Recently, considerable information has been obtained on the behavior of liver homografts, by Moore et al. [8] and in our own laboratory [10,11]. The large and immunologically active liver homografts were rejected in roughly the same time sequence as smaller and less complex tissues. In our studies, the manner of rejection differed from the usual situation in that widespread changes were evoked in the host reticuloendothelial system, involving the lungs, kidneys, lymph nodes, bone marrow and other organs. The functional response of the transplanted liver was substantially the same, both in Moore’s studies [8] and in our own [11]. In all our animals, jaundice developed by the fifth day, and the animals died from one to sixteen days later.
In the present study, other abdominal viscera were added to the liver, to constitute a relatively enormous multiorgan graft consisting of the liver, spleen, pancreas, omentum, stomach, small bowel and colon. The behavior of the liver as a constituent of the multivisceral graft was compared with that previously studied with homotransplantation of the liver alone. Since the graft contains the major portion of the reticuloendothelial system of the body, particular attention was paid to the possibility of a graft versus host reaction.
METHODS
Adult healthy dogs from 10 to 20 kg. in weight were used in thirty-eight transplantation experiments. The animals were dewormed, passively immunized against distemper, and prepared for surgery with a two or three day bowel preparation, using cathartics and 2 gm. of neomycin sulphate per day. All recipient dogs were females, and most donors were males. The donor dogs were 1 to 5 kg. lighter than the recipients. The animals were anesthetized with 25 to 30 mg. sodium pentobarbital and placed on respirators. Arterial pressures were monitored continuously during and after operation.
Blood chemical and hematologic studies were made before and at intervals after surgery. After death, complete autopsies were promptly performed and the tissues prepared for histologic study.
TECHNIC OF OPERATION
Preparation of Donor Tissues
Using two surgical teams, the operation was started in donor and recipient dogs at the same time. The donor animal was first cooled to 28° to 30°C. by immersion in an ice bath. The abdomen was opened and the entire abdominal aorta mobilized, ligating and dividing all branches, except the superior mesenteric artery and coeliac axis. (Fig. 1.) Mesenteric and other posterior parietal connections, including the afferent fibers to the coeliac and superior mesenteric ganglia, were severed. The stomach was transected at the esophagogastric junction (Fig. 1) and closed in two layers. The colon was transected in the descending or sigmoid portions. A No. 17 gauge needle was inserted into the portal vein, and the liver was perfused with 1,000 cc. iced lactated Ringer’s solution. During the last half of the perfusion, the dog was bled to death. The vena cava was then transected above and below the liver. The aorta was cut free well above the coeliac axis. The specimen was immediately removed and immersed in a bath of iced lactated Ringer’s solution for at least three minutes.
Fig. 1.
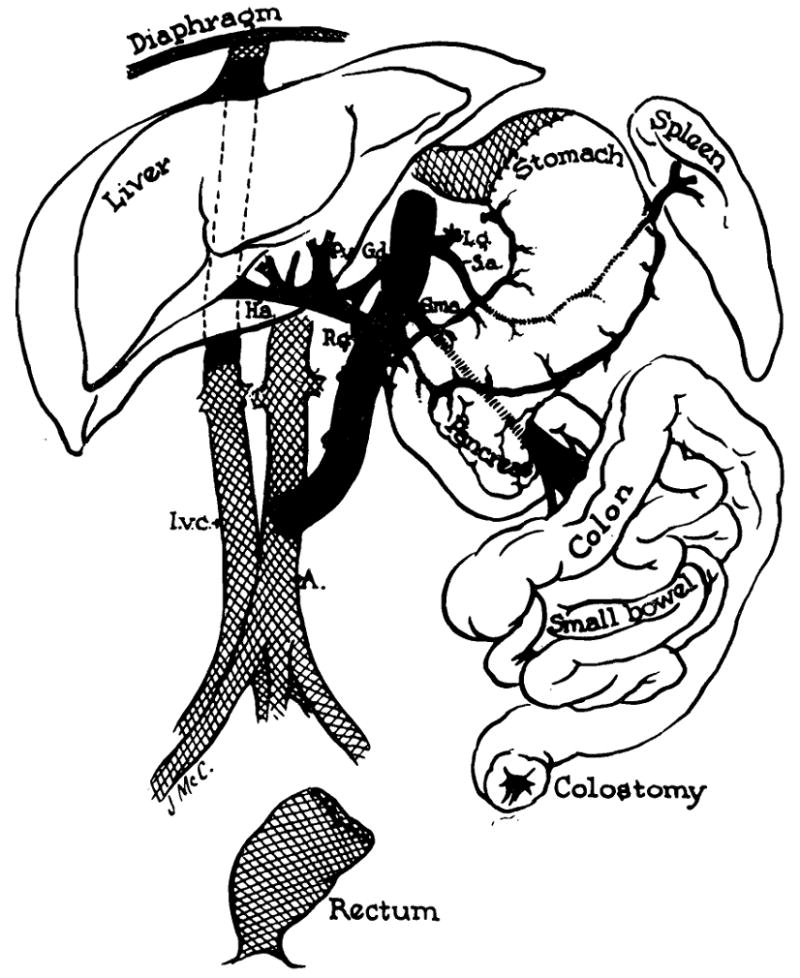
Schematic view of the transplanted tissues and their anatomic relation to the host. The grafted tissues are not shaded.
Procedure in the Recipient Dog
While the homograft was being prepared, the recipient animal was opened with a long midline abdominal incision. The rectum was mobilized, transected and closed in two layers within 1 or 2 cm. of the anus. (Fig. 1.) The visceral attachments to the posterior parietes were severed, and the coeliac axis and superior mesenteric artery were ligated and divided. The stomach was divided leaving a small cuff at the diaphragm for subsequent anastomosis to the donor stomach. (Fig. 1.) The femorojugular venous bypass described by Kaupp et al. [4] was inserted, and the vena cava was grasped with Potts clamps and severed above and below the liver. The liver, stomach, pancreas, spleen, small bowel, colon and omentum were then removed en bloc.
The donor organs were removed from the ice bath, positioned and revascularized. The vena cava was anastomosed above and below the liver (Fig. 1), following which, the occluding clamps were released. The aortic homograft was attached to the abdominal aorta by an end to side anastomosis, and the specimen was arterialized. (Fig. 1.)
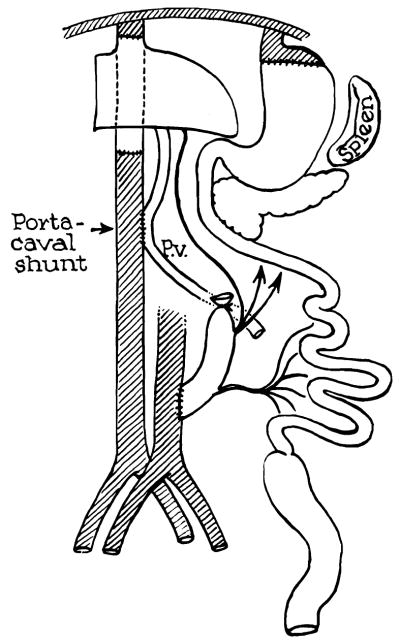
In five of the thirty-eight dogs, a portacaval anastomosis was also constructed between the recipient vena cava and the donor portal vein. (Fig. 2.) Gastrointestinal continuity was reestablished with a two layer gastrogastrostomy proximally and an end colostomy distally.
Fig. 2.
Addition of portacaval shunt to operation depicted in Figure 1.
Postoperative Care
The animals required constant nursing care for the first twelve to twenty-four hours after surgery. Frequent endotracheal suctioning was necessary. Large quantities of blood and fluid were required continuously during this period. Although demonstrable blood loss was often less than 300 or 400 cc., no less than 2,000 cc. were given in any of the successful experiments in addition to 500 to 1,000 cc. saline solution. Infusion with levoarterenol was commonly employed during the first few postoperative hours. The administration of intravenous fluids was discontinued after the first two days, and the dogs were allowed to eat a diet consisting of sugar water and baby food. One million units of aqueous penicillin were given every twelve hours until death.
RESULTS
Period of Ischemia of the Homograft
In most cases, the period of devascularization was from sixty to seventy minutes. There were no survivors when ischemia exceeded ninety minutes.
Operative Mortality
Thirty-eight experiments were performed. Only five dogs lived longer than twenty-four hours after operation. All animals which did not die on the first day lived for five and a half days or longer. The remaining thirty-three died as an immediate consequence of surgery. In every early death, massive gastrointestinal hemorrhage was the primary cause of death.
Early Course After Homografting
The recipient animals generally remained in good condition during evisceration, placement of the graft and anastomosis of the vena cava. With restoration of the arterial supply to the graft, all dogs became profoundly hypotensive, usually with a blood pressure of 60 mm. Hg or lower, despite rapid transfusion. At least 1,000 cc. of whole blood was necessary to restore the blood pressure. Initially, the color of the stomach and bowel was pink, but after thirty or forty minutes, faint cyanosis developed as blood sequestration occurred in the graft. Large quantities of clear or straw-colored fluid transuded the lumen of the gut. Lymphatics on the surface of the stomach and intestine became distended and formed serous blisters. After a time, the liver became dark in color and firmer than normal. Secondary bleeding from vascular anastomoses often developed. Slow infusion with levoarterenol was sometimes effective in combating the shock state. Chlorpromazine, Arfonad,® reserpine and Pitressin® were of no value.
After closure, a profuse serous diarrhea ensued necessitating continuous blood and fluid therapy. In most animals, the diarrhea became bloody, and death followed from massive gastrointestinal hemorrhage in one to twenty-four hours. In the five surviving dogs, the diarrhea abated after twelve to eighteen hours. Further intensive care was not required.
In the early deaths, hemorrhagic gastroenteritis was invariably present at autopsy. All levels of the gastrointestinal tract were involved, but the most profound changes were usually in the duodenum. Histologic studies showed congestion, extravasation of blood, edema and sloughing of the alimentary tract. The other graft organs were also congested. Multiple punched-out duodenal ulcers were common.
The gastrointestinal hemorrhages were apparently not due to acute elevations of portal pressure. In five dogs, a portacaval shunt (Fig. 2) was placed between the donor portal vein and the recipient vena cava. Four of these animals died of alimentary bleeding.
Maximal Survival
The five dogs surviving the immediate postoperative period lived for five and a half, six, sevens nine and nine days, respectively. Fluid requirements abruptly decreased after the first day, and no parenteral therapy was given after forty-eight hours. High carbohydrate diet consisting of brown sugar solution and baby food was started on the third day. Vomiting was rare until just before death.
Physical activity of the dogs was somewhat reduced, but otherwise they seemed normal. Liquid colostomy drainage was continuous but not excessive in quantity. Terminally, in all dogs tarry or frankly bloody fecal drainage developed. The transit time of ingested material was rapid, and food was often undigested. Abdominal roentgenograms usually did not show an abnormal gas pattern. (Fig. 3.)
Fig. 3.
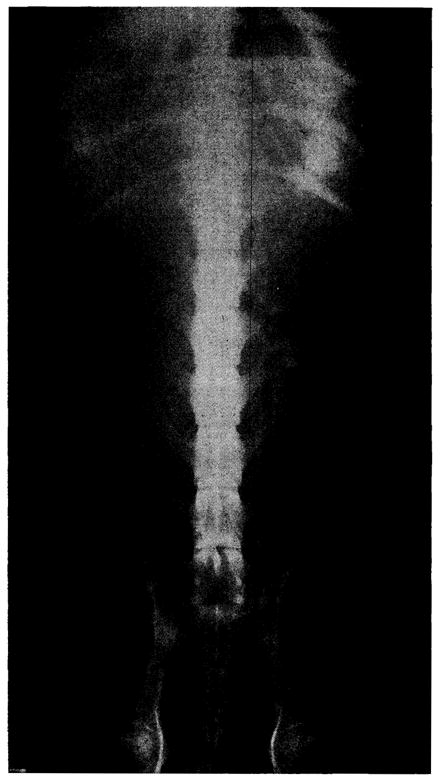
Abdominal roentgenogram of Dog No. 18 on the sixth postoperative day. Dog had been on oral intake for four days.
Most animals were febrile during their entire course. Pulse rates rose just before death. The dogs lost weight rapidly. After four to eight days, activity became sharply reduced, followed by vomiting and death in twelve to twenty-four hours.
Chemical Studies
In three of the five long-surviving dogs, jaundice developed on the fourth or fifth postoperative day. (Fig. 4.) Jaundice was progressive and involved parallel increases in both the direct and indirect reacting bilirubin. Corresponding rises occurred in the alkaline phosphatase. (Fig. 4.) In the other two animals which lived for nine days, the bilirubin remained within normal limits (Fig. 5), although the alkaline phosphatase increased. (Fig. 5.)
Fig. 4.
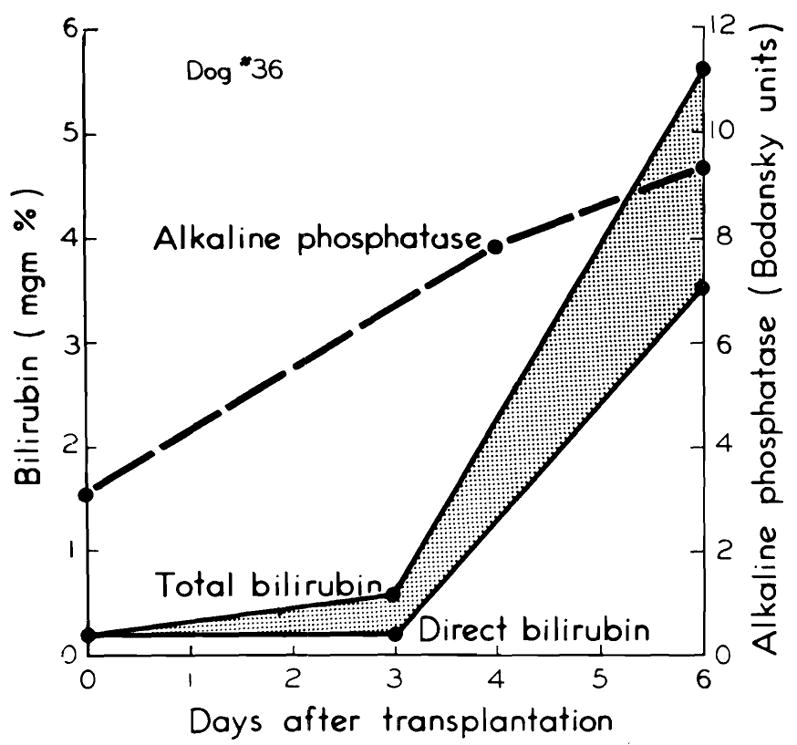
Development of chemical jaundice with rise in alkaline phosphatase, seen in three of the five long-surviving dogs.
Fig. 5.
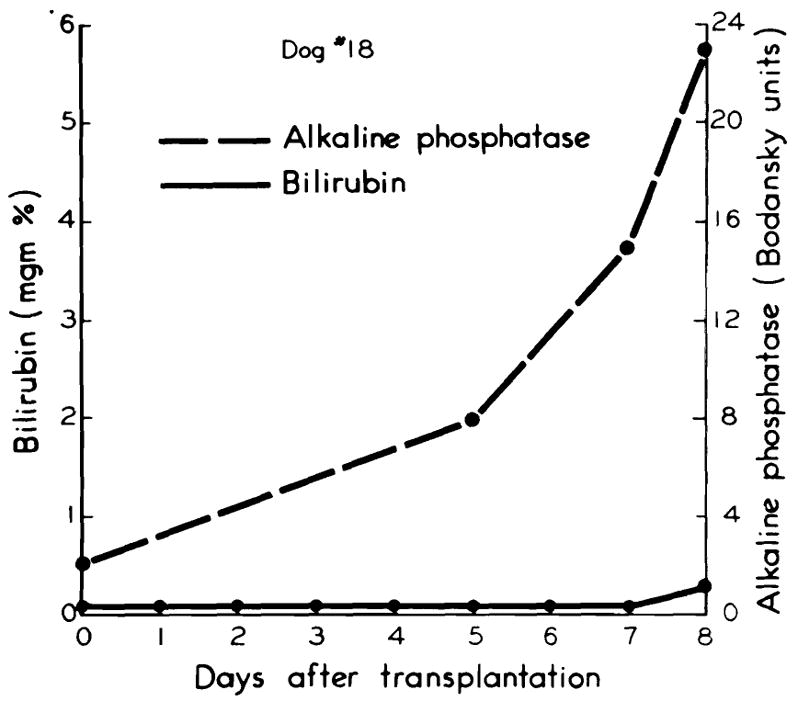
Absence of jaundice in two of the five long-surviving dogs. Note rise in alkaline phosphatase.
Determinations of fasting blood sugars were performed at frequent intervals postoperatively. Hypoglycemia occurred uncommonly. (Fig. 6.)
Fig. 6.
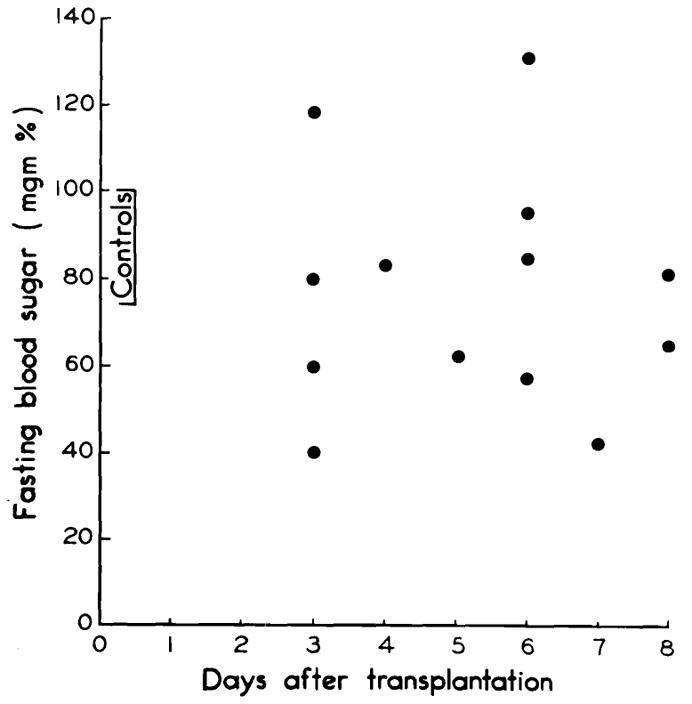
Fasting blood sugars in the five long-surviving dogs. Note usual absence of pronounced hypoglycemia.
Amylase values were abnormally high in three of five dogs. In all five animals, cholesterol levels rose, and in every case at least 30 mg. per cent esters were present at the time of the last determination before death. Protein and albumin:globulin ratios followed an unpredictable pattern. Blood urea nitrogen was elevated in only one animal. Calcium and phosphorus levels were within normal limits.
In all five dogs extreme electrolyte aberrations developed with severe hyperchloremic acidosis. Terminally, bicarbonate fell to as low as 7 mEq./L. and chlorides were as high as 120 or 130 mEq./L.
Urine Studies
Persistent albuminuria developed by the third day. The albumin loss was 4 plus at all times from the third day on. Concentrating power was retained, however, throughout the entire course, and shifting urinary pH’s were demonstrated as late as the eighth day.
In the three dogs with jaundice, urobilinogen disappeared from the urine by the fourth day, and bile appeared. In two dogs without jaundice, urinary bile was not detected, and urobilinogen was excreted until death.
Hematologic Studies
Since the circulating red blood cell mass was maintained by blood transfusions, the peripheral erythroid values could not be used as parameters of myeloid erythropoietic activity or blood loss. In the immediate postoperative period, each animal manifested a moderate leukocytosis due to a relative and absolute neutrophilia. Rather prompt subsidence of the leukocytosis was observed. In the animals which survived for nine days, the leukocyte counts had fallen on the sixth and seventh days to 4,500 and 4,700 per cu. mm., respectively. On the day before death, the white blood cell count was 4,200 per cu. mm. in one of these dogs. However, it had risen to 17,000 per cu. mm. in the other. Alterations in thrombocytes were not detected in these relatively short-term experiments.
Autopsy Findings
In the long-term survivals, postmortem examinations revealed abnormalities in both donor and recipient tissues. Two of the five dogs had small pleural effusions. Despite the fact that no intravenous fluid therapy was given after forty-eight hours, each dog had severe generalized pulmonary edema. In two cases, there was also focal atelectasis. In all animals, the right ventricle had patchy granular areas, principally subepicardial. These lesions were previously shown to be due to the trauma of massive surgery [10].
The abdominal cavities contained 250 to 800 cc. of fluid. In some animals, this material was serous, and in others it was bile or blood stained. The gastrointestinal tract had profound changes. In all cases, there were mucosal or submucosal hemorrhagic areas at some level of the alimentary tract, usually associated with slough or edema. The changes were most marked in the duodenum and were accompanied by multiple deep ulcers in four dogs. In three dogs, the bowel was constricted; in the other two, it was dilated and appeared almost necrotic. In two animals, there was fat necrosis and other evidence of pancreatitis.
The transplanted livers were moderately enlarged and cut with a firm granular sensation. The spleens were slightly enlarged, somewhat mushy and generally dark. The kidneys and adrenals were normal in four dogs and congested in the fifth.
The gastrointestinal anastomoses healed well. There were no ruptutres of the suture line. Venous anastomoses remained patent, but in two dogs there were partially occluding thrombi at the aortic anastomoses.
Mesenteric lymph nodes were greatly enlarged and edematous. In two animals, the mesenteric lymphatics were injected with methylene blue, but no lymphatico-venous communications could be demonstrated. The recipient lymph nodes, of which the mediastinal group were most extensively dissected, were not enlarged.
Histologic Findings in Homograft Tissues
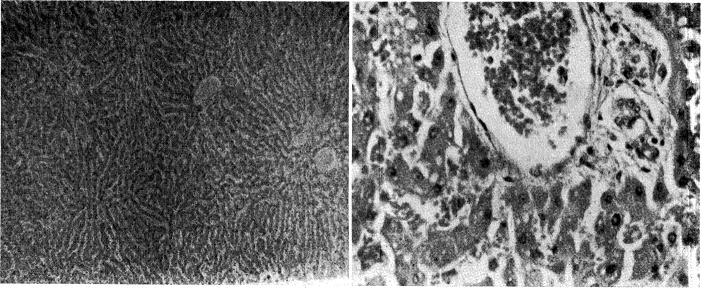
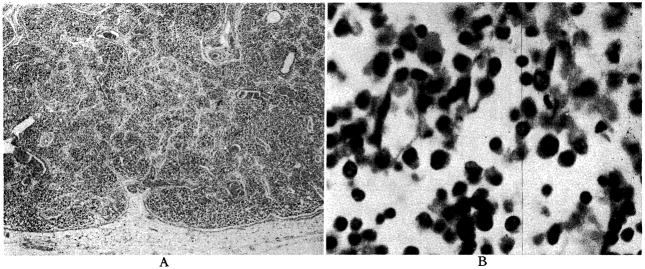
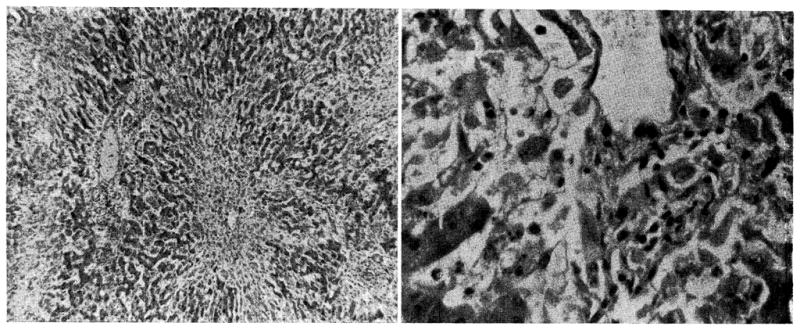
The architecture of the liver was relatively well preserved. The two dogs which lived for nine days and did not become jaundiced had almost normal livers. There was slight central cell loss in some areas,; and none at all in others. (Fig. 7.) The periportal mononuclear infiltrate, which was so prominent with homografts of the liver alone [11], was absent in some sections and present to a rather minimal degree in others. (Fig. 7.) Fatty metamorphosis which was thought to be due to nutritional depletion was present.
Fig. 7.
Liver after nine days, from Dog No. 18, in which jaundice did not develop. A, magnification × 65; B, magnification × 350.
The three dogs which survived for five and a half, six and seven days; and in which jaundice developed had more pronounced histologic abnormalities. There was a significant degree of central cell loss. (Fig. 8.) Periportal aggregates of mononuclear cells were more prominent. (Fig. 8.) However, even in this group, architectural distortion was far less than has been observed with transplantation of the liver alone.
Fig. 8.
Liver after seven days, from Dog No. 4, in which jaundice developed. A, magnification × 65; B, magnification × 350.
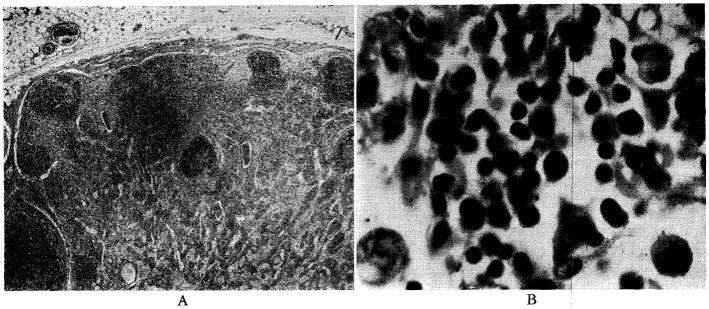
Architecture of the spleen was preserved, although considerable congestion was present in four of the five animals. (Fig. 9.) Malpighian corpuscles were present (Fig. 9), but were sometimes compressed by congestion of the red pulp or partially replaced with a multitude of plasma cells. In the dogs which survived nine days, giant cells (Fig. 9B), which resembled megakaryocytes, and increased numbers of normoblasts were observed. In two dogs diffuse sheets of mononuclear plamocytoid cells were seen in the red pulp.
Fig. 9.
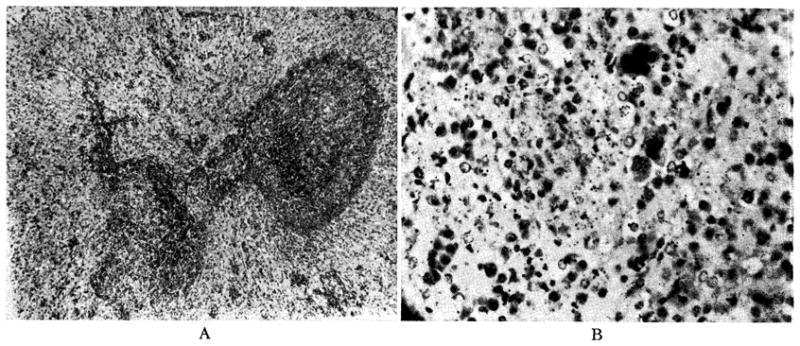
Donor spleen, after nine days, from Dog No. 19. A, magnification × 65; B, magnification × 350.
The lymph nodes in the mesentery of the graft were studied. There was some distortion of the peripheral lymph channels because of the necessary ligation of the central drainage pathways near the coeliac and mesenteric ganglia. Proteinaceous material and numerous macrophages filled the distended lymph vessels. The cortices were anatomically preserved with demonstrable follicles. (Fig. 10.) However, the follicles were reduced in size and number and infiltrated with a variable number of plasma and reticulum cells. (Fig. 10B.) The pulp also contained an increased number of plasma and reticulum cells. Throughout the nodes in two dogs, anomalous plasmacytoid cells with an abundant pink cytoplasm were present.
Fig. 10.
Donor lymph node from mesentery of graft in Dog No. 26. Animal lived five and a half days. A, magnification × 30; B, magnification × 600.
All portions of the gastrointestinal tract had similar changes, although these were most extensive in the duodenum. There was congestion of the entire wall of the vise us, usually with edema. (Fig. 11.) The mucosa was commonly ulcerated focally (Fig. 11), and in some cases there was massive slough. The duodenum in four dogs had deep punctate ulcers. (Fig. 12.) The wall was infiltrated with polymorphonuclear cells in the cases with slough, and there was always some degree of infiltration with mononuclear cells. In four of the five dogs, there was acute pancreatitis which was indistinguishable from that produced by a variety of experimental methods. In the fifth animal, there was pancreatic perivascular infiltration of plasma cells and evidence of fixed tissue proliferation.
Fig. 11.
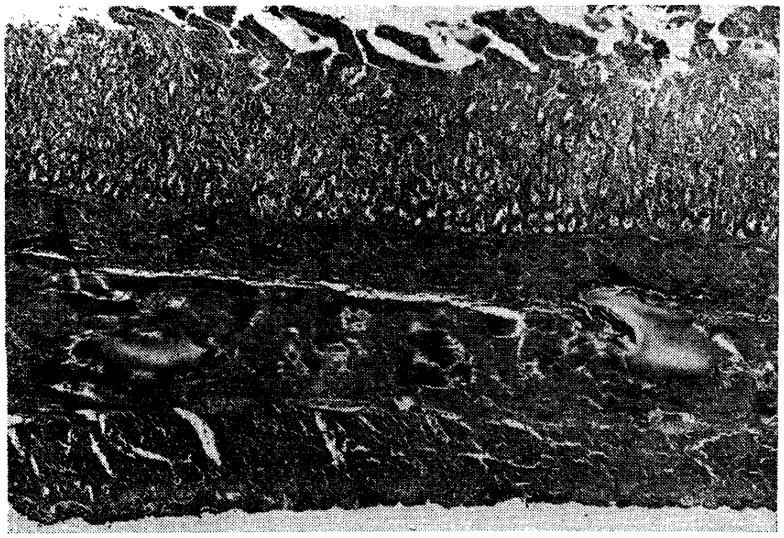
Small intestine of Dog No. 4, seven days after transplantation (magnification × 18). Note congestion, edema and superficial slough.
Fig. 12.
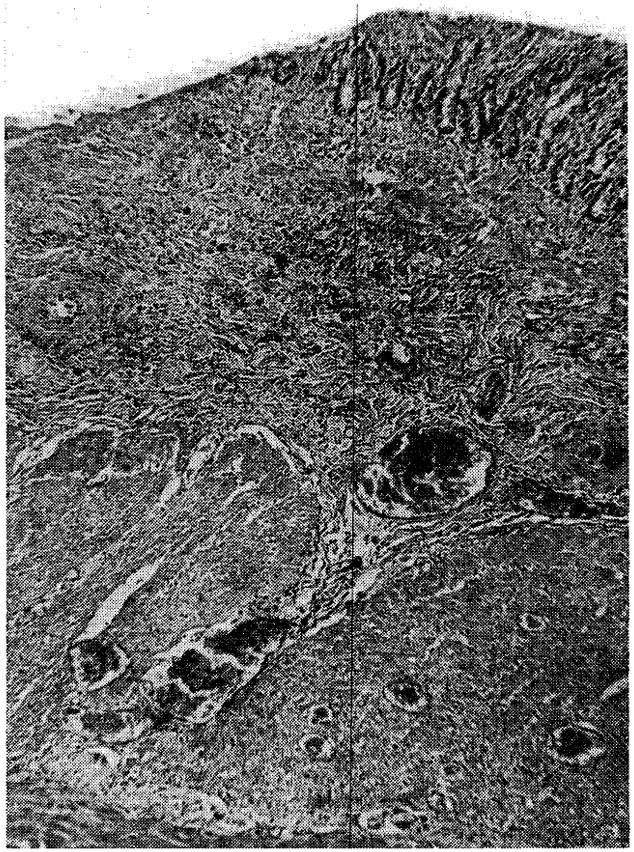
Duodenal ulcer in Dog No. 18, after nine days (magnification × 25).
Histologic Findings in Recipient Tissues
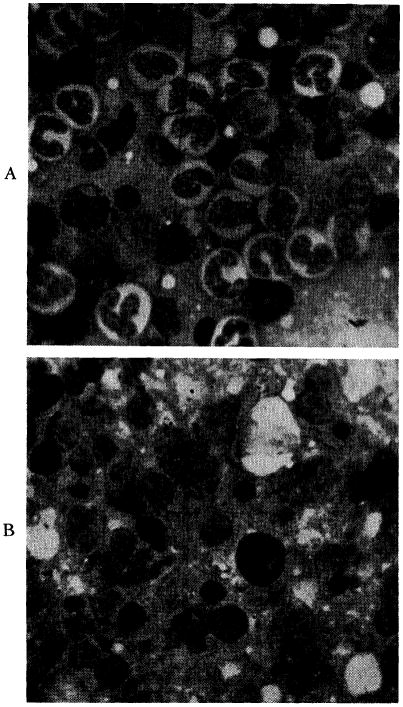
Marrow examinations revealed cellular specimens with striking abnormalities. (Fig. 13.) Normal myelopoiesis was largely replaced by relative and absolute increases in plasma cells, lymphocytes and reticulum cells. Plasma cells comprised approximately 15 to 20 per cent of all nucleated cells. In addition to the marked decrease in erythropoiesis and granulopoiesis, megakaryocytes although present were relatively infrequent.
Fig. 13.
A, bone marrow from normal dog showing active granulopoiesis and erythropoiesis (magnification × 900). B, marrow from Dog No. 26, showing a cellular specimen with extensive replacement of normal myeloid elements by a relative and absolute increase in lymphocytes, reticulum cells and plasma cells (magnification × 900).
Pulmonary edema was present in all animals. (Fig. 14.) Proliferation and thickening of the alveolar septa were present. In one animal, alveolar thickening was marked (Fig. 14), and multinucleated cells resembling megakaryocytes were seen. The kidneys showed no perivascular infiltration of mononuclear cells. However, there were occasional plasma cell aggregates in the perirenal and periadrenal tissues. There were focal myocardial infarcts with varying degrees of organization.
Fig. 14.
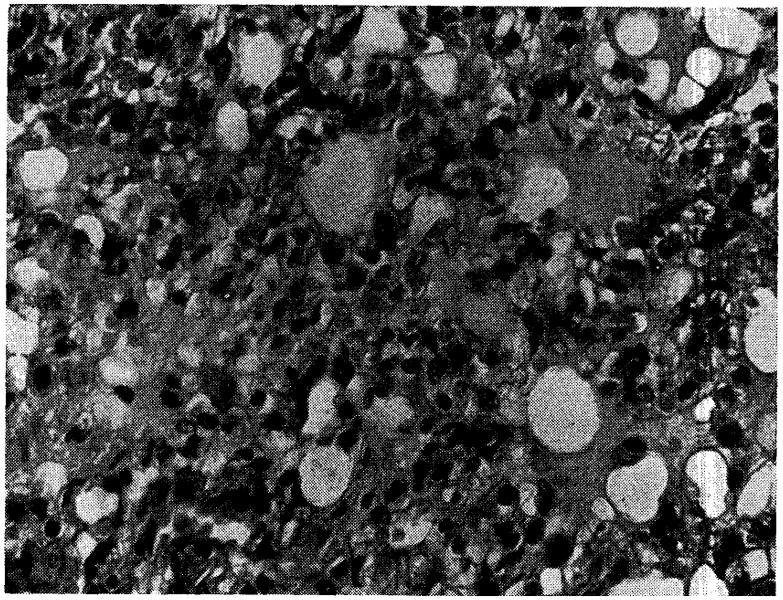
Lung from Dog No. 18, nine days after visceral transplantation (magnification × 350). Note pulmonary edema and proliferative thickening of alveolar septa.
Recipient nodes from the mediastinum showed thinning of the cortex and a decreased number or total absence of follicles. (Fig. 15.) The nodes contained increased numbers of plasma and reticulum cells, often atypical. (Fig. 15B.) Skeletal muscle appeared normal.
Fig. 15.
Recipient lymph node from mediastinum of Dog No. 36 after six days. A, magnification × 30; B, magnification × 600.
COMMENTS
Previous studies in several laboratories have clarified the behavior of homografts of the individual organs which make up the complex graft used in the present study. The bowel [6], liver [8,11] and spleen [8] are usually rejected in five to ten days. The technical problems of transferring the multivisceral graft are less than with transplantation of the individual organs. Only three vascular anastomoses are involved, and these are of large caliber vessels. Despite this, the rate of success in obtaining dogs for long-term study was only five in thirty-eight, or 13 per cent.
In the failures, the usual cause of death was congestion and hemorrhage in the intestinal tract. The bowel congestion was apparently not due to acute portal hypertension, because the addition of portacaval shunts did not prevent its development. Similarly, the congestion could not be explained by excessive ischemia. Lillehei et al. [6] have shown that segments of the bowel can tolerate three or four times more ischemia than was inflicted under the conditions of these experiments.
The failures may be explained by the denervation of the graft. The neuroanatomic state of the graft is depicted in Figure 16. All preganglionic fibers of the parasympathetic system were severed. The sympathetic ganglionic neurons were transported with the graft (Fig. 16) but were separated from all connections with the central nervous system. Popielski [9], Berger and Lium [1,7] and Lillehei and Wangensteen [5] have studied the effects of sympathetic denervation of the bowel in dogs. After extirpation of the coeliac and mesenteric ganglia, mucous or bloody diarrhea commonly developed. The bowel became edematous and hyperemic with mucosal and submucosal petechial hemorrhages and slough. These changes are comparable to those seen in the present study. An additional contributory factor may have been the obstruction to outflow of lymph in the graft.
Fig. 16.
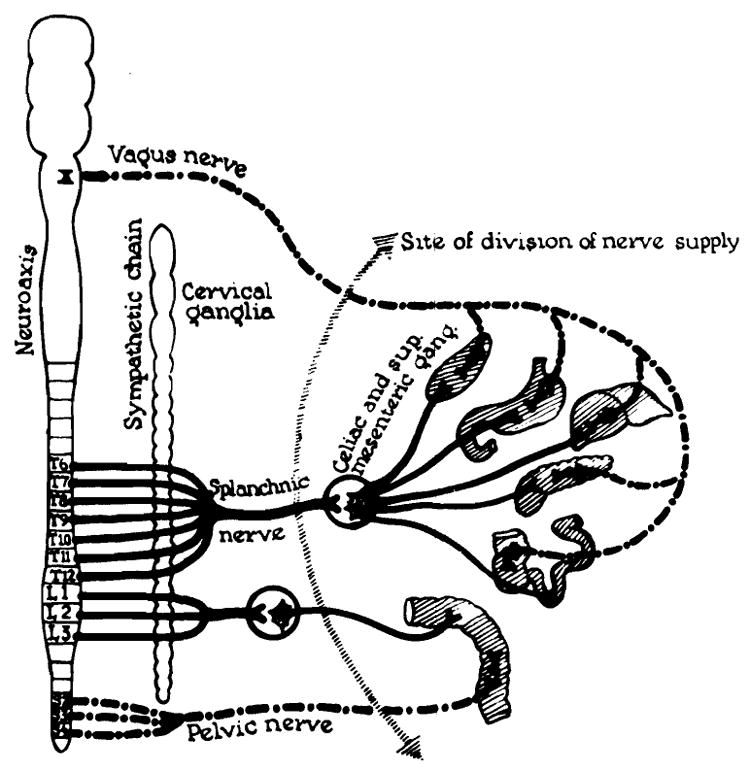
State of denervation of multiple organ graft.
The behavior of the liver in the present studies is of interest when compared with the fate of single whole organ hepatic homografts as studied by Goodrich [3], Moore [8], Starzl [11] and their associates. In both types of experiment, most of the dogs which survived surgery lived for five to ten days. The events leading to death were not, however, entirely similar. When the liver was transplanted alone, bile production ceased after four or five days and, in our experience, jaundice invariably developed by the fifth or sixth day. In most cases, death was thought to be due to cessation of function of the graft. In three of the multiorgan grafts, a similar pattern of early jaundice was seen. In the other two animals, however, jaundice did not develop during the nine days of survival. Urobilinogen persisted in the urine of these two dogs.
Some differences in the histologic appearance of the two kinds of liver homografts were also noted. The liver, when transplanted alone, was in our experience [11] commonly the site of extensive parenchymal destruction at the time of death. In every case, periportal aggregates of mononuclear cells were prominent. As part of the multiorgan homograft, hepatic parenchymal destruction was extensive in only one animal. Mononuclear cell aggregates in the periportal area were usually not prominent, and in the dogs in which jaundice did not develop, this histologic feature was virtually nonexistent.
The response of the host to the presence of the multiple organ graft also differed in some respects from the host reaction to the single organ liver homograft. After homografts of the liver, pulmonary edema occurred uncommonly [11]. Prompt and sustained leukocytosis developed. Despite cortical thinning and an increased number of plasma cells, the recipient lymph nodes retained a recognizable architecture [11]. In dogs with the multiple organ graft, pulmonary edema of such severity developed eventually that it may have been the direct cause of death in every case. The majority of these dogs ultimately had decreases in the white blood cell count. The increases in bone marrow plasma and reticulum cells were more striking, and there was apparent suppression of normal bone marrow activity. Finally, the architecture of the recipient lymph nodes was altered markedly. A definite cortex could rarely be identified, and the follicles were entirely absent in some dogs.
The difficulty of evaluating the inter-reaction between immunologically competent host and graft tissues has been emphasized by Billingham [2]. Histologic changes in donor or recipient organs could represent activity by either host or recipient tissues or both. Despite this limitation in the interpretation of data, there is evidence that the relation to the host of the multiple organ graft is quantitatively different than that of the single organ liver graft. The greater degree of structural and functional preservation of the liver in the multiple organ graft suggests mitigation of the rejection process.
Conversely, evidence for a graft versus host rejection response is stronger in the recipients of multiple organs than in those receiving the liver alone. In animals receiving the liver alone, there was no evidence of functional deterioration of any of the host organ systems. After multiple organ grafts, there was evidence of host organ failure. Examples included suppression of bone marrow activity and the invariable development of pulmonary edema. However, the precise roles of graft and host tissues in the production of these changes cannot be ascertained from our data. Evaluation of the extent of host versus graft and graft versus host reactions will depend on studies in which either the host or the graft is rendered immunologically incompetent by radiation or other means.
SUMMARY
It was technically possible to perform simultaneous homotransplantation of multiple visceral organs including the liver, spleen, pancreas, omentum and the entire gastrointestinal tract. Arterialization of the cooled graft was accomplished through the donor aorta which was removed with the graft and attached to that of the recipient dog. Gastrointestinal hemorrhage after surgery accounted for a high operative mortality and was thought to be due to denervation of the graft.
The five dogs which survived the immediate trauma of surgery lived for five and a half to nine days. After the second day, these animals were physically active and able to resume oral alimentation. In three dogs, there was metabolic evidence of rejection of the liver. In two others, jaundice did not develop.
These observations were compared with chemical, hematologic and pathologic data obtained in previous experiments involving homotransplantation of the liver alone. In some cases, there was less evidence of host versus graft rejection after the multiple organ transplants. Other data in the present study suggested the possibility that a significant graft versus host reaction may have been an important contributory cause of death.
Acknowledgments
This work was aided by a grant from the American Heart Association and by Grant A–3176 from the United States Public Health Service, National Institutes of Health, Bethesda, Maryland.
References
- 1.Berger RL, Lium R. Abdominal post-ganglionic sympathectomy: a method for the production of an ulcerative colitis-like state in dogs. Ann Surg. 1960;152:266. doi: 10.1097/00000658-196008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billingham RE. Reactions of grafts against their hosts. Science. 1959;130:947. doi: 10.1126/science.130.3381.947. [DOI] [PubMed] [Google Scholar]
- 3.Goodrich EO, Welch HF, Nelson JA, Beecher TS, Welch CS. Homotransplantation of the canine liver. Surgery. 1956;39:244. [PubMed] [Google Scholar]
- 4.Kaupp HA, Jr, Starzl TE. The use of an external by-pass during experimental total hepatectomy. Surgery. 1960;48:330. [PMC free article] [PubMed] [Google Scholar]
- 5.Lillehei CW, Wangensteen OH. Effect of celiac ganglionectomy upon experimental peptic ulcer formation. Proc Soc Exper Biol & Med. 1948;68:369. doi: 10.3181/00379727-68-16489. [DOI] [PubMed] [Google Scholar]
- 6.Lillihei RC, Goot B, Miller FA. The physiologic response of the small bowel of the dog to ischemia including prolonged in vitro preservation of the bowel with successful replacement and survival. Ann Surg. 1959;150:543. doi: 10.1097/00000658-195910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lium R. Peptic ulcer and diarrhea following the removal of prevertebral ganglia in dogs. Surgery. 1941;9:538. [Google Scholar]
- 8.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 9.Popielski L. Zur Physiologie des Plexus Coeliacus. Arcb f Anat u Physiol. 1903:338. [Google Scholar]
- 10.Starzl TE, Kaupp HA, Jr, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynec & Obst. in press. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Kaupp HA, Jr, Brock DR, Linman JW. Studies on the rejection of the transplanted homologous dog liver. Surg Gynec & Obst. in press. [PMC free article] [PubMed] [Google Scholar]