater (by 1.6- to 1.8-fold) for low-horm-rTg, regardless of the labeling. When biotin-labeled Tgs were applied to FRTL-5 cells with increasing concentrations of the other Tg preparation unlabeled, transcytosis was reduced (Fig. 1d). However, unlabeled low-horm-rTg reduced transcytosis of biotin-labeled horm-rTg to a much greater extent (by ≈75% at the highest concentration) than the opposite (≈15%), suggesting that when cells are exposed to both Tgs, low-horm-rTg is transcytosed preferentially.
Fig. 1.
(a–d) Transcytosis of Tg across FRTL-5 cells. Horm-rTg and low-hormrTg were used unlabeled (a and b), 125I-labeled (c), or biotin-labeled (d). Tg was detected by WB (a), ELISA (b and d), or γ counting (c). (a) Lane 1, horm-rTg; lane 2, low-horm-rTg. (b and c) Tg was applied alone or in the presence of antimegalin 1H2. Transcytosis via megalin (specific) = total transcytosis – transcytosis in the presence of 1H2. (d) Each biotin-labeled Tg preparation was applied alone or in the presence of the other preparation unlabeled. (e) Uptake of unlabeled Tg by FRTL-5 cells. Tgs were applied alone or with 1H2. Uptake via megalin (specific) = total uptake – uptake in the presence of 1H2.
As shown in Fig. 1e, uptake of Tgs by FRTL-5 cells was reduced by antimegalin 1H2, but not by mIgG (not shown), but reduction was greater for low-horm-rTg (≈72%) than for horm-rTg (≈32%), suggesting that preferential transcytosis is a function of megalin-mediated uptake.
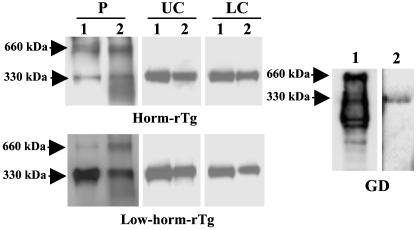
Our results offer an explanation for previous findings in rats and patients with GD showing that serum Tg has a low hormone content (25, 26) and argue against the possibility that Tg undergoes partial lysosomal proteolysis before transcytosis, as proposed by Rousset et al. (27). These conclusions are also supported by the knowledge that inhibition of lysosomal function does not affect transcytosis significantly (28) and by experiments showing that transcytosed Tg has electrophoretic properties and a conformational state similar to Tg before transcytosis. Thus, 330-kDa Tgs transcytosed across FRTL-5 cells migrated similarly and were precipitated to a similar extent (by ≈70%) by an anti-Tg antibody as Tgs added to the upper chamber or collected from the upper chamber after transcytosis (Fig. 2). In addition, serum Tg from GD patients resolved as a single 330-kDa band, was precipitated by anti-Tg, and was devoid of bands of lower molecular mass, most of which representing degradation products, which were instead seen in thyroid extracts from the same patients.
Fig. 2.
Analysis of transcytosed and serum Tg. WB (lane 1) or immunoprecipitation (lane 2) of purified Tgs (P), of Tgs collected from the upper (UC) or lower (LC) chamber of FRTL-5 cells in transcytosis assays. Immunoprecipitation of Tg in the thyroid extract (lane 1) or serum sample (lane 2) from a GD patient. Similar results were obtained in three additional patients.
Although our results argue against partial lysosomal proteolysis, some degree of proteolysis due to cell surface proteases must occur. Thus, unlike purified Tgs, which resolved as 660- (the Tg dimer) and 330-kDa bands (the Tg monomer), transcytosed Tgs and Tgs in the upper chamber resolved as a single 330-kDa band (Fig. 2), suggesting disruption of Tg dimers. Partial cell surface proteolysis was also supported by results reported below showing that there was some degree of hormone release from horm-rTg in the upper chamber of FRTL-5 cells.
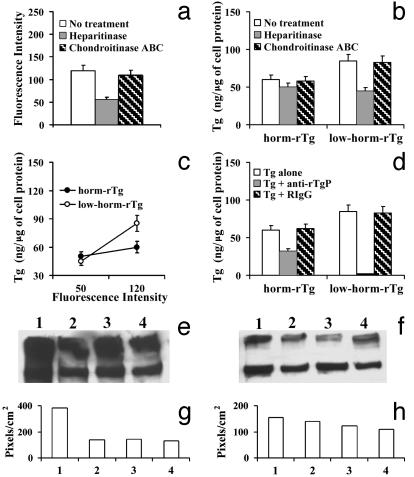
Role of HSPGs and of the Sequence 2489-2503. We studied the role of HSPGs in transcytosis, because binding to HSPGs facilitates Tg binding to megalin (18, 19). Treatment of FRTL-5 cells with heparitinase resulted in a ≈55% reduction of surface HSPGs, as determined by flow cytometry (Fig. 3a), whereas chondroitinase ABC (used as a control) had no effect. Heparitinase reduced minimally (by ≈18%) transcytosis of horm-rTg and markedly (by ≈47%) transcytosis of low-horm-rTg (Fig. 3b), and there was a relation between transcytosis and surface HSPGs, which was more pronounced for low-horm-rTg (Fig. 3c), indicating that interactions with HSPGs are especially important for transcytosis of low-horm-rTg. Because the heparin-binding sequence of rat Tg Arg-2489-Lys-2503 is involved in interactions with megalin and HSPGs (18, 19), we investigated its role in transcytosis. An antibody against this sequence (anti-rTgP), but not RIgG, reduced transcytosis of horm-rTg (by ≈47%) and almost abolished transcytosis of low-horm-rTg (reduction, ≈98%) (Fig. 3d), indicating that 2489–2503 is important for transcytosis, especially of low-horm-rTg.
Fig. 3.
(a–c) Effect of heparitinase, but not of chondroitinase ABC, on: (a) HSPGs expression (assessed by flow cytometry), (b) transcytosis of unlabeled Tgs, and (c) relation between HSPGs expression and transcytosis in FRTL-5 cells. (d) Effect of an antibody against the sequence 2489–2503 (anti-rTgP) on Tg transcytosis across FRTL-5 cells. Tgs were applied alone or with anti-rTgP or RIgG. (e–h) The sequence 2489–2503 is exposed to a greater extent in low-horm-rTg. Horm-rTg (e and g), and low-horm-rTg (f and h) were subjected to WB after preadsorption with RIgG (lanes 1 and bars 1), preadsorption with RIgG and precipitation with anti-rTgP (lanes 2 and bars 2), preadsorption with rabbit anti-T4 (lanes 3 and bars 3), or preadsorption with anti-T4 and precipitation with anti-rTgP (lanes 4 and bars 4). (g and h) Pixel density of the bands in e and f.
We performed immunoprecipitation experiments to investigate whether the sequence 2489–2503 is more exposed in low-horm-rTg, which could account for its greater transcytosis. First, Tgs were subjected to adsorption with RIgG or anti-T4 (to remove T4-containing molecules). Then, Tgs were subjected to WB, either directly or after precipitation with anti-rTgP. Tgs were recovered and revealed by WB after adsorption with RIgG (Fig. 3 e–h, lanes 1, bars 1). Anti-rTgP precipitated the Tgs recovered (Fig. 3 e–h, lanes 2, bars 2), but low-horm-rTg was precipitated to a greater extent (≈90%) than horm-rTg (≈35%), suggesting that 2489–2503 is more exposed in low-horm-rTg. After adsorption with anti-T4, a lower proportion of horm-rTg (≈37%, estimated by comparison with RIgG adsorption) was recovered compared with low-horm-rTg (≈79%), as expected from their T4 content (Fig. 3 e–h, lanes 3, bars 3). However, the Tgs recovered after anti-T4 adsorption were precipitated by anti-rTgP in similar proportions (horm-rTg ≈93%, low-horm-rTg ≈90%) (Fig. 3 e–h, lanes 4, bars 4), indicating that, within horm-rTg, molecules with low T4 content have the sequence 2489–2503 exposed to a similar extent as in low-horm-rTg. No Tg was precipitated by RIgG (not shown)
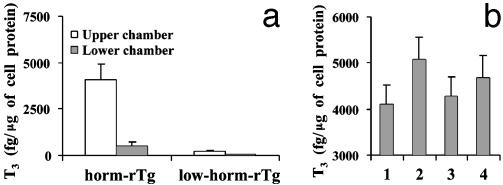
Hormone Release Experiments and Studies in Meg–/– Mice. To study the impact of transcytosis on hormone release, Tgs were applied to the upper chamber of FRTL-5 cells on filters alone, together, or with antimegalin 1H2. At 37°C, T3 was released in a polarized manner from horm-rTg but not, or to a negligible extent, from low-horm-rTg (Fig. 4a). The relatively low amounts of T3 in the upper chamber probably reflect some Tg degradation by extracellular proteases (6). As in previous studies (7), 1H2, but not mIgG (not shown), increased T3 release from horm-rTg (Fig. 4b). When Tgs were added together, T3 release was minimally, but not significantly, increased. When 1H2 was also added, the increase observed with 1H2 alone was rescued in part.
Fig. 4.
(a) Release of T3 by FRTL-5 cells on filters, from horm-rTg, but not from low-horm-rTg. (b) Release of T3 in the lower chamber from horm-rTg alone (bar 1) or in the presence of antimegalin 1H2 (bar 2), low-horm-rTg (bar 3), or both (bar 4).
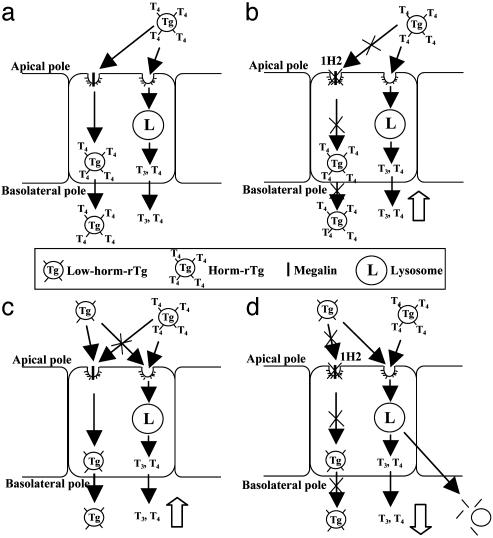
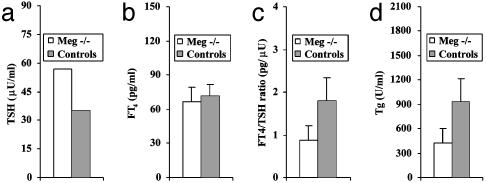
Our interpretation of the results is outlined in Fig. 5. When cells are exposed to horm-rTg, some horm-rTg is degraded in lysosomes, and some is transcytosed (Fig. 5a). When megalin is blocked by 1H2, transcytosis is inhibited, and degradation of horm-rTg (T3 release) is increased (Fig. 5b). When both Tgs are added, low-horm-rTg is transcytosed preferentially, which reduces transcytosis of horm-rTg and slightly increases its degradation (T3 release) (Fig. 5c). When megalin is blocked by 1H2 (Fig. 5d), transcytosis is inhibited, and low-horm-rTg enters lysosomes and competes with horm-rTg, thereby reducing the increase in T3 release from horm-rTg produced by megalin blocking as in Fig. 5b. Fig. 5 c and d probably resemble physiological conditions or megalin deficiency in vivo. Indeed, findings in a small number of Meg–/– mice confirmed that megalin is required for effective hormone release. Thus, compared with three Meg+/– control littermates, in which megalin function is known to be conserved (22), three Meg–/– mice had higher median serum TSH levels (Fig. 6a), similar FT4 (Fig. 6b) and a reduced FT4/TSH ratio (Fig. 6c), resembling mild hypothyroidism, in which increased TSH rescues from impaired hormone release (1). As expected, serum Tg was reduced in Meg–/– mice, reflecting impaired transcytosis (Fig. 6d). The presence of detectable levels of Tg in Meg–/– mice probably reflects increased Tg passage into the circulation by means other than transcytosis, such as direct secretion by thyrocytes, which may occur due to increased Tg synthesis triggered by enhanced TSH stimulation. Due to the small number of samples available, the results have no statistical relevance.
Fig. 5.
Interpretation of results in Fig. 4. Tg transcytosis and lysosomal degradation (T3 release) after exposure of FRTL-5 cells to horm-rTg alone (a) or in the presence of antimegalin 1H2 (b), low-horm-rTg (c), or both (d).
Fig. 6.
Serum TSH (a), FT4 (b), FT4/TSH ratio (c), and Tg (d) in three Meg–/– and three control (Meg+/–) mice.
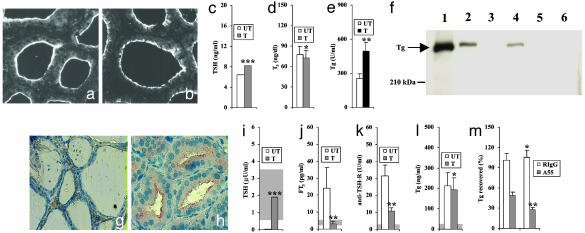
In Vivo Studies in Rats and Humans. To study reduced hormone formation within Tg in vivo, we treated rats with aminotriazole, which inhibits thyroperoxidase activity (29). To prevent increased megalin expression by thyrocytes, rats were also given LT4, because aminotriazole alone provokes hypothyroidism with increased TSH, which enhances thyroid megalin expression (7). Rats treated with aminotriazole and LT4 had levels of megalin expression similar to untreated rats (Fig. 7 a and b), as were serum TSH (Fig. 7c) and T3 levels (Fig. 7d). In contrast, serum Tg was 1.9-fold higher (P < 0.05) in treated rats (Fig. 7e), suggesting that reduced hormone formation within Tg results in increased transcytosis. We investigated by immunoprecipitation with anti-Tg or antimegalin antibodies whether and to what extent serum Tg was complexed with megalin secretory components. As shown in Fig. 7f, Tg was precipitated by anti-Tg both in treated (lane 1) and untreated (lane 4) rats and by antimegalin only in treated rats (lane 2), indicating that Tg is transcytosed to a greater extent by megalin when its hormone content is reduced. No Tg was precipitated by RIgG (Fig. 7f, lanes 3 and 6).
Fig. 7.
In vivo studies. (a–f) Rats. Immunofluorescence staining for megalin of thyroid sections from an untreated rat (a) and a rat treated with aminotriazole and LT4 (b). TSH (c), total T3 (d), and Tg (e) serum levels in untreated (UT) or treated (T) rats. Mann—Whitney, ***, P = NS. t test, *, P = NS; **, P < 0.05. (f) Precipitation of serum Tg with an antimegalin antibody. Sera from six treated (lanes 1–3) or six untreated (lanes 4–6) rats were pooled and precipitated with rabbit anti-Tg (lanes 1, 4), rabbit antimegalin (lanes 2, 5), or RIgG (lanes 3, 6), followed by WB for Tg. (g–m) Patients with GD. Immunohistochemistry for megalin in thyroid sections: (g) normal thyroid and (h) GD. Serum TSH (i), free-T3 (j), anti-TSH-R antibodies (k), and Tg (l) in GD patients untreated (UT) or treated with methimazole (T). Gray area, normal range. (m) Serum Tg after preadsorption with antimegalin A55 or RIgG. Tg recovered: % of Tg before preadsorption. Wilcoxon Signed Rank test, ***, P < 0.05. t test, *, P = NS; **, P < 0.05.
We studied the effect of reduced hormone formation within Tg in patients with GD, an autoimmune condition in which anti-TSH-R-stimulating autoantibodies increase thyroid function, resulting in hyperthyroidism (30) and enhanced Tg transcytosis (8). Methimazole is used for treatment of hyperthyroidism, because it restores euthyroidism by inhibiting thyroperoxidase activity and hormone formation within Tg (31). As in a previous study (8), we found increased megalin in GD thyroid glands, due to TSH-R stimulation, compared with normal thyroid tissue (Fig. 7 g and h). We then studied GD patients evaluated before and 3 mo after beginning methimazole. Treatment resulted in normalization of serum TSH (Fig. 7i), free-T3 (Fig. 7j), and, as reported (32), reduction of anti-TSH-R autoantibodies (Fig. 7k). Serum Tg did not change significantly (Fig. 7l), and, because it did not parallel TSH-R stimulation (deduced from anti-TSH-R levels), we concluded that reduced hormone formation within Tg sustains transcytosis under methimazole. To estimate the contribution of megalin-mediated transcytosis, we measured the proportion of serum Tg complexed with megalin secretory components. After preadsorption of sera with an antimegalin antibody, Tg was recovered to a lower extent in treated than in untreated patients (P < 0.05), whereas there was no difference after adsorption with RIgG (Fig. 7m), suggesting that in humans, as in rats, reduced hormone formation within Tg results in increased transcytosis via megalin.
Discussion
Transcytosis of Tg, which was first postulated by Unger et al. (33) and then demonstrated by Herzog (34), is mediated by the endocytic receptor megalin (7), but the physiological role of this pathway was unclear until now. Here we provide evidence that transcytosis serves to divert Tg molecules with a low hormone content from lysosomes, thereby favoring lysosomal degradation of hormone-rich Tg molecules, resulting in a more effective hormone release.
In support of these conclusions, we found that transcytosis across cultured thyrocytes (FRTL-5 cells) is greater and preferential for low-horm-rTg. In addition, reduced hormone formation within Tg in vivo, due to treatment of rats with aminotriazole and of GD patients with methimazole, resulted in increased megalin-mediated transcytosis. Our findings are in keeping with the observations that low iodine availability and impaired iodide uptake, transport, or coupling are associated with elevated serum Tg (35), and that serum Tg originated from transcytosis has a low hormone content (25, 26).
Greater interactions of Tg with surface HSPGs, due to greater exposure of the heparin-binding sequence 2489–2503, accounted for preferential transcytosis of low-horm-rTg. In this regard, an effect of hormone content on conformation had been demonstrated in studies on immune epitopes of Tg (36). Indeed, a major hormonogenic site, which is close to 2489–2503 (37), may account for the conformational state of the region.
We found that preferential transcytosis of low-horm-rTg affects hormone release from horm-rTg. As outlined in Fig. 5, concomitant exposure of FRTL-5 cells to low-horm-rTg reduced hormone release from horm-rTg when megalin was blocked, indicating that when transcytosis is inhibited, low-horm-rTg competes for the lysosomal pathway. This finding implies that reduced transcytosis should result in hypothyroidism with increased TSH levels, which may cause thyroid enlargement (i.e., goiter). This hypothesis was supported by serological findings in a small number of Meg–/– mice that had reduced serum Tg, as expected from impaired transcytosis, increased TSH, and normal FT4 levels, resembling mild hypothyroidism in humans (1). Due to the limited availability of Meg–/– mice, we could not examine thyroid tissue at this time and determine whether they have goiter. Further studies in a larger number of animals are needed to investigate this issue and to confirm our present findings.
Whether megalin deficiency can cause thyroid dysfunction in humans is unknown. The renal alterations in Meg–/– mice resemble those of Fanconi's syndrome, in which reduced megalin expression in the kidney has been proposed to be responsible for low molecular weight proteinuria (13, 38). Interestingly, Fanconi's syndrome can be associated with hypothyroidism (39), and therefore studies on megalin dysfunction in humans should be focused on this condition.
Our study points to a previously undescribed role of megalin in the control of body homeostasis, in addition to its role in the kidney. Our findings raise the possibility that further studies in the remaining organs where megalin is expressed may reveal additional important functions.
Supplementary Material
Acknowledgments
This work was supported by grants from Ministero dell'Istruzione dell'Università e della Ricerca (2001-068454) (to A.P., C.M., and M.M.); from Associazione Italiana per la Ricerca sul Cancro (2001) (to A.P. and M.M.); and from National Institutes of Health (Grant DK15070) (to S.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: T4, thyroxine; T3, triiodothyronine; TSH, thyroid-stimulating hormone; Tg, thyroglobulin; Meg–/–, megalin-deficient mice; horm-rTg, hormonogenic rat Tg; low-horm-rTg, low-hormonogenic rat Tg; WB, Western blotting; anti-rTgP, antibody to rat Tg 2489–2503; 1H2, mouse antimegalin antibody; HSPGs, heparan sulfate proteoglycans; mIgG, mouse IgG; RIgG, rabbit IgG; GD, Graves' disease; FT4, free T4; TSH-R, TSH receptor.
References
- 1.Scanlon, M. F. & Toft, A. D. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 234–253.
- 2.Dunn, J. T. & Dunn, A. D. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 91–104.
- 3.Taurog, A. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 61–84.
- 4.Marinò, M. & McCluskey, R. T. (2000) Am. J. Physiol. Cell Physiol. 279, C1295–C1306. [DOI] [PubMed] [Google Scholar]
- 5.Schneider, P. B. (1964) Endocrinology 74, 973–980. [DOI] [PubMed] [Google Scholar]
- 6.Brix, K., Linke, M., Tepel, C. & Herzog, V. (2001) Biol. Chem. 382, 717–725. [DOI] [PubMed] [Google Scholar]
- 7.Marinò, M., Zheng, G., Chiovato, L., Pinchera, A., Brown, D., Andrews, D. & McCluskey, R. T. (2000) J. Biol. Chem. 275, 7125–7138. [DOI] [PubMed] [Google Scholar]
- 8.Marinò M., Chiovato L., Mitsiades, N., Latrofa, F., Andrews, D., Tseleni-Balafouta, S., Collins, A. B., Pinchera, A. & McCluskey, R. T. (2000) J. Clin. Endocrinol. Metab. 85, 3458–3467. [DOI] [PubMed] [Google Scholar]
- 9.Kerjaschki, D. & Farquhar, M. G. (1982) Proc. Natl. Acad. Sci. USA 79, 5557–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raychowdhury, R., Niles, J. L., McCluskey, R. T. & Smith, J. A. (1989) Science 244, 1163–1165. [DOI] [PubMed] [Google Scholar]
- 11.Saito, A., Pietromonaco, S., Loo, A. K. C. & Farquhar, M. G. (1994) Proc. Natl. Acad. Sci. USA 91, 9725–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjalm, G. E., Murray, G., Crumley, W., Harazim, S., Lundgren, I., Onyango, B., Ek, M., Larsson, C., Juhlin, P., Hellman, H., et al. (1996) Eur. J. Biochem. 239, 132–137. [DOI] [PubMed] [Google Scholar]
- 13.Christensen, E. I. & Birn, H. (2002) Nat. Rev. Mol Cell. Biol. 3, 256–266. [DOI] [PubMed] [Google Scholar]
- 14.Marinò, M., Andrews, D., Brown, D. & McCluskey, R. T. (2001) J. Am. Soc. Nephrol. 12, 637–648. [DOI] [PubMed] [Google Scholar]
- 15.Marinò, M., Lisi, S., Pinchera, A., Mazzi, B., Latrofa, F., Sellari-Franceschini, S., McCluskey, R. T. & Chiovato, L. (2001) Thyroid 11, 177–185. [DOI] [PubMed] [Google Scholar]
- 16.Ambesi-Impiombato, F-S., Parks, L. A. & Coon, H. G. (1980) Proc. Natl. Acad. Sci. USA 77, 3455–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiovato, L. & Pinchera, A. (1991) Autoimmunity 10, 319–331. [DOI] [PubMed] [Google Scholar]
- 18.Marinò, M., Friedlander, J. A., McCluskey, R. T. & Andrews, D. (1999) J. Biol. Chem. 274, 30377–30386. [DOI] [PubMed] [Google Scholar]
- 19.Marinò, M., Andrews, D. & McCluskey, R. T. (2000) Thyroid 10, 551–559. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein, L. J., Basu, S. K., Brunschede, G. Y. & Brown, M. S. (1976) Cell 7, 85–95. [DOI] [PubMed] [Google Scholar]
- 21.Marinò, M., Zheng, G. & McCluskey, R. T. (1999) J. Biol. Chem. 274, 12898–12904. [DOI] [PubMed] [Google Scholar]
- 22.Willnow, T. E., Hilpert, J., Armstrong, S. A., Rohlmann, A., Hammer, R. E., Burns, D. K. & Herz, J. (1996) Proc. Natl. Acad. Sci. USA 93, 8460–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss, R. E., Forrest, D., Pohlenz, J., Cua, K., Curran, T. & Refetoff, S. (1997) Endocrinology 138, 3624–3629. [DOI] [PubMed] [Google Scholar]
- 24.Marinò, M., Chiovato, L., Friedlander, J. A., Latrofa, F., Pinchera, A. & McCluskey, R. T. (1999) J. Clin. Endocrinol. Metab. 84, 2468–2474. [DOI] [PubMed] [Google Scholar]
- 25.Ikekubo, K., Kishihara, M., Sanders, J., Jutton, J. & Schneider, A. B. (1981) Endocrinology 109, 427–432. [DOI] [PubMed] [Google Scholar]
- 26.Druetta, L., Bornet, H., Sassolas, G. & Rousset, B. (1999) Eur. J. Endocrinol. 140, 457–467. [DOI] [PubMed] [Google Scholar]
- 27.Rousset, B., Selmi, S., Bornet, H., Bourgeat, P., Rabilloud, R. & Munari-Silem, Y. (1989) J. Biol. Chem. 264, 12620–12626. [PubMed] [Google Scholar]
- 28.Marinò, M., Lisi, S., Pinchera, A., Chiovato, L. & McCluskey, R. T. (2003) J. Endocrinol. Invest. 26, 222–229. [DOI] [PubMed] [Google Scholar]
- 29.Strum, J. M. & Karnovsky, M. J. (1971) Lab. Invest 24, 1–12. [PubMed] [Google Scholar]
- 30.Davies, T. F. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 518–530.
- 31.Cooper, D. S. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 691–718.
- 32.Kuo, S. W., Huang, W. S., Hu, C. A., Liao, W. K., Fung, T. C. & Wu, S. Y. (1994) Eur. J. Endocrinol. 131, 125–130. [DOI] [PubMed] [Google Scholar]
- 33.Unger, J., Boeynaems, J. M., Van Herle, A., Van Sande, J., Rocmans, P. & Mockel, J. (1979) Endocrinology 105, 225–231. [DOI] [PubMed] [Google Scholar]
- 34.Herzog, V. (1983) J. Cell Biol. 97, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vijlder, J. J. M. & Vulsma, T. (2000) in Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, eds. Braverman, L. E. & Utiger, R. D. (Lippincott, Williams & Wilkins, Philadelphia), pp. 733–742.
- 36.Rose, N. R. & Burek, C. L. (2000) Appl. Biochem. Biotechnol. 83, 245–251. [DOI] [PubMed] [Google Scholar]
- 37.Kim, P. S., Ding, M., Menon, S., Jung, C.-G., Cheng, J.-M., Miyamoto, T., Li, B., Furudate, S. I. & Agui, T. (2000) Mol. Endocrinol. 14, 1944–1953. [DOI] [PubMed] [Google Scholar]
- 38.Norden, A. G., Lapsley, M., Igarashi, T., Kelleher, C. L., Lee, P. J., Matsuyama, T., Scheinman, S. J., Shiraga, H., Sundin, D. P. & Thakker, R. V. (2002) J. Am. Soc. Nephrol. 13, 125–133. [DOI] [PubMed] [Google Scholar]
- 39.McLean, R. H., Kennedy, T. L., Rosoulpour, M., Ratzan, S. K., Siegel, N. J., Kauschansky, A. & Genel, M. (1982) J. Pediatr. 101, 72–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.