Abstract
Obesity and diabetes have been associated with increased consumption of highly processed foods, and reduced consumption of whole grains and nuts. It has been proposed, mainly on the basis of observational studies, that nuts may provide superior satiation, may lead to reduced calorie consumption, and may decrease the risk of type 2 diabetes; but evidence from randomized, interventional studies is lacking. Twenty men and women with the metabolic syndrome participated in a randomized, double blind, cross-over study of walnut consumption. Subjects had two four-day admissions to the clinical research center where they were fed an iso-caloric diet. In addition, they consumed shakes for breakfast containing either walnuts or placebo (shakes were standardized for calories, carbohydrate, and fat content). Appetite, insulin resistance, and metabolic parameters were measured. We found an increased level of satiety (overall p-value 0.0079) and sense of fullness (p=0.05) in pre-lunch questionnaires following the walnut breakfast as compared to the placebo breakfast, with the walnut effect achieving significance on day 3 and 4 (p=0.02 and p=0.03). We did not find any change in resting energy expenditure, hormones known to mediate satiety, or insulin resistance when comparing the walnut versus placebo diet. Walnut consumption over 4 days increased satiety by day 3. Long term studies are needed to confirm the physiologic role of walnuts, the duration of time needed for these effects to occur, and to elucidate the underlying mechanisms.
Keywords: Walnuts, Diet, Metabolic Syndrome
Introduction
Obesity and diabetes are among the more significant public health epidemics of the 21st century around the world. Both obesity and diabetes have been associated with increased consumption of highly processed foods and reduced consumption of whole grains and nuts. Nuts, an important component of the Mediterranean diet, have been shown through several large epidemiological studies to have beneficial health effects. Nuts have been shown to reduce the risk of cardiovascular disease, sudden death, and diabetes mellitus (1-4). These benefits are thought to be due, in part, to improvements in lipid profile, inflammation, and endothelial function (5;6).
Walnuts, similar to other nuts, are rich in fat. Although they have a low content of saturated fatty acids (SFA) and a high content of polyunsaturated fatty acids (PUFA) (7), concerns have been raised that because of their high fat content, increased walnut consumption would lead to weight gain. Epidemiological studies have shown a consistent inverse association between nut consumption and weight change (2;8-10). A randomized, cross over study recently found an increase in post-meal energy expenditure, but no difference in satiety between subjects fed walnuts versus fat-rich dairy products. This was based on reported appetite satisfaction after one meal in an outpatient setting (11). Thus, although it has been proposed that whole foods, such as nuts, may provide superior satiation and lead to reduced calorie consumption (12), there have been no blinded and controlled feeding studies to evaluate the effects of walnuts on satiety. Similarly, no studies have been conducted where subjects are closely observed and fed over several days in a clinical research center. In addition, the effect of walnuts on energy expenditure remains unknown. Molecules important in regulating energy homeostasis, including pancreas, gut, and adipose tissue-derived peptides, have also not been studied in relation to walnut consumption. It would be reasonable to hypothesize that these molecules may provide a link between increased satiety and energy expenditure or the observed lower incidence of type 2 diabetes in habitual walnut consumers. Many of these effects would be of particular benefit to individuals with the metabolic syndrome, a condition associated with insulin resistance and increased risk of cardiovascular disease.
We performed a randomized, double-blind, cross over study of nut consumption in men and women with the metabolic syndrome, who were studied during two randomly assigned, four day-long inpatient study periods in our institution's clinical research center. The primary aim of this study was to examine the effects of walnuts on satiety over a four day period in the context of a blinded, randomized, interventional trial. The secondary aims were to investigate the effects of walnut consumption on molecules that regulate appetite, as well as glucose tolerance and endocrine function.
Methods and Procedures
Subjects
The study was conducted between March 2007 and November 2008 at Beth Israel Deaconess Medical Center's General Clinical Research Center. Participants were volunteers recruited from the community and were deemed eligible to participate if they were between the ages of 40 and 75 years old and had the metabolic syndrome, as defined by the 2006 International Diabetes Federation criteria (13). Subjects were excluded if they had diabetes mellitus requiring pharmacotherapy, were pregnant or breastfeeding, and/or had anemia or malabsorbtion from any cause. Subjects with current alcoholism or drug abuse or use of medications that could interfere with the study, such as corticosteroids, growth hormone, or antiretroviral therapy were also excluded. In addition, individuals with any history of a nut allergy were excluded. These conditions were screened for by a detailed history and physical examination.
Sixty three subjects were screened with an evaluation of medical history, physical examination, and laboratory parameters. Of these, thirty one qualified to participate. Eleven subjects withdrew consent prior to randomization. The remaining twenty individuals participated in the study and were included in the per protocol screening analysis. A total of fifteen subjects completed both arms of the study. The study was approved by the institutional review board of the Beth Israel Deaconess Medical Center and all subjects provided written informed consent to participate. The clinical trial registration number is NCT00525629.
Study Design
The study was a randomized, double blind, cross-over study of walnut consumption. Eligible subjects were counseled by a research dietitian prior to starting the study and were asked to abstain from walnuts for two weeks prior to their first study visit. They also received standardized advice on the American Heart Association Therapeutic Lifestyle change diet and were asked to maintain a stable diet and exercise pattern throughout the study. After two weeks of washout, subjects were admitted for their first study visit at 9pm prior to the first day of testing. Subjects were randomly assigned by a blinded statistician to either receive walnut-containing diet or placebo diet on the first visit. During both visits, subjects followed an identical iso-caloric diet based on foods that they like to eat. Subjects could only eat food provided by the nutritionist. They were encouraged to eat the entire meal provided, and any food they did not eat was weighed back and accounted for in the metabolic kitchen. Following a baseline assessment and completion of appetite questionnaire on the first study day, subjects received a liquid breakfast meal. They were permitted to ambulate freely around the hospital during their stay, but were not permitted to exercise or leave the hospital. Subjects consumed a liquid breakfast each morning and had repeated assessment on the morning of the fourth day. Following completion of this assessment, subjects were discharged and asked to avoid walnuts for one month. After one month, they returned for a second, 4-day stay in the General Clinical Research Center.
Intervention
In order to allow for blinding of subjects and study staff, 48g of walnuts were incorporated into a liquid meal with similar macronutrient composition. The walnut/placebo shakes were standardized for calories (walnuts 586 kcal v. placebo 585 kcal), carbohydrate, and fat content. The walnut meal contained 7% protein, 41% fat, and 52% carbohydrates. The placebo meal contained 2% protein, 41% fat, and 52% carbohydrates. The walnut meal was rich in PUFA (22.82g PUFA, 4.4g MUFA); while the placebo meal was rich in MUFA (4.79g PUFA, 24.01g MUFA). Remaining fat content consisted of small amounts of SFA's. The walnut-containing liquid meal contained 48g of walnuts, 50g of frozen mango, 50g of frozen strawberries, 60g of banana, 100g of frozen berries, 250g of pineapple juice. The placebo liquid meal contained 32g safflower oil, 60g of frozen mango, 50g of frozen strawberries, 80g of banana, 100g of frozen berries, 260g of pineapple juice and 40 drops of walnut flavoring. Walnut halves and pieces were used and blended with the other ingredients into a milk-shake consistency drink which was consumed cold for each morning meal. A pilot study in a group of 10 representative subjects demonstrated that subjects could not determine which shake contained walnuts. Subjects were fed an iso-caloric diet for the 4 days of each visit. All food was weighed back to ensure compliance with the diet.
Measurements
Subjects were weighed on the first day of each admission. Body composition was measured using Tanita Bioelectrical Impedence Analysis (BIA). Appetite was assessed by a visual analog scale before breakfast and lunch on each day of both visits as previously described (14). Resting energy expenditure was measured using indirect calorimetry on the first and final day of each visit (Vmax sensor).
Assessment of Insulin Resistance and Laboratory Parameters
On the first and last day of each visit, following a 12-hour fast, subjects had a baseline blood draw after which they were requested to consume their liquid breakfast within 5 minutes. Repeat blood sampling was performed every 30 minutes for 3 hours. Glucose and lipids (LDL, triglycerides, HDL, total cholesterol) were measured in the central clinical laboratory at BIDMC using standard laboratory techniques. All other samples were stored as serum at −80°C until assayed in duplicate. Samples for the same subject were run in the same assay. Standard techniques were used to measure insulin as previously described (15). Glucagon-like peptide (GLP-1) was measured using enzyme-linked immunosorbent assay (ELISA) (LINCO, St. Charles, Missouri) with sensitivity 2.0 pM/l, intra-assay CV 6-9 %, and inter-assay CV <1.0-13 %. Glucose-dependent insulinotropic polypeptide (GIP) was measured by ELISA (LINCO, St. Charles, Missouri) with sensitivity 8.2pg/mL, intra-assay CV 1.8-6.1 %, and inter-assay CV 3.0-8.8 %. Active ghrelin (AGh) and total ghrelin (TGh) were measured by ELISA (LINCO, St. Charles, Missouri) as described previously (16). Leptin and adiponectin were measured using radioimmunoassays (LINCO, St. Charles, Missouri) as described previously (17-19). Peptide YY (PYY) was measured by ELISA (LINCO, St. Charles, Missouri), with sensitivity 1.4 pmol/l, intra-assay CV 0.86-5.78%, inter-assay CV 3.65-16.5%, and 100% cross reactivity for the full-length peptide (PYY1–36) and the truncated PYY3–36, both of which have biological activity.
Statistical Analysis
The results are presented as mean values ± standard error of the mean. SAS (version 9.1, SAS Institute, Cary, NC) and SPSS (version 11.5, SPSS Inc., Chicago, IL) was used for statistical analysis, and P<0.05 (two-tailed) was considered statistically significant for all analyses. We used one-way ANOVA followed by the protected least significant-differences technique for continuous variables, and Fisher's Exact test for categorical variables, to compare subjects who completed the study with subjects who did not complete the study. As a primary analysis, we used Wilcoxon's signed rank (paired tests), paired T-test and mixed model controlled for sequence to compare change under walnut-containing diet versus a placebo diet (on-treatment analysis, n=15 completing both visits) and Wilcoxon's rank sum test and mixed model controlled for sequence (independent-samples tests) as secondary analysis (intention-to-treat analysis, n=20).
Results
Twenty subjects were randomized and participated in the study. Eighteen completed at least one 4-day visit, and fifteen subjects completed both study visits. Baseline demographic characteristics of the study sample are presented in Table 1. Completers versus non-completers had significantly lower HDL cholesterol (41.3mg/dL vs 58.6mg/dL, p=0.02) at baseline. All other baseline characteristics were not significantly different. Subjects' weight was not statistically different between the first day of each admission (p=0.33). Similarly, there was no difference in macronutrient intake during either admission (96.01% target kcal/day on walnut diet, 97.73% target kcal/day on placebo diet, p=0.594). There was no difference in any outcome between the total sample (all 18 subjects who completed at least 1 visit) and those who completed both visits. Only data for completers is shown.
Table 1. Baseline characteristics of study subjects.
| Total Sample (N=20) |
Completed (N=15) |
Did Not Complete (N=5) |
P* | |
|---|---|---|---|---|
| Age | 59.0 ± 2.0 | 58.0 ± 2.5 | 62.0 ± 2.8 | 0.41 |
| Height (cm) | 169.1 ± 2.2 | 171.1 ± 2.5 | 163.3 ± 4.3 | 0.13 |
| Weight (kg) | 104.1 ± 3.6 | 106.2 ± 4.4 | 97.9 ± 5.4 | 0.33 |
| BMI | 37.0 ± 1.4 | 36.9 ± 1.7 | 37.1 ± 2.6 | 0.96 |
| Waist Circumference (cm)1 | 114.8 ± 2.3 | 117.0 ± 2.7 | 109.2 ± 3.8 | 0.14 |
| Glucose (mg/dL) | 89.9 ± 3.5 | 91.3 ± 4.4 | 85.4 ± 4.0 | 0.47 |
| TC (mg/dL) | 207.0 ± 11.8 | 208.5 ± 12.8 | 202.4 ± 30.1 | 0.83 |
| LDL (mg/dL) | 122.3 ± 10.7 | 124.6 ± 10.9 | 115.4 ± 29.8 | 0.72 |
| Triglycerides (mg/dL) | 199.2 ± 22.5 | 218.3 ± 26.1 | 142.0 ± 36.3 | 0.15 |
| HDL (mg/dL) | 45.7 ± 3.6 | 41.3 ± 3.1 | 58.6 ± 9.1 | 0.03 |
| Systolic BP (mmHg) | 138.4 ± 3.0 | 138.9 ± 3.8 | 136.8 ± 4.7 | 0.78 |
| Diastolic BP(mmHg) | 80.6 ± 2.0 | 81.5 ± 2.3 | 77.8 ± 4.2 | 0.43 |
| Exercise (hrs/week) | 4.1 ± 1.4 | 2.9 ± 1.1 | 7.8 ± 4.6 | 0.13 |
| N (%) | N(%) | N(%) | ||
| Male | 10 (50%) | 9 (60%) | 1 (20%) | 0.30 |
| Caucasian | 15 (75%) | 13 (87%) | 2 (40%) | 0.07 |
| African American2 | 4 (21%) | 2 (13%) | 2 (50%) | 0.18 |
| Blood Pressure Medications3 | 4 (22%) | 4 (27%) | 0 (0%) | 1 |
| Cholesterol Medications3 | 9 (50%) | 6 (40%) | 3 (100%) | 0.21 |
Abbreviations: BMI, body mass index; TC, total cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein
Values are means ± SE.
p-value is based on one-way ANOVA followed by the protected least significant-differences technique for continuous variables and Fisher's Exact test for categorical variables.
information is only available for n=18
information is only available for n=19
information is only available for n=18
Appetite
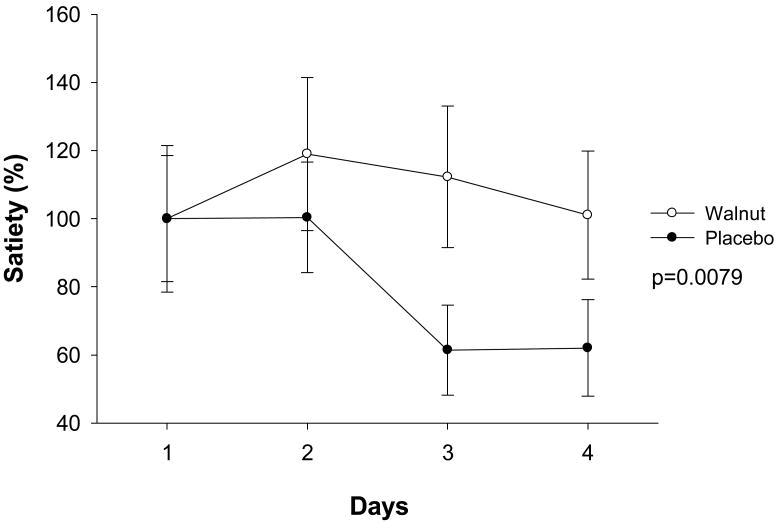
On all days of both placebo and walnut visits, the subjects reported no difference in their feelings of fullness, satiety, or hunger on pre-breakfast questionnaires (data not shown). Subjects reported feeling more satiated pre-lunch on a walnut-containing diet (overall p=0.0079) with the walnut effects achieving significance on day 3 and day 4 (p=0.02 and p=0.03 respectively) (Table 2). Subjects also had a significantly higher rate of feeling “full” during the walnut compared to placebo diet (p=0.05) (Table 2) (Figure 1). There was a significant difference in reporting of feeling full between the two groups (Table 2) on day 3 (p=0.03) and the difference was borderline on day 4 (p=0.1).
Table 2. Results of pre-lunch appetite questionnaires on days 1-4 for subjects who completed both visits (using LOCF*).
| Walnut | Placebo | P1 | P2 | P3 | |
|---|---|---|---|---|---|
| Full | 0.05 | ||||
| Day1 | 32.3 ± 6.5 | 23.6 ± 6.8 | 0.26 | 0.3 | |
| Day2 | 37.5 ± 8.3 | 24.6 ± 4.4 | 0.2 | 0.33 | |
| Day3 | 27.6 ± 6.8 | 14.6 ± 3.8 | 0.03 | 0.03 | |
| Day4 | 30.7 ± 5.1 | 22.3 ± 5.4 | 0.14 | 0.13 | |
| Satiety | 0.01 | ||||
| Day1 | 38.5 ± 6.7 | 33 ± 7.3 | 0.56 | 0.9 | |
| Day2 | 45.7 ± 8.2 | 33.1 ± 5.3 | 0.25 | 0.37 | |
| Day3 | 43.1 ± 7.5 | 19.4 ± 4.4 | 0.01 | 0.02 | |
| Day4 | 38.8 ± 6.8 | 19.6 ± 4.7 | 0.02 | 0.03 | |
| Hungry | 0.17 | ||||
| Day1 | 68.2 ± 6.9 | 63.5 ± 6.8 | 0.5 | 0.64 | |
| Day2 | 55.7 ± 8.2 | 59.4 ± 7.2 | 0.68 | 0.86 | |
| Day3 | 62.3 ± 7.7 | 68.9 ± 7.7 | 0.45 | 0.27 | |
| Day4 | 59.8 ± 7.2 | 52.7 ± 8.3 | 0.16 | 0.31 |
Values are millimeters on a visual analog scale; means ± SE are presented
p-value is based on Wilcoxon signed rank test
p-value is based on paired T-test and mixed model controlled for sequence which have same results
p-value is based on repeated measures analysis to compare differences between walnut and placebo groups over four days of period.
LOCF: Last observation carried forward
Figure 1. Satiety changes between day 1 to day 4 in response to either walnut or placebo breakfasts (LOCF).
Metabolic Profile
Fasting glucose and insulin levels were measured on day 1 and day 4 of each visit. There was no significant difference between the two groups (Table 3A). There was no difference in HOMA-IR on day 1 of both visits (data not shown). Similarly, after 4 days of a walnut containing diet, insulin resistance was no different than the placebo diet (data not shown). We also performed glucose and insulin assessments every 30 minutes after walnut-containing and placebo breakfast. The difference between the area under the curve for mean glucose was similar between walnut and placebo on day 4 of each visit (Table 3A). 40% of subjects were on statin treatment during the study. No subject had a change in their statin dose during the study period. There was no difference in LDL, HDL, total cholesterol, or triglyceride values on walnut containing diet.
Table 3.
| Table 3A. Biomarkers (paired results) for subjects who completed both visits | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 4 | |||||||
| Area Under Curve (AUC) | Walnut | Placebo | P1 | P2 | Walnut | Placebo | P1 | P2 |
| Glucose | 18332 ± 1091 | 20540 ± 1230 | 0.01 | 0.02 | 17576 ± 834.1 | 18332 ± 1091 | 0.12 | 0.09 |
| Insulin | 9533 ± 1182 | 10756 ± 1078 | 0.30 | 0.25 | 11533 ± 1127 | 11935 ± 1118 | 0.56 | 0.58 |
| FFA | 80.79 ± 5.2 | 78.1 ± 3.8 | 0.60 | 0.48 | 68.14 ± 4.2 | 67.2 ± 4 | 0.93 | 0.71 |
| AGh | 35712 ± 5429 | 35077 ± 4626 | 0.46 | 0.82 | 29020 ± 5202 | 25478 ± 3902 | 0.33 | 0.28 |
| TGh | 160619 ± 6708 | 162588 ± 6005 | 0.76 | 0.63 | 142714 ± 7916 | 140390 ± 8820 | 0.90 | 0.72 |
| GLP | 723.06 ± 81.43 | 755.76 ± 72.34 | 0.54 | 0.36 | 791.45 ± 75.74 | 811.31 ± 90.47 | 0.95 | 0.68 |
| GIP | 40188 ± 4379 | 49657 ± 6031 | 0.00 | 0.01 | 35643 ± 3678 | 45517 ± 6580 | 0.06 | 0.04 |
| PYY | 19231 ± 2191 | 16254 ± 1518 | 0.01 | 0.01 | 19626 ± 2632 | 15688 ± 1639 | 0.02 | 0.03 |
| Baseline level | ||||||||
| Glucose (mg/dL) | 97.43 ± 3.85 | 101.64 ± 3.74 | 0.16 | 0.17 | 97.79 ± 3.08 | 97.43 ± 3.851 | 0.80 | 0.87 |
| Insulin (μU/mL) | 18.25 ± 1.88 | 19.82 ± 2.55 | 0.52 | 0.50 | 22.58 ± 2.49 | 27.44 ± 3.46 | 0.30 | 0.18 |
| FFA (mmol/L) | 0.7 ± 0.06 | 0.73 ± 0.05 | 0.50 | 0.50 | 0.46 ± 0.04 | 0.52 ± 0.05 | 0.05 | 0.06 |
| AGh (pg/mL) | 253.49 ± 38.61 | 250.68 ± 36.43 | 0.81 | 0.89 | 193.32 ± 36.79 | 181.17 ± 27.96 | 0.43 | 0.61 |
| TGh (pg/mL) | 955.79 ± 48.23 | 956.2 ± 49.1 | 0.39 | 0.99 | 838.84 ± 54.83 | 829.97 ± 61.11 | 1.00 | 0.80 |
| GLP (pmol/mL) | 3.44 ± 0.36 | 3.46 ± 0.29 | 0.95 | 0.92 | 3.44 ± 0.322 | 3.32 ± 0.343 | 0.46 | 0.32 |
| GIP (pg/mL) | 55.04 ± 9.5 | 69.27 ± 11.74 | 0.13 | 0.29 | 46.66 ± 6.05 | 52.26 ± 9.59 | 0.79 | 0.43 |
| PYY (pg/mL) | 56.6 ± 7.61 | 70.58 ± 14.33 | 0.68 | 0.38 | 52.57 ± 9.82 | 52.62 ± 8.49 | 0.85 | 0.99 |
| Leptin (ng/mL) | 28.38 ± 5.42 | 27.01 ± 5.23 | 0.25 | 0.30 | 26.75 ± 5.03 | 28.32 ± 6.40 | 0.93 | 0.51 |
| Adiponectin (ug/mL) | 3.96 ± 0.51 | 3.81 ± 0.46 | 0.30 | 0.28 | 3.48 ± 0.46 | 3.56 ± 0.44 | 0.36 | 0.53 |
| LDL(ng/mL) | 398.01 ± 65.06 | 414.92 ± 54.65 | 0.90 | 0.41 | ||||
| sCD40L (ng/mL) | 1.37 ± 0.47 | 1.43 ± 0.46 | 0.72 | 0.93 | ||||
| Abbreviations: FFA, free fatty acids; AGh, active ghrelin; TGh, total ghrelin; GLP, glucagon-like peptide; GIP, glucose-dependent insulinotropic polypeptide; PYY, peptide YY | ||||||||
| Values are means ± SE | ||||||||
| 1 p-value is based on Wilcoxon signed rank test | ||||||||
| 2 p-value is based on paired T-test and mixed model controlled for sequence | ||||||||
| Table 3B. Change in biomarkers after 4 days of treatment with walnut or placebo (data based on subjects who completed both visits) | ||||
|---|---|---|---|---|
| Walnut | Placebo | P1 | P2 | |
| Area Under the Curve | ||||
| Glucose | -785.09 ± 657.60 | -2208.21 ± 456.59 | 0.09 | 0.08 |
| Insulin | 2000.64 ± 812.30 | 1178.89 ± 854.01 | 0.25 | 0.37 |
| FFA | -12.65 ± 5.64 | -10.87 ± 3.28 | 0.93 | 0.71 |
| AGh | -6691.38 ± 3055.74 | -9598.96 ± 3953.04 | 0.76 | 0.47 |
| TGh | -17904.84 ± 3969.25 | -22198.79 ± 7133.54 | 0.50 | 0.57 |
| GLP | 68.39 ± 24.02 | 55.55 ± 61.58 | 0.63 | 0.83 |
| GIP | -4544.81 ± 3815.86 | -4139.68 ± 4540.23 | 0.84 | 0.92 |
| PYY | 395.87 ± 1288.87 | -566.11 ± 1168.63 | 0.80 | 0.46 |
| Baseline level | ||||
| Glucose (mg/dL) | -0.86 ± 1.70 | -4.21 ± 1.70 | 0.17 | 0.14 |
| Insulin (μU/mL) | 4.33 ± 2.49 | 7.62 ± 2.93 | 0.49 | 0.33 |
| FFA (mmol/L) | -0.23 ± 0.06 | -0.20 ± 0.05 | 0.82 | 0.59 |
| AGh (pg/mL) | -60.18 ± 19.44 | -69.51 ± 32.40 | 0.50 | 0.74 |
| TGh (pg/mL) | -116.95 ± 41.83 | -126.24 ± 37.30 | 0.86 | 0.86 |
| GLP (pmol/mL) | 0.00 ± 0.14 | -0.14 ± 0.16 | 0.71 | 0.57 |
| GIP (pg/mL) | -8.38 ± 8.29 | -17.01 ± 8.55 | 0.27 | 0.52 |
| PYY (pg/mL) | -4.03 ± 6.90 | -17.96 ± 16.09 | 0.64 | 0.39 |
| Leptin (ng/mL) | -1.62 ± 1.07 | 1.31 ± 2.84 | 0.37 | 0.56 |
| Adiponectin (ug/mL) | -0.48 ± 0.17 | -0.26 ± 0.11 | 0.11 | 0.21 |
Abbreviations: FFA, free fatty acids; AGh, active ghrelin; TGh, total ghrelin; GLP, glucagon-like peptide; GIP, glucose-dependent insulinotropic polypeptide; PYY, peptide YY
Values are means ± SE
p-value is based on Wilcoxon signed rank test
p-value is based on paired T-test and mixed model controlled for sequence
Molecules of Energy Homeostasis
We performed measurements of gut peptides following walnut and control meals on day 1 and day 4 of each visit. Levels of GLP-1, GIP, PYY, total ghrelin, and active ghrelin were unchanged between day 1 and day 4 (Table 3B). We also performed measurements of adipokines on day 1 and day 4, but leptin and adiponectin levels were also unchanged.
Discussion
We demonstrate, herein, that a walnut-containing breakfast improves satiation over a 3-4 day period. It has been proposed, mostly on the basis of epidemiological studies, that whole foods, such as nuts, may provide superior satiation and lead to reduced calorie consumption (12). Casa-Agustench et al. recently performed a randomized, cross over trial comparing satiety between three high fat meals administered once (11). One meal was high in PUFA derived from walnuts, another rich in MUFA derived from olive oil, and the third meal was high in SFA derived from dairy products. There was no difference in satiety between any of the groups. Interestingly, this study assessed satiety after only one meal, whereas our study found differences in satiety, which started achieving significance after 3 days of walnut treatment. Importantly, although subjects in the prior study were instructed to follow an iso-caloric diet at home, we provided the meals in a blinded and randomized manner, as well as in the controlled environment of an inpatient setting where diet and activity could be closely monitored. A possible explanation for the differing results of these studies is that the mechanism by which walnuts increase satiety may not manifest in an extremely short term basis and are difficult to assess in an uncontrolled environment.
Interestingly, the same epidemiological evidence that suggests walnuts improve satiation also suggests that they may lead to reduced calorie consumption (12). This theory is supported by epidemiology and interventional studies, which demonstrate a consistent inverse association between nut consumption and weight change (10;11). Some of the satiation effects of walnuts are thought to be due, in part, to a compensatory reduction in energy consumption. This accounts for up to 75% of the calories contained in the nuts so that when nuts are added to the diet, without any other intervention, the resulting weight gain is far less than expected (20). We could not assess caloric intake because, by study design, subjects were instructed to consume all food provided to them to maintain an iso-caloric diet for the duration of the study. We did not find any resting energy expenditure changes on day 4 of admission; we only report an effect of walnuts on satiety on the third and fourth days of walnut-enriched breakfast. We then hypothesized that peripherally secreted hormones such as leptin, adiponection, or recently discovered gut peptides may underlie the observed effect of walnut consumption. Several gut peptides such as GLP-1, PYY, and GIP are increased with meal consumption, and act to slow gastric emptying, stimulate insulin secretion, inhibit glucagon secretion, and control body weight (21;22). Recent evidence indicates that meal content may have a direct effect on the secretion of gut peptides such as GLP-1 from intestinal L cells through duodenal taste receptors (23). We found no change in levels of GLP-1 and/or total or active ghrelin. Ghrelin is a hormone secreted by the P/D1 cells that line the fundus of the human stomach and epsilon cells of the pancreas, which stimulate hunger (24). Ghrelin increases food intake by an action exerted at the level of the hypothalamus (25). If the satiation caused by walnuts was due to changes in gut hormones, we would have expected a decreased level of active ghrelin in the walnut group during the time frame of our study. Our data does not support this hypothesis, but perhaps frequent sampling or more long term studies may help to elucidate this further. Further, we did not see any changes in the levels of leptin or adiponectin after the 4 days of treatment. A finding consistent with the more long term role of these adipokines is the regulation of energy homeostatsis. It is possible that there is an undiscovered protein, either peripherally or centrally derived, which mediates the induced satiety. Alternatively, the fatty acid composition of walnuts could, by acting centrally, alter appetite, as previously suggested (26). Further investigation is needed. Thus, based on our analysis, short term satiety from walnut consumption does not appear to be mediated through gut hormones and/or the adipocyte secreted hormones, leptin, and adiponectin.
Walnuts have been shown to have beneficial effects in diabetic patients and those with the metabolic syndrome. A large prospective cohort found that increased nut intake is associated with decreased risk of type 2 diabetes (3). A recent randomized interventional study showed that diets containing walnuts result in significantly lower fasting glucose levels at one year (27). In contrast, in a randomized controlled study of 8 weeks duration, a high walnut content diet did not result in any benefit in metabolic parameters, or any of the markers of the metabolic syndrome (28). Another randomized study showed that diets containing 30g of walnuts improve the lipid profile in diabetic subjects after six months, in addition to showing a trend towards reduced body fat (6). Our study was of significantly shorter duration, only 4 days of a walnut containing diet, and is consistent with the prior literature by not demonstrating a large change in metabolic or hormonal measures within a few days, including changes in insulin resistance, fasting glucose, or lipid levels. In the future, more long term studies need to be performed to more completely understand both the exact timing and the specific long term effects and/or benefits of walnuts on patients with type 2 diabetes mellitus and/or the metabolic syndrome.
In conclusion, this randomized, cross over study found that satiation is increased within 3-4 days of a walnut containing diet. We did not detect effects on body weight, insulin resistance, or metabolic hormone levels. Long term studies are needed to further elucidate the underlying mechanisms and the physiologic role of walnuts on these outcomes.
Acknowledgments
We would like to acknowledge Mollie Wood for her help with statistical analysis. We would also like to acknowledge Rianna Stefanakis, Geetha Mylvaganam, and Huizhi Gong for conducting laboratory measurements.
The project described was supported by Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The study was also supported in part from NIH grant M01-RR-01032 and an investigator-initiated grant from the California Walnut Commission. CSM is supported by NIDDK grants DK58785, DK79929, DK 081913, and a discretionary grant from Beth Israel Deaconess Medical Center (ClinicalTrials.com NCT00525629). None of the authors had a personal or financial conflict of interest.
Footnotes
Disclosure: None of the authors had a personal or financial conflict of interest.
Reference List
- 1.Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians' Health Study. Arch Intern Med. 2002;162:1382–7. doi: 10.1001/archinte.162.12.1382. [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Manson JE, et al. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–5. doi: 10.1136/bmj.317.7169.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288:2554–60. doi: 10.1001/jama.288.20.2554. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JH, Jr, Sabate J. Nuts and coronary heart disease: an epidemiological perspective. Br J Nutr. 2006;96 2:S61–S67. doi: 10.1017/bjn20061865. [DOI] [PubMed] [Google Scholar]
- 5.Ros E, Nunez I, Perez-Heras A, et al. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–14. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 6.Tapsell LC, Gillen LJ, Patch CS, et al. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27:2777–83. doi: 10.2337/diacare.27.12.2777. [DOI] [PubMed] [Google Scholar]
- 7.Sabate J. Nut consumption and body weight. Am J Clin Nutr. 2003;78:647S–50S. doi: 10.1093/ajcn/78.3.647S. [DOI] [PubMed] [Google Scholar]
- 8.CD ROM. US Department of Agriculture, Agricultural Research Service, 2000. Continuing Survey of Food Intake by Individuals CSFII 1994-96. 1998. [Google Scholar]
- 9.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study Arch Intern Med. 1992;152:1416–24. [PubMed] [Google Scholar]
- 10.Mattes RD, Kris-Etherton PM, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr. 2008;138:1741S–5S. doi: 10.1093/jn/138.9.1741S. [DOI] [PubMed] [Google Scholar]
- 11.Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Gomez-Flores A, Salas-Salvado J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28:39–45. doi: 10.1016/j.clnu.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Burton-Freeman B. Dietary fiber and energy regulation. J Nutr. 2000;130:272S–5S. doi: 10.1093/jn/130.2.272S. [DOI] [PubMed] [Google Scholar]
- 13.Holt RI. International Diabetes Federation re-defines the metabolic syndrome. Diabetes Obes Metab. 2005;7:618–20. doi: 10.1111/j.1463-1326.2005.00519.x. [DOI] [PubMed] [Google Scholar]
- 14.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 16.Chan JL, Bullen J, Lee JH, Yiannakouris N, Mantzoros CS. Ghrelin levels are not regulated by recombinant leptin administration and/or three days of fasting in healthy subjects. J Clin Endocrinol Metab. 2004;89:335–43. doi: 10.1210/jc.2003-031412. [DOI] [PubMed] [Google Scholar]
- 17.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 18.Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003;88:4823–31. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 19.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. J Clin Endocrinol Metab. 2000;85:4511–4. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 20.Sabate J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain? Br J Nutr. 2005;94:859–64. doi: 10.1079/bjn20051567. [DOI] [PubMed] [Google Scholar]
- 21.Riddle MC, Drucker DJ. Emerging therapies mimicking the effects of amylin and glucagon-like Peptide 1. Diabetes Care. 2006;29:435–49. doi: 10.2337/diacare.29.02.06.dc05-1267. [DOI] [PubMed] [Google Scholar]
- 22.Zwirska-Korczala K, Konturek SJ, Sodowski M, et al. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol. 2007;58 1:13–35. [PubMed] [Google Scholar]
- 23.Karhunen LJ, Juvonen KR, Huotari A, Purhonen AK, Herzig KH. Effect of protein, fat, carbohydrate and fibre on gastrointestinal peptide release in humans. Regul Pept. 2008;149:70–8. doi: 10.1016/j.regpep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–56. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 25.Lall S, Tung LY, Ohlsson C, Jansson JO, Dickson SL. Growth hormone (GH)-independent stimulation of adiposity by GH secretagogues. Biochem Biophys Res Commun. 2001;280:132–8. doi: 10.1006/bbrc.2000.4065. [DOI] [PubMed] [Google Scholar]
- 26.Kelesidis T, Kelesidis I, Mantzoros C. Enivronmental inputs, intake of nutrients and endogenous molecules contributing to the regulation of energy homeostasis. In: Mantzoros C, editor. Nutrition and Metabolism Aristides Daskalopoulos Foundation. 2007. pp. 49–94. [Google Scholar]
- 27.Tapsell LC, Batterham MJ, Teuss G, et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009 doi: 10.1038/ejcn.2009.19. [DOI] [PubMed] [Google Scholar]
- 28.Mukuddem-Petersen J, Stonehouse OW, Jerling JC, Hanekom SM, White Z. Effects of a high walnut and high cashew nut diet on selected markers of the metabolic syndrome: a controlled feeding trial. Br J Nutr. 2007;97:1144–53. doi: 10.1017/S0007114507682944. [DOI] [PubMed] [Google Scholar]