Abstract
The class Cephalopoda (Phylum Mollusca), encompassing squids and octopuses, contains multiple species that are characterized by the presence of specialized organs known to emit light. These complex organs have a variety of morphological characteristics ranging from groups of simple, light-producing cells, to highly specialized organs (light organs) with cells surrounded by reflectors, lenses, light guides, color filters, and muscles. Bacteriogenic light organs have been well characterized in sepiolid squids, but a number of species in the family Loliginidae are also known to contain bacteriogenic light organs. Interest in loliginid light organ structure has recently arisen because of their potential as ecological niches for Vibrio harveyi, a pathogenic marine bacterium. This also implies the importance of loliginid light organs as reservoirs for V. harveyi persistence in the ocean. The present study utilized transmission and scanning electron microscopy to characterize the morphology of loliginid light organs and determined the location of bacterial symbiont cells within the tissue. It was determined that the rod-shaped loliginid symbionts lack flagella, as similarly observed in other light organ-associated bacteria. Also, the interaction of individual cells to light organ tissue is not as defined as reported for other squid-Vibrio systems. In addition, SEM observations show the presence of two pores leading to the bacterial chamber. Data presented here offer support for the hypothesis of environmental transfer of bacterial symbionts in loliginid squids.
Keywords: CEPHALOPODA, LIGHT ORGAN, VIBRIO, SYMBIOSIS, UROTEUTHIS, LOLIGINIDAE
INTRODUCTION
Several families within the class Cephalopoda (Mollusca) are characterized by the presence of light-producing organs, also known as photophores (Young 1972). Cephalopod photophores have a wide range of morphological features ranging from simple groups of photogenic cells, to organs with photogenic cells surrounded by reflectors, lenses, light guides, color filters, and muscles. These luminescent organs vary from very small, simple photophores (less than 0.2 mm in diameter) such as those of the mesopelagic squid Abralia trigonura (Young & Arnold 1982), to larger, more complex organs from squid of the family Sepiolidae (McFall-Ngai & Montgomery 1990). Complex photophores, also referred to as light organs, are found in the widely studied sepiolid squid Euprymna scolopes (McFall-Ngai & Montgomery 1990, Nishiguchi et al. 2004), as well as a number of other species in the families Sepiolidae and Loliginidae (Naef 1912a). Light organs found in sepiolids are oftentimes comparable in complexity to the compound eyes of many animals, including cephalopods themselves.
Light organs are able to readily adjust color, intensity, and angular distribution of light produced from within (Ferguson & Messenger 1991). In oceanic cephalopods, photophores emit intrinsic luminescence (autogenic) with light emanating from their own photocytes (Pringgenies & Jorgensen 1994). These cells are also able to emit different spectra of light (Herring et al. 1992). In contrast, photophores of most neritic cephalopods have extrinsic luminescence (bacteriogenic), with light produced by bacteria housed in a specialized light organ complex within the mantle cavity of their host (Naef 1912b, McFall-Ngai & Montgomery 1990, McFall-Ngai & Ruby 1991). Studies observing E. scolopes demonstrate that down-welling light intensity can be matched by the squids luminescence in a behavior termed counterillumination (Jones & Nishiguchi 2004). This confirms the hypothesis proposed by Young & Roper (1977) that production of light ventral to the squid mantle cavity is used for counter-shading down-welling light from the moon. Light organs of similar morphology are also found in oceanic teuthid squids (epipelagic and mesopelagic), such as Chiroteuthis spp., Chiropsis spp. and Taningia spp. However, according to Herring (1977), these anal light organs never house luminous bacteria.
Within the family Loliginidae, a number of species are known to posses bacteriogenic light organs (Alexeyev 1992). Loliginids are neritic, muscular squids, ranging from 3 to 100 cm in total body length. In contrast to sepiolids, loliginids have a gladius that extends along the mantle. They are also characterized by the existence of a branchial canal in the gill (Vecchione 2008). Light organs in these squids are bilobed and situated on the ventral side of the ink sac near the anus. They are known to harbor luminous bacteria from the family Vibrionaceae, which have been characterized both by molecular methods (Guerrero-Ferreira & Nishiguchi 2009, 2007) and biochemical and morphological assays (Guerrero-Ferreira et al. in review). These studies have provided evidence confirming that the pathogenic marine bacterium V. harveyi colonizes light organs in loliginid squid, and posed questions regarding the role of loliginid light organs as a reservoir for V. harveyi persistence in the ocean. However, the morphology of loliginid light organs has not been well described in the scientific literature, most likely because their potential as V. harveyi reservoir was not entirely understood. Thus, the objective of this study was to describe the anatomy and ultrastructure of loliginid light organs using transmission electron microscopy and scanning electron microscopy techniques. In addition, the presence of symbiotic bacteria in the light organ would clarify whether loliginid light organs resembled those of sepiolid squids. The results of this study describe in detail the morphology of light organs in loliginid squids from the genus Uroteuthis, as well as the location of symbiotic bacteria existing inside the light organ complex.
MATERIALS AND METHODS
Sample collection and fixation
Specimens (~10 of each species) of the loliginid squids Uroteuthis chinensis and Uroteuthis etheridge were collected off the coasts of Cairns and Townsville (Australia) respectively, by trawl netting at daytime. Light organs were dissected from the mantle cavity and immediately fixed in 4 % paraformaldehyde and 0.5 % glutaraldehyde (Electron Microscopy Sciences, Hartfield, PA, USA) in 0.1 M marine phosphate buffer (MPB, 0.66 M NaCl, 0.2 M phosphate buffer, pH 7.2). After fixation, light organs were kept at 4°C until processed for light microscopy and transmission electron microscopy. Tissue samples were simultaneously collected for confirmation of squid species identity by cytochrome c oxidase gene (COI) sequencing using universal primers (Folmer 1994) under the following amplification conditions: initial denaturation at 94°C followed by 25 cycles of 94°C for 50 s, 50°C for 75 s, and 72°C for 90 s. Sequences were compared with the National Center for Biotechnology Information (NCBI) database using BLAST 2.2.11 (Basic Local Alignment Search Tool, NCBI, NLM, NIH, Bethesda, MD) to confirm species identity.
Sample preparation for scanning and transmission electron and light microscopy
Scanning electron micrographs were directly taken using a Hitachi TM-1000 tabletop microscope, without further sample preparation. For preparation of light organs for transmission electron microscopy and light microscopy, samples were rinsed with 0.1 M MPB and post-fixed with 0.5 % osmium tetroxide in 0.1 M MPB for 1 hour, stained with % tannic acid (in dH202) for 1.5 hours, rinsed with water and subsequently dehydrated using a 50–100 % ethyl alcohol gradient. Light organs were incubated for 1 hour in each ethanol solution and finally incubated in 100 % ethyl alcohol overnight. Samples were then infiltrated with 3:1 ethanol: Spurr’s resin for 1 hour (Electron Microscopy Sciences, Hartfield, PA, USA), and then transferred to a 1:1 ethanol: Spurr’s solution overnight. A 1:3 ethanol: Spurr’s media was used to infiltrate the samples on the second day overnight, and then finally transferred to 100 % Spurr’s resin overnight for the last infiltration. All infiltration steps were completed on a rotating table at room temperature (~22°C). Tissue was embedded in Spurr’s resin in plastic molds. Resin was polymerized at 68°C overnight.
Thick Sectioning and imaging
Polymerized blocks were trimmed and sectioned using a Leica UC6 Ultramicrotome (Leica, Bannockburn, IL, USA). Thick sections (2 μm) were collected onto droplets of double distilled water on ethanol-washed glass slides. Slides were dried on a heat block for approximately five minutes to guarantee complete attachment of the sections to the slide. Subsequently, sections were broadly covered with 1 % toluidine blue epoxy stain (EMS, Hatfield, PA) for 1 minute. Sections were then extensively washed with double distilled water to ensure that all excess stain was removed from the slides. Samples were then viewed with a Nikon E800 upright epifluorescence microscope (Nikon Inc., Melville, NY).
Thin sectioning and imaging
Thin sections were collected onto a 300 mesh (squared) copper grid (Electron Microscopy Sciences, Hatfield, PA, USA) previously washed with a mild solution of hydrochloric acid. Subsequently, sections were positively stained with 2 % uranyl acetate (EMS, Hatfield, PA) for 10 min. and then with 1 % lead citrate (Polysciences Inc., Warrington, PA) for 10 min. Sections were washed with distilled water for one minute between stains. Stained samples were viewed through a Hitachi H7650 Transmission Electron Microscope (Hitachi, Schaumburg, IL, USA) at an accelerating voltage of 80kV.
RESULTS
Specimens were identified through sequencing of the cytochrome c oxidase subunit one gene (COI) and when compared with the NCBI database using BLAST 2.2.11, were confirmed as two species of the family Loliginidae, Uroteuthis chinensis and Uroteuthis etheridge.
Light organs in these species of squid are located on the ventral face of the ink sac. With respect to the size of U. chinensis and U. etheridge specimens (approximately 30 cm of dorsal mantle length), the light organs are considerably smaller when compared to the homologous structures in members of the family Sepiolidae, such as the Hawaiian bobtail squid Euprymna scolopes (McFall-Ngai & Montgomery 1990). Ventral dissection of the squid U. etheridge reveals the relatively small, bilobed light organ situated within the mantle cavity (Fig. 1).
Fig. 1.
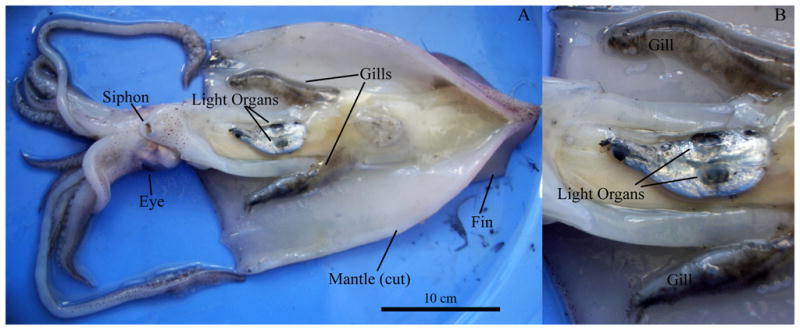
Ventral view of the mantle cavity of an adult Uroteuthis etheridge. A: Whole specimen B: closeup of the light organs. Dorsal mantle length: 30 cm.
A histological cross-section of one lobe of the light organ reveals the existence of labyrinths of crypts where bacteria are housed (Fig. 2). Light microscopy also illustrates the location of the reflector tissue (RT) with respect to the light organ crypts within the bacterial chamber (BC). These observations confirmed results made by Pringgenies & Jorgensen (1994), which illustrated a layer of tissue representing a reflector that was immediately in contact with the bacterial chamber (Fig. 2). Their results were also similar to earlier descriptions of light organ morphology in sepiolid squids (summarized in Nyholm & Nishiguchi 2008), where bacterial crypts are also surrounded by reflector tissue (dorsal) as well as a lens (ventral).
Fig. 2.
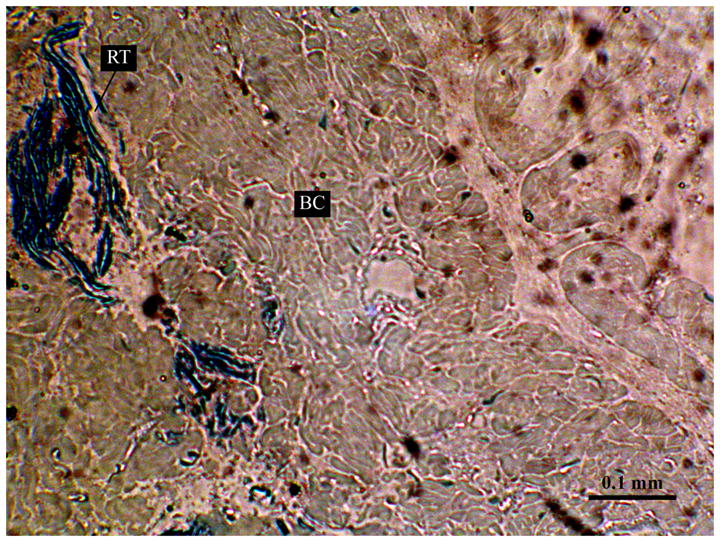
Light micrograph of a section showing the bacterial chamber (BC) of the loliginid squid Uroteuthis etheridge. Highly electron dense material corresponds to the reflector tissue (RT). Scale bar: 0.1 mm
By completing transmission electron microscopy of the reflector tissue area, it was possible to confirm the presence of flat, structural platelets or lamellae organized in alternate layers (Fig. 3). Secreted ink granules from the ink sac are also clearly visible in the micrograph. The lamellae have a constant width of approximately 100 nm, but their length varies considerably. These structures have been studied in E. scolopes and have been found to contain proteins called reflectins (Crookes et al. 2004) which makes them an exception to most aquatic animals that contain reflectors made of crystals of purinic nucleotides, specially guanine and hypoxanthine (Denton & Land 1971).
Fig. 3.
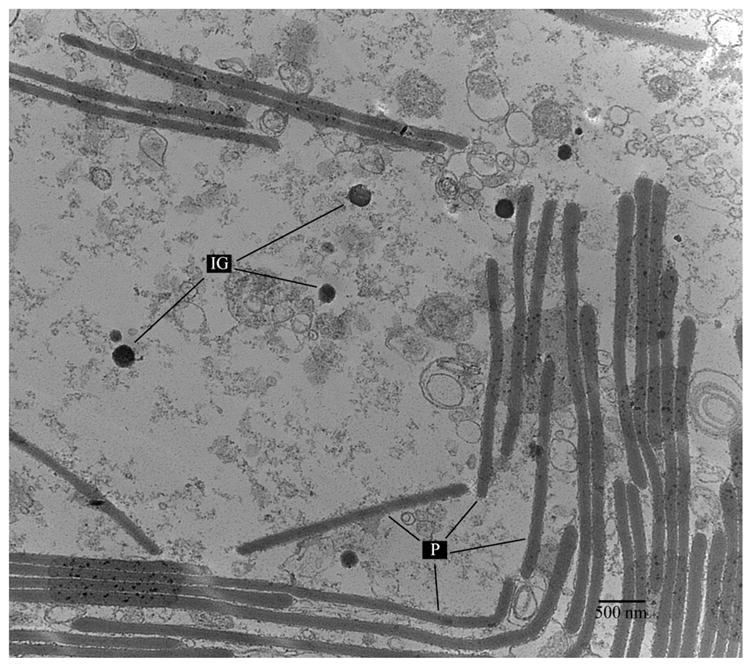
TEM image of a region of U. etheridge light organs. Ink granules (IG) and platelets (P) are shown in the field. Scale bar: 500 nm.
Uroteuthis etheridge light organs (Fig. 1) are known to house luminescent bacteria of the family Vibrionaceae (Guerrero-Ferreira & Nishiguchi 2007). Because recent studies in our lab have demonstrated that at least one member of the bacterial symbiotic population within these organs is the marine pathogen Vibrio harveyi, it was of great interest to structurally describe the state of the interaction between the two partners. A particular goal of this study was to determine where in the light organ crypts these bacterial symbionts reside, as well as to gather information regarding the symbiotic morphology of the bacteria, particularly the presence of bacterial cell appendages which might facilitate adhesion to squid tissues.
Figure 4 portrays the location of the bacterial cells when associated with U. chinensis squid tissue. The most noticeable feature of the symbionts is the absence of flagella. Even though bacteria from the family Vibrionaceae are known to possess either monotrichous or peritrichous flagella (Baumann 1984), bacterial cells in U. chinensis light organ lack flagella. Previous research studying the association between E. scolopes and V. fischeri has also shown that loss of flagella is a characteristic process that occurs upon establishment of the association (Ruby & Asato 1993).
Fig. 4.

Vibrio bacteria (B) associated to Uroteuthis chinensis squid tissue (CT for connective tissue). Cells are located inside crypt spaces. Scale bar: 500 nm.
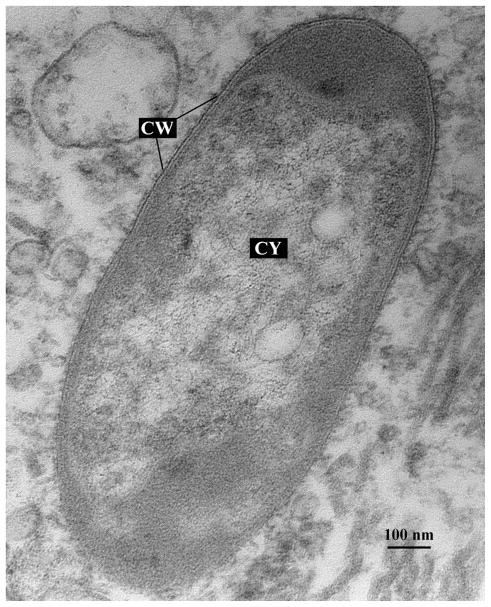
The morphology of the bacteria colonizing light organs was the characteristic rod shape of members in the family Vibrionaceae. Figure 5 illustrates one of these symbionts and clearly defines the cell wall (CW) and cytoplasm (CY) of the bacterium. In general, transmission electron microscopy of the bacteria reveals they have the ovoid or oblong shape, characteristic of vibrios. Their sizes vary, with smaller bacteria measuring approximately 0.5 μm in length, whereas the largest having dimensions of up to 3.0 μm in length. Symbiotic bacteria residing in the light organ exhibit a dense cytoplasm, with the presence of vacuoles approximately 100 nm in diameter (Fig. 6). In addition, cell appendage pili are present that might allow for bacterial attachment to squid tissue, as it is known to occur in V. fischeri bacteria colonizing E. scolopes (Nair and Nishiguchi 2009, McFall-Ngai & Ruby 1991).
Fig. 5.
Bacterial symbiont within the crypt region of Uroteuthis etheridge light organ. CW: Cell wall. CY: Cytoplasm. Scale bar: 100 nm.
Fig. 6.
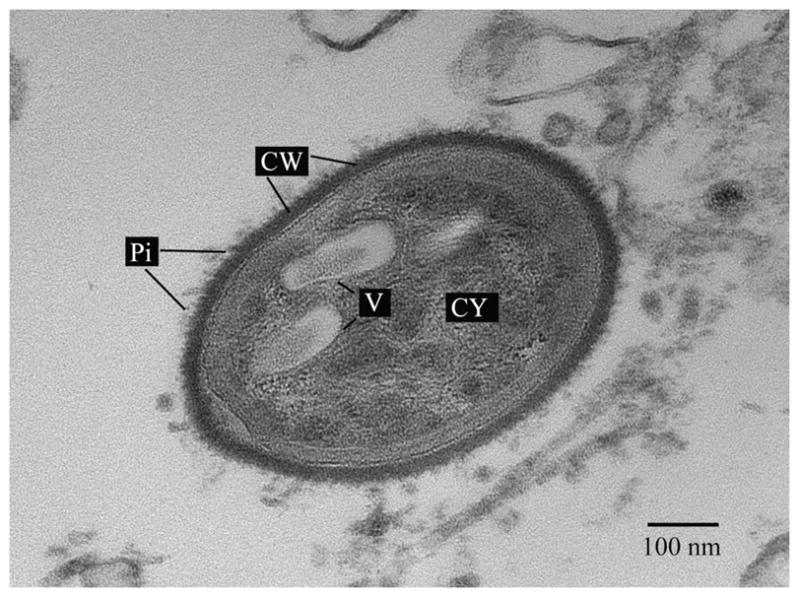
Bacterial cell associated to the squid Uroteuthis etheridge with pili (P) and vacuoles (V) present. Scale bar: 100 nm.
Scanning electron microscopy was also used to determine the presence of pores that are necessary for initial colonization by symbionts infecting the crypt region of the light organ. In the E. scolopes light organ complex, three pores exist in the juvenile squid after hatching. Upon successful colonization by their symbiotic partner V. fischeri, developmental changes occur leading to the formation of a single pore that allows for the dial cycle of bacterial venting that occurs throughout the life of the mollusc (Foster et al. 2002). Interestingly, SEM illustrates that U. chinensis has a light organ with two pores leading into the bacterial chamber in the adult squid (Fig. 7). The size of these pores is approximately 5 μm, which is similar to the size reported by Montgomery and McFall-Ngai in sepiolid squids (1993). Previous results have shown that pore number varies among sepiolid squids; the genus Sepiola has 4 pores on each light organ lobe prior to infection by their Vibrio bacteria (Foster et al. 2002). The functional advantage for the occurrence of two pores in adult U. chinensis is still unclear, but their presence may be required for similar venting behaviors to those found occurring in adult sepiolids.
Fig. 7.
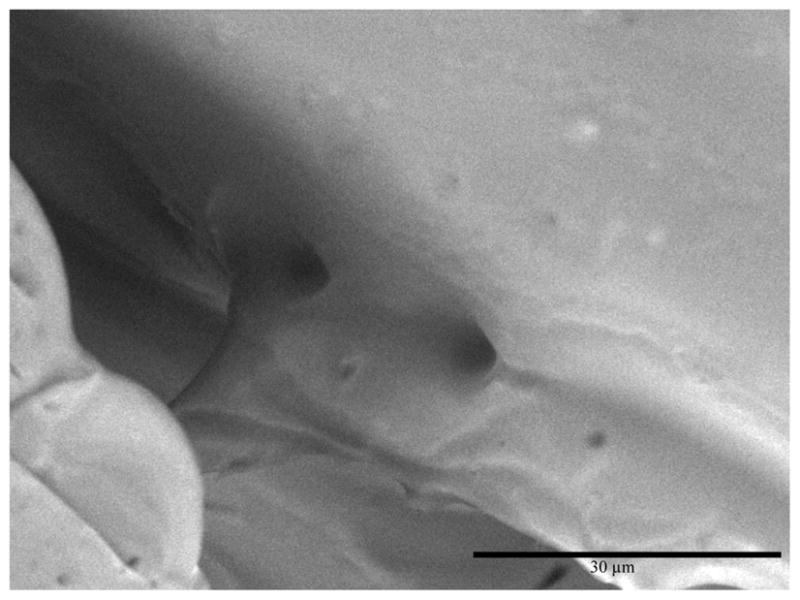
Scanning electron micrograph of Uroteuthis chinensis light organ showing two pores leading into the bacterial chamber. Scale bar: 30 μm.
DISCUSSION
This study provides evidence that physical interactions between bacterial cells and loliginid squid light organs is somewhat sporadic. Electron micrographs illustrate an infrequent distribution of bacterial cells with respect to each other (i.e., no aggregation) and with the squid host light organ tissue. This is in agreement to the description given by Pringgenies and Jorgensen (1994). According to their study, scarcity of bacterial cells was due to the crypts in the light organ being used exclusively for expulsion of excretion products from both symbiont and host. However, it is currently understood that the symbiont community in sepiolid light organs has a diurnal fluctuation (McFall-Ngai & Ruby 1991, Ruby & McFall-Ngai 1992), which may account for the low density of bacteria observed in the micrographs presented here. In addition, absence of a strong physical connection between host cells and symbionts may account for the lack of species-specificity found in loliginid light organ symbiosis and may explain the diversity of bacterial genotypes found in their crypts. Recent studies have established that more than one species of Vibrionaceae bacteria colonize light organs of squids in the family Loliginidae (Guerrero-Ferreira & Nishiguchi 2007). Further studies would need to address whether light organ venting occurs with the onset of dawn in loliginid squids, and if the lack of pili corresponds to a less specific interaction on the cellular level.
This is also true in the case of light organs in the leiognathid fish Nuchequula nuchalis, where Vibrio harveyi was recently found as a member of the bacterial community (Dunlap et al. 2008). In this species, more than two distinct ecological variants of Photobacterium leiognathi were also found colonizing the light organ tissue. Transmission electron micrographs of N. nuchalis show a similar scattered distribution of symbionts within the light organ tubules, which supports the aforementioned idea of a relationship between lack of specificity and limited physical attachment to host tissue.
In addition, vacuole-like, refractive granules were observed within the symbionts in quantities varying from one to five. These granules have been previously proposed to be poly-β-hydroxybutyrate (PHB) (Dunlap et al. 2008), a compound that is produced in V. harveyi cells at high density in the presence of endogenous lux autoinducer, n-(3-D-hydroxybutanoyl) homoserine lactone (Sun et al. 1994). This restricts the production of PHB to V. harveyi strains living under confined conditions (such as the light organ of loliginid squids) which might account for the classification of V. harveyi cells as PHB negative (Baumann 1981). Because of this, it is possible that PHB containing bacteria living in conditions such as those of fish and squid light organs were misclassified as species different than V. harveyi, as observed by Miyamoto et al. (1998).
Tissue organization in loliginid light organs observed through TEM resembles the structure of sepiolid light organs with reflector and lens tissue surrounding the chambers where the bacterial symbionts are located (McFall-Ngai & Montgomery 1990). In sepiolid squids, the arrangement of these various tissue types allows the animal to utilize light produced by the bacteria in a behavior known as counterillumination (Jones & Nishiguchi 2004). Our results present morphological evidence suggesting that Uroteuthis spp. use their association with Vibrionaceae bacteria for the same purpose. Further behavioral studies investigating light production under controlled down welling light intensity will contribute to our understanding of the evolutionary significance of this association for loliginids. Additionally, temporal studies with light organs from squids collected at several times of the day would help determine whether low bacterial densities within light organ crypts are due to diurnal variation in bacterial populations in loliginid squids.
Moreover, we can argue that association of loliginid squids with their bacterial symbionts is currently in an ancestral state. Nishiguchi et al. (2004) presented a hypothesis for the origin of bacteriogenic light organs from the accessory nidamental gland (ANG) complex of sepiolid squids. This organ complex has more than 20 strains of bacteria present that are acquired from the surrounding seawater environment (Kaufman et al. 1998). If ANG in loliginid squids contributes to the symbiont population in loliginid light organs, this may explain the diversity of bacteria found in these complexes and the lack of a strong physical association between partners (Guerrero-Ferreira & Nishiguchi 2007). Strong selection by the host for particular strains or species of Vibrio bacteria will need to occur in order to accommodate strain specificity and cospeciation (Nishiguchi et al. 1998, Nishiguchi 2002, Nyholm & Nishiguchi 2008). Likewise, these environmentally transmitted symbionts that colonize loliginid light organs will need to be successful in their free-living stages in order to out-compete other bacteria for their niche in this mutualism. Trade-offs between these two life history strategies appear to be the ultimate driving forces for the evolution of this dynamic and evolutionary significant symbiosis; whether multiple or single strains can coexist needs to be taken into careful consideration when contemplating the origin of bacteriogenic light organs (Nishiguchi et al. 2008, Nyholm & Nishiguchi 2008).
Acknowledgments
The authors thank R Al-Katib from the NMSU Electron Microscopy Lab for his assistance with this project. Field work for RGF was in part funded by a travel grant from the Associated Students of New Mexico State University. This work was supported by NSF-IOS 0744498 and NIH-NIAID 1SC1AI081659-01 to MKN.
References
- Alexeyev DO. The systematic position of bioluminescent squids of the family Loliginidae (Cephalopoda: Myopsida) Zool Zhurnal. 1992;71:12–23. [Google Scholar]
- Baumann P, Baumann L. The marine Gram-negative eubacteria. In: Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG, editors. The Prokaryotes, a Handbook on Habitats, Isolation, and Identification of Bacteria. Springer Verlag; New York: 1981. pp. 1352–1394. [Google Scholar]
- Baumann P, Schubert RHW. Family II. Vibrionaceae. In: Krieg RN, Holt JG, editors. Bergy’s Manual of Systematic Bacteriology. Vol. 1. The Williams and Wilkins Co; Baltimore: 1984. pp. 516–517. [Google Scholar]
- Crookes WJ, Ding LL, Huang QL, Kimbell JR, Horwitz J, McFall-Ngai MJ. Reflectins: The unusual proteins of squid reflective tissues. Science. 2004;303:235–238. doi: 10.1126/science.1091288. [DOI] [PubMed] [Google Scholar]
- Denton EJ, Land MF. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc R Soc Lond B Biol Sci. 1971;178(50):43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Dunlap PV, Davis KM, Tomiyama S, Fujino M, Fukui A. Developmental and microbiological analysis of the inception of bioluminescent symbiosis in the marine fish Nuchequula nuchalis (Perciformes: Leiognathidae) Appl Environ Microbiol. 2008;74(24):7471–7481. doi: 10.1128/AEM.01619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GP, Messenger JB. A countershading reflex in cephalopods. Proc R Soc B. 1991;243(1306):63–67. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Foster JS, Boletzky Sv, McFall-Ngai MJ. A comparison of the light organ development of Sepiola robusta Naef and Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Bull Mar Sci. 2002;70(1):141–153. [Google Scholar]
- Guerrero-Ferreira RC, Gorman C, Willie S, Nishiguchi MK. Identification of Vibrio harveyi bacterial isolates from light organs of loliginid squids (Mollusca: Cephalopoda) from Thailand. Microb Ecol. in review. [Google Scholar]
- Guerrero-Ferreira RC, Nishiguchi MK. Differential gene expression in bacterial symbionts from loliginid squids demonstrates variation between mutualistic and environmental niches. Environ Microbiol Rep. 2009 doi: 10.1111/j.1758-2229.2009.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Ferreira RC, Nishiguchi MK. Biodiversity among luminescent symbionts from squid of the genera Uroteuthis, Loliolus and Euprymna (Mollusca: Cephalopoda) Cladistics. 2007;23(5):497–506. doi: 10.1111/j.1096-0031.2007.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring PJ. Luminescence in cephalopods and fish. Symp Zool Soc Lond. 1977;38:127–159. [Google Scholar]
- Herring PJ, Widder EA, Haddock SHD. Correlation of bioluminescence emissions with ventral photophores in the mesopelagic squid Abralia veranyi (Cephalopoda: Enoploteuthidae) Mar Biol. 1992;112:293–298. [Google Scholar]
- Jones BW, Nishiguchi MK. Counterillumination in the bobtail squid, Euprymna scolopes Berry (Mollusca: Cephalopoda) Mar Biol. 2004;144:1151–1155. [Google Scholar]
- Kaufman MR, Ikeda Y, Patton C, van Dykhuizen G, Epel D. Bacterial symbionts colonize the accessory nidamental gland of the squid Loligo opalescens via horizontal transmission. Biol Bull. 1998;194:36–43. doi: 10.2307/1542511. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Montgomery MK. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae) Biol Bull. 1990;179:332–339. doi: 10.2307/1542325. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Ruby EG. Symbiont recognition and subsequent morphogenesis as early events in an animal-bacterial symbiosis. Science. 1991;254:1491–1494. doi: 10.1126/science.1962208. [DOI] [PubMed] [Google Scholar]
- Miyamoto CM, Sun W, Meighen EA. The LuxR regulator protein controls synthesis of polyhydroxybutyrate in Vibrio harveyi. Biochim Biophys Acta (BBA) - Protein Struct Mol Enzym. 1998;1384(2):356–364. doi: 10.1016/s0167-4838(98)00028-4. [DOI] [PubMed] [Google Scholar]
- Montgomery MK, McFall-Ngai MJ. Embryonic development of the light organ of the Sepiolid squid Euprymna scolopes Berry. Biol Bull. 1993;184:296–308. doi: 10.2307/1542448. [DOI] [PubMed] [Google Scholar]
- Naef A. Teuthologische Notizen. 2. Gattungen der Sepioliden. Zool Anz. 1912a;39:244–248. [Google Scholar]
- Naef A. Teuthologische notizen. 3. Die Arten der Gattungen Sepiola und Sepietta. Zool Anz. 1912b;39:262–271. [Google Scholar]
- Nair VS, Nishiguchi MK. Biological properties (in vitro) exhibited by free-living and symbiotic Vibrio isolates. Vie Milieu. 2009;59(3/4):277–285. [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK, Devinney R, Hirsch AM, Riley M, Mansky L, Vendatum G. Perspective: Evolution of virulence: Deciphering the mechanisms between pathogenic and benign symbioses. Vie Milieu. 2008;58:87–106. [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK. The use of physiological data to corroborate cospeciation events in symbiosis. In: DeSalle R, Wheeler W, Giribet G, editors. Molecular Systematics and Evolution: Theory and Practice. Birkhäuser; Basel, Germany: 2002. pp. 237–246. [DOI] [PubMed] [Google Scholar]
- Nishiguchi MK, Lopez JE, Boletzky Sv. Enlightenment of old ideas from new investigations: more questions regarding the evolution of bacteriogenic light organs in squids. Evol Dev. 2004;6(1):41–49. doi: 10.1111/j.1525-142x.2004.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi MK, Ruby EG, McFall-Ngai MJ. Competitive dominance among strains of luminous bacteria provides an unusual form of evidence for parallel evolution in sepiolid squid-Vibrio symbioses. Appl Environ Microbiol. 1998;64:3209–3213. doi: 10.1128/aem.64.9.3209-3213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm SV, Nishiguchi MK. The evolutionary ecology of a sepiolid squid-Vibrio association: From cell to environment. Vie Milieu. 2008;58:175–184. [PMC free article] [PubMed] [Google Scholar]
- Pringgenies D, Jorgensen JM. Morphology of the luminous organ of the squid Loligo duvauceli d’Orbigny, 1839. Acta Zool-Stockholm. 1994;75(4):305–309. [Google Scholar]
- Ruby EG, Asato LM. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- Ruby EG, McFall-Ngai MJ. A squid that glows in the night: Development of an animal-bacterial mutualism. J Bacteriol. 1992;174(15):4865–4870. doi: 10.1128/jb.174.15.4865-4870.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Cao JG, Teng K, Meighen EA. Biosynthesis of poly-3-hydroxybutyrate in the luminescent bacterium, Vibrio harveyi, and regulation by the lux autoinducer, N-(3-hydroxybutanoyl) homoserine lactone. J Biol Chem. 1994;269(32):20785–20790. [PubMed] [Google Scholar]
- Vecchione M. 2008 Loliginidae Lesueur, 1821. Version 04. The Tree of Life Web Project. 2008 March; http://tolweb.org.
- Young RE. Function of extra-ocular photoreceptors in bathypelagic cephalopods. Deep-Sea Res. 1972;19:651–660. [Google Scholar]
- Young RE, Arnold JM. The functional morphology of a ventral photophore from the mesopelagic squid, Abralia trigonura. Malacologia. 1982;23:135–183. [Google Scholar]
- Young RE, Roper CFE. Intensity regulation of bioluminescence during countershading in living midwater animals. Fish Bull. 1977;75:239–252. [Google Scholar]