Abstract
Purpose
The expression of Nitric oxide Synthase (NOS) and aquaporin (AQP) water channels in rat bladder is recently reported. The aim of this study is to evaluate the expression of inducible NOS (iNOS), aquaporin-3 (AQP-3) in cyclophosphamide (CYP) induced rat bladder.
Materials and Methods
The 32 Sprague-Dawley rats were divided into cystitis group (n=20) and control group (n=12). In cystitis group, 100mg/kg CYP was injected every second day for 1 week whereas in control group, normal saline was injected. After extracting of the bladder and dividing dome, body and trigone of the bladder, independently H&E staining and immunohistochemical staining for iNOS and AQP-3 were performed. Expressions of iNOS and AQP-3 were analyzed with a confocal laser scanning microscope and an image analyzer.
Results
The expression of iNOS significantly increased in the mucosa, submucosa layer of dome in cystitis group (p<0.05). The expression of AQP-3 significantly increased in the mucosa, submucosa, vessel layer of dome in cystitis group (p<0.05).
Conclusions
These results suggest that inflammatory change activates NOS and AQP-3 expression in the bladder tissue of rats. These may imply that NOS and AQP-3 have a pathophyiological role in the cyclophophamide induced interstitial cystitis. Further study on the NOS and AQP-3 in bladder is needed for clinical application.
Keywords: Nitric oxide synthase, Aquaporin-3, Interstitial cystitis, Cyclophosphamide
Introduction
Interstitial cystitis is chronic disease of unknown etiology characterized by vague bladder pain and nonspecific urinary symptoms such as urgency and frequency. Its prevalence has significantly increased [1], but many studies for it have used animals because of limitations in clinical research. As of today, bladder inflammation, urethra inflammation, stimuli in lower urinary tract, mechanical bulge within uterus or vagina, or urethra obstruction have been presented as the animal models of interstitial cystitis or overactive bladder syndrome and detrusor overactivity [2,3]. The experiment model using cyclophosphamide (CYP) has some strength: inflammation is limited to bladder; inflammation can occur within several hours after agent administration; and types of pain and voiding symptoms are similar to those of human because CYP also causes cystitis for human [4,5]. Many researches on NOS in terms of bladder and urethra tissue have focused on the induction of relaxation of smooth muscles, or in particular, reduction in urethral pressure in urination, and NOS is also known to be an important substance related to penile erection [6-8].
Auaporin (AQP), a passage channel of water, is related to quick water movement through cell membrane, and 13 kinds including AQP 0, AQP 1, and to AQP 12 had been known as of today. There is AQP 1-4 in kidney, and recently AQP 6-8 and AQP 11 are known to be expressed; as for bladder, AQP 1-3 is known to be expressed [9,10]. However, it is still controversial on the expression and roles of NOS and AQP in changes within bladder tissue by interstitial cystitis. In this context, we studied changes in expression of iNOS and AQP-3 within bladder tissue of rats with interstitial cystitis induced by CYP to identify the pathophysiology of interstitial cystitis. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences.
Materials and Methods
Experiment animal
The experiment animals of this study were Sprague-Dawley female white rats (200-250g, Daehan Biolink Co. Ltd), and they were raised by two in a cage covered with sawdust. Water and feed were presented for free eating, the temperature was controlled to be 22℃, and the illumination was changed at an interval of twelve hours. The rats were randomly divided into two groups: the control group (n=12), the cystitis-induced group (n=20).
Induction of interstitial cystitis
The rats of the cystitis group were intraperitoneally injected of 100 mg/kg of CYP by using of syringe (26gauge, 1cc) once per two days for three sessions to maintain the intraperitoneal pain and inflammation, and those of thecontrol group were injected of normal saline instead of CYP with the same method and at the same day.
Histologic examination
After the experiment, in order to observe histologic findings of cystitis by CYP, we made paraffin blocks, conducted H&E staining, and compared findings of interstitial edema, deposition of fibrin, thickness of urothelium, desquamation, and interstitial hemorrhage.
Immunohistochemical staining
Operation was performed at the seventh day after the initial administration of CYP. We conducted vertical incision to expose the bladder, which was excised at the bladder neck. Then, the removed bladder was divided into three partsdome, body, and trigone-to be fixed by 10% of neutral formalin, to be washed by flowing water to remove fixative, and to be dehydrated. After clearing, the blocks were paraffin-embedded, and the tissue blocks were adhered to sections, which was made to slide glasses coated by silane with 4 µm of thickness. For deparaffiinization and hydration, the blocks were reacted to Xylene I and II for 10 minutes and 100%, 95%, 90%, 80%, and 70% of alcohol in order for five minutes, were washed by distilled water for five minutes, and washed by 0.01M PBS (pH 7.4) three times for 10 minutes. In order to expose antigens, the blocks were dip into 1X sodium citrate buffer solution to be pretreated by microwave oven for four minutes and then were washed by 0.01M PBS (pH 7.4) three times for 10 minutes. For removing non-specific reactions, the blocks were treated by 10% normal donkey serum at 37℃ for one hour. Then, they were reacted to anti-iNOS mouse monoclonal antibody (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, USA) and anti-Aquaporin-3 rabbit polyclonal antibody (1:200, Santa Cruz Biotechnology Inc., Santa Cruz, USA) as primary antibodies at 4℃ for overnight, and were washed by 0.01M PBS (pH 7.4)three times for 10 minutes. Donkey anti-mouse IgG FITC (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, USA), Donkey anti-rabbit IgG Texas Red (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, USA), and DAPI (4'6-Diamidino-2-phenyindole, dilactate, 1:400, Sigma, St. Louis, USA) were used as secondary antibodies, and iNOS and aquaporin-3 were stained on one same slide. The slides were washed by 0.01M PBT containing 0.1% Triton X-100 (Sigma-Aldrich Corp., St. Louis, USA)twice for 30 minutes, and washed by PBS fifteen times for 20 minutes. The slides after the staining were mounted by mounting media (Dako, Carpinteria, USA).
Image analysis and statistical process
The intensity of iNOS and AQP-3 staining was estimated by using confocal microscope and image analyzer (Zeiss LSM 5.0, Jena, Germany) in terms of expression of fluorescence. We observed at least five parts of each tissue, and as for staining intensity between each tissue, it was considered significant difference when p value was less than 0.05 by using parametric test (Independent T-test). The SPSS for Window (version 12.0, SPSS Inc., Chicago, USA) was used for statistical analysis.
Results
Results of histological evaluation
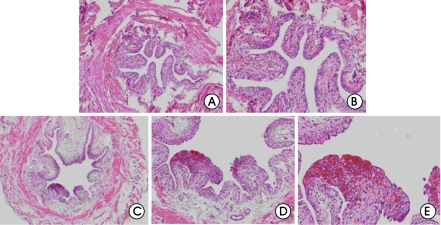
When the bladder tissues were stained by H&E, interstitial edema, hemorrhage, increase in tissue thickness, and infiltration of inflammatory cells were found on all the three parts (dome, body, and trigone) administrated by CYP, but they were not found on those of the control group administered of normal saline (fig. 1).
Figure 1.
Histological findings (Hematoxylin and Eosin staining) of the normal and cyclophophamide induces cystitis bladders. (A) Histologic findings of the normal bladder (×40). (B) Histologic findings of the normal bladder (×100). (C) Interstitial edema and hemorrhage in the cyclophosphamide-induced cystitis bladder (×40). (D) Magnified findings of focal hemorrhage (×100). (F) Magnified findings of focal hemorrhage (×200).
Results of immunohistochemical staining
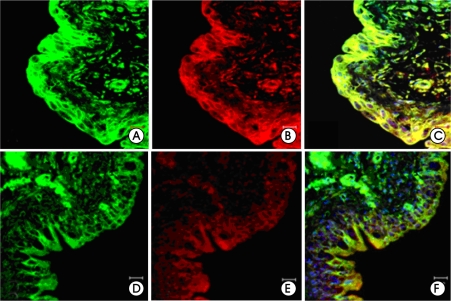
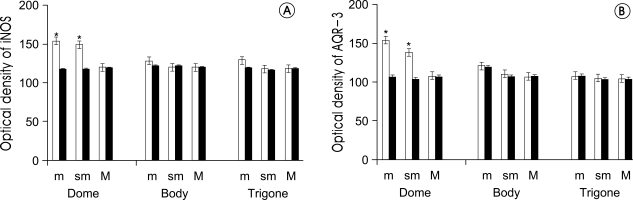
As for iNOS expression in the bladder mucosa, there was no significant difference on the body and trigone parts of the bladders but significant increase was observed in the dome parts, when compared to those of the control group. Also, the expression was significantly increased on the submucosal layer tissues and vessels (p<0.05) (fig. 2A, D, fig. 3A).
Figure 2.
Expressions of inducible nitric oxide synthase (iNOS, green) and aquaporin-3 (AQP-3, red) (×400). Merged images of iNOS and AQP-3 (C, F). The expressions of iNOS and AQP-3 in cystitis group showed significantly stronger immunoreactivity in mucosa and submucosal layer than control group. A-C; Cystitis group, D-F; Control group.
Figure 3.
Optical density of inducible nitric oxide synthase (iNOS) and aquaporin-3 (AQP-3) between cystitis group and control group. Bars are mean±SE. White bars are control group, Black bars are interstitial cystitis group. A: iNOS, B: AQP-3, m: mucosa, sm: submucosa, M: muscle, *: p<0.05 vs. control group.
The expression of AQP-3 was similar to that of iNOS. When compared to the control group, the expression of AQP-3 failed to show significant difference on the body and the trigone parts but was significantly increased on the dome parts (p<0.05) (fig. 2B, E, fig. 3B). The expression of AQP-3 and iNOS was increased in the mucosal, submucosal layer with almost similar distribution (fig. 2C, F).
Discussion
In interstitial cystitis, damage of the protective bladder lining leads to impaired urothelial barrier function. Consequently, urinary solutes penetrate the epithelium and activate sensory nerve endings, leading to pain and inflammation [1]. Interstitial cystitis involves primarily changes in urothelial permeability, along with mast cell activation and neurogenic inflammation [11], but the underlying pathophysiology of interstitial cystitis is incompletely understood. This indefiniteness gives difficulties in developing animal models, and until recently bladder inflammation, urethra inflammation, stimuli in lower urinary tract, mechanical bulge within uterus or vagina have been presented as animal models of cystitis with pain and inflammation. However such models have problems, including errors in experiment because of sufficient stress caused by intense labor that experimenters give to models and such pain or inflammation can be specific for organ [12]. CYP is known to be the cause for hemorrhagic cystitis characteristic of plasma extravasation, vessel dilatation and edema within bladder, leukocyte infiltration, and urothelium damage [13,14]. It is converted to acrolein in kidney to be accumulated in bladder, and the consistent stimulus by acrolein within bladder by urine causes painful cystitis, and such inflammatory reaction causes overactive bladder because expression of receptors in capsaicin sensitive afferent nerves is increased by increase in activity of iNOS and nerve growth factors by action of acrolein as CYP metabolite or direct action of CYP [15,16].
This experiment model is significant in that the inflammation is limited to bladder, inflammation occurs within several hours after agent administration, and detrusor overactivity occurs only by intraperitoneal injection without operation or intubation [5]. In this study, CYP caused manual and histological inflammation in bladder effectively and quickly, and cystitis by CYP causes changes in neurochemical, electrophysiological, and voiding reflex composition [17] to induce chronic pathologic status. These changes transform courses of sensory nerves and reduce pain threshold to cause abnormal pain or hyperalgesia. In this study, as observed by Boucher et al. [18], rats administered of CYP showed characteristic changes in actions such as eye closing, piloerection, and reduction in respiratory rate, or hump-backed posture, indicating CYP induces pain in internal organs. Therefore, intraperitoneal injection of CYP can serve as interstitial cystitis model without difficulty and the findings can be shown from movement of animal during experiment.
NO is a kind of endothelial derived relaxation factor, first reported by Furchgott and Zawadzki in 1980 [19]. It is known to have three in vivo types, with extremely short half-life (about 20 minutes). It is generated by transformation of guanidine nitrogen of L-arginine by nitric oxide synthase (NOS), playing various roles in vessels, immune system, and nervous system. NOS is divided into constitutive NOS (cNOS) and inducible NOS (iNOS), and cNOS is divided into endothelial NOS (eNOS) acting on vessels and neuronal NOS (nNOS) that is considered a main transmitter in nerves [6]. The type I, or nNOS, is found mainly in brain, spinal cord, and peripheral nervous system, considered a neurotransmitter in nonadrenergic noncholinergic (NANC) nervous system of nervous cells to relax smooth muscles. The type III, or eNOS, is found mainly in endothelial cells of vessels, considered to relax vessel smooth muscles, to extend vessels, and to inhibit agglutination of platelets and leukocytes. The type II, or iNOS, is secreted from activated macrophagocytes, and is known to be promoted of the secretion by inflammatory reaction or stress [20]. There are many studies of NO in penile erection or proximal urethra but its role in bladder has not been clearly established. According to report by Huang et al. [21], it is regarded to maintain tone of local vessels or to involve in muscle relaxation, growth regulation of epithelial tissue, and control of synthesis & secretion of extracellular matrix protein in normal bladder. As for stomach, NO is reported to protect gastric cells by stimulating mucin secretion, and, by similar mechanism, it is regarded to inhibit cell damage by urine by making transitional epithelium of bladder secrete glycoprotein [22,23]. Also, in animal experiment models, eNOS and nNOS are increased with increase in iNOS in the group of chronic renal occlusion, and this increase is reported to cause vessel extension of muscle layers and inhibition of fibrosis and, as for bladder, is known to induce inflammatory reaction by increasing synthesis of cGMP by stimulating nerves of bladder basal layers [24].
Austin et al. [25] reported that they confirmed the activity of iNOS and collagen type III related to tissue fibrosis and inflammatory reaction was increased when inflammation in smooth muscle cells of human bladder caused by bacterial lipopolysaccharide and inflammatory cytokine and that aminoganidine as iNOS inhibitor reduced inflammatory reaction in bladder. Jezernik et al. [26] reported that there was significant correlation between activity of iNOS and apoptosis and proliferation of urothelial cells in the rat urothelium induced by CYP, but its detailed mechanism has not been clearly established. Logadottir et al. [27] reported that, as for NOS activity of 17 patients with interstitial cystitis, NO was increased in the patients showing severe abnormality and infiltration of inflammatory cells in bladder epithelial cells in histological examination and it was significantly increased at the earlier stage of interstitial cystitis. Hosseini et al. [28] reported that, when 15 patients with classic interstitial cystitis were treated by prednisolone for 8 weeks to be observed of NO, it expressed degrees of disease inflammation and served as a objective reaction factor for treatment. In this study, we divided the bladder of rats with interstitial cystitis into three (dome, body, and trigone)for comparison; activity of iNOS was higher in all the three parts of the cystitis group and in particular, the activity was statistically significantly higher in the mucosa and submucosa of the dome. This results may indicate inflammation of the dome was more than that of the other parts, requiring further studies on the mechanism.
Aquaporin is cell membrane protein through which water pass selectively. Since AQP-1 has first found in human erythrocytes by Denker et al. [29] in 1988, various subtypes have been found in several organs such as collection duct in kidney, epithelial cells in gallbladder, pneumonocytes, and bronchus, known to play an important role in water movement through cells. Most of them were studied in kidney and few studies have been conducted in bladder. Spector et al. [10] reported that AQP-2 and 3 were much expressed in urothelium cells in ureter and bladder of rats and AQP-1 localized only in endothelial cells. Kim et al. [30] reported that AQP-1 expression was significantly increased in rat bladder with partial bladder outlet obstruction and that AQP-1 has a functional role in the detrusor instability that occurs in association with bladder outlet obstruction. Krane et al. [9] reported AQP-2, 3 expression was increased in dehydration on rat urothelium, and AQP-1 expression was decreased. In this study, AQP-3 was increased at the interstitial cystitis model, and especially was significantly increased at the mucosa, submucosa, and vessel layers of the bladder dome part. Such increase in expression may be by increase in AQP-3 synthesis within cells by connection of vasopressin-its concentration is enhanced for compensation of increased water exposure within bladder due to inflammatory reaction-to the V2 receptors of granular cells in bladder.
In a study regarding all the subtypes of AQP 13 in cultured normal human urothelium cells and human tissues of bladder and ureter, it is reported that AQP 3, 4, 7, 9, and 11 were expressed but AQP 0, 1, 2, 5, 6, 8, 10, and 12 were not, and that they were different from the subtypes expressed in rats [31]. From a functional perspective, the AQPs can be divided into two subfamilies: AQP 0, 1, 2, 4, 5, 6, and 8 move water directly; AQP 3, 7, 9, and 10 as aquaglyceroporins move water with additional small neutrally charged solute such as glycerol, urea, and pyrimidines [32]. Human urothelium is known to channel complex with the two functions and rat bladder can have similar physiological function. In human urothelium, AQP-3 among the various AQP subtypes is mostly expressed at the borders of intermediate cells and basal cells, and AQP-3 whose function is aquaglyceroporin is increased most maybe because bladder epithelial cells control components and osmolarity of urine [31]. In this study the increase in AQP-3 may be regarded to compensation to response to osmolarity by urothelium cells on inflammatory reaction by interstitial cystitis. However, researches on bladder AQP have been insufficient and started recently. Further studies may clearly establish roles of AQP in bladder.
Conclusions
The iNOS and AQP-3 expression was increased on the dome part within the bladder tissues of the interstitial cystitis models induced by CYP in white rats. In particular, iNOS expression was increased on the mucosa and submucosal layer of the bladder dome and AQP-3 expression was increased on the mucosa, submucosal layer, and vessels. Researches on AQP-3 in bladder are still insufficient and require further study, which may support the underlying pathophysiology and development of treatment of interstitial cystitis.
Footnotes
The authors have nothing to disclose.
References
- 1.Moutzouris DA, Falagas ME. Interstitial cystitis: an unsolved enigma. Clin J Am Soc Nephrol. 2009;4:1844–1857. doi: 10.2215/CJN.02000309. [DOI] [PubMed] [Google Scholar]
- 2.Mcmahon SB, Abel C. A model for the study of visceral pain states: chronic inflammation of the chronic decerebrate rat urinary bladder by irritant chemicals. Pain. 1987;28:109–127. doi: 10.1016/0304-3959(87)91065-7. [DOI] [PubMed] [Google Scholar]
- 3.Berkley KJ, Wood E, Scofield SL, Little M. Behavioral responses to uterine or vaginal distension in the rat. Pain. 1995;61:121–131. doi: 10.1016/0304-3959(94)00150-D. [DOI] [PubMed] [Google Scholar]
- 4.Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophophamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox-24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- 5.Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs. 1991;42:781–795. doi: 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Ho MH, Bhatia NN, Khorram O. Physiologic role of nitric oxide and nitric oxide synthase in female lower urinary tract. Curr Opin Obstet Gynecol. 2004;16:423–429. doi: 10.1097/00001703-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Bennett BC, Kruse MN, Roppolo JR, Flood HD, Fraser M, de Groat WC. Neural control of urethral outlet activity in vivo: role of nitric oxide. J Urol. 1995;153:2004–2009. [PubMed] [Google Scholar]
- 8.Hallen K, Gustafsson LE, Wiklund NP. Nerve-induced release of nitric oxide from the rabbit corpus cavernosum is modulated by cyclic guanosin 3',5'-monophosphate. Neuroscience. 2005;133:169–174. doi: 10.1016/j.neuroscience.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Krane CM, Goldstein DL. Comparative functional analysis of aquaporins/glyceroporins in mammals and anurans. Mamm Genome. 2007;18:452–462. doi: 10.1007/s00335-007-9041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spector DA, Wade JB, Dillow R, Steplock DA, Weinman EJ. Expression, localization, and regulation of aquaporin-1 to-3 in rat urothelia. Am J Physiol Renal Physiol. 2002;282:F1034–F1042. doi: 10.1152/ajprenal.00136.2001. [DOI] [PubMed] [Google Scholar]
- 11.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: Role in pathophysiology and pathogenesis. Urology. 2007;69:34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 12.Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- 13.Grinberg-Funes DJ, Sheldon C, Weiss M. The use of prostaglandin F2 alpha for the prophylaxis of cyclophosphamide induced cystitis in rats. J Urol. 1990;144:1500–1504. doi: 10.1016/s0022-5347(17)39786-0. [DOI] [PubMed] [Google Scholar]
- 14.Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliani S. Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol. 1994;111:1017–1022. doi: 10.1111/j.1476-5381.1994.tb14845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfieri AB, Malave A, Cubeddu LX. Nitric oxide synthases and cyclophosphamide-induced cystitis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:353–357. doi: 10.1007/s002100000371. [DOI] [PubMed] [Google Scholar]
- 16.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 17.Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000;420:335–348. [PubMed] [Google Scholar]
- 18.Boucher M, Meen M, Codron JP, Coudore F, Kemeny JL, Eschalier A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol. 2000;164:203–208. [PubMed] [Google Scholar]
- 19.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 20.Forstermann U, Pollock JS, Tracey WR, Nakane M. Isoforms of nitric-oxide synthase: purification and regulation. Methods Enzymol. 1994;233:258–264. doi: 10.1016/s0076-6879(94)33029-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang A, Palmer LS, Hom D, Valderrama E, Trachtman H. The role of nitric oxide in obstructive nephropathy. J Urol. 2000;163:1276–1281. [PubMed] [Google Scholar]
- 22.Whittle BJ. Nitric oxide in physiology and pathology. Histochem J. 1995;27:727–737. [PubMed] [Google Scholar]
- 23.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 24.Smet PJ, Jonavicius J, Marshall VR, de Vente J. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–348. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- 25.Austin PF, Casale AJ, Cain MP, Rink RC, Weintraub SJ. Lipopolysaccharide and inflammatory cytokines cause an inducible nitric oxide synthase-dependent bladder smooth muscle fibrotic response. J Urol. 2003;170:645–648. doi: 10.1097/01.ju.0000068727.22429.e8. [DOI] [PubMed] [Google Scholar]
- 26.Jezernik K, Romih R, Mannherz HG, Koprivec D. Immunohistochemical detection of apoptosis, proliferation and inducible nitric oxide synthase in rat urothelium damaged by cyclophosphamide treatment. Cell Biol Int. 2003;27:863–869. doi: 10.1016/s1065-6995(03)00175-6. [DOI] [PubMed] [Google Scholar]
- 27.Logadottir YR, Ehren I, Fall M, Wiklund NP, Peeker R, Hanno PM. Intravesical nitric oxide production discriminates between classic and nonulcer interstitial cystitis. J Urol. 2004;171:1148–1150. doi: 10.1097/01.ju.0000110501.96416.40. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini A, Ehrén I, Wiklund NP. Nitric oxide as an objective marker for evaluation of treatment response in patients with classic interstitial cystitis. J Urol. 2004;172:2261–2265. doi: 10.1097/01.ju.0000144761.69398.be. [DOI] [PubMed] [Google Scholar]
- 29.Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem. 1988;263:15634–15642. [PubMed] [Google Scholar]
- 30.Kim SO, Song SH, Ahn K, Kwon D, Park K, Ryu SB. Changes in aquaporin 1 expression in rat urinary bladder after partial bladder outlet obstruction: preliminary report. Korean J Urol. 2010;51:281–286. doi: 10.4111/kju.2010.51.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenwolf PC, Georgopoulos NT, Clements LA, Feather S, Holland P, Thomas DF, et al. Expression and Localisation of Aquaporin Water Channels in Human Urothelium In Situ and In Vitro. Eur Urol. 2009;56:1013–1024. doi: 10.1016/j.eururo.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Fu D, Lu M. The structural basis of water permeation and proton exclusion in aquaporins. Mol Membr Biol. 2007;24:366–374. doi: 10.1080/09687680701446965. [DOI] [PubMed] [Google Scholar]