Abstract
A large body of documented evidence shows that smoking during pregnancy is harmful to both the mother and fetus. Prenatal exposure to nicotine in various forms alters neurologic development in experimental animals and may increase the risk for neurologic conditions in humans. There is a direct association between maternal smoking and SIDS; however the connection with depression, attention disorders, learning and behavior problems, and nicotine addiction in humans is not straightforward. Nicotine’s action on the production and function of neurotransmitters makes it a prime suspect in the pathology of these diseases. Nicotine accentuates neurotransmitter function in adults but desensitizes these functions in prenatally exposed infants and children. This desensitization causes an abnormal response throughout the lifespan. Furthermore, nicotine use in the adolescent and adult can alleviate some of the symptoms caused by these neurotransmitter problems, but it also increases the risk for nicotine addiction. Although nicotine replacement drugs are allowed for pregnant women, there is no clear indication that they improve outcomes during pregnancy, and they may add to the damage to the developing neurologic system. Understanding the effects of nicotine exposure is important in providing safe care for pregnant women, children and families and for developing appropriate smoking cessation programs during pregnancy.
Keywords: Nicotine, Smoking, Pregnancy, SIDS, ADHD
INTRODUCTION
Exposure to cigarette smoking is one of the most modifiable causes of morbidity and mortality for both the mother and fetus. Damage from maternal smoking has a direct adverse effect on placental development, which decreases the transfer of nutrients and oxygen to the fetus and can result in premature delivery, fetal growth restriction, and smaller head size.1 Exposure to cigarette smoke during gestation has also been associated with problems beyond the perinatal period that can last well into adulthood.2, 3
Cigarette smoke is made up of more than 4000 compounds.4 Nicotine, carbon monoxide, and aldehydes are all likely candidates for causing perinatal damage. In the developing fetus, nicotine crosses both the placental and the blood-brain barriers, and is found in the fetal compartment in a concentration 15% higher than in maternal tissues.5 Smoking during pregnancy accounts for as many as 161,000 perinatal deaths and 4800 infant deaths in the United States each year, with more than half of these classified as sudden infant death syndrome (SIDS).3 Maternal smoking is currently the leading risk factor for SIDS. Although paternal smoking and exposure to other forms of environmental tobacco smoke (ETS) have been shown to increase SIDS risk, the risk is highest when the mother smokes during pregnancy.6
Much of the current research on the pathophysiology of nicotine exposure has been done in animal models. Research in humans has focused primarily on epidemiologic study of human behaviors. The biggest issue in human studies of nicotine exposure is defining and accurately assessing maternal smoking status. Both self-report and past recall of smoking status during pregnancy introduces significant concern of an underreporting bias. The better-designed studies use biologic measures of smoking, which may increase reliability but are still subject to underreporting. When pregnant women know in advance that they will be tested for cotinine (a nicotine metabolite), they can alter their smoking habits and change their reported incidence. Cotinine has a half-life of 17 to 21 hours in non-pregnant women7 but only 9 hours in pregnant women between 16 and 40 weeks gestation.8 Changes in smoking behavior prior to cotinine sampling can thus alter test results.9 This alone may explain discrepancies across studies in reported incidence of maternal smoking which have yielded estimates varying from 7%9 to 33%10, with most commonly reported values ranging between 22 and 27%, subject to variation by socioeconomic group and maternal age.11, 12
Because of the high nicotine exposure to the fetus from maternal smoking, this paper will review animal and human research associated with the effect of nicotine on the developing nervous system and the implications for clinical practice. We will focus on data examining the role of prenatal nicotine exposure as a contributing factor to subtle long-term effects on neurodevelopment, learning disorders, attention deficits, addiction, and behavioral changes. Although these outcomes are more difficult to attribute to maternal smoking, the action of nicotine on the production and function of neurotransmitters makes it a prime suspect in their underlying pathology. Other tobacco-related risks for children (including the irritant effect of ETS and associated increase in chronic otitis media, asthma, and respiratory infections)13 will not be discussed in this paper.
THE EFFECTS OF NICOTINE ON THE ADULT
Nicotine accentuates neurotransmitter function in adults but desensitizes these functions in prenatally exposed infants and children. In adults, nicotine directly affects the central nervous system by stimulating the sympathetic nervous system to release epinephrine from the adrenal cortex. This is accomplished through its action on the nicotinic acetylcholine receptor (nAChR), and results in an increase in blood pressure and heart rate.14 Small frequent doses of nicotine produce alertness and arousal, whereas sustained exposure has a sedative action, reduces anxiety, and induces euphoria.14 At commonly used doses nicotine enhances intellectual performance, decreases depression and anxiety, and activates the dopamine reward system, which is important in addiction.15–17
THE EFFECTS OF PRENATAL NICOTINE EXPOSURE ON NERVOUS SYSTEM DEVELOPMENT
Central Nervous System Development
In order to understand the effects of cigarette smoking during pregnancy, it is necessary to describe the processes by which the central nervous system develops and how nicotine exposure can change that development. During the first and second trimesters of pregnancy neurological development progresses from the spinal cord to brain stem, midbrain, and cerebral cortex. The cerebral cortex is a layer of gray matter that covers the cerebral hemispheres of the frontal, temporal, parietal, and occipital lobes of the brain, and is responsible for voluntary muscle activity, learning, language, and memory (Figure 1).18 The higher functions of the brain involving the hypothalamus and associated structures of the precortical areas (areas under the cortex), the limbic system, and cerebellar function continue to develop during the first few years of post-neonatal life in the human infant, and during the first two weeks of life in the laboratory rodent (Figure 2).19 The effect of a neurotoxin such as nicotine depends on the dose and timing of exposure. Obviously, chronic exposure throughout pregnancy will affect many different functions in the developing brain, whereas exposure that is limited to a specific time of pregnancy may only affect the specific functions developing during that precise interval.1
Figure 1.
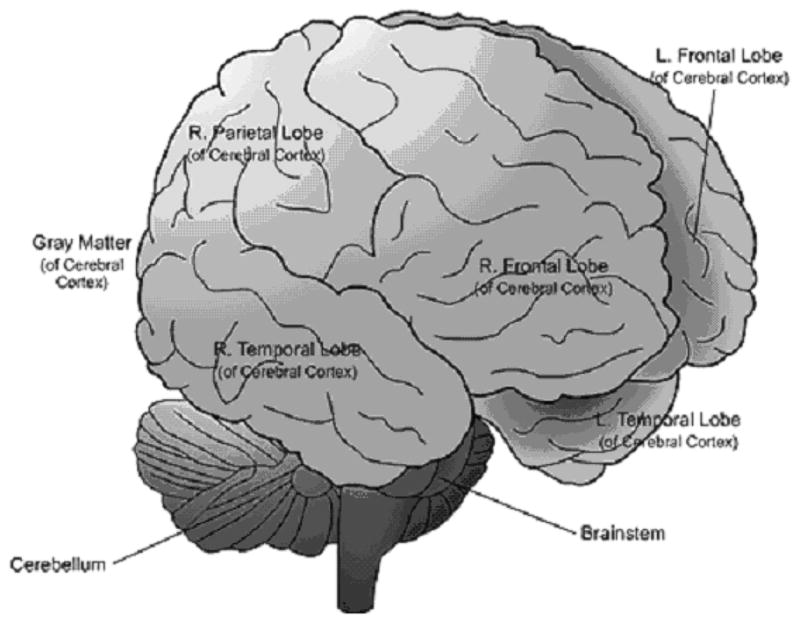
Cerebral cortex and areas of the outer brain 49
Figure 2.

Interior structures of the brain affected by perinatal nicotine exposure
Prenatal Nicotine Exposure and Nervous System Development
It has been determined from animal studies that the nicotinic acetylcholine receptor (nAChR) is functional early in fetal development, by the time the neural tube is being formed.20 From the standpoint of nervous system development, the most important physiologic characteristic of nicotine is its ability to stimulate the nAChR and trigger neurodevelopmental events that are normally ascribed to the action of acetylcholine.21–24 In animal models it has been demonstrated that acetylcholine has a very active role in brain development and is responsible for the proliferation, maturation, and differentiation of multiple types of brain cells.25, 26
Nicotine exposure changes the intensity and timing of brain cell development and the programming of neurodevelopmental events on a cellular level. When the timing of these events is perturbed, it alters the orderly processes by which neurons are replicated and differentiate into functional neuronal cells.27 These processes include initiation of axons and dendrites, migration of nerve cells, synapse function, and localization of specific nerve cell populations. Later in development, exposure to nicotine changes higher sensory, memory, and motor functions through its effects on hippocampal, cerebellar, and sensory cortex development.28
EFFECTS OF PRENATAL NICOTINE EXPOSURE ON AUTONOMIC NERVOUS SYSTEM FUNCTION
Effects on Autonomic Nervous System Function in Animals
In animal studies prenatal nicotine exposure alters both central and peripheral autonomic tone, which is important in the regulation of protective cardiac and respiratory functions in the neonate.24, 29, 30 Neonatal rat pups exposed to nicotine during gestation lose their ability to mount a protective response to a hypoxic challenge. This lack of hypoxia tolerance is assumed to be related to nicotine-induced reduction in the ability of the adrenal medulla to secrete adequate levels of epinephrine and norepinephrine.30 These catecholamines are responsible for heart rate, cardiorespiratory functions of arousal, breathing, apnea response, heart rate variability, and autoresuscitation.31
In addition to reducing the levels of epinephrine and norepinephrine, prenatal nicotine exposure delays the development of beta receptors in the heart which are important for increasing heart rate responsiveness to hypoxia and other cardiovascular challenges. Nicotine-exposed rat pups not only show decreased binding at the cardiac beta receptor, but also have no protective tachycardia when exposed to 5% O2 for 10 minutes.32 These animals respond with an immediate and rapid drop in heart rate. This effect is related to an increase in inhibitory cardiac M2-muscarinic cholinergic receptors and a down-regulation of the stimulatory beta-adrenergic receptor. Both factors effectively down-regulate the protective sympathetic response to hypoxia.32
Thus prenatal nicotine exposure appears to change autonomic responses by decreasing the production of epinephrine and norepinephrine in the adrenal medulla, and by down-regulating receptor function in the heart. In rats, this alteration in autonomic response persists into adulthood, with decreased levels of norepinephrine in the brain and an inability of acute cholinergic stimulation to evoke a normal adult norepinephrine response.33
Effects on Autonomic Nervous System Function in Humans
As in animal models, infants prenatally exposed to nicotine have lower epinephrine and norepinephrine levels in cord blood at birth than unexposed infants.34 These catecholamines play a critical role in autonomic responses. Concerns about imbalance in autonomic tone are well documented in the SIDS literature, because it may decrease the infant’s ability to respond to cardiovascular and respiratory challenges, resulting in death.35 Although SIDS is likely to have many causes, infants of smoking mothers have a 2- to 4- fold increased vulnerability compared to unexposed infants.6, 36
EFFECTS OF PRENATAL NICOTINE EXPOSURE ON NEUROTRANSMITTERS
Neurotransmitters are brain chemicals that are critical for brain function. Table 1 describes the normal function of neurotransmitters and the changes found in animal models following prenatal nicotine exposure. Nicotine exposure early in fetal development adversely affects the synaptic development and function of serotonin systems as well as those of other monoamines (dopamine, norepinephrine), eliciting neuronal damage and cell death, as well as suppressing both presynaptic and postsynaptic elements required for neurotransmission.37–40
Table 1.
Neurotransmitters are influenced by nicotine exposure during development
| Neurotransmitter | Location | Function | Effect of Prenatal Nicotine Exposure |
|---|---|---|---|
| Acetylcholine | Neuromuscular junction of skeletal muscle, autonomic nerve synapses, central nervous system, spinal cord | Important for normal development of the nervous system 25 | Nicotine is a direct stimulant of the nAChR. Its action on the receptor during brain development alters proliferation, maturation, and differentiation of brain cells.25, 26 |
| Serotonin | Central nervous system, spinal cord, GI tract | Regulation of mood and depression.41 Regulates heart rate, respiration and arousal from sleep in brain stem42 |
Causes a significant reduction in serotonin turnover in many areas of the brain, including vital areas of the brainstem. 37 Decreased serotonin function will increase risk of depression and SIDS.42 |
| Catecholamines: Epinephrine |
Produced in the adrenal medulla and sympathetic nerve terminals.41 |
Diverse effects, but primarily involved in autonomic responses of “fight and flight”. Increases blood pressure, pulse, and respiration.41 |
Infants of smokers have lower epinephrine levels in cord blood at birth than infants of non-smoking mothers.34 This can change the regulation of autonomic responses. |
| Norepinephrine | Most postganglionic sympathetic fibers, spinal cord, and the adrenal medulla41 | Same as epinephrine, plus norepinephrine is involved in the pathophysiology of inattention and distractibility 43, 44 | Infants of smokers have lower norepinephrine levels in cord blood at birth than infants of non-smoking mothers.34 This is important for autonomic function and ADHD. |
| Dopamine | Adrenal medulla, central nervous system, autonomic nervous system synapses41 | Regulating blood pressure and prolactin release; suppressing inappropriate behavioral impulses.42, 44 Dopamine is important in addiction | In animals, prenatal nicotine exposure has been shown to lower the levels at the receptor site. This could be important for symptoms in ADHD and addiction.45 |
Prenatal Nicotine Exposure and Serotonin in Animals
Serotonin is important for regulation of mood and depression; however, in the brain stem it also regulates heart rate, respiration, and arousal from sleep.42, 46 Fetal nicotine exposure in rats alters the ability of the serotonin transporter to function effectively, which results in significant reduction of serotonin turnover in many areas of the brain, including vital areas of the brainstem.37, 47 Decreased serotonin turnover results in a lower level of serotonin in the neural synapse, similar to the mechanism thought to occur in depression.48
Prenatal Nicotine Exposure and Serotonin in Infants
In human infants, serotonin defects have been best studied as they relate to SIDS. On autopsy, SIDS infants have a significantly higher number of serotonin-producing neurons but a lower density of serotonin receptor binding sites in regions of the medulla that control some homeostatic functions. This pathology in serotonin function in SIDS is fairly extensive within the central nervous system and includes abnormal neuronal synthesis, release, and clearance.35 It is hypothesized that the damage in the serotonergic system found in some SIDS infants is directly related to maternal smoking, which could support a possible biologic basis for the association between maternal smoking and increased risk of SIDS. 3 However, quantifying the relationship of dose to outcome for such a pathway would be very difficult.42 Not all SIDS infants have a history of nicotine exposure, and other environmental insults as well as differences in genetic vulnerability are likely to contribute to risk.
Prenatal Nicotine Exposure and Animal Data Support a Role in ADHD
Both norepinephrine and dopamine have been linked to the etiology of ADHD.49 They appear to be important in the ability of the brain to accept or repress the normal stimulation of daily living.42–44 Optimal brain function requires a balance between stimulation and suppression of stimuli that varies by function, timing and need. In animal studies, nicotine exposure during pregnancy has been shown to reduce levels of norepinephrine and dopamine function in the brain that may be important in controlling activity and impulsive behaviors.37, 47 Prenatal nicotine exposure of rat pups significantly increases hyperactive behavior; however more sophisticated studies of hyperactivity and learning problems are difficult to do in an animal model.50 .
Prenatal Nicotine Exposure and Human Data to Support a Role in ADHD
In studies of children with ADHD, the aberrant focus appears to be in subcortical pathways of the brain that are normally rich in dopamine and norepinephrine.49 The frontal cortex is important in regulating impulse control, executive functions, and the modulation of reward pathways. Affected children have a wide range of symptoms involving some degree of inability to ignore the environment and suppress input.49
A systematic analysis of 24 studies of prenatal substance exposure demonstrated an increased risk for ADHD-related disorders among children whose mothers smoked during pregnancy.2 Children with a specific dopamine transporter polymorphism and exposure to maternal smoking have significantly higher incidence of hyperactivity-impulsivity than children without this combination of environmental and genetic risk.43 This studies provides evidence of an association but also the complex interaction of factors involved in maternal smoking risks.
Medications that increase the activity of dopamine and norepinephrine reduce ADHD symptoms by blocking reuptake of these neurotransmitters.49 Nicotine treatment and cigarette smoking can also decrease the symptoms of ADHD and other psychiatric disorders, which may explain the prevalence of nicotine self-medication in adolescents and adults with ADHD or depression.51–53 In prenatally nicotine exposed adolescent rats, deficiencies in content and turnover of these two neurotransmitters were found in the midbrain, an area most closely associated with addiction.45
Addiction
Although smoking addiction has been blamed on the social influences of familial smoking and peers, current thinking is that there is also a biologic basis for these behaviors.54, 55 There is a high correlation between smoking behavior and symptoms of depression, inattention and hyperactivity in adolescents and adults.54, 55 These symptoms are often intensified during nicotine deprivation.53, 56, 57 Nicotine use in adolescents and adults appears to partially correct for the symptoms caused by chronic nicotine exposure to the fetus.
As indicated earlier, in animal studies, prenatal nicotine exposure has been shown to decrease the function of several neurotransmitters at birth.58–62 The decrease in function resolves somewhat in the first few months of life only to recur during adolescence.58, 62 When adolescent rats are given the opportunity, those exposed prenatally to nicotine will self administer larger amounts than non exposed rats, with the effect being more pronounced in female rats.59–62 If a comparable pattern occurs in humans, it could explain both the increase in smoking prevalence in adolescents at risk for depression and ADHD, and the increased risk that these adolescents will develop nicotine addiction.63
BEHAVIOR, LEARNING AND SENSORY DEVELOPMENT
The hippocampus is an important area of the brain responsible for short-term memory and sequential learning, as well as a part of the limbic system which has been associated with pathology in ADHD. The cerebellum plays a role in the integration of sensory input and coordination of motor control.41 Damage to these two areas of the brain can change how a person perceives and responds to their environment. Such damage can also alter the developing sensory cortex, inducing changes in visual, somatosensory, and auditory function.64, 65 Changes in the hippocampus are likely to affect memory, behavior, motor and cognitive function.66
Prenatal Nicotine Exposure, Hippocampal and Cerebellar Damage in Animals
In rats, neonatal nicotine exposure during the development of the hippocampus and cerebellum induces changes related to increased motor activity as they mature.50 Chronic neonatal nicotine exposure causes cell death and changes in cell morphology in the hippocampus and cerebellum, damage that lasts well into adulthood.28, 67 These areas are important in learning and integration of higher functions of mental capacity. Damage to these structures in animals from prenatal nicotine exposure could indicate damage of higher functions in humans.
Prenatal Nicotine Exposure, Hippocampal and Cerebellar Damage in Humans
Because the hippocampus develops near the end of pregnancy and postnatally in the first few years of life, damage occurs with all environmental exposures including maternal smoking. 19 Some exposed children have been shown to have an increased incidence of auditory-cognitive deficits that cause problems with understanding speech and verbally presented information, particularly in noisy settings. They may be unable to tell differences between similar sounds although their hearing is not impaired. Auditory processing problems are most prominent in males, whereas females demonstrate both auditory and visual cognitive impairments.68
There is compelling evidence that the children of women who smoke may be more likely to develop cognitive and learning deficits that in addition to ADHD, impaired attention and orientation, and poor impulse control;69 however, the literature is mixed with studies that ascribe these changes to the detrimental effects of impaired social and environmental factors as well.70 The role of prenatal and neonatal nicotine exposure in causing neurodevelopmental is subtle and highly likely to be associated with many other complex risk factors.
CHALLENGES OF SMOKING CESSATION PROGRAMS
Reducing tobacco use by pregnant women is a public health priority because of its contribution to poor pregnancy outcomes.71 Smoking cessation during pregnancy can counteract some of the risks to the fetus, but successful compliance with cessation protocols is challenging and can often be as problematic as active smoking. The risk from prenatal nicotine exposure is additionally increased because smoking women often live in social situations where smoking is common, thus increasing their chronic exposure to environmental tobacco smoke and making quitting more difficult.
The standard prenatal practice of brief behavioral counseling at a prenatal visit produces only a modest rate of smoking cessation.71 In an attempt to increase efficacy, prenatal treatment has incorporated the use of smoking cessation medications such as bupropion (Zyban) or nicotine replacement therapies (NRT).71 In a sample of 296 women, only 29% reported that their obstetric provider discussed using cessation medication during pregnancy, whereas 46% reported being offered a non-pharmacological cessation aid, booklets, or referral to a smoking cessation program. These practice patterns appear to be consistent with clinical guidelines that recommend considering medication for heavier smokers and for smokers who fail non-pharmacologic methods.71 When medications were discussed, nicotine replacement was talked about more than twice as often as bupropion (Zyban). Of the women offered a pharmacologic treatment, only 10% actually used medication during their pregnancy.71
Pharmacologic Treatments for Smoking Cessation
Bupropion (Zyban) and varenicline (Chantix) are commonly used medications for smoking cessation in the general adult population. There is no human data to support the use of varenicline (Chantix) during pregnancy; however, bupropion (Zyban) appears to have no more side effects in the fetus than the selective serotonin reuptake inhibitor medications used for depression. The Bupropion Registry examined 1597 pregnancies with exposure in one or more trimesters. The prevalence of malformations associated with 1213 first trimester exposures was 2.3%. Outside the first trimester, prevalence was 2.2%.72 This report suggested no increase in birth defects over baseline 3% reported for the United States; however, the use of buproprion (Zyban) as with any medications during pregnancy should be recommend only if the potential for benefit outweighs the potential unknown risk.73
Nicotine replacement therapies (NRT) in pregnancy have been studied more extensively than the pharmacologic therapies described above. However, there are few large randomized control studies, and the data on safety and efficacy are inconclusive. A recent study of NRT use during pregnancy was terminated early due to an increase in adverse events in the treatment group.74 Though the data safety monitoring board reported that these adverse events were likely not associated with NRT use, they were potentially serious: preterm birth <37 weeks, low birth weight<2500 g, pre-eclampsia, placental abruption, placental previa, neonatal intensive care unit admission, fetal demise, and infant death.74 The assumption of the monitoring board that these events were likely unrelated to NRT use was based on the lower cotinine levels in women using NRT than those found among women actively smoking, and on the higher proportion of women in the NRT arm having a prior history of preterm birth.74 The numbers of participants included were relatively small so further study on the safety of NRT in pregnancy is indicated.74
A different study examining the use of NRT found that the use of nicotine gum did not increase smoking cessation rates, but did increase birth weight and prolonged gestational age.75 In contrast, yet another study concluded that the risks of low birthweight and preterm birth were highest in women using NRT.76 It must be kept in mind that heavier smokers, who have the most difficulty with cessation, are also more likely to use NRT, which can confound comparisons between these studies. In order to establish a significant connection between NRT during pregnancy and subtle outcomes it will be important to have larger numbers of subjects, longer follow-up, and good information on potential confounding factors
There has been some discussion that NRT may be less hazardous than active smoking during pregnancy.77 This assumption raises several questions. A smoker’s dose of nicotine is intermittent; however, when nicotine is administered in the form of a patch the dose is continuous, so that the total dose of nicotine delivered to the fetus is higher than the amount delivered by actual smoking. In animal studies using continuous delivery methods, levels of nicotine in the fetal rat brain are about 2.5 times higher than in the mother’s blood.78 Nicotine appears to accumulate in the fetal compartment in higher concentrations than in maternal tissues.5 This suggests that continuous nicotine exposure could be more hazardous than intermittent smoking, although there are no direct data to prove or disprove this assumption.
Gerald Briggs, the author of Drugs in Pregnancy and Lactation, recommends that counseling is the preferred treatment for smoking cessation during pregnancy and lactation.79 Bupropion (Zyban) appears to be the least toxic of the cessation drugs, is as effective as NRT, and does not expose the fetus to nicotine.79 When NRT is used during pregnancy and lactation, intermittent dosing is most likely better than continuous use. Nicotine replacement therapies should probably be avoided in the first trimester and used with caution for the remaining pregnancy. Removal of the patch at night will decrease the nicotine exposure to the fetus. Gum, lozenges, or nasal spray also decrease the amount of nicotine exposure to the fetus; however, they have been associated with problems of decreased compliance due to poor taste and oral-pharyngeal irritation.79
Non-Pharmacologic Treatments for Smoking Cessation
Non-pharmacologic treatments for pregnant women include cognitive behavioral therapies, support groups, self-help aids and hypnosis. Hypnosis is one of the more popular non-pharmacologic aids, although its effectiveness is not well established.71 Cognitive behavioral therapy is felt to be of some benefit during pregnancy, but the literature is mixed regarding its effectiveness. Cognitive behavioral therapy should be considered as the first choice because it does not increase the risk of nicotine exposure to the fetus and has been shown to have few side effects. Unfortunately, the greatest success in smoking cessation has been obtained with a combination of both cognitive behavioral therapy and pharmacologic treatment.74
The Adolescent and Smoking Cessation During Pregnancy
It is important to remember that a smoking adolescent female often becomes a smoking adolescent mother. In the U.S., 17% of pregnant adolescents between 15 and 19 years of age smoke.80 The majority (60–80%) of these adolescents continue to smoke throughout their pregnancies. In many instances, smoking behaviors actually increase as the pregnancy advances.81
The smoking cessation interventions used for adolescents are the same as for adults.81 Cognitive behavioral strategies in adolescents directed at decreasing smoking based on peer-enhanced concepts of social support and therapeutic relationships, addressing goal setting, re-education, and urge control have been the most effective. However, the use of peer-enhanced programming has not been shown to sustain smoking cessation beyond the postpartum period.81 These results are similar those seen in to studies of adults, who also often return to smoking after pregnancy.74 Additional factors that make dealing with the adolescent particularly difficult are the issues of peer influence, cognitive development in regards to risk-taking behavior, and testing of independence.81
For all age groups, further consideration should be directed toward pursuing smoking cessation in women prior to pregnancy or immediately postpartum. These women are usually healthy and often receive regular medical care. A formal, aggressive approach to providing cognitive behavioral support as well as smoking cessation medication could improve outcomes, by increasing the proportion of women who quit smoking before they become pregnant again.
SMOKING DURING LACTATION
Nicotine is excreted in breast milk, in a dose-dependent manner, with breast milk levels 2.9 times higher than maternal plasma.82 Nicotine has also been shown to lower prolactin levels, which could decrease milk supply in some women and may account for the lower incidence of breastfeeding among smoking women.83 Given that women who smoke are less likely to intend to breastfeed, 84 it cannot be assumed that the relationship between smoking and duration of breastfeeding is purely a physiological one. There is a wide variation in breastfeeding rates among smoking mothers. Therefore, psychosocial factors are likely to contribute to the lower rates of breastfeeding found in women who smoke.84
CONCLUSION
A large body of documented evidence shows that smoking during pregnancy is harmful to both the mother and fetus. Controlled animal studies have confirmed some of the mechanisms of pathology. In animals, prenatal exposure to nicotine has been shown to alter autonomic functioning and protective responses that could be involved in the pathophysiology of SIDS. Significant epidemiologic data in humans supports the potentially devastating effects of nicotine on fetal growth and development. In humans, there is evidence that prenatal nicotine exposure is associated with subtle changes in learning and behavior problems. Nicotine addiction is also increased in people who were exposed to nicotine in utero.
Understanding the effects of nicotine exposure on neurologic development and the consequences for long-term outcomes is the direct responsibility of providers caring for pregnant women and children. Childhood and adolescent morbidity from cognitive difficulties, ADHD, conduct disorders, behavioral problems, depression, and other smoking related concerns are a significant public health issue.
Biographies
Jane Blood-Siegfried RN, CPNP, DNSc, is an Associate clinical professor in the School of Nursing at Duke University in Durham, North Carolina.
Elizabeth K Rende RN, CPNP, MSN, is a pediatric nurse practitioner in the Department of Pediatric Neurology at Duke University in Durham, North Carolina and instructor for the University of Phoenix.
Footnotes
This paper will review some of the animal and human research associated with the effects of maternal smoking on the developing nervous system and their implications for practice.
References
- 1.Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med. 1999;16:208–215. doi: 10.1016/s0749-3797(98)00089-0. [DOI] [PubMed] [Google Scholar]
- 2.Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 3.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 4.Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl) 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- 5.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 6.Anderson ME, Johnson DC, Batal HA. Sudden Infant Death Syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. [Accessed Jan 11, 2008];BMC Medicine. 2005 3(1):4. doi: 10.1186/1741-7015-3-4. [serial online] Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15644131. [DOI] [PMC free article] [PubMed]
- 7.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey D, Jacob P, 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301:594–598. doi: 10.1124/jpet.301.2.594. [DOI] [PubMed] [Google Scholar]
- 9.George L, Granath F, Johansson AL, Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. 2006;85:1331–1337. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 10.Bardy AH, Seppala T, Lillsunde P, Kataja JM, Koskela P, Pikkarainen J, et al. Objectively measured tobacco exposure during pregnancy - neonatal effects and relation to maternal smoking. Br J Obstet Gynaecol. 1993;100:721–726. doi: 10.1111/j.1471-0528.1993.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 11.Kohler E, Bretschneider D, Rabsilber A, Weise W, Jorch G. Assessment of prenatal smoke exposure by determining nicotine and its metabolites in maternal and neonatal urine. Hum Exp Toxicol. 2001;20:1–7. doi: 10.1191/096032701669841404. [DOI] [PubMed] [Google Scholar]
- 12.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tobacco Res. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 13.Witschi H, Joad JP, Pinkerton KE. The toxicology of environmental tobacco smoke. Annu Rev Pharmacol Toxicol. 1997;37:29–52. doi: 10.1146/annurev.pharmtox.37.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Parrott AC. Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research. Psychopharmacology (Berl) 2006;184:567–576. doi: 10.1007/s00213-005-0294-y. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, et al. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine Tob Res. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- 16.Breslau N, Kilbey M, Andreski P. Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry. 1991;48:1069–1074. doi: 10.1001/archpsyc.1991.01810360033005. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard WS, Robinson JH, Guy TD. Enhancement of continuous performance task reaction-time by smoking in nondeprived smokers. Psychopharmacology (Berl) 1992;108:437–442. doi: 10.1007/BF02247417. [DOI] [PubMed] [Google Scholar]
- 18.Kailasanath V, Fu S. The HOPES brain tutorial. The Huntington's Outreach Program for Education; Stanford: 2003. [Accessed August 8, 2008]. Available from: http://www.stanford.edu/group/hopes/basics/braintut/ab0.html. [Google Scholar]
- 19.Kuhn C, Mailman R. Developmental neurotoxicology. In: Abou-Donia M, editor. Neurotoxicology. Ann Arbor: CRC Press; 1992. pp. 293–318. [Google Scholar]
- 20.Atluri P, Fleck MW, Shen Q, Mah SJ, Stadfelt D, Barnes W, et al. Functional nicotinic acetylcholine receptor expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Dev Biol. 2001;240:143–156. doi: 10.1006/dbio.2001.0453. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Res. 2000;853:41–48. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- 22.Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- 23.Navarro HA, Seidler FJ, Schwartz RD, Baker FE, Dobbins SS, Slotkin TA. Prenatal exposure to nicotine impairs nervous-system development at a dose which does not affect viability or growth. Brain Res Bull. 1989;23:187–192. doi: 10.1016/0361-9230(89)90146-9. [DOI] [PubMed] [Google Scholar]
- 24.Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, et al. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- 25.Pugh PC, Margiotta JF. Nicotinic acetylcholine receptor agonists promote survival and reduce apoptosis of chick ciliary ganglion neurons. Mol Cell Neurosci. 2000;15:113–122. doi: 10.1006/mcne.1999.0810. [DOI] [PubMed] [Google Scholar]
- 26.Kolb B. Brain-development, plasticity, and behavior. Am Psychol. 1989;44:1203–1212. doi: 10.1037//0003-066x.44.9.1203. [DOI] [PubMed] [Google Scholar]
- 27.Yanai J. Neurobehavioral Teratology. Amsterdam: Elseveir; 1984. [Google Scholar]
- 28.Roy TS, Sabherwal U. Effects of gestational nicotine exposure on hippocampal morphology. Neurotoxicol Teratol. 1998;20:465–473. doi: 10.1016/s0892-0362(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 29.Navarro HA, Mills E, Seidler FJ, Baker FE, Lappi SE, Tayyeb MI, et al. Prenatal nicotine exposure impairs beta-adrenergic function - persistent chronotropic subsensitivity despite recovery from deficits in receptor-binding. Brain Res Bull. 1990;25:233–237. doi: 10.1016/0361-9230(90)90066-9. [DOI] [PubMed] [Google Scholar]
- 30.Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull. 1995;38:69–75. doi: 10.1016/0361-9230(95)00073-n. [DOI] [PubMed] [Google Scholar]
- 31.Hafstrom O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respiratory Physiology and Neurobiology. 2005;149:325–341. doi: 10.1016/j.resp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Seidler FJ, Levin ED, Lappi SE, Slotkin TA. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat-brain regions. Dev Brain Res. 1992;69:288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- 34.Oncken CA, Henry KM, Campbell WA, Kuhn CM, Slotkin TA, Kranzler HR. Effect of maternal smoking on fetal catecholamine concentrations at birth. Pediatr Res. 2003;53:119–124. doi: 10.1203/00006450-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, et al. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. Jama-Journal of the American Medical Association. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 36.Blair PS, Sidebotham P, Berry PJ, Evans M, Fleming PJ. Major epidemiological changes in sudden infant death syndrome: a 20-year population-based study in the UK. Lancet. 2006;367:314–319. doi: 10.1016/S0140-6736(06)67968-3. [see comment] [DOI] [PubMed] [Google Scholar]
- 37.Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 38.Imperato PJ, Mitchell G. Cigarette-smoking - a chosen risk. N Y State J Med. 1986;86:485–489. [PubMed] [Google Scholar]
- 39.Brazell MP, Mitchell SN, Gray JA. Effect of acute administration of nicotine on invivo release of noradrenaline in the hippocampus of freely moving rats - a dose-response and antagonist study. Neuropharmacology. 1991;30:823–833. doi: 10.1016/0028-3908(91)90116-s. [DOI] [PubMed] [Google Scholar]
- 40.Lena C, Changeux JP. Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus. J Neurosci. 1997;17:576–585. doi: 10.1523/JNEUROSCI.17-02-00576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugarman R. Structure and function of the neurologic system. In: McCance K, Huether S, editors. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 5. St Louis: Mosby Inc; 2006. pp. 441–446. [Google Scholar]
- 42.Kinney HC, Filiano JJ, White WF. Medullary serotonergic network deficiency in the sudden infant death syndrome: Review of a 15-year study of a single dataset. J Neuropathol Exp Neurol. 2001;60:228–247. doi: 10.1093/jnen/60.3.228. [DOI] [PubMed] [Google Scholar]
- 43.Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of dopamine transporter genotype with prenatal smoke exposure on ADHD symptoms. J Pediatr. 2008;152:263–269. doi: 10.1016/j.jpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Stein DJ, Fan J, Fossella J, Russell VA. Inattention and hyperactivity-impulsivity: psychobiological and evolutionary underpinnings of ADHD. Cns Spectrums. 2007;12:190–197. doi: 10.1017/s1092852900020903. [DOI] [PubMed] [Google Scholar]
- 45.Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- 46.Panigrahy A, Filiano J, Sleeper LA, Mandell F, Valdes-Dapena M, Krous HF, et al. Decreased serotonergic receptor binding in rhombic lip-derived regions of the medulla oblongata in the sudden infant death syndrome. J Neuropathol Exp Neurol. 2000;59:377–384. doi: 10.1093/jnen/59.5.377. [DOI] [PubMed] [Google Scholar]
- 47.Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction - developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- 48.Slotkin TA, Pinkerton KE, Tate CA, Seidler FJ. Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain Res. 2006;1111:30–35. doi: 10.1016/j.brainres.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 49.Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 2005;57:1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 50.Thomas JD, Garrison ME, Slawecki CJ, Ehlers CL, Riley EP. Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicol Teratol. 2000;22:695–701. doi: 10.1016/s0892-0362(00)00096-9. [DOI] [PubMed] [Google Scholar]
- 51.Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- 52.Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit hyperactivity disorder. Psychopharmacology (Berl) 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- 53.Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 54.Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, et al. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196:461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- 55.Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet. 1995;25:95–101. doi: 10.1007/BF02196920. [DOI] [PubMed] [Google Scholar]
- 56.Jacobsen LK, Slotkin TA, Westerveld M, Mencl WE, Pugh KR. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. 2006;31:1550–1561. doi: 10.1038/sj.npp.1300981. [DOI] [PubMed] [Google Scholar]
- 57.Glassman AH. Cigarette smoking: implications for psychiatric illness. Am J Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 58.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: serotonin receptors and cell signaling. Neuropsychopharmacology. 2006;31:2462–2475. doi: 10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- 59.Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, et al. Adolescent vs adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl) 2003;169:141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- 61.Levin ED, Lawrence S, Petro A, Horton K, Seidler FJ, Slotkin TA. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacol Biochem Behav. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- 63.Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, Buka S. Maternal transmission of nicotine dependence: Psychiatric, neurocognitive and prenatal factors. Am J Addict. 2001;10:16–29. doi: 10.1080/105504901750160420. [DOI] [PubMed] [Google Scholar]
- 64.Metherate R. Nicotinic acetylcholine receptors in sensory cortex. Learn Memory. 2004;11:50–59. doi: 10.1101/lm.69904. [DOI] [PubMed] [Google Scholar]
- 65.Liang K, Poytress BS, Chen YL, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24:857–866. doi: 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- 66.Huang LZ, Abbott LC, Winzer-Serhan UH. Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum. Neuroscience. 2007;146:1854–1868. doi: 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdel-Rahman A, Dechkovskaia AM, Sutton JM, Chen WC, Guan X, Khan WA, et al. Maternal exposure of rats to nicotine via infusion during gestation produces neurobehavioral deficits and elevated expression of glial fibrillary acidic protein in the cerebellum and CA1 subfield in the offspring at puberty. Toxicology. 2005;209:245–261. doi: 10.1016/j.tox.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 68.Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 69.Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacol Biochem Behav. 2006;85:835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 70.D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rigotti NA, Park ER, Chang Y, Regan S. Smoking cessation medication use among pregnant and postpartum smokers. Obstet Gynecol. 2008;111:348–355. doi: 10.1097/01.AOG.0000297305.54455.2e. [DOI] [PubMed] [Google Scholar]
- 72.GlaxoSmithKline. The bupropion pregnancy registry. Wilmington, NC: Kendle International Inc; 2008. [Google Scholar]
- 73.Coleman T. Recommendations for the use of pharmacological smoking cessation strategies in pregnant women. CNS Drugs. 2007;21:983–993. doi: 10.2165/00023210-200721120-00003. [DOI] [PubMed] [Google Scholar]
- 74.Pollak KI, Oncken CA, Lipkus IM, Lyna P, Swamy GK, Pletsch PK, et al. Nicotine replacement and behavioral therapy for smoking cessation in pregnancy. Am J Prev Med. 2007;33:297–305. doi: 10.1016/j.amepre.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oncken C, Dornelas E, Greene J, Sankey H, Glasmann A, Feinn R, et al. Nicotine gum for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2008;112:859–867. doi: 10.1097/AOG.0b013e318187e1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaither KH, Huber LR, Thompson ME, Huet-Hudson YM. Does the use of nicotine replacement therapy during pregnancy affect pregnancy outcomes? Matern Child Health J. 2008 doi: 10.1007/s10995-008-0361-1. [DOI] [PubMed] [Google Scholar]
- 77.Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Sarasin A, Schlumpf M, Muller M, Fleischmann L, Lauber ME, Lichtensteiger W. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod Toxicol. 2003;17:153–162. doi: 10.1016/s0890-6238(02)00119-3. [DOI] [PubMed] [Google Scholar]
- 79.Briggs GG. Cigarette Smoking Cessation. [Accessed 2/20/2009];OBGyn News. 2008 43(22) [serial online] Available from: http://www.obgynnews.com//article/S0029-7437(08)70689-X/fulltext.
- 80.US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. The health consequences of smoking: a report of the Surgeon General.Washington, DC2004.
- 81.Albrecht SA, Caruthers D, Patrick T, Reynolds M, Salamie D, Higgins LW, et al. A randomized controlled trial of a smoking cessation intervention for pregnant adolescents. Nurs Res. 2006;55:402–410. doi: 10.1097/00006199-200611000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Luck W, Nau H. Exposure of the fetus, neonate, and nursed infant to nicotine and cotinine from maternal smoking. N Engl J Med. 1984;311:672. doi: 10.1056/NEJM198409063111014. [DOI] [PubMed] [Google Scholar]
- 83.Andersen AN, Lund-Andersen C, Larsen JF, Christensen NJ, Legros JJ, Louis F, et al. Suppressed prolactin but normal neurophysin levels in cigarette smoking breast-feeding women. Clin Endocrinol (Oxf) 1982;17:363–368. doi: 10.1111/j.1365-2265.1982.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 84.Amir LH, Donath SM. Does maternal smoking have a negative physiological effect on breastfeeding? The epidemiological evidence. Breastfeed Rev. 2003;11:19–29. [PubMed] [Google Scholar]


