Abstract
Elevations of sperm Ca2+ seem to be responsible for an asymmetric form of motility called hyperactivation, which is first seen near the time of fertilization. The mechanism by which intracellular Ca2+ concentrations increase remains unknown despite considerable investigation. Although several prototypical voltage-gated calcium channels are present in spermatozoa, they are not essential for motility. Furthermore, the forward velocity and percentage of motility of spermatozoa are associated with infertility, but their importance relative to hyperactivation also remains unknown. We show here that disruption of the gene for a recently described sperm-specific voltage-gated cation channel, CatSper2, fails to significantly alter sperm production, protein tyrosine phosphorylation that is associated with capacitation, induction of the acrosome reaction, forward velocity, or percentage of motility, yet CatSper2–/– males are completely infertile. The defect that we identify in the null sperm cells is a failure to acquire hyperactivated motility, which seems to render spermatozoa incapable of generating the “power” needed for penetration of the extracellular matrix of the egg. A loss of power is suggested also by experiments in which the viscosity of the medium was increased after incubation of spermatozoa in normal capacitating conditions. In high-viscosity medium, CatSper2-null spermatozoa lost the ability to swim forward, whereas wild-type cells continued to move forward. Thus, CatSper2 is responsible for driving hyperactivated motility, and, even with typical sperm forward velocities, fertilization is not possible in the absence of this highly active form of motility.
Keywords: hyperactivation, spermatozoa, infertility
Elevations of flagellar Ca2+ seem to be responsible for a hyperactivated form of sperm motility (1) first seen in the female, but the means by which Ca2+ is modulated are unknown. Because millimolar concentrations of extracellular calcium are needed to develop and maintain hyperactivated motility in vitro, plasma membrane channels have been proposed to mediate the apparent Ca2+ mobilization. A variety of widely expressed calcium-permeable channels (voltage-gated, cyclic nucleotidegated, and transient receptor potential) have been identified in sperm cells, each localized to discrete regions of the sperm cell (2–5). However, genetic disruption of these channels in mice has failed to cause infertility or apparent sperm motility deficiencies in animals that reach reproductive age (6–9). Recently, Ho and Suarez offered a second model for Ca2+ mobilization in which the Ca2+ that is required for induction of hyperactivation is speculated to originate from intracellular stores (10, 11). Extracellular calcium is proposed to be required for motility maintenance.
Recently, we and others discovered a family of sperm-specific cation channels, consisting of CatSper1 through CatSper4 (12–15; GenBank accession no. AAP21831). These proteins are putative six-transmembrane voltage-gated ion channels located principally on the sperm flagellum, where they may act as regulators of sperm motility. Whether the four CatSper proteins form a single heterotetrameric channel remains unknown; however, attempts to coimmunoprecipitate CatSper1 and CatSper2 from detergent extracts have failed (12). Also, these two proteins are expressed at different levels along the flagellum, as determined by immunocytochemistry (12, 13). The gene for CatSper1 has been disrupted, and the male mice are infertile (13), but a prominent phenotype of reduced basal velocity of sperm may have accounted for this infertility.
Here, we disrupt the gene for CatSper2 and find that CatSper2–/– males, but not females, are infertile. In contrast to the substantial reductions found in CatSper–/– sperm motility (13), we found little effect of the CatSper2 deletion on percentage of motility or basal velocity. However, noticeably absent in CatSper2–/– spermatozoa was a transition to a calcium-dependent, hyperactivated form of motility, normally seen near the time of fertilization. This defect appears to render the spermatozoa incapable of penetrating the extracellular matrix of the egg and, thus, incapable of fertilization. We conclude that activation of CatSper2 drives a hyperactivated form of motility that is essential for fertilization.
Materials and Methods
Construction of the Targeting Vector and Analysis of Embryonic Stem Cell (ES Cell) Clones. CatSper2 gene DNA fragments were isolated from a mouse 129/SvJ genome bacmid clone, identified previously in fluorescence in situ hybridization experiments (12). The targeting vector was prepared by subcloning a 2.2-kb EcoRI–XhoI gene fragment (containing exon 1) and a 6.0-kb KpnI gene fragment (containing exons 6 and 7) into the pKO Scrambler NTKV 1904 plasmid flanking the neomycin resistance (Neor) coding sequence (Stratagene). An ES cell line (SM1) that was derived from 129/SvEvTac mice was transfected by electroporation with 20 μg of the NotI-linearized targeting vector. After 24 h of recovery without selection, transfected ES cells were selected in medium supplemented with 200 μg/ml G418 and 0.2 μM ganciclovir. Genomic DNA was extracted from the drug-resistant ES cells and screened by PCR to identify apparent correctly targeted clones. The PCRs contained 15 ng of genomic DNA template in a 50-μl volume with 10 pmol each of gene-specific sense (GTGGTGGCTATACGCTCAGCATTTGGG), antisense (AAAGCTGAGCCTGTGCCTATTAGTACGC) and Neor (GGGTGTGGCGGATCGCTATCAGG) primers, and 2.5 units of Pfu Turbo DNA polymerase (Stratagene). Cycling conditions were five cycles at 95°C for 30 sec, 68°C for 30 sec, and 72°C for 5 min, followed by 30 cycles at 95°C for 30 sec, 65°C for 30 sec, and 72°C for 5 min. The identified candidate clones were confirmed by Southern blotting. Briefly, genomic DNA (1–2 μg) was digested with SphI (5′ external probe), BamHI/SacI (3′ external probe), or EcoRV/SpeI (neomycin phosphotransferase II probe), electrophoresed, and blotted to Hybond-N membranes (Amersham Biosciences). The blots were hybridized with 32P-labeled gene-specific probes flanking the expected recombination event (5′ 700-bp EcoRI CatSper2 gene fragment and 3′ 730-bp CatSper2 genomic DNA PCR fragment containing exons 8–10; Fig. 1a, P5 and P3, respectively), as well as a neomycin phosphotransferase II DNA probe. Hybridization was done overnight at 65°C in RapidHyb solution (Amersham Biosciences). Filters were washed at 65°C in 0.1× SSC (0.15 M sodium chloride/0.015 M sodium citrate, pH 7) with 0.1% SDS.
Fig. 1.
Targeted disruption of the mouse CatSper2 gene. (a) A schematic representation of a portion of the endogenous CatSper2 gene, the targeting vector, and the expected recombinant mutation. Exons are indicated by the numbered bars. The gene-specific probes (P5 and P3), restriction sites used for Southern blot analysis of homologous recombination (SphI for P5 and BamHI and SacI for P3), and expected restriction fragments are shown. The underlined restriction sites originate from the targeting vector. Neomycin (N) and thymidine kinase (TK) selection cassettes are shown. WT, wild type. (b Upper) Southern blot analysis of SM1 genomic DNA showing restriction fragments with the predicted sizes (kb) using both gene-specific probes. Data are representative of three independent clones. (b Lower) Mouse genotyping by PCR with genotype-specific primers showing the expected products. (c) SDS polyacrylamide gel electrophoresis (4–20% gradient) of mouse sperm total protein (1 million cells per lane) stained with Coomassie blue (Left) and the same samples (electrophoresed on an 8% SDS polyacrylamide gel) probed with the CatSper2 C-terminal antibody (Right), demonstrating the absence of CatSper2 in homozygous null (–/–) mice. Relative migration of protein standards (Mr × 103) is shown. (d) Immunocytochemistry of methanol-fixed mouse sperm with the C-terminal CatSper2 antibody. In b–d, the genotypes are wild type (+/+), heterozygote (+/–), and null (–/–).
Production and Genotyping of Mutant Mice. Three independent ES cell clones carrying the disrupted CatSper2 allele were used to generate chimeric mice, which were crossed with C57BL/6J females to obtain heterozygous animals. Genotype analysis was performed by PCR on isolated genomic DNA. Reactions were prepared as described above with the following primers: gene-specific sense (10 pmol, GTCAGGCTGTTGCTTTGTCC), gene-specific antisense (5 pmol, CAGCCTAGTGAAGGACAGCC), and Neor (5 pmol, GGGTGTGGCGGATCGCTATCAGG). Cycling conditions were 40 cycles at 94°C for 30 sec, 63°C for 30 sec, and 72°C for 1 min. The mice used in this study were the offspring of F1 and/or F2 animals (129/SvEvTac;C57BL/6J genetic background).
In Vivo and in Vitro Fertilization. For analysis of fertility after normal mating, sexually mature male animals were housed with three mature female animals for 1–3 months. Vaginal plugs, pregnancies, and litter sizes were recorded for matings of wild-type males with wild-type or null females and for matings of null males with wild-type females. Under in vitro conditions, caudal epididymal spermatozoa were capacitated for 90 min then added at 1 million cells per ml to ovulated eggs (recovered 13 h after human chorionic gonadotropin stimulation) in the same medium (16). In cases where the zona pellucida was removed, the ovulated mouse eggs were treated with hyaluronidase (100 units/ml; Type I-S, Sigma) to remove cumulus cells, washed, and then exposed briefly to acid Tyrodes solution to dissociate the zona pellucida (17). Gametes were cocultured at 37°C under 5% CO2 for 5 h, and then the eggs were washed into an embryo-culturing medium containing 5 mg/ml BSA. Successful fertilization was scored as two-cell embryos 25–28 h after insemination.
Sperm Cell Motility Assays. Spermatozoa were obtained by applying gentle pressure to excised caudal epididymal tissue nicked at four sites with scissors. Cells that dispersed into the modified Krebs–Ringer solution at 37°C were collected for 15 min (18). The epididymal tissue was then removed with forceps, and this point was designated time 0. Subsequently, sperm cells were maintained at 37°C on a slide warmer for ≤10 min or in a tissue culture incubator under 5% CO2 for longer periods. The IVOS Sperm Analyzer (Version 12, Hamilton Thorne Research, Beverly, MA) was used for all motility analyses. Images of sperm cell tracks were captured in an 80-μm chamber for 0.5 s at 60 Hz. Parameters that were examined included percentage of motile sperm cells, path velocity (the distance traveled along a smoothed average path divided by the elapsed time), linear velocity (the straight-line distance between the first and final location of the sperm head divided by the elapsed time), track velocity (the total distance traveled by the sperm head from image to image divided by the elapsed time), and straightness (linear velocity divided by path velocity multiplied by 100; higher values indicate a straighter trajectory). For each sample and time point, 40 sperm tracks were selected that exhibited unimpeded motion that could be tracked accurately over the entire 0.5-sec recording period (30 data points). Four arbitrary and independent fields were chosen for sperm-motility examination. Within each field, selection of cells to view started at the 12 o'clock position and moved clockwise. In the viscosity experiments, long chain polyacrylamide (Scientific Polymer Products, Ontario, NY) was dissolved directly in medium by stirring overnight at room temperature to result in a 2% stock solution (viscosity of 4,000 cP, according to the manufacturer's instructions; 1 P = 0.1 Pa·sec). This medium was diluted to 0.75% with freshly prepared medium, and 18 μl was overlaid onto 2 μl of spermatozoa in medium on a prewarmed 80-μm chamber slide. A coverslip was then placed over the sample, and analysis was performed with the IVOS Sperm Analyzer, as described above.
Sperm Flagellar Beat Analysis. For beat-frequency measurement, spermatozoa were obtained as described above except in BSA-free medium. The cells were diluted with an equal volume of the same medium, and 20 μl of medium was spotted onto a glass coverslip in a tissue culture dish at 37°C. After 5 min, the dish was flooded with medium. Live spermatozoa that had attached to the coverslip at the posterior head were then viewed with an inverted microscope (IX-70, Olympus, Melville, NY) and recorded at 53 Hz with a digital charge-coupled device camera (Hamamatsu, Bridgewater, NJ). Recorded images were analyzed manually. Beat frequency was calculated as one-third of the time elapsed for three complete flagellar beats. For flagellar beat envelope measurement, spermatozoa that were released into capacitation medium were placed in an 80-μm chamber, and tracks of swimming cells were recorded on the inverted microscope, as described above. One-second images were overlaid to visualize the flagellar beat envelope, which was measured at the widest point perpendicular to the cell path.
SDS/PAGE and Immunoblotting. SDS/PAGE was performed according to the method of Laemmli (19). Immunoblotting and immunocytochemistry of CatSper2 were done as reported (12). Anti-phosphotyrosine immunoblots were performed similarly to the CatSper2 procedure, except that the nitrocellulose membranes were blocked with 5% IgG-free BSA (Sigma) before probing with PY-Plus monoclonal antibodies (Zymed) at 50 ng/ml in the blocking solution.
Results and Discussion
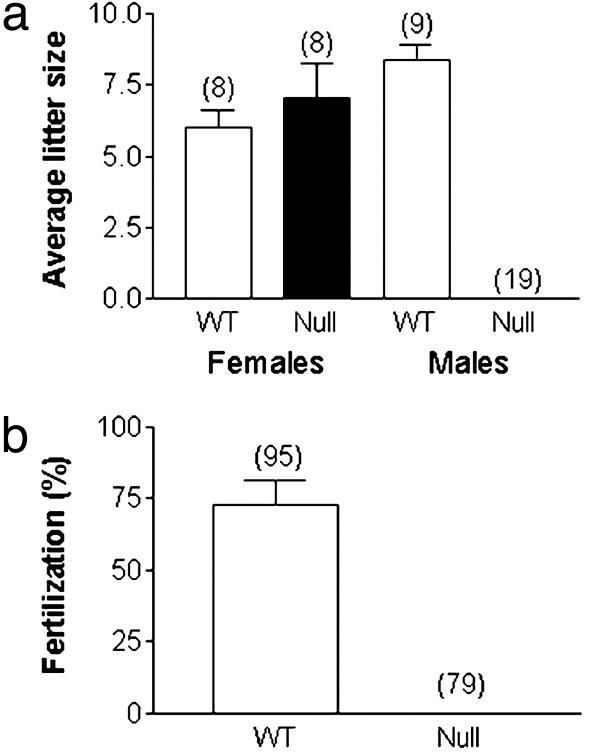
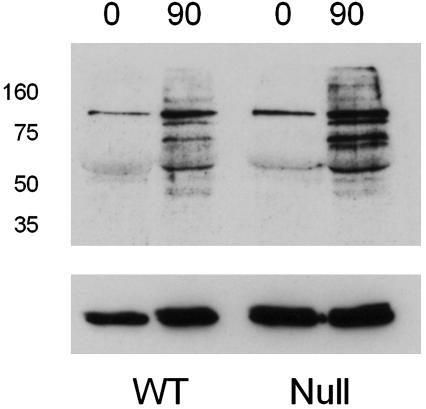
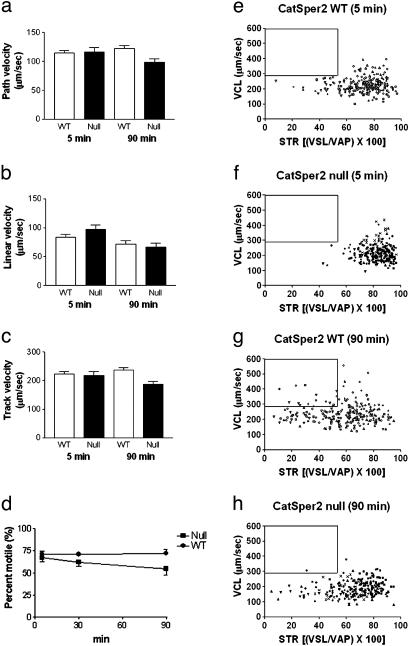
We inactivated the CatSper2 gene in mice by substituting exons 2–5, which encode the initiation methionine through the first three transmembrane helices, with the neomycin phosphotransferase II-coding DNA sequence in reverse orientation relative to the CatSper2 transcript (Fig. 1 a and b). The null spermatozoa contained no detectable CatSper2, as determined by Western blotting (Fig. 1c) and immunocytochemistry (Fig. 1d). The null allele was inherited with the expected Mendelian ratio, suggesting that embryonic development was unaffected (data not shown). There were no gross abnormalities in size, behavior, or viability of the null animals. Consistent with the late spermatogenic expression of CatSper2 mRNA, disruption of the gene did not alter testis weights (120 ± 7.8 mg and 109 ± 11.2 mg for wild-type and null animals, respectively), testis histology, the caudal epididymal sperm cell numbers (1.9 ± 0.3 × 107 and 1.7 ± 0.2 × 107 for wild-type and null animals, respectively), or sperm cell ultrastructural morphology (see Fig. 7, which is published as supporting information on the PNAS web site) (20). However, disruption of the CatSper2 gene resulted in complete and specific male infertility (Fig. 2a). The complete loss of male fertility seen with normal mating was also replicated under in vitro conditions (Fig. 2b), showing that fertility failure was not limited to a defect in sperm cell transport in the female reproductive tract. Whether elimination of the gene also disrupts transport in the female has not been examined. As a consequence of the fertilization failure, we asked whether the CatSper2–/– cells could undergo capacitation and the acrosome reaction, events thought to be absolutely required for successful fertilization (21). Sperm cells normally phosphorylate a specific subset of cellular proteins on tyrosine residues during in vitro capacitation by a calcium- and cAMP-dependent signaling mechanism (22). The CatSper2–/– spermatozoa continued to display the same pattern of phosphorylation as seen in wild-type spermatozoa (Fig. 3). This finding suggested that capacitation (defined as the ability to undergo an acrosome reaction) was normal. By the end of the capacitation period, spermatozoa are competent to undergo a ligand-induced acrosome reaction. This requisite secretory event depends also on extracellular calcium and calcium influx and has been suggested to involve voltage-gated calcium channels and a store-operated channel, based principally on pharmacological evidence (5, 23). We examined the ability of the null and wild-type sperm cells to initiate the acrosome reaction in response to intact zona pellucida and found no significant differences in the percentage of acrosome-reacted spermatozoa between the two genotypes (wild type, 46 ± 3.8%; null, 58 ± 10.5%). Thus, incubation of null or wild-type spermatozoa under conditions known to capacitate cells in vitro seems to result in normal capacitation (defined here as the ability to initiate an acrosome reaction), and, thus, capacitation failure does not seem to account for male infertility. We then asked whether sperm motility was normal in the null animals. Both the forward velocity parameters (Fig. 4 a–c) and percentage of motility (Fig. 4d) were approximately the same in wild-type and CatSper2-null spermatozoa. Closer examination showed that normal velocity is maintained in the null spermatozoa in the face of slight decreases in the average flagellar amplitude (26.1 ± 6.5 μm vs. 37.5 ± 5.8 μm) and small increases in the rate of the flagellar beat (10.4 ± 2.5 Hz vs. 6.3 ± 1.4 Hz) (n = 40 cells; mean ± SD.). The striking observation, however, was the failure to generate a hyperactivated form of sperm motility that was coincident with capacitation (Fig. 4 e–h). Spermatozoa swimming with a large amplitude and asymmetric flagellar beat define hyperactivated motility in the mouse (24). These changes of flagellar beat cause spermatozoa to increase apparent velocity (path and track) and frequency of abrupt turning. The time-dependent appearance of sperm cells with both of these two movement characteristics (high track velocity and nonlinear trajectory) is evident in wild-type but not null sperm cell populations as indicated by boxes in Fig. 4 e–h. All of the cells within the boxes displayed hyperactivated motility. The computer-defined proportions of hyperactivated cells are underestimates in that manual analysis of the wild-type sperm cell recordings taking into account large amplitude flagellar beats indicated that ≈70% of the cells were hyperactivated, whereas hyperactivation was never observed in the null population. Given that null spermatozoan velocity and percentage of motility were normal, the opportunity arose to determine the significance of hyperactivated motility specifically under in vitro conditions.
Fig. 2.
CatSper2 inactivation results in complete male infertility under normal breeding and in vitro conditions. (a) Failure of fertilization after normal matings. Numbers above the bars indicate the number of animals mated. (b) In vitro fertility of CatSper2 homozygous null male mice (n = 3 animals for each genotype). The numbers above the bars indicate the total number of eggs examined.
Fig. 3.
Incubation under capacitating conditions results in normal protein tyrosine phosphorylation patterns in both wild-type (WT) and CatSper2-null spermatozoa. (Upper) Anti-phosphotyrosine immunoblot of spermatozoa from CatSper2 wild-type and null males that were incubated for t = 0 and t = 90 min in conditions that promote capacitation. Relative migration of protein standards (M × 103 r) is shown. (Lower) The same immunoblot probed with an anti-tubulin antibody as a loading control after removing the anti-phosphotyrosine and secondary antibodies.
Fig. 4.
CatSper2 wild-type (WT) and null sperm cell motility parameters. (a–c) Average forward velocity of WT and null spermatozoa at 5 min and 90 min after swim-out. (d) Average percentage of motility of WT and null spermatozoa. The data shown in a–d are the sperm cell population means ± SEM (n = 6 animals for each genotype). (e–h) Plots of sperm cell track velocity (VCL) vs. straightness of trajectory (STR), defined as the linear velocity (VSL) divided by the path velocity (VAP), multiplied by 100. Higher values indicate a straighter trajectory. Each data point represents an individual sperm cell (n = 240 from six animals for each genotype). The different symbols represent spermatozoa from different animals. The boxes define regions containing high-velocity nonlinear trajectory spermatozoa delimited by the 90th percentile track velocity and 10th percentile STR values from the noncapacitated wild-type spermatozoa at t = 5 min. All spermatozoa within these boxes were hyperactivated.
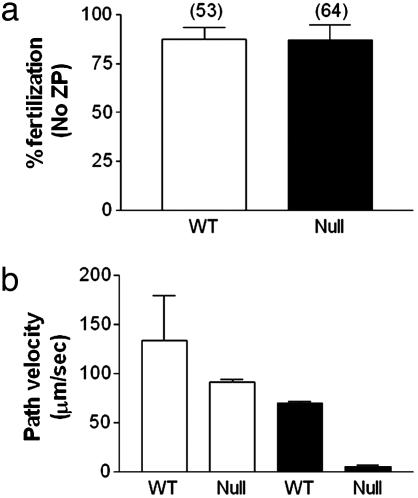
Removal of the extracellular matrix corrected the infertility of null sperm cells (Fig. 5a), suggesting that the zona pellucida of the egg represented an absolute barrier to CatSper2 spermatozoa. We then found that null spermatozoa failed to penetrate the zona pellucida of intact eggs (data not shown). This finding suggests that despite normal forward velocity and percentage of motile cells, the spermatozoa lack the “power” to penetrate the zona pellucida. To examine this possibility, we tested the effects of elevating the viscosity of the medium on the motility of wild-type and null spermatozoa. Previous studies had documented that hyperactivated sperm cells swim more effectively in higher-viscosity media (25). In normal cell medium containing 0.75% (wt/vol) long chain polyacrylamide, we found that, whereas wild-type cells remained progressively motile with reductions in forward velocity of ≈50%, null spermatozoa failed to move forward (Fig. 5b).
Fig. 5.
CatSper2 null spermatozoa can fertilize eggs without an extracellular matrix but cannot penetrate a viscous environment. (a) Removal of the extracellular matrix of the egg allows CatSper2 null mice to fertilize eggs in vitro (n = 3 animals for each genotype). Numbers above the bars indicate the total number of eggs examined. ZP, zona pellucida. (b) Increased viscosity of the medium does not block wild-type (WT) sperm from swimming but does block the motility of CatSper2 null spermatozoa after incubation in conditions that promote capacitation. The values indicated are expressed as the mean ± SD sperm cell path velocities of the various samples. The open bars represent normal medium, and the filled bars represent medium containing 0.75% (wt/vol) long chain polyacrylamide.
We conclude from this work that CatSper2 is essential for the generation of a hyperactivated form of motility and that this motility is necessary for successful fertilization, despite a normal forward velocity and percentage of motile cells. A simple model representing our current hypothesis concerning the CatSper2 signaling pathway and the transition to hyper-activated motility is shown in Fig. 6. Our general conclusions are the same as those conclusions now reported for CatSper1 in the work of Carlson et al. (26). It is not known whether CatSper1 and CatSper2 are subunits of the same channel or whether they are completely independent entities, but the failure of hyperactivated motility after disruption of both CatSper1 and CatSper2 suggests that they participate in a common signaling pathway and may be partners of the same channel. Arguments against their being a part of the same channel include an inability to coimmunoprecipitate the two proteins, their different apparent channel localization in the flagellum, and the expression of the CatSper1 and CatSper 2 transcripts at different times during spermatogenesis (12, 13, 20). Intriguingly, four testicular ESTs from the urochordate Ciona intestinalis with high homology to CatSper2 (two ESTs) and CatSper4 (two ESTs) have been identified in a large-scale sequencing project (27). Thus, it is conceivable that the CatSper proteins form at least two independent heterotetrameric channels that are critical to sperm cell motility. The possibility that CatSper1 and CatSper2 mediate hyperactivation is in agreement with observations that show higher Ca2+ concentrations in hyperactivated sperm cells (10) and that Ca2+, but not cAMP, causes hyperactivation of demembranated bull spermatozoa with an accessible axoneme (28). Even in sea urchin sperm cells, Ca2+ is known to increase flagellar beat asymmetry (29), which is a characteristic of most hyperactivated forms of motility in mammalian spermatozoa. These and many other observations in spermatozoa and cilia seem to place Ca2+ at the heart of motility regulation (24, 30). Although a large number of ion channels can be listed as potential regulators of sperm flagellar Ca2+ (2–4), of these channels, only CatSper1 and CatSper2 are known to cause infertility (this report and ref. 13). Another interesting aspect of motility hyperactivation is an apparent requirement to raise intracellular pH to enable Ca2+ to induce changes in flagellar curvature (28). This finding suggests that, in addition to activation of CatSper2 near the time of fertilization, elevations in intracellular pH may also be required.
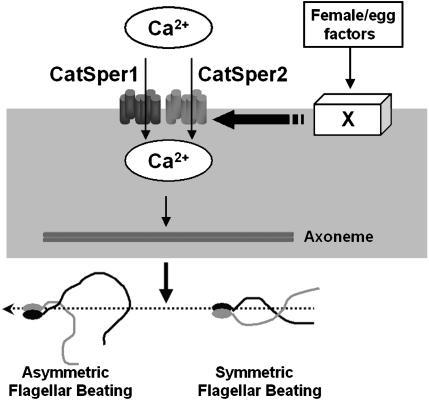
Fig. 6.
A hypothetical model for initiation of sperm cell hyperactivated motility. Undefined female factors are proposed to initiate a signaling mechanism that opens that CatSper channel(s), allowing calcium entry into the sperm cell flagellum. Calcium then interacts with components of the axoneme to modify the flagellar beat from a symmetric to an asymmetric form.
In the model shown (Fig. 6), we speculate that, when sperm cells enter the female, factor(s) in the female activate a signaling process that leads to the opening of the CatSper1 and CatSper2 channels. The activation of CatSper1 or CatSper2 appears to occur independent of events such as the acrosome reaction or specific protein tyrosine phosphorylation. Thus, the original definition of the term “capacitation” as “some physiological changes of the spermatozoa in the female genital tract before they are capable of penetrating and fertilizing the eggs” (31) is more accurate than “the acquisition of the ability to undergo an acrosome reaction,” given that we show here that hyperactivation is essential for fertilization.
The CatSper2–/– infertile phenotype is impenetrant, and, because others have recently attributed infertility in a human family to a mutation in CatSper2 (32), the channel becomes a particularly attractive target for male germ cell-directed contraception. Our work also raises further questions on the validity of fertility assays such as the zona pellucida-free hamster egg assay (33), which measures the ability of human sperm cells to penetrate the naked hamster egg. This assay would fail to detect sperm cells defective in initiating hyperactivated motility.
Supplementary Material
Acknowledgments
We thank Liz Lummus and Sara Love Swaney for excellent technical assistance and Dr. Donner F. Babcock for helpful discussions. Testicular histology and electron microscopy were performed in the Molecular Pathology Core Laboratory and the Molecular and Cellular Imaging Facility at the University of Texas Southwestern Medical Center, respectively. This research was supported by the Cecil H. and Ida Green Center for Reproductive Biology Sciences, the Howard Hughes Medical Institute, and National Institutes of Health Grant HD 36022.
Abbreviation: ES cell, embryonic stem cell.
References
- 1.Suarez, S. S., Varosi, S. M. & Dai, X. (1993) Proc. Natl. Acad. Sci. USA 90, 4660–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wennemuth, G., Westenbroek, R. E., Xu, T., Hille, B. & Babcock, D. F. (2000) J. Biol. Chem. 275, 21210–21217. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner, B., Weiner, J., Middendorff, R., Hagen, V., Kaupp, U. B. & Weyand, I. (1998) J. Cell Biol. 142, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellano, L. E., Trevino, C. L., Rodriguez, D., Serrano, C. J., Pacheco, J., Tsutsumi, V., Felix, R. & Darszon, A. (2003) FEBS Lett. 541, 69–74. [DOI] [PubMed] [Google Scholar]
- 5.Jungnickel, M. K., Marrero, H., Birnbaumer, L., Lemos, J. R. & Florman, H. M. (2001) Nat. Cell Biol. 3, 499–502. [DOI] [PubMed] [Google Scholar]
- 6.Muth, J. N., Varadi, G. & Schwartz, A. (2001) Trends Pharmacol. Sci. 22, 526–532. [DOI] [PubMed] [Google Scholar]
- 7.Stowers, L., Holy, T. E., Meister, M., Dulac, C. & Koentges, G. (2002) Science 295, 1493–1500. [DOI] [PubMed] [Google Scholar]
- 8.Freichel, M., Suh, S. H., Pfeifer, A., Schweig, U., Trost, C., Weissgerber, P., Biel, M., Philipp, S., Freise, D., Droogmans, G., et al. (2001) Nat. Cell Biol. 3, 121–127. [DOI] [PubMed] [Google Scholar]
- 9.Biel, M., Seeliger, M., Pfeifer, A., Kohler, K., Gerstner, A., Ludwig, A., Jaissle, G., Fauser, S., Zrenner, E. & Hofmann, F. (1999) Proc. Natl. Acad. Sci. USA 96, 7553–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, H. C. & Suarez, S. S. (2001) Biol. Reprod. 65, 1606–1615. [DOI] [PubMed] [Google Scholar]
- 11.Ho, H. C. & Suarez, S. S. (2003) Biol. Reprod. 68, 1590–1596. [DOI] [PubMed] [Google Scholar]
- 12.Quill, T. A., Ren, D., Clapham, D. E. & Garbers, D. L. (2001) Proc. Natl. Acad. Sci. USA 98, 12527–12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren, D., Navarro, B., Perez, G., Jackson, A. C., Hsu, S., Shi, Q., Tilly, J. L. & Clapham, D. E. (2001) Nature 413, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arias, J. M., Murbartian, J. & Perez-Reyes, E. (2003) Biochem. Biophys. Res. Commun. 303, 31–36. [DOI] [PubMed] [Google Scholar]
- 15.Lobley, A., Pierron, V., Reynolds, L., Allen, L. & Michalovich, D. (2003) Reprod. Biol. Endocrinol. 1, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howlett, S. K. & Bolton, V. N. (1985) J. Embryol. Exp. Morphol. 87, 175–206. [PubMed] [Google Scholar]
- 17.Fraser, L. R. (1993) Methods Enzymol. 225, 239–253. [DOI] [PubMed] [Google Scholar]
- 18.Si, Y. & Olds-Clarke, P. (2000) Biol. Reprod. 62, 1231–1239. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. (1970) Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 20.Schultz, N., Hamra, F. K. & Garbers, D. L. (2003) Proc. Natl. Acad. Sci. USA 100, 12201–12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talbot, P., Shur, B. D. & Myles, D. G. (2003) Biol. Reprod. 68, 1–9. [DOI] [PubMed] [Google Scholar]
- 22.Osheroff, J. E., Visconti, P. E., Valenzuela, J. P., Travis, A. J., Alvarez, J. & Kopf, G. S. (1999) Mol. Hum. Reprod. 5, 1017–1026. [DOI] [PubMed] [Google Scholar]
- 23.Arnoult, C., Kazam, I. G., Visconti, P. E., Kopf, G. S., Villaz, M. & Florman, H. M. (1999) Proc. Natl. Acad. Sci. USA 96, 6757–6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez, S. S. & Ho, H. C. (2003) Reprod. Domest. Anim. 38, 119–124. [DOI] [PubMed] [Google Scholar]
- 25.Suarez, S. S. & Dai, X. (1992) Biol. Reprod. 46, 686–691. [DOI] [PubMed] [Google Scholar]
- 26.Carlson, A. E., Westenbroek, R. E., Quill, T., Ren, D., Clapham, D. E., Hille, B., Garbers, D. L. & Babcock, D. F. (2003) Proc. Natl. Acad. Sci. USA 100, 14864–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inaba, K., Padma, P., Satouh, Y., Shin, I. T., Kohara, Y., Satoh, N. & Satou, Y. (2002) Mol. Reprod. Dev. 62, 431–445. [DOI] [PubMed] [Google Scholar]
- 28.Ho, H. C., Granish, K. A. & Suarez, S. S. (2002) Dev. Biol. 250, 208–217. [DOI] [PubMed] [Google Scholar]
- 29.Brokaw, C. J. (1991) Cell Motil. Cytoskeleton 18, 123–130. [DOI] [PubMed] [Google Scholar]
- 30.Plattner, H. & Klauke, N. (2001) Int. Rev. Cytol. 201, 115–208. [DOI] [PubMed] [Google Scholar]
- 31.Chang, M. C. (1984) J. Androl. 5, 45–50. [DOI] [PubMed] [Google Scholar]
- 32.Avidan, N., Tamary, H., Dgany, O., Cattan, D., Pariente, A., Thulliez, M., Borot, N., Moati, L., Barthelme, A., Shalmon, L., et al. (2003) Eur. J. Hum. Genet. 11, 497–502. [DOI] [PubMed] [Google Scholar]
- 33.Wolf, J. P., Bulwa, S., Ducot, B., Rodrigues, D. & Jouannet, P. (1996) Fertil. Steril. 65, 1196–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.