Nattokinase, a protein found in high levels in the traditional Japanese food natto, has been reported to have high thrombolytic activity. In the present study, the crystallization of native nattokinase and the collection of X-ray diffraction date from a nattokinase crystal to a resolution of 1.74 Å are reported.
Keywords: nattokinase, Bacillus subtilis natto, fibrinolysis
Abstract
Nattokinase is a single polypeptide chain composed of 275 amino acids (molecular weight 27 724) which displays strong fibrinolytic activity. Moreover, it can activate other fibrinolytic enzymes such as pro-urokinase and tissue plasminogen activator. In the present study, native nattokinase from Bacillus subtilis natto was purified using gel-filtration chromatography and crystallized to give needle-like crystals which could be used for X-ray diffraction experiments. The crystals belonged to space group C2, with unit-cell parameters a = 74.3, b = 49.9, c = 56.3 Å, β = 95.2°. Diffraction images were processed to a resolution of 1.74 Å with an R merge of 5.2% (15.3% in the highest resolution shell) and a completeness of 69.8% (30.0% in the highest resolution shell). This study reports the first X-ray diffraction analysis of nattokinase.
1. Introduction
Natto is a popular traditional food in Japan and is made from soybeans fermented by Bacillus subtilis natto. The health benefits of eating natto are well known and have been attributed to several chemical constituents such as nattokinase (NK; Sumi et al., 1987 ▶) and vitamin K2 (menaquinone-7; MK-7; Sakano et al., 1988 ▶). NK consists of a single polypeptide chain composed of 275 amino acids (molecular weight 27 724), containing no disulfide bonds, and displays strong fibrinolytic activity (Sumi et al., 1992 ▶). In previous studies, we have revealed that NK not only has fibrinolysis activity itself but also activates other fibrinolytic enzymes such as pro-urokinase (Sumi et al., 1996 ▶) and tissue plasminogen activator (Urano et al., 2001 ▶; Yatagai et al., 2008 ▶). Currently, NK is used as a dietary supplement, mainly in Japan, as both a prophylactic and a curative medicine. NK belongs to the serine protease family and its homology to subtilisin E (SE) from B. subtilis is 99%. The crystal structure of SE, which was overexpressed in Escherichia coli, has been reported previously at a resolution of 2.0 Å (Jain et al., 1998 ▶). The three-dimensional structure of NK can be predicted based on the crystal structure of SE (Zheng et al., 2004 ▶, 2006 ▶), but the X-ray crystal structure of NK is not yet available. Structural information may help in elucidating the molecular mechanisms of its strong fibrinolytic properties, which are not yet understood. We have recently found that native NK extracted from B. subtilis natto may be modified by glycosylation or other chemical reactions (Chiba-Kamoshida et al., 2010 ▶). In the present study, purification, crystallization and preliminary X-ray experiments were carried out with the aim of better understanding this protein.
2. Materials and methods
2.1. Purification by hydrophobic interaction chromatography and crystallization
In the present study, two types of native nattokinase (NK), solution and powder, were provided by Honda Trading CC. Using these, purifications and crystallizations were performed at the TL building in the Research Reactor Institute of Kyoto University.
In the first experiment, purification of NK was carried out by a combination of the ammonium sulfate (AS) precipitation method and hydrophobic interaction chromatography (HIC) using a butyl-Sepharose column, as reported previously (Fujita et al., 1993 ▶). The supernatant was extracted from the culture medium of B. subtilis natto by ultrafiltration. The crude NK was precipitated by adding 1.5 M AS to 1 ml supernatant solution at 277 K and the precipitate was then dissolved in a large quantity (50 ml) of buffer solution consisting of 25 mM Tris–HCl pH 6.8, 2.0 M AS and 2 mM phenylmethanesulfonylfluoride (PMSF). The solution was purified using a GE butyl-Sepharose FF (1 ml) column mounted on an ÄKTAprime FPLC system (GE Healthcare). A gradient from 2.00 to 0 M AS was used, with a flow rate of 1.0 ml min−1 and a total elution period of 100 min. NK eluted as a single peak between 1.75 and 1.50 M AS. The NK-containing fractions were desalted using GE Sephadex G-75 (1.5 × 36 cm) and were concentrated to 8.0 mg ml−1 using UltraFree-15 and UltraFree-0.5 centrifugal filter units with a nominal molecular-weight limit (NMWL) of 10 and 5 kDa, respectively.
NK purified by HIC (NK-HIC) was crystallized at 293 K by the sitting-drop vapour-diffusion method using Crystal Screen (CS; Hampton Research). During crystallization, 2 mM CaCl2 was added to all crystallization droplets as Ca2+ is important for the activity of serine proteases. Two types of crystals were obtained after one week. Many needle-like microcrystals were produced in solution Nos. 9, 10, 15, 18, 22 and 28 of CS; however, they were too small for use in X-ray experiments. A plate-like crystal of NK-HIC was grown in condition No. 22 of CS (8.0 mg ml−1 NK-HIC, 0.2 M sodium acetate trihydrate, 0.1 M Tris–HCl pH 8.5 and 30% polyethylene glycol 4000; Fig. 1 ▶). This crystal was soaked in 50% glycerol for 5 s and flash-frozen using nitrogen gas at 100 K. A diffraction experiment using synchrotron radiation was carried out to assess the quality of the crystal. The diffraction pattern indicated that the crystal was indeed a protein crystal and the maximum resolution achieved was 3.5 Å. However, this crystal was a polycrystal consisting of multiple layers of thin crystals; therefore, the quality of the diffraction images was insufficient for determination of the crystallographic parameters.
Figure 1.
Plate-like crystals of nattokinase purified using hydrophobic interaction chromatography.
2.2. Purification by gel-filtration chromatography and crystallization
Next, NK powder containing dextrin as a diluent was purified by gel-filtration chromatography using a solution consisting of 50 mM Tris–HCl pH 7.5, 5 mM CaCl2 and 100 mM NaCl. The diluent was removed by an initial gel filtration on GE Sephadex G10 (2.0 × 15 cm) and the protein solution was incubated for 12 h at 277 K in a solution consisting of 50 mM Tris–HCl pH 7.5, 5 mM CaCl2 and 2 mM PMSF. It was further purified by a second gel filtration using GE Sephacryl-S200 (1.5 × 40 cm) and the resulting protein (NK-GC) was concentrated using UltraFree-15 (NMWL 3 kDa) and UltraFree-0.5 (NMWL 5 kDa) centrifugal filter units.
A solution consisting of 6.4 mg ml−1 NK-GC, 50 mM Tris–HCl pH 7.5, 5 mM CaCl2 and 100 mM NaCl was used in crystallization screening using Crystal Screen and Crystal Screen 2 (CS and CS2). Crystallization was carried out at 293 K using the sitting-drop vapour-diffusion method. 4 mM Pefabloc SC (Roche) was added to the droplets to avoid self-digestion of NK-GC.
2.3. X-ray experiment
Needle-like crystals of NK-GC were used for X-ray diffraction studies. A preliminary X-ray experiment was carried out using a synchrotron X-ray source. The crystal was soaked in a solution consisting of 50 mM HEPES pH 7.5, 5% polyethylene glycol 8000, 4% ethylene glycol and 50% glycerol for 5 s. After soaking, the crystal was flash-frozen using nitrogen gas at 100 K. X-ray diffraction images were collected using synchrotron radiation on BL44XU at SPring-8. The X-ray beam was monochromated at 0.9 Å. The oscillation angle for each diffraction image was 1.0° and the crystal was rotated through a total of 100°. The diffraction images were indexed, integrated and merged into independent reflections with HKL-2000 (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
3.1. Activity measurements and crystallization of NK-GC
The fibrinolytic activity of NK-GC was assessed using the fibrin-plate method (Astrup & Mullertz, 1952 ▶) and colorimetric methods (Sumi et al., 2009 ▶, 2010 ▶) using a synthetic substrate as detailed below. 30 µl of a 5.4 mg ml−1 solution of NK-GC was added to fibrin plates containing 0.5% fibrin (generated from fibrinogens), borate-buffered saline pH 7.8 and thrombin. After incubation for 4 h at 310 K, the lysis area (30.2 mm2) was measured. The activity of NK-GC was estimated using an assay with a 5.4 mg ml−1 solution of NK-GC and the synthetic substrate Bz-lle-Glu-(Or)-Gly-Arg-pNA. The reaction mixture (1 ml) contained 100 µl substrate (at a final concentration of 5 × 10−4 M) and 0.17 M borate-buffered saline solution pH 7.8. The amount of pNA released was determined based on the absorbance observed at 405 nm. NK-GC was shown to have an activity of 1.53 IU ml−1. Both methods therefore confirmed the fibrinolytic activity of NK-GC.
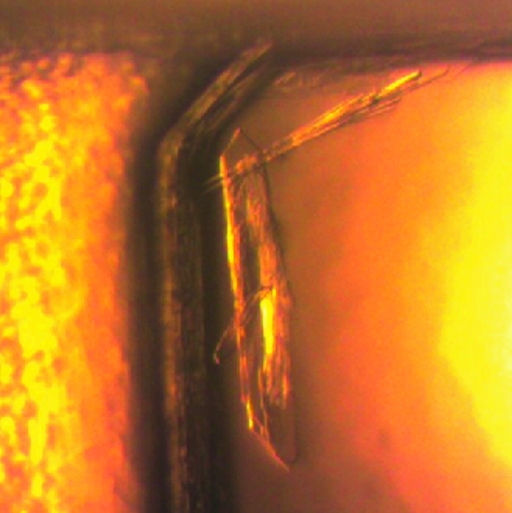
Needle-like crystals were obtained in the crystallization of NK-GC. The shapes of the crystals were similar to the needle-like microcrystals of NK-HIC. The NK-GC crystals were grown in different crystallization solutions (CS No. 41 and CS2 Nos. 30, 32, 37, 38 and 48) from those that produced NK-HIC crystals (CS Nos. 9, 10, 15, 18, 22 and 28). The NK-GC crystals were larger in size than the needle-like NK-HIC crystals and the largest crystal was obtained from the crystallization solution CS2 No. 37 (6.4 mg ml−1 NK-GC, 50 mM HEPES pH 7.5, 10% polyethylene glycol 8000 and 8% ethylene glycol; Fig. 2 ▶). 15% SDS electrophoresis was performed to check the chemical composition of the crystal and showed the presence of NK-GC in the crystals.
Figure 2.
Needle-like crystals of nattokinase purified using gel-filtration chromatography.
3.2. X-ray experiment
The crystal belonged to space group C2, with unit-cell parameters a = 74.3, b = 49.9, c = 56.3 Å, β = 95.2°. The effective resolution was 1.74 Å. R merge and the completeness of the data were 5.2% and 69.8%, respectively. Assuming the presence of one NK molecule in the asymmetric unit, the Matthews coefficient was 1.87 Å3 Da−1. The statistics of the X-ray experiment are summarized in Table 1 ▶.
Table 1. Crystallographic data and statistics of data collection.
Values in parentheses are for the highest resolution shell.
| Crystallographic data | |
| Space group | C2 |
| Unit-cell parameters (Å, °) | a = 74.3, b = 49.9, c = 56.3, β = 95.2 |
| Z† | 4 |
| Data collection | |
| Wavelength (Å) | 0.9 |
| Temperature (K) | 100 |
| No. of frames | 1 |
| Δω (° per frame) | 210 |
| Resolution (Å) | 1.74 (1.77–1.74) |
| Unique reflections | 14847 |
| Rmerge (%) | 5.2 (15.3) |
| Completeness (%) | 69.8 (30.0) |
| 〈I/σ(I)〉 | 31.1 (12.1) |
| Multiplicity | 2.2 (1.4) |
No. of NK molecules in a unit cell.
The initial phases of the X-ray data set were determined by molecular replacement using the program AMoRe (Navaza, 1994 ▶). The atomic coordinates of amino-acid residues 1–275 of SE from B. subtilis (PDB entry 1scj; Jain et al., 1998 ▶) were used as a probe. The molecular-replacement solution was determined with an R factor of 32.0% at a resolution of 3.0 Å. Atomic parameters were refined to a resolution of 1.74 Å using the program PHENIX (Adams et al., 2010 ▶). After three cycles of positional refinement and individual B-factor refinement, the R factor and R free were 24.2% and 29.6%, respectively. Further refinement, including model building and water location, is in progress.
4. Conclusions
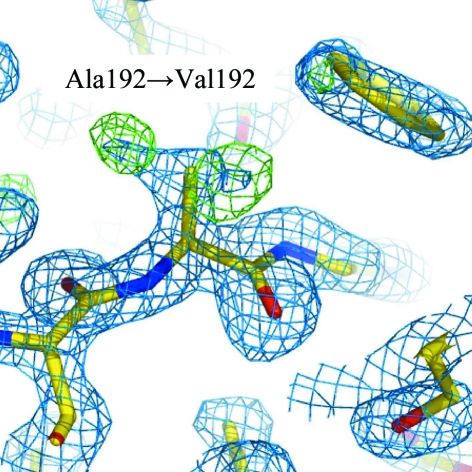
In the present study, native NK was purified by hydrophobic interaction chromatography and gel-filtration chromatography and used for crystallization. NK purified by gel filtration showed fibrinolytic activity and sufficient purity to obtain good-quality crystals for X-ray diffraction experiments. These needle-like crystals were obtained from a solution consisting of 6.4 mg ml−1 NK-GC, 50 mM HEPES pH 7.5, 10% polyethylene glycol 8000 and 8% ethylene glycol. Diffraction images were collected from a single crystal at 100 K using synchrotron radiation. This represents the first report of an X-ray data set for NK. The diffraction images were processed to a resolution of 1.74 Å with an R merge of 5.2% (15.3% in the highest resolution shell) and a completeness of 69.8% (30.0% in the highest resolution shell). Initial phases for the X-ray data set were derived using the molecular-replacement method based on a previously reported X-ray crystal structure of SE and the first X-ray Fourier map was calculated with an R factor of 24.2% (R free = 29.6%) (Fig. 3 ▶). More detailed structural analysis will be reported at a later date.
Figure 3.
2|F o| − |F c| and |F o| − |F c| Fourier maps superimposed on the 192nd amino-acid residue calculated after molecular replacement using subtilisin E (SE) as an initial model. Blue and green contours show 2|F o| − |F c| density at the 1.5σ level and |F o| − |F c| density at the 3.5σ level, respectively. The 192nd residue differs between NK (Val) and SE (Ala). In the |F o| − |F c| map, two strong densities corresponding to the Cδ1 and Cδ2 atoms of valine were observed.
Acknowledgments
This work was supported in part by the Research Project of the Research Reactor Institute of Kyoto University (1921P9-3, 22P6-2) and an Education Research 2008–2009 grant from Chiba Institute of Sciences.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Astrup, T. & Mullertz, S. (1952). Arch. Biochem. Biophys.40, 346–351. [DOI] [PubMed]
- Chiba-Kamoshida, K., Yanagisawa, Y., Chatake, T., Ukibe, M., Shiki, S., Saito, J., Sumi, H. & Ohkubo, M. (2010). Seibutsu Butsuri, 50, Suppl. 2, S48. In Japanese.
- Fujita, M., Nomura, K., Hong, K., Ito, Y., Asada, A. & Nishimuro, S. (1993). Biochem. Biophys. Res. Commun.197, 1340–1347. [DOI] [PubMed]
- Jain, S. C., Shinde, U., Li, Y., Inoue, M. & Berman, H. M. (1998). J. Mol. Biol.284, 137–144. [DOI] [PubMed]
- Navaza, J. (1994). Acta Cryst. A50, 157–163.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Sakano, T., Matumoto, S., Nagoya, T., Morimoto, A., Fujimoto, K., Masuda, S., Suzuki, Y., Fujimoto, K. & Masuda, S. (1988). Vitamins, 62, 393–398. In Japanese.
- Sumi, H., Banba, T. & Kishimoto, N. (1996). Nippon Shokuhin Kagaku Kaishi, 43, 1124–1127. In Japanese.
- Sumi, H., Hamada, H., Tsushima, H., Mihara, H. & Muraki, H. (1987). Experientia, 43, 1110–1111. [DOI] [PubMed]
- Sumi, H., Naito, S., Yatagai, C., Ohsugi, T., Yanagisawa, Y. & Maruyama, M. (2010). FOOD Style 21, 143, 52–54. In Japanese.
- Sumi, H., Taya, H., Nakajima, N. & Hiratani, H. (1992). Fibrinolysis, 6, Suppl. 2, 86.
- Sumi, H., Yatagai, C., Naito, S., Ohsugi, T. & Saito, J. (2009). J. Thromb. Haemost.7, Suppl. 2, PP-WE-148.
- Urano, T., Ihara, H., Umemura, K., Suzuki, Y., Oike, M., Akita, S., Tsukamoto, Y., Suzuki, I. & Takada, A. (2001). J. Biol. Chem.276, 24690–24696. [DOI] [PubMed]
- Yatagai, C., Maruyama, M., Kawahara, T. & Sumi, H. (2008). Pathophysiol. Haemost. Thromb.36, 227–232. [DOI] [PubMed]
- Zheng, Z., Ye, M., Zuo, Z., Liu, Z., Tai, K. & Zou, G. (2006). Biochem. J.395, 509–515. [DOI] [PMC free article] [PubMed]
- Zheng, Z., Zuo, Z., Liu, Z., Tsai, K., Liu, A. & Zou, G. (2004). J. Mol. Graph. Model.23, 373–380. [DOI] [PubMed]