Abstract
Direct cardiac stimulation was conducted in the open chest. In normal animals, auricular stimulation at frequencies faster than the spontaneous rate caused little change in vascular pressures or cardiac output. Comparable ventricular stimulation in the same animals caused falls in cardiac output and blood pressure, with elevations in venous pressure. In contrast, ventricular stimulation in animals with complete heart block caused elevations in cardiac output and blood pressure, and declines in venous pressure. A study was also made of repetitive stimulation in experimental cardiac arrest. Occasionally pacemaking was of value in the resuscitation, but in most cases effective contractions could not be induced with stimulation.
Repetitive electric stimulation of the animal heart for investigative purposes has been standard laboratory procedure for many decades. The interest of clinicians has been sporadically directed to electric pacemaking as a tool in the treatment, of clinical cardiac arrest since it could be, in theory, a means of dealing with this disastrous situation by meeting a specific need with a specific therapeutic provision. Several reports are available, concerned with the evaluation of pacemaker cardiac resuscitation under various conditions, in experimental animals3–7 and in man.1,2
With increasing interest in this form of therapy, it becomes desirable to obtain a more precise understanding of the potentialities and limitations of cardiac stimulation, as well as a more definitive understanding of the type of arrest in which its use will give a reasonable hope of beneficial effect. The present study was undertaken with these objectives. During the performance of the experiments, data were obtained which may have wider application relevant to some of the physiological mechanisms involved in serial auricular and ventricular extrasystoles.
General Methods
Sixty-six mongrel dogs were used, ranging in weight from 7 to 20 Kg, and satisfactory technical conditions were realized in 51 experiments. Twenty-three to 30 mg. of Nembutal per kilogram were given intravenously, tracheostomy was performed, and the animal placed on a positive pressure respirator (supplied with 100 per cent oxygen in experiments where cardiac outputs were measured). Cardiac stimulation was usually conducted through a left thoracotomy in the fifth intercostal space. In experiments requiring an auriculoventricular block, this procedure was done through a right thoracotomy in the fourth intercostal space: the incision was closed with water seal drainage, and at least an hour was allowed to pass before reopening the chest for pacemaking.
In cases where it was desirable, cardiac output was obtained by the dye dilution method of Stewards9 and Hamilton.10, 11 A repeatedy calibrated photometer was employed which continuously measured the dye concentration of flowing arterial blood.12 Standard limb electrocardiogram, blue-dye cardiac output, and arterial and venous pressures were recorded on a multichannel direct writing oscillograph. Vascular pressures were detected with inductance type transducers, the natural frequency of the measuring and recording systems being greater than 30 cycles per second, when tested by recording pressure within a balloon during explosion.
Bipolar concentric electrodes were used for stimulation. These consisted of an insulated stainless steel syringe tubing, which contained an insulated stainless steel wire, the enamel at the tips of both components being filed off. Polar distance was 1 to 3 mm., and electrode resistance in position in the heart was 5000 to 8000 ohms. Current source was a Goodwin condensor stimulator. Three to 5 volts were routinely used, with a 1 millisecond falling phase. The stimulating electrode was placed in position like a dart so that both poles were in the myocardium.
Blood loss from surgical procedures, or tests, was replaced with plasma expander or whole blood, as soon as possible after the exact moment of loss. Specific details of methods of creating arrest, or alterations in the above technique, are given under the appropriate section.
Results
Repetitive Auricular and Ventricular Stimulation in Animals with an Intact Conduction System
Stimulation was conducted in seven animals. Cardiac output, electrocardiogram, arterial blood pressure, and, in most cases, femoral venous pressure were studied. Stimulation was at 10 to 70 impulses per minute faster than the prevailing spontaneous rate. It was not possible to drive the heart slower than the spontaneous beat. Records were obtained first with the spontaneous rhythm, then with ventricular stimulation at different locations, then with auricular stimulation, and finally after cessation of stimulation. In any given series (fig. 1A, B) auricular and ventricular pacemaking was conducted at the same rate. The entire sequence was usually performed in 8 to 10 minutes. In no case did the stimulation produce ventricular fibrillation.
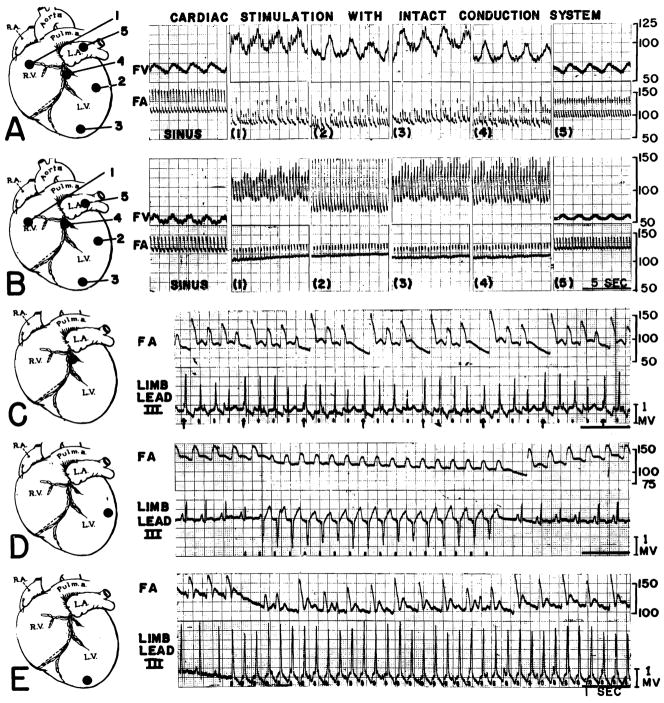
Fig. 1. Repetitive stimulation of intact heart with 3 to 5 volts. Stimulation sites are indicated by black circles on the insets. Femoral venous (FV) and arterial (FA) calibrations are in millimeters water and mercury respectively.
A. Ventricular (1–4) and left atrial (5) stimulation at 240 per minute. Sinus rate (at left) was 185. Note similarity of effect on venous arid arterial pressures irrespective of the ventricular stimulation site.
B. Variation of the effects noted in A. Sinus rate was 160, and stimulation at 190 per minute.
C. Alteration of ventricular driving pattern, by persistance of active sinus mechanism. Sinus rate was 154 and stimulation 190 per minute.
D. More complete control with ventricular stimulation. Sinus rate was 148 and stimulation 190 per minute.
E. Alternating pulse obtained with 240 per minute stimulation. Sinus rate was 150 per minute.
Repetitive stimulation of the auricles, irrespective of site, produced a total picture comparable to that occurring with sinus control, but at a faster rate. The electrocardiogram was easily controlled, with cessation of spontaneously occurring P waves, and unchanged QRS complexes. The pulse was regular, and mean blood pressure was maintained with a slight fall in pulse pressure (fig. 1 A5, B5). The configuration of the venous pressure was usually unchanged (fig. 1 A5, B5). The cardiac output (fig. 2) usually remained essentially unchanged, with a consequent reduction in stroke volume.
Fig. 2.
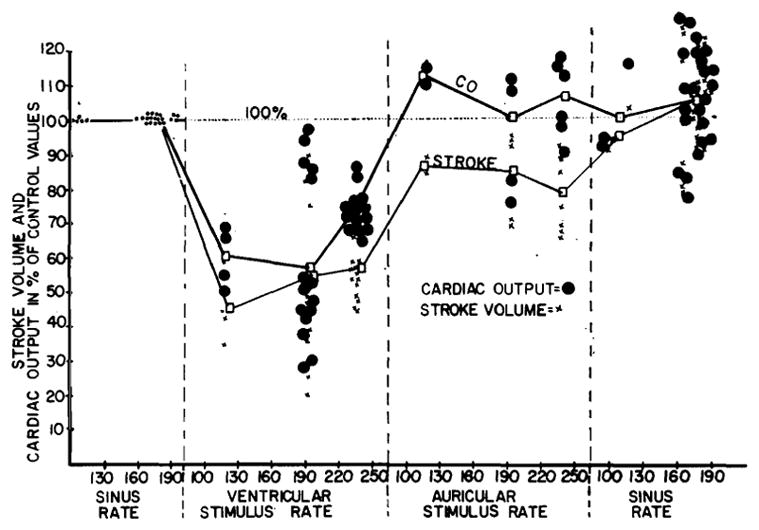
The effect of left auricular and ventricular repetitive stimulation upon cardiac output and stroke volume in intact heart, expressed as changed from the initial sinus values. Ventricular driving in a given animal yielded similar results irrespective of stimulation site.
In contrast to the minor changes induced by auricular driving, repetitive ventricular stimulation precipitated marked alterations in cardiocirculatory function. Electrical events of the cardiogram could rarely be totally controlled. In the majority of cases P waves continued to appear (fig. 1C, D, E), often at their original rate (C, E). The difficulty, in the normal animal, of completely dominating auricular rhythm by retrograde conduction from the ventricle has been recognized before.13, 14 The form of the artificially produced QRS complexes depended upon the site of stimulation, and was in conformity with known rules of electrocardiographic localization.15 However, details of QRS duration, amplitude, and timing were subject to considerable variation, a variation which often was obviously related to the independently continuing rhythm. Thus in figure 1C, in which four P waves corresponded to every five driven ventricular beats, shifts in the duration, amplitude, and time of onset of the QRS complexes occurred in cycles of four with resultant inconstancy of the period of diastolic filling. That this electrical shift related to the effectiveness of the individual myocardial contractions is evident from the record of the simultaneous arterial pressure (fig. 1C), in which the strength of the pulses also varied in cycles of four beats. In the case shown, the strongest mechanical beats occurred, after the periods of longest diastole, when the ventricular stimulus came just after the P wave (indicated with arrows in the figure). The weakest pulses were found when the ventricular stimulus was delivered after the shortest diastole (fig. 1C), approximately where the auricular driven QRS would have occurred. In all cases in which analyzable auricular interference of this sort was encountered, the decisive factor in determining the strength of the beat was not the source of excitation, whether it be spontaneous auricular or artificial ventricular. Of critical importance, however, was the element of timing in ventricular excitation as it related to the duration of diastole.
During ventricular stimulation, marked changes were detected in vascular pressures. Large falls in blood pressure were recorded, irrespective of whether the driven pulse was regular (fig. 1B) or irregular (fig. 1A), the latter being more common. Moderate or marked elevations in venous pressure were found (fig. 1A, B), often with the development of bizarre huge venous pulses with each electrically driven beat (fig. 1B).
Ventricular driving with an intact conduction system caused a reduction in the cardiac output and stroke volume which ranged from a small decline to a precipitous drop (fig. 2).
The foregoing data indicates that repetitive auricular stimulation in normal animals exerts a basically different influence on cardiac function than comparable ventricular stimulation. Pacemaking of any portion of the auricles caused minor alterations of function which were explicable solely in terms of change of rate. Comparable ventricular stimulation at different sites uniformly produced injurious changes, with incomplete control of electrocardiographic pattern, irregular pulse, decline of blood pressure, rise in venous pressure, and fall in cardiac output and stroke volume. It is thought that the difference is due chiefly to double ventricular excitation during ventricular pacemaking, through the normal auriculoventricular pathways and from the ventricular stimulation site.
Repetitive ventricular Stimulation in Animals with Acute Complete Heart Block
In animals with acute complete heart block the occurrence, during stimulation, of dual excitation of the ventricles from competing auricular and ventricular sources is eliminated. Repetitive ventricular stimulation was carried out in seven animals in which a complete heart block had been established 60 to 90 minutes previously. Rates in this group ranged from 24 to 45 per minute. It was not possible to drive the heart at a rate slower than the spontaneous rhythm. Auricular pacemaking was of course without effect on the ventricular pattern. After studies were obtained at the idioventricular rate, ventricular stimulation was performed at various frequencies and this was followed by a final control study at the cessation of stimulation. There were no instances of ventricular fibrination precipitated by the electrical pacemaking.
In a given animal, stimulation at different points on both ventricles yielded similar results except for the predictable differences in the electrocardiogram (fig. 3). With the onset of pacemaking, electrical events of the ventricle were instantly and easily brought under complete control, the driven QRS complexes being uniform in appearance and timing with no tendency for spontaneous ventricular discharges (fig. 3). The ventricles relinquished their automaticity to such an extent that, after cessation of stimulation, it often required many seconds before the ventricles again took up their original spontaneous rate (fig. 3D). During ventricular stimulation, the P waves continued at their original rate (fig. 3), indicating absence of retrograde conduction.
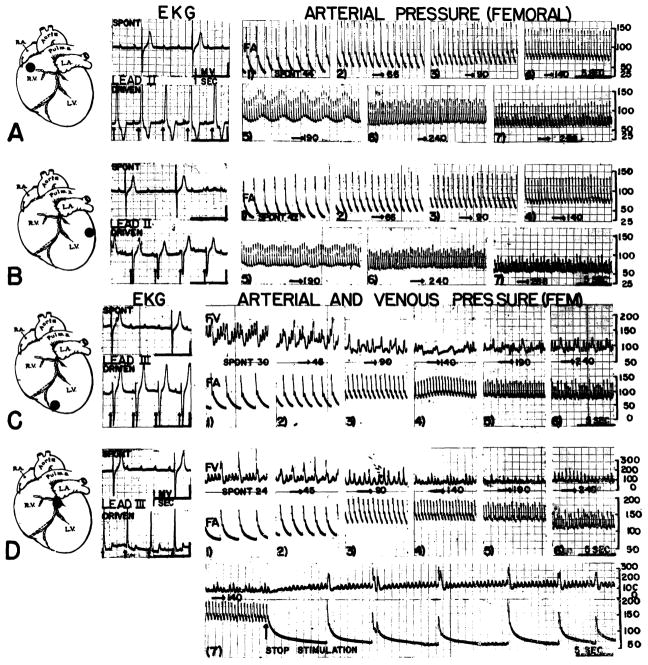
Fig. 3.
Effect of stimulation at varying rates and at different ventricular sites in animals with complete heart block. In each case locus of stimulation is marked in the inset, spontaneous and driven electrocardiograms shown on the left, and arterial pressure (FA) recordings on the right Femoral venous (FV) records are also included in C–D. Note marked slowing of pulse rate at cessation of stimulation in D. Arterial and venous calibrations are in mm mercury and water respectively. Voltages were 3 to 5.
In contrast to the irregular arterial pulse often produced by ventricular stimulation in the intact animal. regular pulses could be driven to the rate of 190 and frequently to 240 per minute (fig. 3A–D). In general, the systolic blood pressure remained at about the same level as before, with marked elevation of the diastolic and mean pressures (fig. 3A–C), the optimal mean blood pressure generally being with stimulation at 144 per minute. When starting with very slow spontaneous rates, however, both systolic and diastolic pressures were increased (fig. 3D), by ventricular pacemaking at faster rates.
Venous pressure regularly declined until the stimulus frequency of 190 per minute was exceeded, beyond which it rose again (fig. 3C, D). Similar central venous pressure falls were recorded from the right auricle in two animals.
It has been shown16 under conditions similar to those prevailing in the present study, that cardiac output is diminished in animals with heart block because of a reduction in rate so extreme that a complete stroke volume adjustment is not possible. In the presence of such a low output due to bradycardia, ventricular stimulation at faster frequencies increased the cardiac output (fig. 4). The output gains were most striking when the spontaneous rates were slowest. Starting with idioventricular rates of 24 to 27 per minute, the cardiac output was increased an average of almost 400 per cent with ventricular pacemaking at faster rates. With initial rates of 30 to 33 per minute the gain in output was more than 200 per cent. With initial rates of 42 to 45 per minute, the output rose 100 per cent. Although the pre-existing idioventricular rate determined the magnitude of gain, the pattern of cardiac output rise was the same starting with all rates from 24 to 45 per minute. Almost all the output augmentation occurred by the time the stimulus frequency reached 90 per minute, with an additional small rise to the stimulus frequency of 144 or 190 per minute (fig. 4). Further increments in stimulus rates caused declines in the cardiac output.
Fig. 4.
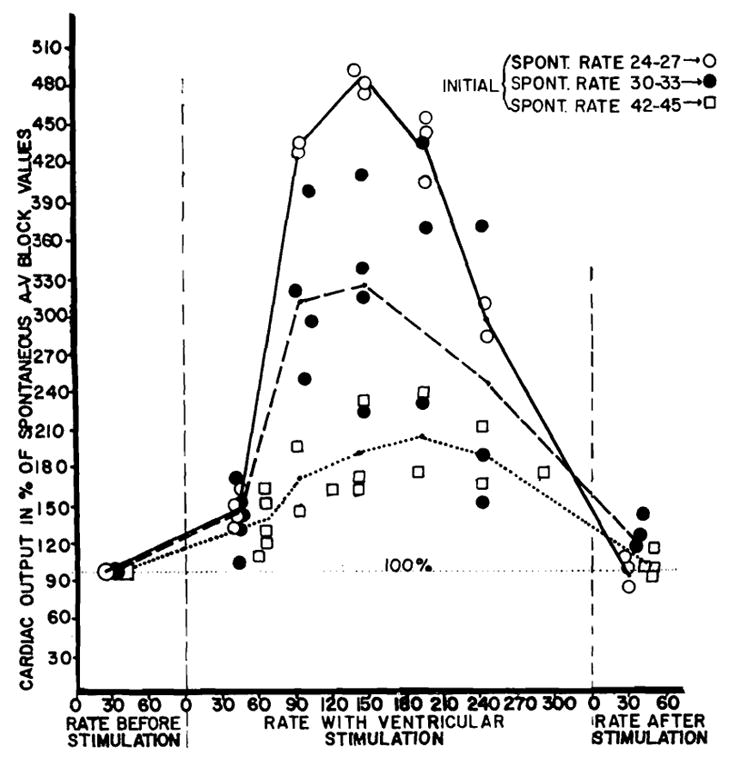
Effect of ventricular stimulation at different rates on cardiac output of dogs with acute complete heart block, expressed in per cent of initial spontaneous idioventricular value. Note how the spontaneous rate determined the magnitude of increase.
The mechanism of the beneficial effect of ventricular pacemaking on the cardiac output in these cases is evident from fig. 5. There is a definite tendency, as the rate is artificially increased by stimulation, for the stroke volume to remain fixed until the rate of 90 is attained, above which the stroke volume progressively diminishes. Accordingly, cardiac output rises linearly with the rate up to a stimulus frequency of 90 per minute.
Fig. 5.
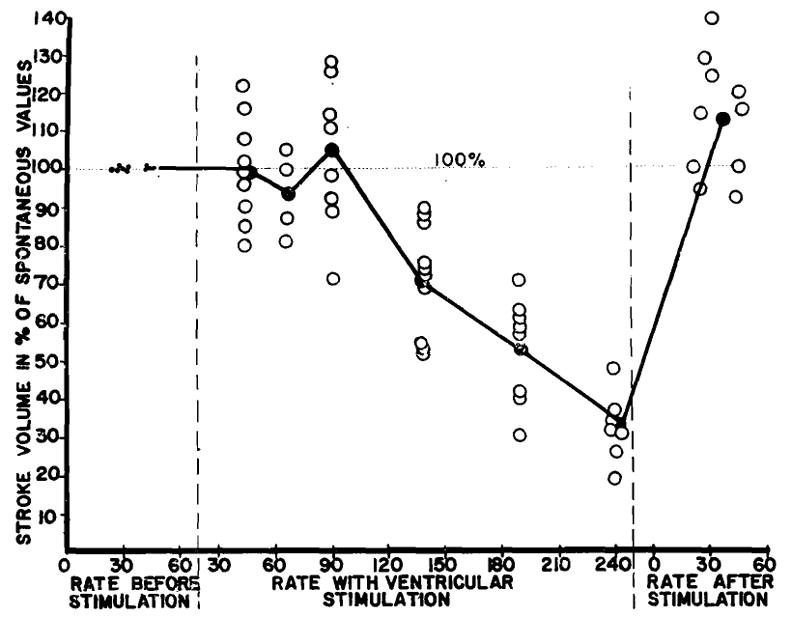
Effect of ventricular stimulation at different rates on stroke volume of dogs with acute complete heart block, expressed in per cent of initial spontaneous value. Note tendency of stroke to be fixed up to the rate of 90 per minute.
In summary, the cardiovascular status of dogs with acute heart block is brought by ventricular driving from a low to a higher level of effective function, as judged by measures of vascular pressures and cardiac output. In contrast to ventricular pacemaking in animals with an intact conduction system, the rate and electrocardiographic patterns are easily controlled and regular arterial pulses are induced to frequencies of 190 to 240 per minute.
Repetitive Ventricular Stimulation in Animals with Acute Complete Heart Block in Shock
A controlled shock state established in three dogs with acute heart block by permitting the animals to bleed from an arterial cannula into a reservoir suspended at a height calculated to give the desired blood pressure. Fifteen to 30 minutes were allowed for equilibration. Experiments were done with systolic pressure levels of 90,60, and 30 mm. Hg. Idioventricular rates in these cases were 30, 39, and 21 per minute with cardiac outputs of 50, 40, and 20 cc per kilogram, respectively. In the first two cases stimulation at faster rates did not affect the greatly reduced cardiac output, and in the last case caused a 30 per cent fall.
Repetitive Ventricular Stimulation in Animals with Experimental Cardiac Arrest
Cardiac arrest, with absent recordable pulse, or with a heart rate of less than 10 per minute, was created 45 times in 34 animals. Thoracotomy had been previously performed. Stimulation was accomplished as described elsewhere in this study. In all the cases included in this report in which electrical pacemaking was tested, stimulation was started in the absence of ventricular fibrillation. Because of the poor condition of the heart, fibrillation frequently began spontaneously, with stimulation or with minimal handling of the heart. It was our impression that fibrillation was somewhat less likely to be precipitated by stimulation than by massage.
Acute asphyxia, accomplished by clamping the endotracheal tube, was used to produce arrest 12 times in 8 animals under light Nembutal anesthesia. After the animals had been pulseless for at least two minutes, they were placed back on the respirator, and stimulation was started. The pattern of arrest in general followed that illustrated in figure 6C. In the example shown, effective myocardial contractions, as evidenced by absence of recordable arterial, pulses, were abolished after four and one half minutes of asphyxia. With inspection of the heart, contractions were virtually indiscernible. Sinus driven QRS complexes, however, continued for eight more minutes until the animal fibrillated after 13 minutes of acute anoxia (fig. 6C). In none of these animals was there electrocardiographic evidence of an absolute conduction failure until fibrillation began terminally. Repetitive ventricular stimulation, before the onset of fibrillation, did not alter the existing course in a single case. After stimulation had failed, the application of massage, appropriate drug therapy, and defibrillation (when necessary) was successful in reviving the animals in 6 of the 12 arrests.
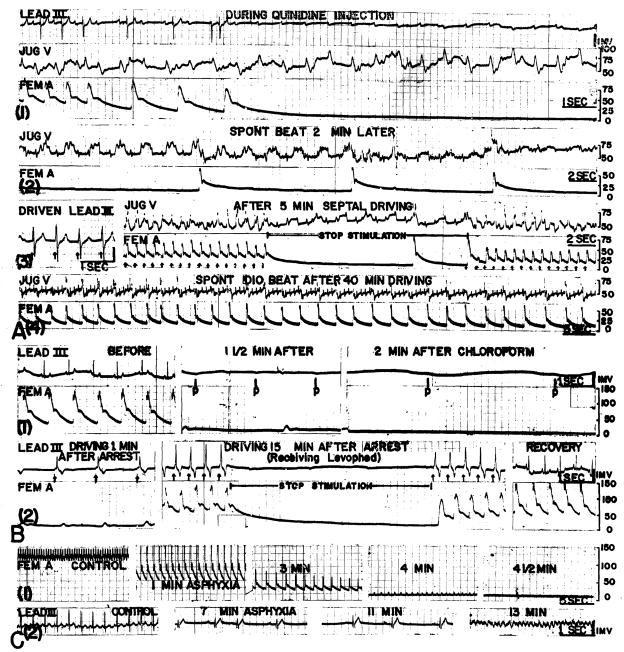
Fig. 6. Experimental cardiac arrest. Electrocardiogram, jugular venous (Jug V) and/or femoral arterial (Fem A) pressures are included and calibrated in millimeters water or mercury respectively.
A. Animal had received 21 mg per kilogram 15 minutes before with consequent hypotension. Additional 21 mg per kilogram injected as indicated. Ventricular driving at 90 per minute, 5 volts.
B. Arrest with endotracheal chloroform. Ventricular driving at 5 volts, 50 or 140 per minute.
C. Arrest with asphyxia, showing persistance of sinus driven electrocardiogram (2) long after blood pressure was not recordable (1).
Using chloroform inhalation, or chloroform with basal Nembutal anesthesia, 20 arrests were produced in 14 animals. After the animals had been pulseless for at least two minutes, the respirator was changed to air and stimulation started. In 17 cases, the type of arrest was similar to that described with asphyxia, with electrocardiographic evidence of a functional conduction mechanism long after the blood pressure had approached 0. Ventricular pacemaking did not alter the course in any of these instances. In 9 of the 17 arrests, massage was instituted after stimulation had failed, and recovery accomplished five times. The remaining three arrests were of a different type, and were characterized by a sudden unexpected complete heart block and ventricular asystole immediately after a time when arterial pulses were still present (fig. 6B). P waves continued, but there was no electrocardiographic indication of ventricular activity. In this state, ventricular contractions could be induced by mechanically stimulating the ventricle with the tip of the finger. Ventricular pacemaking in these three cases induced contractions of approximately the same vigor as had been present just before the standstill, as gauged from the arterial pulse (fig. 6B). Pacemaking alone did not, however, ensure recovery, for although contractions were being initiated, they mere feeble, as gauged from the low blood pressure (B-2). The use of diluted intravenous levarterenol bitartrate, in addition to stimulation, resulted in the restoration of a normal blood pressure (B-2) in all three instances. During the maintenance of beats with ventricular pacemaking, the stimulator was turned off at intervals. When complete heart block with ventricular asystole persisted during these test periods (B-2), P waves were the only electrical activity in the electrocardiogram, and arterial pulses promptly ceased In these three dogs, it was necessary to maintain the cardiac rhythm with the pacemaker for 5 to 20 minutes before a spontaneous beat was reestablished.
Sixteen to 64 mg. per kilogram of intravenous quinidine gluconate was given to 12 dogs, 6 of which had had an acute complete heart block established surgically a few minutes before. Nine of the dogs showed a fall of blood pressure with continuing QRS complexes in the electrocardiogram. As with similar cases in the other two forms of arrest, ventricular stimulation did not in any way change the course. All died despite pacemaking attempts. In the other three animals, one of which had a previous surgical heart block, sudden complete ventricular asystole occurred (fig. 6A), with an isoelectric electrocardiogram except for P waves. Within a few seconds an extreme ventricular bradycardia developed and stabilized at 5 to 10 per minute (fig. 6A-2). After a minute or two pulse vigour began to deteriorate. It was possible to provoke additional ventricular contractions at this time by poking the ventricles with the tip of a finger. In these three animals, ventricular pacemaking at 50 to 100 per minute was conducted for 15 to 60 minutes before the hearts established sufficient automaticity to maintain life, sustaining rates (A-4) of 24 to 39 per minute. Frequent tests were made (A-3) to determine if the heart could initiate its own beats and when this occurred, the stimulator was permanently turned off.
It is self–evident that the only condition in which an electrical pacemaking needle could aid in cardiac resuscitation would be a primary defect in the initiation of ventricular excitation. The pacemaking current as used in this study has no potentiality beyond depolarizing a trigger zone of myocardium from which the wave of excitation might spread, causing general myocardial contraction. The ideal experimental environment for the use of such a pacemaking instrument would be complete ventricular asystole with a relatively normal myocardium. In our hands, as indicated, these conditions were imperfectly realized in only 6 of 45 cases of cardiac arrest. In this small group repetitive ventricular stimulation exerted a beneficial effect.
The most impressive feature of the other 39 arrests was the tenacity with which organized ventricular electrical activity persisted long after effective or even easily discernible myocardial contractions had ceased. It would have been surprising if repetitive stimulation of such hearts could exert any helpful therapeutic effect, for it merely furnishes what is already present, namely a pacemaking mechanism. Under such circumstances, the only hope for survival lies in the standard measures which are aimed at restoring myocardial contractility—massage and appropriate drug therapy.
Discussion
The complete heart block preparation affords unusual conditions for studying ventricular function during ectopic ventricular stimulation. It was observed long ago17, 18, and in the present study as well, that serial ectopic ventricular beats caused inhibition of spontaneous impulse initiation in animals with idioventricular rhythm. It is thus possible, by stimulating the ventricle at faster than existing rates, to usurp and retain absolute control of the heart without interference from spontaneous impulses such as were demonstrated when stimulating the ventricles in the face of an intact conduction system.
The effect of such controlled rate augmentation in animals with complete heart block deserves comment. The changes in cardiac output with ventricular stimulation are essentially a reversal of the changes demonstrated in recent work on the physiology of acute heart block in dogs. It was shown16 that reduction of rate down to 90 per minute was without significant effect on cardiac output because of larger stroke volumes at the slower rates. However, when the rate fell below 90 per minute, increases of stroke volume ceased and the cardiac output was reduced roughly as the fraction expressed by the ratio of idioventricular rate divided by 90.
In a recent study by Sarnoff and Berglund19 concerned with in vivo analysis of Starling’s law in dogs with a normal myocardium, it was disclosed that no descending limb could be demonstrated in the curves drawn by plotting auricular pressures against stroke work (Starling curves). With controlled elevation of auricular pressure by fluid infusion, a point was reached beyond which further elevation of auricular pressure produced neither a rise nor fall in stroke work. It is thought that under the conditions of the present study, a similar behavior is manifest. The central venous pressure is controlled in the present work by rate rather than by infusion and as the rates fall toward 90 both the venous pressure and stroke volume increase. With rates below 90 per minute, the heart is operating at maximum stroke volume on the flat portion of the Starling curve where further increases on central venous pressure are without effect on stroke work. Thus the cardiac output is placed under direct control of the rate between the range of the idioventricular rhythm and 90 per minute.
Based chiefly on work done by Wiggers,20 it has often been suggested that ectopic ventricular beats are inherently less effective than those in which excitation is through normal routes—a concept which derives some support from the fact that patients with ventricular tachycardia decompensate more frequently than patients with auricular tachycardia at comparable rates. Wiggers states21 that “this is due in part to a less perfect summation of fractionate contractions when the excitation waves spread from a ventricular focus, and, in part, to a less efficient mechanical twist of the ventricular muscle scrolls when the septum is not excited first”.
The information obtained in the present study does not comply with this concept. In a given animal with acute heart block, approximately the same changes in cardiac output and vascular pressures are obtained irrespective of stimulation sites and without regard to their proximity to the septum. With ventricular pacemaking, stroke output tends to remain the same as it had been during spontaneous idioventricular rhythm despite considerable speeding of the rate. In addition, it has been shown16 that if the ventricles are stimulated at the same rate as the heart had been beating before the block, full restoration of the cardiac output results. These findings, singly arid together, suggest that stimulation of any portion of the isolated ventricles provokes contractions comparable in effectiveness with those initiated via the normal pathways.
Auricular and ventricular pacemaking in animals with a normal conduction system of course allows a more analogous comparison to the clinical conditions of auricular and ventricular tachycardia, respectively. In such animals in the present study, the ventricular ectopic beats were shown to be highly ineffective in comparison with the contractions evoked with similar auricular stimulation. Evidence has been presented which indicates that this inefficiency of ectopic ventricular beats is due primarily to ventricular excitation from more than one source, rather than to an inherent inferiority of the excitation or contractile mechanism of ventricular extrasystoles.
This study of experimental cardiac arrest allows several observations about the use of pacemaking therapy in cardiac standstill. From a practical point of view, myocardial failure preceded cessation of conduction in more than 85 per cent of the cases, a finding which is in accord with older studies on the selective effects of anoxia on myocardial and conduction tissue.22 In this group, stimulation was of no value. In the remaining few arrests, there was an early complete heart block and an absence or limitation of ventricular impulse initiation. It should obviously have done no good to stimulate the auricles under these circumstances. Ventricular pacemaking was of assistance in the treatment of this group, but in half the cases had to be combined with drug therapy for recovery. It is thought that the clinical circumstances under which repetitive cardiac stimulation will be of benefit can be prognosticated from the behavior of these animals. Arrest or extreme bradycardia must have been sudden and preceded by reasonably good clinical condition. Pacemaking must be instituted immediately before the myocardium becomes unresponsive. If the client is open, it should be possible to evoke test beats with mechanical stimulation. One must be absolutely certain that a feeble sinus-governed beat does not exist, since it was shown that even in normal hearts ventricular stimulation in the face of an intact conduction system is injurious. It is thought that occasional cases which will respond to pacemaking can be recognized. To attempt pacemaking therapy in the absence of the characteristics outlined would, however, be wasteful of time that could be better spent in the application of standard methods of cardiac resuscitation.
Summary
A study of the effects of repetitive electrical cardiac stimulation was conducted in dogs with normal hearts, complete heart block, and experimental cardiac arrest. In intact animals, auricular stimulation at faster than spontaneous rates caused alterations in cardiac output and vascular pressure explicable solely in terms of rate change. Ventricular stimulation, under similar conditions, produced injurious effects explicable primarily on the basis of double excitation of the ventricles from continuing auricular and artificial ventricular sources.
In animals with acute heart block, cardiac output is diminished because of bradycardia so extreme that an adequate compensatory increase of stroke volume is not possible.16 Ventricular stimulation at faster rates raises the cardiocirculatory status from a low to a higher level of function as judged from measures of cardiac output and vascular pressures. In such blocked hearts, in which it is possible to have ventricular excitation solely from the stimulation site (without interference from spontaneously initiated beats), electrically induced beats compared favorably with those initiated through normal pathways.
Experimental cardiac arrest was created by asphyxia, injection of quinidine, and by chloroform inhalation to test the effectiveness of pacemaker stimulation in cardiac standstill. In more than 85 per cent of the cases, the myocardial contractility was lost long before failure of spontaneous initiation of electrical impulses. Pacemaking was valueless in the treatment of these animals. The remaining few animals developed an early complete heart block with ventricular asystole, at a time when the myocardium retained contractile potentiality. In this small group, repetitive ventricular stimulation was therapeutically useful.
Acknowledgments
Aided by a grant from the United States Public Health Service, National Institutes of Health, Bethesda, Maryland.
Footnotes
Presented at the fall meetings, American Physiology Society, Madison, Wisconsin, September, 1954.
References
- 1.Zoll PM. Resuscitation of the heart in ventricular standstill by external electric stimulation. New England J Med. 1952;247:768. doi: 10.1056/NEJM195211132472005. [DOI] [PubMed] [Google Scholar]
- 2.Zoll PM, Linenthal AJ, Normal LR. Treatment of Stokes-Adams disease by external electric stimulation of the heart. Circulation. 1954;9:482. doi: 10.1161/01.cir.9.4.482. [DOI] [PubMed] [Google Scholar]
- 3.Hyman AS. Resuscitation of the stopped heart by intracardial therapy. AMA Arch Int Med. 1932;50:283. [Google Scholar]
- 4.Hyman AS. Resuscitation of the stopped heart by intracardial therapy. IV. U S Navy M Bull. 1935;33:205. [Google Scholar]
- 5.Bigelow WG, Callaghan JC, Hopps JA. General hypothermia for intracardiac surgery. Ann Surg. 1950;132:531. doi: 10.1097/00000658-195009000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaghan JC, Bigelow WG. An electrical artificial pacemaker for standstill of the heart. Ann Surg. 1951;134:8. doi: 10.1097/00000658-195107000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herod CE, Lee RH, Goggans WH, McCombs RK, Gerbode F. Control of heart action by repetitive electrical stimuli. Ann Surg. 1952;136:510. doi: 10.1097/00000658-195209000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Gaertner RA, Kelley E. Chronic Heart Block in Dogs. A method for producing experimental heart failure. Circulation. doi: 10.1161/01.cir.12.2.259. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steward GN. The pulmonary circulation time, the quantity of blood in the lungs, and the output in the heart. Am J Physiol. 1921;58:20. [Google Scholar]
- 10.Kinsman JM, Moore JW, Hamilton WF. Studies on the circulation. I Injection method: physical and mathematical considerations. Am J Physiol. 1929;89:322. [Google Scholar]
- 11.Moore JW, Kinsman JM, Hamilton WF, Spurling RG. Studies on the circulation. II. Cardiac output determinations: Comparison of the injection method with the direct Fick procedure. Am J Physiol. 1929;89:331. [Google Scholar]
- 12.Friedlich A, Heimbecker R, Bing RJ. A device for continuous recording of concentration of Evans Blue Dye in whole blood and its application to the determination of cardiac output. J Appl Physiol. 1950;3:12. doi: 10.1152/jappl.1950.3.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Hukuhara T, Komita S. Pflanzt sich die Reizung der Kammer bei Säugetieren auf die Vorhofe fort? P Arch ges Physiol. 1938;241:444. [Google Scholar]
- 14.Lewis T, Oppenheimer BS. The influence of certain factors on asphyxial heart block. Quart J Med. 1911;4:145. [Google Scholar]
- 15.Einthoven W. Weiteres ueber das Elektrokardiogramm. P Arch ges Physiol. 1908;122:517. [Google Scholar]
- 16.Starzl TE, Gaertner RA, Baker RR. Acute heart block in dogs. Circulation. doi: 10.1161/01.cir.12.1.82. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis T, White PW, Meakins J. The effects of premature contractions in vagotomized dogs with especial reference to atrioventricular rhythm. Heart. 1914;5:335. [Google Scholar]
- 18.Miki Y, Rothberger CJ. Experimentelle untersuchungen ueber die Pause nach Vorhofextrasystolen. Ztschr ges exper Med. 1922;30:347. [Google Scholar]
- 19.Sarnoff SJ, Berglund E. Ventricular Function. I Starling’s law of the heart studied by means of simultaneous right and left ventricular function curves in the dog. Circulation. 1954;9:706. doi: 10.1161/01.cir.9.5.706. [DOI] [PubMed] [Google Scholar]
- 20.Wiggers CJ. The muscular reactions of the mammalian ventricles to artificial surface stimuli. Am J Physiol. 1925;73:346. [Google Scholar]
- 21.Wiggers CJ. Physiology in Health and Disease. 5. Philadelphia: Lea and Febiger; 1949. pp. 720–1. [Google Scholar]
- 22.Rothrerger CJ. Beiträge zur normalen und pathologischen Physiologie der spezifischen Herzmuskulatur. Cardiologia. 1937;1:234. [Google Scholar]
- 23.Scherf D, Schott A. Extrasystoles and allied arrhythmias. London: William Heinemann; 1953. [Google Scholar]