Abstract
Protozoan parasites of the genus Leishmania alternate between flagellated, elongated extracellular promastigotes found in insect vectors, and round-shaped amastigotes enclosed in phagolysosome-like Parasitophorous Vacuoles (PVs) of infected mammalian host cells. Leishmania amazonensis amastigotes occupy large PVs which may contain many parasites; in contrast, single amastigotes of Leishmania major lodge in small, tight PVs, which undergo fission as parasites divide. To determine if PVs of these Leishmania species can fuse with each other, mouse macrophages in culture were infected with non-fluorescent L. amazonensis amastigotes and, 48 h later, superinfected with fluorescent L. major amastigotes or promastigotes. Fusion was investigated by time-lapse image acquisition of living cells and inferred from the colocalization of parasites of the two species in the same PVs. Survival, multiplication and differentiation of parasites that did or did not share the same vacuoles were also investigated. Fusion of PVs containing L. amazonensis and L. major amastigotes was not found. However, PVs containing L. major promastigotes did fuse with pre-established L. amazonensis PVs. In these chimeric vacuoles, L. major promastigotes remained motile and multiplied, but did not differentiate into amastigotes. In contrast, in doubly infected cells, within their own, unfused PVs metacyclic-enriched L. major promastigotes, but not log phase promastigotes - which were destroyed - differentiated into proliferating amastigotes. The results indicate that PVs, presumably customized by L. major amastigotes or promastigotes, differ in their ability to fuse with L. amazonensis PVs. Additionally, a species-specific PV was required for L. major destruction or differentiation – a requirement for which mechanisms remain unknown. The observations reported in this paper should be useful in further studies of the interactions between PVs to different species of Leishmania parasites, and of the mechanisms involved in the recognition and fusion of PVs.
Author Summary
Many non-viral intracellular pathogens lodge within cell vesicles known as “parasitophorous vacuoles” (PVs), which exhibit a variety of pathogen-dependent functional and compositional phenotypes. PVs of the protozoan Leishmania are similar to the digestive organelles known as phagolysosomes. We asked if, in phagocytes infected with two different Leishmania species, would the two parasites be found in the same or in separate vacuoles? Of the species chosen, Leishmania amazonensis develops within large vacuoles which shelter many parasites; in contrast, Leishmania major lodges in small PVs containing one or two parasites. In the present experiments, the species and their life-cycle stages (extracellular promastigotes, and intracellular amastigotes) were distinguished by means of fluorescent markers, and the intracellular localization of the parasites was examined in living cells. We report here that, whereas L. major amastigotes remained within their individual vacuoles, L. major promastigotes were delivered to L. amazonensis vacuoles, in which they survived and multiplied but were unable to differentiate into amastigotes. A species-specific vacuole was thus required for L. major differentiation. The model should be useful in cellular and molecular studies of the biology of these parasites and of their parasitophorous vacuoles.
Introduction
In a classic review of intracellular parasitism, James Moulder proposed that microbial parasites customize the morphology, composition and function of parasitophorous vacuoles (PVs) in which they are sheltered [1]. Considering the implications of Moulder's proposal we asked if pathogens could survive and multiply within PVs that sheltered a different organism. In some instances it was possible to generate chimeric vacuoles in cells coinfected with different pathogens [2], [3], [4], [5]. In the present studies macrophages were coinfected with two species of Leishmania parasites normally lodged in PVs that differ in their biogenesis, morphology and parasite occupancy.
Leishmania are dimorphic trypanosomatid parasites, which induce cutaneous, muco-cutaneous or visceral disease in man and other animals. Elongated, proliferating, extracellular procyclic promastigote forms colonize the midgut of sandfly vectors. These forms, which can be grown axenically, differentiate into infective, stationary phase metacyclics promastigotes that can be released into the dermis of mammalian hosts in the course of the insect bloodmeal. Macrophages and other mammalian cells internalize infective promastigotes within PVs, in which parasites differentiate into the smaller, internally flagellated, oval-shaped amastigote forms. Amastigotes divide intracellularly and spread the infection in the mammal host [6].
Leishmania PVs are bound by a membrane - initially derived from the host cell plasma membrane - which undergoes compositional changes as they fuse with late endosomes/lysosomes and possibly with other vesicles. The phagolysosome-like nature of Leishmania PVs, initially supported by the acquisition of electron dense colloids by fusion of parasite-containing phagosomes with vesicles carrying the markers [7], [8], [9], [10], and by the demonstration that PVs were acidified, was reinforced by the detection of lysosomal markers such as lysosome-associated membrane proteins (LAMPs) and Rab GTPases in the PV membranes of a few Leishmania species examined [11], [12], [13]. Thus Leishmania PVs are considered acidic organelles, contain lysosomal enzymes and present a vacuolar pH in the range of 4.7–5.2 [12], [14].
Most studies on Leishmania PVs were performed with parasites of the mexicana group - L. amazonensis and L. mexicana – both sheltered in spacious PVs that may contain many amastigotes. These large, communal PVs were shown to selectively fuse with phagosomes containing large particles or microorganisms [15], [16], [17], [18]. Time-lapse microcinematographic studies revealed that most incoming zymosan-containing phagosomes remained in contact with L. amazonensis PVs for several hours before fusion took place, which itself lasted for only a few minutes [19]. More recently, fusion between L. amazonensis PVs was examined in macrophage cultures infected with amastigotes for 48 hours and reinfected with labeled amastigotes or promastigotes of the same species. Two hours after reinfection, the initially tight incoming PVs contacted the large recipient PVs; however, while both vacuoles stained positively for LAMP1 markers, fusion was only detected by 12 hours of reinfection, when both vacuoles were spacious. Thus, both labeled amastigotes or promastigotes could be transferred to large PVs which sheltered the same parasite species [20].
Most of Leishmania species studied, however, are lodged in small, membrane-bound PVs which display lysosomal markers, usually contain a single parasite and undergo fission as parasites divide [21], [22], [23]. It was reported that the pH within L. donovani membrane-bound PV is about 5.5, and the increase in vacuolar pH to 5.8 was not only tolerated by the parasites but exacerbated intracellular infection [24].
In the present studies, macrophages infected with L. amazonensis were challenged with L. major lesion amastigotes or promastigotes and coinfected cells observed by multidimensional live imaging. We found that whereas L. major amastigotes were excluded, L. major promastigotes were delivered into L. amazonensis PVs where they survived and multiplied but did not differentiate into amastigote forms.
Materials and Methods
Ethics statement
All experiments involving animal work were conducted under guidelines approved by UNIFESP and Institut Pasteur ethics committees, which are in accordance with international recommendations.
Mice and parasites
BALB/c female mice, 8 weeks of age, were used as source of bone marrow cells. BALB/c nude mice, 8 weeks of age, were used as source of lesion-derived amastigotes after 2 months of the inoculum of wild-type L. (L.) amazonensis LV79 (MPRO/BR/72/M1841), or DsRed2-transfected L. (L.) major NIH173 (MHOM/IR/-/173) on mice footpad. Isolation of amastigotes from footpad lesions was performed as described previously [19].
Leishmania major-DsRed2 or GFP-transfected (MRHO/SU/59/P) promastigotes were cultivated at 26°C in an air atmosphere in M199 medium containing 10% fetal calf serum, 100 u/ml of penicillin, 100 µg/ml of streptomycin, and buffered with 10 mM HEPES at pH 7.2. Metacyclic enriched promastigote populations were separated in Ficoll PM type 400 gradients (Sigma-Aldrich Co.), as described elsewhere [25].
In growth studies Leishmania major-DsRed2 log phase promastigotes were seeded at 105 parasites per well in 96 wells plates. Plates were cultivated at 34°C or 26°C in air atmosphere. For growth at pH 5.0 the buffer used was 2-morpholinoethanesulfonic acid (MES) at 10 mM. Wells were examined under an Olympus IX70 inverted microscope (10 x, 0.3 NA, 20 x, 0.4 NA, and 40 x, 0.6 NA objectives) equipped with an Olympus DP71 CCD Camera. Numbers of parasites per field were estimated from randomly acquired images of 10 microscopic fields in each of 3 wells, over periods of up to 10 days. Images were analyzed with Image Pro Plus 6 software (Media Cybernetics Inc.) for algorithm-based quantification. Parasite numbers were corrected to a 10 x objective field area.
Infection of macrophage cultures
Bone marrow-derived macrophages were obtained and cultivated for 7 days in RPMI 1640 medium with 10% fetal calf serum, 5% L929 cell conditioned medium, 100 u/ml of penicillin and 100 µg/ml of streptomycin (complete medium) [26]. Macrophages were replated on round dishes (ibidi, GmbH), suitable for maintenance in incubators coupled to microscopes, or on 13 mm diameter coverslips placed in the wells of tissue culture plates. Before their use for experiments, cultures were kept overnight at 37°C, 5% CO2 in a humidified air atmosphere.
Lesion-derived amastigotes, stationary phase metacyclic-enriched promastigotes or log phase procyclic promastigotes were added to macrophages cultures at a multiplicity of infection of 5∶1 parasites to cell and incubated at 34°C, 5% CO2 in complete medium for different time periods according to the parasite stages used. Cultures were washed with Hanks' Buffered Salt Solution (HBSS) to remove free parasites, and cultivated in complete medium, 34°C, 5% CO2 in air atmosphere. To reduce the proliferation of extracellular promastigotes, cultures were washed and their medium replaced daily.
Design of the experiments
In all experiments macrophage cultures infected for 48 hours with L. amazonensis-WT amastigotes were superinfected with L. major-DsRed2 amastigotes, metacyclic-enriched promastigotes or procyclic promastigotes. The first infection allowed for the development of large recipient PVs, to be distinguished from the small donor PVs that sheltered L. major parasites. Vacuoles containing parasites of the two species were denominated chimeric PVs. After different periods of superinfection, cultures were used for live imaging or fixed for immunolocalization. Superinfected cultures were examined to determine i) the occurrence of fusion between L. amazonensis and L. major PVs as a function of the stages of the later; ii) the proportion of the L. major intracellular parasites that were sheltered in chimeric vacuoles; iii) the proliferation and eventual differentiation of L. major parasites in superinfected compared to that in monoinfected macrophage cultures.
Immunolocalization
Macrophages on coverslips were washed and fixed for 1 hour with 3.5% formaldehyde in phosphate buffered saline (PBS). Parasitophorous vacuoles and other acidic compartments were identified by immunolabeling of membrane proteins LAMP1 and LAMP2, with rat anti-mouse specific antibodies. Leishmania amazonensis amastigotes were identified and distinguished from L. major with the help of the 2A3-26 antibody conjugated to FITC (kindly provided by Dr. Eric Prina, Institut Pasteur, France). Cultures were then stained for 15 minutes with 100 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) and mounted with 50% glycerol in PBS, containing 0.01% p-phenylenediamine. Confocal images were obtained with a Bio-Rad 1024UV system, coupled to a Zeiss Axiovert 100 microscope. Images acquired with a 100 x (1.4 NA) oil immersion objective were renderized by Imaris Software (Bitplane AG) using blend or MIP filters.
Live imaging
Live imaging of cultures was performed by a Nikon Biostation IM Live cell recorder system (Nikon Corporation) and a Perkin-Elmer UltraView RS Nipkow-disk system (PerkinElmer Inc.) attached to a Zeiss Axiovert 200 M microscope with CCD detector Hamamatsu ORCA II ER. To identify Leishmania PVs, Lysotracker green DND-26 (Invitrogen Corporation), a lysosomotropic probe for acidic compartments, was added to complete medium at 10 µM concentration throughout image acquisition. Cultures were maintained at 34°C and 5% CO2, by incubators coupled to microscopes.
Conventional time-lapse acquisition
The Nikon Biostation IMq was used to acquire, in 10 different microscopic fields, serial images of superinfected macrophage cultures in multi-chamber dishes. The Biostation acquired images in phase contrast and in two fluorescent channels (for Lysotracker and DsRed2 labeled parasites), with 40 x objectives (0.8 NA) at intervals of 5 minutes. Time after superinfection is displayed as day-hours:minutes (dhh:mm).
Fluorescent parasites were quantified with Acapella software (Version 2.0 -PerkinElmer Inc.), which recognizes fluorescent patterns by algorithm-based image analysis.
Multidimensional acquisition
The Perkin-Elmer UltraView RS system was used to acquire approximately 20 focal stacks of 2 or more fields of live, superinfected macrophage cultures. Preparations were scanned at intervals of 15 minutes under 63 x (1.3 NA) oil objectives, to minimize phototoxicity and photobleaching of Lysotracker. Time after superinfection is displayed as hours:minutes (hh:mm).
Acquired images were processed by Imaris software (Bitplane AG) for construction of multidimensional images, comprising 3D, time and fluorescent channels. Surface rendering was used to measure parasites' volume, and sphericity (a parameter ranging from non-spherical 0 to spherical 1). Renderization using blend or MIP filters was used to visualize interaction between Leishmania PVs.
Statistics
All experiments were repeated at least twice, with duplicate or triplicate coverslips in the case of fixed samples. Results presented in the form of images are representative of at least 2 other images analyzed. Statistical analyses (ANOVA) and graphs were built using SPSS 15.0 software (SPSS Inc.). Graphs display means and standard errors (s.e.m).
Results
Parasitophorous vacuoles that shelter L. major amastigotes did not fuse with L. amazonensis PVs
To determine if L. amazonensis and L. major PVs could fuse with each other, macrophages infected for 48 hours with L. amazonensis amastigotes were superinfected with L. major-DsRed2 amastigotes. Live cultures were loaded with Lysotracker to identify Leishmania PVs. Both live and fixed cultures were scanned to search for chimeric PVs.
A few hours after superinfection, L. major PVs were found closely apposed to L. amazonensis PVs. However, for up to 11 days after superinfection, fusion between L. major and L. amazonensis PVs was not directly observed or inferred from finding of chimeric PVs (Fig. 1 and Video S1). In live cell recordings, the L. major PV is not visible since it did not take up detectable Lysotracker amounts possibly due to the small vacuolar volume between parasite and PV membranes. The possibility that the intravacuolar environment was less acidic cannot, however, be discarded.
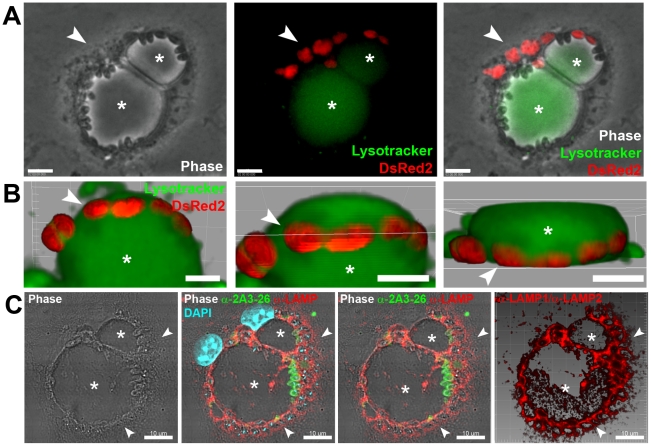
Figure 1. L. major amastigote PVs did not fuse with L. amazonensis vacuoles.
(A) Macrophages were previously infected for 48 h with L. amazonensis-WT and then superinfected with L. major-DsRed2 amastigotes for additional 72 hours. Image shows a macrophage loaded with Lysotracker (green) and hosting the two parasite species under phase contrast channel (Ph2), fluorescence channels (Lysotracker and DsRed2), and merged channels, respectively. Asterisk indicates L. amazonensis PV and arrowheads indicate L. major-DsRed2 amastigotes (red). Bars = 10 µm. (B) Live multidimensional imaging of coinfected macrophages. Asterisk indicates L. amazonensis-WT PV stained with Lysotracker (green), surrounded by membrane-bound PVs with weak Lysotracker signal which shelter L. major-DsRed2 amastigotes (red). Multidimensional images were constructed by Imaris blend filter and each image represents a rotation of approximately 45°. Bars = 5 µm. (C) Immunolocalization of LAMP1/LAMP2 proteins in superinfected macrophages. Leishmania major amastigotes were sheltered by tight LAMP1/LAMP2-positive PVs (arrowheads), close to large recipient L. amazonensis PVs, indicated by asterisks. Image was acquired 11 days after L. major-DsRed2 amastigote addition. LAMP immunolabeling in red, 2A3-26 antibody (specific for L. amazonensis amastigotes) immunolabeling in green, DAPI staining in blue. Images are disposed as phase contrast (Ph3), phase contrast with RGB fluorescence channels, phase contrast with RG channels and 3D reconstruction of red channel with Imaris blend filter. Bars = 10 µm.
Images that initially suggested fusion were later shown by multidimensional live imaging to result from L. major PVs positioned underneath L. amazonensis vacuoles (Fig. 1B and Video S1). Segregation of the two species of amastigotes in separate PVs was also confirmed by LAMP1/LAMP2 immunolabeling of superinfected macrophages (Fig. 1C). Similar results were obtained when macrophages were simultaneously infected with L. amazonensis and L. major amastigotes (data not shown). For up to 6 days after superinfection, L. major amastigotes, sheltered in individual PVs, multiplied at similar rates in monoinfected and superinfected phagocytes. These conclusions were supported by the observation of fixed cell preparations (data not shown).
Leishmania major promastigotes were delivered to L. amazonensis vacuoles through PV fusion
In these experiments, macrophages infected for 48 hours with L. amazonensis were challenged with L. major-DsRed2 metacyclic-enriched promastigotes. In the first hours of superinfection, L. major donor vacuoles were found in video recordings and in fixed preparations to be in contact with L. amazonensis PVs. The contact regions were monitored by live multidimensional imaging. The sequence shown in Fig. 2A and Video S2 begins with the image of an L. major promastigote apparently apposed to an L. amazonensis PV loaded with Lysotracker.
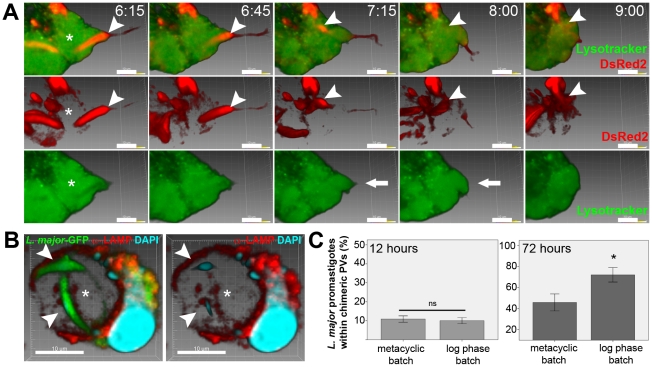
Figure 2. Fusion of L. amazonensis vacuoles with PVs that shelter L. major promastigotes.
(A) Multidimensional imaging of macrophages infected with L. amazonensis-WT for previous 48 hours and superinfected with L. major-DsRed2 metacyclic-enriched promastigotes. Arrowhead indicates L. major-DsRed2 promastigote sheltered by tight PV, weakly stained with Lysotracker (green), interacting with large, Lysotracker-positive, L. amazonensis-WT PV (asterisk). In the first row, merged images of Lysotracker and DsRed2 signals show transfer of L. major-DsRed2 promastigote to L. amazonensis PV; time after promastigote addition is shown (h:mm). The second row shows DsRed2 signal, evidencing the transfer of promastigote by parasite posterior pole. The third row shows Lysotracker signal, showing changes in PV shape (bold arrow) to accommodate the incoming promastigote. Images were constructed using Imaris blend filter. Bars = 10 µm. (B) Immunolocalization of LAMP1 in superinfected macrophages; Leishmania major–GFP procyclic promastigotes (arrowheads) were sheltered by LAMP1-positive chimeric PV (asterisk). Image was acquired 48 h after L. major-GFP promastigote addition. LAMP1 immunolabeling in red, GFP in green, DAPI staining in blue. Three dimensional images, constructed by Imaris blend filter, are disposed as merged RGB fluorescence channels, merged RB channels and red channel. Bars = 10 µm. (C) Percentage of L. major-GFP promastigotes within chimeric PVs in fixed, superinfected macrophages. Samples were fixed 12 and 72 hours after addition of L. major promastigotes from metacyclic-enriched or log phase parasite batches. Columns are representative of 10 microscopic fields (under 100x objective) in triplicate samples. There is no statistical difference in the percentage of procyclic or metacyclic-enriched promastigotes within chimeric PVs at 12 hours post-superinfection. At 72 hours, a higher percentage of promastigotes from log phase superinfection batch within chimeric PVs was observed, comparing to superinfection with metacyclic-enriched batches (Univariate ANOVA, p<0.05).
In the early frames of the recording, the L. major promastigote occupied a PV which displayed a weak Lysotracker signal, contrasting with the stronger signal detected in the recipient L. amazonensis PV (Video S2). In subsequent images, the increase in volume occupied by the Lysotracker confirmed that the recipient PV was reshaped in the course of fusion with the L. major promastigote PV (Fig. 2A, bold arrow). The recording also shows that the posterior pole of the L. major promastigote was the first to be transferred to the recipient PV, in the opposite direction to the movement displayed by free Leishmania promastigotes. The duration of the fusion in this sequence was estimated to be around 80 minutes. The completion of fusion was heralded by the motility of the L. major promastigote within the L. amazonensis PV (Video S2).
Immunolabeling of LAMP1 proteins displayed by L. amazonensis PVs confirmed the existence of chimeric vacuoles containing L. major promastigotes from superinfection batches of procyclic (Fig. 2B) or metacyclic-enriched parasites (data not shown).
Delivery of L. major promastigotes to L. amazonensis PVs did not depend on metacyclogenesis, as approximately 10% of promastigotes (from logarithmic phase cultures or metacyclic-enriched baths of promastigotes) developed chimeric PVs in the first 12 hours of superinfection (Fig. 2C). After 72 hours of superinfection, 45.8% (±8.0 s.e.m., n = 3) of promastigotes from metacyclic-enriched batch were found within chimeric PVs as undifferentiated promastigotes; in these samples, we observed L. major amastigotes hosted within unfused donor vacuoles. At the same period after superinfection, 72% (±6.8 s.e.m., n = 3) of promastigotes from log phase batch were found within chimeric PVs, conserving promastigote morphology.
Leishmania major promastigotes increase in number within chimeric PVs
The total number of L. major-DsRed2 promastigotes in superinfected macrophages was quantified by algorithm-based software analysis (Fig. 3). Images were taken from time-lapse recordings of 10 different microscopic fields, at 5 minutes intervals, and the total number of L. major-DsRed2 parasites per field were quantified at each time point (Fig. 3A–B). The number of L. major promastigotes inside chimeric PVs was accessed by direct observation of the time-lapse video records of each acquired microscopic field.
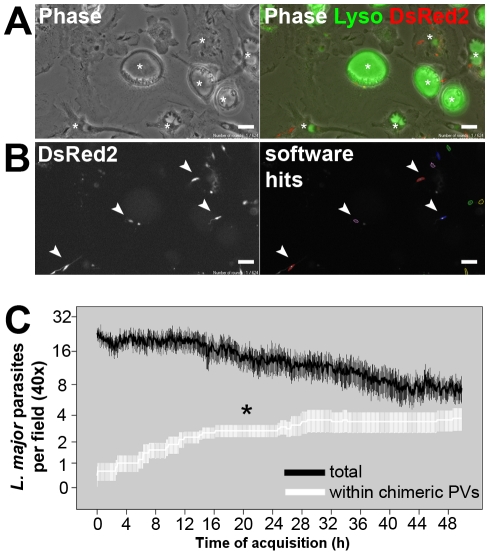
Figure 3. Algorithm-based recognition of L. major-DsRed2 parasites hosted by superinfected macrophages.
(A) Example of an acquired field of macrophages infected for 48 hours with L. amazonensis and superinfected with L. major-DsRed2 metacyclic-enriched promastigotes. First picture is a phase contrast image (Ph2) acquired at 40x magnification, and second is the phase contrast image merged with RG fluorescence channels; Lysotracker in green, L. major-DsRed2 in red. Leishmania amazonensis PVs are indicated by asterisks. (B) Parasite recognition and quantification by Acapella software. The raw data (DsRed2 fluorescence) are shown in the first image and a quantified image is presented in the second (circles represent quantification hits). Examples of parasites recognized by the software are indicated by arrowheads. Bars = 10 µm. (C) Total number of L. major-DsRed2 parasites quantified by software algorithms (black line) and L. major-DsRed2 found within chimeric PVs (white line) quantified by time-lapse videomicrography observation. Acquisition started 2 hours after promastigote addition. Each line represents the mean quantification of 7 microscopic fields (40 x), at logarithmic (base 2) scale, plotted with s.e.m. The total number of promastigotes hosted by superinfected macrophages decreased after approximately 20 hours of experiment. There is a significant increase in the number of L. major parasites found within chimeric PVs in the first 12–20 hours of acquisition (One-way ANOVA with Tamhane's T2, Dunnett's T3 and Games-Howell Post Hoc multiple comparison tests between hourly time points, p<0.05).
While the total number of L. major promastigotes fell, the number of L. major-DsRed2 promastigotes within chimeric PVs increased (Fig. 3C). At 12 hours of image acquisition (16 hours after L. major-DsRed2 promastigote addition), 13.6% (±4.68 s.e.m., n = 7) of promastigotes are found inside chimeric PVs. At 48 hours of acquisition, this percentage raised to 62.79% (±19.9 s.e.m., n = 7). The destruction of L. major promastigote took place exclusively in unfused donor PVs (data not shown). The accumulation of L. major promastigote within chimeric PVs was due to continuous transfer of L. major promastigotes to L. amazonensis PVs in the first 12 hours after superinfection.
Leishmania major promastigotes multiplied within large, acidic L. amazonensis PVs
In the metacyclic-enriched bath of promastigotes administered to macrophages in superinfection, we expected a mixed population of L. major metacyclic and procyclic parasites, which contact and are transferred to L. amazonensis PVs. Thus, we investigated by live imaging if L. major promastigotes within chimeric PVs would be destroyed, behave like procyclic or stationary promastigotes or differentiate into amastigotes.
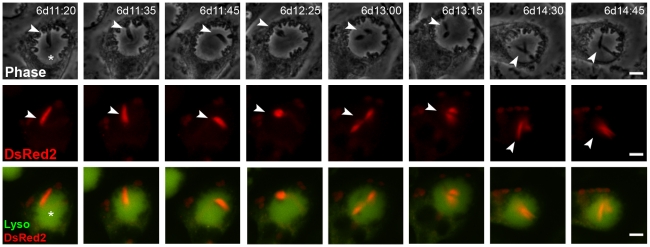
While some promastigotes did not divide, we often observed multiplication of L. major promastigotes within chimeric PVs. In Fig. 4 and Video S3, one L. major-DsRed2 promastigote (6 days after superinfection) was tracked within a large and acidic L. amazonensis PV. The promastigote moved freely in the chimeric vacuoles and kept the DsRed2 fluorescence, then displayed decreased movement and morphological changes at time point 11:45 h; at 12:25 h we identified two promastigote bodies bound by the parasite anterior pole. From time point 13:00 h, the promastigote completed a division, so we could observe two promastigotes moving inside L. amazonensis PV. The division occurred at stabilized temperature of 34°C as shown by temperature log of Nikon Biostation acquisition chamber (data not shown). Division of L. major-DsRed2 promastigotes within chimeric PVs was also observed in other series of images, from 24 hours to 96 hours after promastigotes addition (data not shown).
Figure 4. L. major promastigotes multiply inside chimeric PVs.
Time-lapse recording of macrophages infected with L. amazonensis-WT for 48 hours and superinfected with metacyclic-enriched L. major-DsRed2 promastigotes. Image acquisition started 6 days after L. major-DsRed2 promastigote addition. Division of L. major-DsRed2 promastigote (arrowheads) inside L. amazonensis-WT PV (asterisk) was documented. The figure shows phase contrast (Ph2) in the first row, DsRed2 signal in the second, and Lysotracker merged with DsRed2 signal in the third. Time after promastigote addition is shown (d:h:min). Scale at 10 µm.
To investigate the influence of high temperature and low pH in promastigote multiplication, the growth curves of L. major-DsRed2 promastigotes axenically cultivated under different pH and temperature conditions were compared (Fig. S1). The growth of L. major-DsRed2 promastigote cultures at acidic pH was slower than that at neutral environment at 26°C (paradigmatically the optimal temperature for promastigote cultivation). Morphology and movement were preserved at 26°C, pH 5.0 or 7.2. At 34°C, L. major-DsRed2 promastigotes grew as well in media adjusted to pH 5.0 or 7.2, with no apparent difference for 3–4 days. After that time, at 34°C at pH 5.0 or 7.2, L. major promastigotes enter death phase, presenting altered morphology and DsRed2 emission, and presence of debris.
The growth kinetics of L. major-DsRed2 promastigotes within chimeric vacuoles was not directly demonstrated. However, in additional experiments, L. major promastigotes were isolated from coinfected macrophages after 3 or 5 days of superinfection with log-phase L. major promastigotes. The infectivity of the isolated parasites was tested on fresh macrophage cultures; it was found that L. major promastigotes isolated from coinfected macrophages were infective and that the infectivity was higher at 5 days than at 3 days of coinfection (unpublished data).
Differentiation of L. major promastigotes into amastigotes did not take place within chimeric PVs
We tracked L. major-DsRed2 metacyclic-enriched promastigotes hosted by superinfected macrophage and we observed differentiation into amastigotes forms exclusively within unfused donor PVs. We compared the morphology of L. major parasites within chimeric or donor PVs by multidimensional imaging (Fig. 5). Software surface rendering allowed measurement of parasite features such as volume and sphericity that were used as markers of promastigote-to-amastigote differentiation. Within donor vacuoles, the increase in sphericity and decrease in volume occurs in approximately 2 hours (data not shown). Within chimeric PVs, promastigotes maintained their initial volume and sphericity measurements, and displayed typical promastigote morphology, i.e., flagellated and elongated (Fig. 5A).
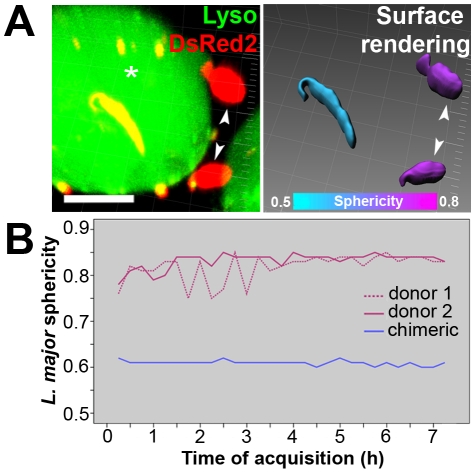
Figure 5. Multidimensional image of metacyclic-enriched L. major-DsRed2 promastigotes in superinfected macrophages.
(A) On the left, multidimensional image of a chimeric PV (asterisk) and L. major-DsRed2 parasites sheltered by unfused donor PVs (arrowheads); Imaris MIP filter. On the right, surface rendering of parasites through DsRed2 channel allowed the software to assign a colorimetric scale to each L. major-DsRed2 parasite: it displays the sphericity parameter, ranging from cyan (less spherical, 0.5) to magenta (more spherical, 0.8); Imaris blend filter. Images were acquired 24 hours after addition of L. major-DsRed2 metacyclic-enriched promastigotes to macrophages. Bar = 10 µm. (B) Sphericity measurements during coinfection, presented by L. major-DsRed2 parasites hosted within unfused donor PVs (magenta lines) or within chimeric PV (blue line). Acquisition of multidimensional images started 12 hours after L. major-DsRed2 metacyclic-enriched promastigotes were added to macrophages.
This site-dependent morphology was maintained for several hours in superinfected macrophages (Fig. 5C). Leishmania major-DsRed2 promastigotes in donor PVs presented a sphericity of 0.8–0.85 while promastigotes within chimeric PVs remained elongated (with sphericity near 0.6) and displayed flagellar movement. Non-dividing L. major promastigotes within chimeric PVs were tracked for 48 hours through live imaging; they kept flagellar movement and elongated morphology, with no apparent signs of differentiation (data not shown). Immunolabeling of LAMP1 in macrophages superinfected with L. major metacyclic-enriched promastigotes for 6 days revealed the presence of amastigotes exclusively in unfused membrane-bound PVs (data not shown).
Discussion
We have shown that the PVs containing L. major amastigotes adhered to but did not fuse with preformed L. amazonensis spacious vacuoles. In contrast, L. major log phase promastigotes and promastigote suspensions enriched in metacyclic parasites were delivered by vacuolar fusion to L. amazonensis PVs. In the chimeric vacuoles thus formed, L. major promastigotes multiplied but did not differentiate into amastigotes, whereas in the same macrophages, L. major promastigotes sheltered in their own vacuoles, either died or differentiated into amastigotes. The biochemical and molecular mechanisms that underlie the lack of fusion of incoming L. major amastigote-containing PVs with L. amazonensis vacuoles and the permissiveness of the latter for fusion with L. major promastigote-carrying PVs, remain to be elucidated.
In contrast with the observation of fusion between intraspecific L. amazonensis PVs [20], the present results show that L amazonensis amastigote-PVs did not fuse with incoming PVs that contained L. major amastigotes (Fig. 1 and Video S1). In the coinfected cells, L. major PVs kept their usual morphology and the parasites multiplied as they did in monoinfected cells. Although Rab5 has been assumed to mediate homotypic fusion of early Rab5-positive L. mexicana PVs [13], we did not detect homotypical fusion between Rab7/LAMP1/LAMP2-positive PVs that sheltered L. major or L. amazonensis amastigotes. Thus, Rab7 may be responsible for the close contact observed between interspecific PVs, but their fusion may require additional factors.
Contrasting with the lack of fusion of L. major amastigotes-containing PVs with preformed L. amazonensis PVs, about 10% of incoming L. major promastigotes (from either log-phase or metacyclic-enriched populations) were found within L. amazonensis PVs. Multidimensional images allowed for the spatial visualization of fusion events in the first 12 hours after superinfection with promastigotes (Fig. 2–3 and Video S2). Thus, interspecific fusion of PVs is parasite-stage dependent.
Interspecific fusion was assumed to be regulated by parasite surface ligands, such as small size glycoconjugates [27], [28], [29], and/or by parasite-secreted macromolecules inserted in PV membranes and/or targeted to the cytosol [30]. The composition and fusogenicity of Leishmania PVs may also depend on the relative contribution to the PV membranes of the plasma membrane, endocytic and autophagic vesicles and/or endoplasmic reticulum [31].
Parasitophorous vacuoles of different Leishmania species have been isolated and compositional studies were initiated [32], [33], [34]. We believe that the characterization of macromolecules and other factors involved in fusion between PVs will require in vitro reconstitution of fusion of isolated Leishmania PVs [35], [36], [37], [38].
Rather surprisingly, L. major promastigotes that reached L. amazonensis PVs, instead of differentiating into amastigote stages or being destroyed by an acidic, phagolysosome-like environment, survived and multiplied while retaining the promastigote morphology and flagellar movement (Fig. 4 and Video S3). This unprecedented observation stands in marked contrast with the results of the intraspecific model, in which Leishmania amazonensis stationary-phase promastigotes survived, did not multiply and differentiated into amastigotes within PVs previously formed by the same species [20].
The extended survival of log-phase L. major procyclic promastigotes in chimeric PVs contrasts with the rapid destruction of log-phase promastigotes in their own, unfused vacuoles. It has been proposed that parasites sheltered in large vacuoles are protected from macrophage microbicidal effectors [26], [39], [40]. Additionally, proteophosphoglycans (PPG) secreted by L. mexicana amastigotes, were shown to attenuate macrophage leishmanicidal activity [27]. It is thus, conceivable that PPG secreted by L. amazonensis amastigotes could account for the survival of L. major log-phase promastigotes in chimeric PVs.
When metacyclic-enriched populations of L. major promastigotes were added to macrophages previously infected with L. amazonensis, differentiation of L. major was only observed within their own unfused, membrane-bound vacuoles (Fig. 5). Stationary-phase L. major promastigotes were found moving within chimeric PVs during time-lapse image acquisitions. The reasons for the lack of L. major differentiation are not understood. One possibility is that the pH within the L. amazonensis PVs is not optimal for differentiation of the L. major promastigotes; another is that such differentiation could require the close contact of the L. major parasites with their vacuolar membranes.
The interspecific PV fusion and transfer of L. major promastigotes into L. amazonensis PVs provides an additional answer to the recurrent question: “to what extent can a microorganism survive and multiply in vacuoles customized by a different pathogen?” There is, however, no reason to assume that a general answer will be necessarily found.
It has been assumed that genetic exchange, rarely found between Leishmania species, might take place in doubly infected vectors [41]. Patients infected with two different Leishmania species have been described in the literature [42]. If the present in vitro findings would mimic the in vivo situation, the isolation of amastigotes in parasite species-specific PVs could restrict the genetic exchange and Leishmania speciation to mixed populations of promastigotes in insect-vectors.
Finally, our results emphasize the usefulness of continuous live recordings in studies of intracellular parasitism [43]. It is hoped that additional experiments will map the fusogenicity of the spacious vacuoles of the mexicana group with each other and with other Leishmania species confined to small parasitophorous vacuoles.
Supporting Information
Algorithm-based recognition of L. major-DsRed2 promastigotes axenically cultivated. (A) Parasite recognition and quantification by Image Pro Plus Software. The raw data are shown on the left (parasites DsRed2 fluorescence) and quantified image on the right (crosses represent quantification hits). Bars = 20 µm. (B–C) Growth curves of L. major-DsRed2 promastigotes at 34°C or 26°C, and pH 7.2 (B) or 5.0 (C). Parasites were counted by software per microscopic field and the numbers were normalized to a 10x field. Each line is representative of 10 microscopic fields per condition, with triplicates. Graphs are associated to images showing the morphological aspect of L. major-DsRed2 promastigotes after 4 and 10 days of cultivation at 34°C. Scale at 20 µm.
(0.47 MB TIF)
Interaction between L. amazonensis vacuoles and L. major amastigote PVs. (A) Live imaging of macrophages previously infected with L. amazonensis-WT for 48 h and then superinfected with L. major-DsRed2 amastigotes. Video shows a macrophage, loaded with Lysotracker (green) and hosting the two species, at phase contrast and fluorescence merged channels. Image acquisition started at 72 hours after L. major-DsRed2 amastigote addition. Time after L. major-DsRed2 amastigote addition is shown (d:h:min:sec). Bar = 20 µm. (B) Live multidimensional imaging of macrophages previously infected with L. amazonensis-WT for 48 h and then superinfected with L. major-DsRed2 amastigotes. Leishmania major-DsRed2 amastigotes, sheltered by tight PV, weakly stained with Lysotracker (green), were observed at the periphery of large, Lysotracker-positive, L. amazonensis-WT PV. Multidimensional images were constructed using Imaris blend filter, and rotated in different angles. Time after L. major-DsRed2 amastigote addition is shown (d:h:min). Bar = 5 µm.
(2.18 MB MOV)
Fusion between L. amazonensis and L. major PVs recorded by multidimensional imaging of macrophages previously infected with L. amazonensis-WT for 48 hours and then superinfected with L. major-DsRed2 metacyclic-enriched promastigotes. (A) Leishmania major-DsRed2 promastigote (red), sheltered within tight PV, weakly stained with Lysotracker (green), fused with large, Lysotracker-positive, L. amazonensis-WT PV. (B) Lysotracker channel, showing changes in PV shape and remodeling at the end of process. (C) Promastigote DsRed2 channel, showing the transfer of promastigote by parasite posterior pole. (D) Image rotation for visualization of fusion process from the bottom of the sample. Time after L. major-DsRed2 promastigote addition is shown (h:min). Multidimensional images were constructed using Imaris blend filter. Bar = 10 µm.
(0.82 MB MOV)
Multiplication of L. major-DsRed2 promastigote within chimeric PV. Time-lapse recording of macrophages previously infected with L. amazonensis-WT for 48 hours and then superinfected with metacyclic-enriched L. major-DsRed2 promastigotes. Image acquisition started 6 days after L. major-DsRed2 promastigote addition. (A) Phase contrast (Ph2); (B) DsRed2 channel; (C) Lysotracker channel (green) merged with L. major-DsRed2 channel (red); (D) all channels merged. Time after promastigote addition is shown (d:h:min:sec). Bar = 10 µm.
(1.60 MB MOV)
Acknowledgments
This work was mainly developed at Plate-form Imagerie Dynamique (PFID), Institut Pasteur, France. We would like to thank Dr. Spencer Shorte, Emmanuelle Perret (live cell recordings at Nikon Biostation), Christophe Machu (multidimensional imaging at Spinning disk system), Anne Danckaert (algorithm-based quantifications) and Pascal Roux (confocal imaging at IP). We also thank, Dr. Eric Prina, Dr. Thierry Lang and Dr. Marcello Barcinski for fruitful discussions, insights and brainstormed coffee-breaks. Finally, the authors express their immense gratitude to Dr. Geneviève Milon, for her generosity and rigorous advisorship.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by funds from Institut Pasteur AMMRAA DAMD1 02 0018 (PI G. Milon), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Brazil. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moulder JW. Comparative biology of intracellular parasitism. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veras PS, Moulia C, Dauguet C, Tunis CT, Thibon M, et al. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect Immun. 1995;163:3502–3506. doi: 10.1128/iai.63.9.3502-3506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovitch M, Veras PS. Cohabitation of Leishmania amazonensis and Coxiella burnetii. Trends Microbiol. 1996;4:158–161. doi: 10.1016/0966-842x(96)10027-5. [DOI] [PubMed] [Google Scholar]
- 4.de Chastellier C, Thibon M, Rabinovitch M. Construction of chimeric phagosomes that shelter Mycobacterium avium and Coxiella burnetii (phase II) in doubly infected mouse macrophages: an ultrastructural study. Eur J Cell Biol. 1999;78:580–592. doi: 10.1016/S0171-9335(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 5.Andreoli WK, Taniwaki NN, Mortara RA. Survival of Trypanosoma cruzi metacyclic trypomastigotes within Coxiella burnetii vacuoles: differentiation and replication within an acidic milieu. Microbes Infect. 2006;8:172–182. doi: 10.1016/j.micinf.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Alexander J, Satoskar AR, Russell DG. Leishmania species, models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 7.Alexander J, Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana infected macrophages. J Protozool. 1975;22:502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- 8.Berman JD, Fioretti TB, Dwyer DM. In vivo and in vitro localization of Leishmania within macrophage phagolysosomes: use of colloidal gold as a lysosomal label. J Protozool. 1981;28:239–242. doi: 10.1111/j.1550-7408.1981.tb02839.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd VL, Stahl PD, Bernd P, Rabinovitch M. Receptor-mediated entry of β-glucuronidase into the parasitophorous vacuoles of macrophages infected with Leishmania mexicana amazonensis. J Exp Med. 1983;157:1471–1482. doi: 10.1084/jem.157.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoine JC, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;7:392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 11.Duclos S, Diez R, Garin J, Papadopoulou B, Descoteaux A, et al. Rab5 regulates the kiss and run fusion between phagosomes and endosomes and the acquisition of phagosome leishmanicidal properties in RAW 264.7 macrophages. J Cell Sci. 2000;113:3531–3541. doi: 10.1242/jcs.113.19.3531. [DOI] [PubMed] [Google Scholar]
- 12.Courret N, Frehel C, Gouhier N, Pouchelet M, Prina E, et al. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasite. J Cell Sci. 2002;115:2303–2316. doi: 10.1242/jcs.115.11.2303. [DOI] [PubMed] [Google Scholar]
- 13.Lippuner C, Paape D, Paterou A, Brand J, Richardson M, et al. Real-time imaging of Leishmania mexicana-infected early phagosomes: a study using primary macrophages generated from green fluorescent protein-Rab5 transgenic mice. FASEB J. 2009;23:483–491. doi: 10.1096/fj.08-108712. [DOI] [PubMed] [Google Scholar]
- 14.Antoine JC, Prina E, Jouanne C, Bongrand P. Parasitophorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect Immun. 1990;58:779–787. doi: 10.1128/iai.58.3.779-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veras PS, de Chastellier C, Rabinovitch M. Transfer of zymosan (yeast cell walls) to the parasitophorous vacuoles of macrophages infected with Leishmania amazonensis. J Exp Med. 1992;176:639–646. doi: 10.1084/jem.176.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell DG, Xu S, Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J Cell Sci. 1992;103:1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- 17.Collins HL, Schaible UE, Ernst JD, Russell DG. Transfer of phagocytosed particles to the parasitophorous vacuole of Leishmania mexicana is a transient phenomenon preceding the acquisition of annexin I by the phagosome. J Cell Sci. 1997;110:191–200. doi: 10.1242/jcs.110.2.191. [DOI] [PubMed] [Google Scholar]
- 18.Schaible UE, Schlesinger PH, Steinberg TH, Mangel WF, Kobayashi T, et al. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J Cell Sci. 1999;112:681–693. doi: 10.1242/jcs.112.5.681. [DOI] [PubMed] [Google Scholar]
- 19.Veras PS, Topilko A, Gouhier N, Moreau MF, Rabinovitch M, et al. Fusion of Leishmania amazonensis parasitophorous vacuoles with phagosomes containing zymosan particles: cinemicrographic and ultrastructural observations. Braz J Med Biol Res. 1996;29:1009–1018. [PubMed] [Google Scholar]
- 20.Real F, Pouchelet M, Rabinovitch M. Leishmania (L.) amazonensis: fusion between parasitophorous vacuoles in infected bone-marrow derived mouse macrophages. Exp Parasitol. 2008;119:15–23. doi: 10.1016/j.exppara.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Chang KP, Dwyer DM. Leishmania donovani. Hamster macrophage interactions in vitro: cell entry, intracellular survival, and multiplication of amastigotes. J Exp Med. 1978;147:515–530. doi: 10.1084/jem.147.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro R, Scott K, Jordan T, Evans B, Craig J, et al. The ultrastructure of the parasitophorous vacuole formed by Leishmania major. J Parasitol. 2006;92:1162–1170. doi: 10.1645/GE-841R.1. [DOI] [PubMed] [Google Scholar]
- 23.Körner U, Fuss V, Steigerwald J, Moll H. Biogenesis of Leishmania major-harboring vacuoles in murine dendritic cells. Infect Immun. 2006;74:1305–1312. doi: 10.1128/IAI.74.2.1305-1312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Späth GF, Schlesinger P, Schreiber R, Beverley SM. A novel role for Stat1 in phagosome acidification and natural host resistance to intracellular infection by Leishmania major. PLoS Pathog. 2009;5:e1000381. doi: 10.1371/journal.ppat.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Späth GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 26.Zamboni DS, Rabinovitch M. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect Immun. 2003;71:1225–1233. doi: 10.1128/IAI.71.3.1225-1233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters C, Stierhof YD, Ilg T. Proteophosphoglycan secreted by Leishmania mexicana amastigotes causes vacuole formation in macrophages. Infect Immun. 1997;65:783–786. doi: 10.1128/iai.65.2.783-786.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scianimanico S, Desrosiers M, Dermine JF, Méresse S, Descoteaux A, et al. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell Microbiol. 1999;1:19–32. doi: 10.1046/j.1462-5822.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 29.Vinet AF, Fukuda M, Turco SJ, Descoteaux A. The Leishmania donovani lipophosphoglycan excludes the vesicular proton-ATPase from phagosomes by impairing the recruitment of synaptotagmin V. PLoS Pathog. 2009;5:e1000628. doi: 10.1371/journal.ppat.1000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, et al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 31.Ndjamen B, Kang BH, Hatsuzawa K, Kima PE. Leishmania parasitophorous vacuoles interact continuously with the host cell's endoplasmic reticulum; parasitophorous vacuoles are hybrid compartments. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01483.x. doi: 10.1111/j.1462-5822.2010.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cell Microbiol. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 33.Kima PE, Dunn W. Exploiting calnexin expression on phagosomes to isolate eishmania parasitophorous vacuoles. Microb Pathog. 2005;38:139–145. doi: 10.1016/j.micpath.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Cortázar TM, Hernández J, Echeverry MC, Camacho M. Role of the parasitophorous vacuole of murine macrophages infected with Leishmania amazonensis in molecule acquisition. Biomedica. 2006;26:26–37. [PubMed] [Google Scholar]
- 35.Oates PJ, Touster O. In vitro fusion of Acanthamoeba phagolysosomes. III. Evidence that cyclic nucleotides and vacuole subpopulations respectively control the rate and the extent of vacuole fusion in Acanthamoeba homogenates. J Cell Biol. 1980;85:804–810. doi: 10.1083/jcb.85.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer A. Membrane fusion in eukaryotic cells. Annu Rev Cell Dev Biol. 2002;18:289–314. doi: 10.1146/annurev.cellbio.18.032202.114809. [DOI] [PubMed] [Google Scholar]
- 37.Haluska CK, Riske KA, Marchi-Artzner V, Lehn JM, Lipowsky R, et al. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci U S A. 2006;103:15841–15846. doi: 10.1073/pnas.0602766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott P, Sher A. A spectrum in the susceptibility of leishmanial strains to intracellular killing by murine macrophages. J Immunol. 1986;136:1461–1466. [PubMed] [Google Scholar]
- 40.Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, et al. Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science. 2009;324:265–268. doi: 10.1126/science.1169464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastrenta B, Mita N, Buitrago R, Vargas F, Flores M, et al. Human mixed infections of Leishmania spp. And Leishmania-Trypanosoma cruzi in a sub Andean Bolivian area: identification by polymerase chain reaction/hybridization and isoenzyme. Mem Inst Oswaldo Cruz. 2003;98:255–264. doi: 10.1590/s0074-02762003000200015. [DOI] [PubMed] [Google Scholar]
- 43.Lang T, Lecoeur H, Prina E. Imaging Leishmania development in their host cells. Trends Parasitol. 2009;25:464–473. doi: 10.1016/j.pt.2009.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Algorithm-based recognition of L. major-DsRed2 promastigotes axenically cultivated. (A) Parasite recognition and quantification by Image Pro Plus Software. The raw data are shown on the left (parasites DsRed2 fluorescence) and quantified image on the right (crosses represent quantification hits). Bars = 20 µm. (B–C) Growth curves of L. major-DsRed2 promastigotes at 34°C or 26°C, and pH 7.2 (B) or 5.0 (C). Parasites were counted by software per microscopic field and the numbers were normalized to a 10x field. Each line is representative of 10 microscopic fields per condition, with triplicates. Graphs are associated to images showing the morphological aspect of L. major-DsRed2 promastigotes after 4 and 10 days of cultivation at 34°C. Scale at 20 µm.
(0.47 MB TIF)
Interaction between L. amazonensis vacuoles and L. major amastigote PVs. (A) Live imaging of macrophages previously infected with L. amazonensis-WT for 48 h and then superinfected with L. major-DsRed2 amastigotes. Video shows a macrophage, loaded with Lysotracker (green) and hosting the two species, at phase contrast and fluorescence merged channels. Image acquisition started at 72 hours after L. major-DsRed2 amastigote addition. Time after L. major-DsRed2 amastigote addition is shown (d:h:min:sec). Bar = 20 µm. (B) Live multidimensional imaging of macrophages previously infected with L. amazonensis-WT for 48 h and then superinfected with L. major-DsRed2 amastigotes. Leishmania major-DsRed2 amastigotes, sheltered by tight PV, weakly stained with Lysotracker (green), were observed at the periphery of large, Lysotracker-positive, L. amazonensis-WT PV. Multidimensional images were constructed using Imaris blend filter, and rotated in different angles. Time after L. major-DsRed2 amastigote addition is shown (d:h:min). Bar = 5 µm.
(2.18 MB MOV)
Fusion between L. amazonensis and L. major PVs recorded by multidimensional imaging of macrophages previously infected with L. amazonensis-WT for 48 hours and then superinfected with L. major-DsRed2 metacyclic-enriched promastigotes. (A) Leishmania major-DsRed2 promastigote (red), sheltered within tight PV, weakly stained with Lysotracker (green), fused with large, Lysotracker-positive, L. amazonensis-WT PV. (B) Lysotracker channel, showing changes in PV shape and remodeling at the end of process. (C) Promastigote DsRed2 channel, showing the transfer of promastigote by parasite posterior pole. (D) Image rotation for visualization of fusion process from the bottom of the sample. Time after L. major-DsRed2 promastigote addition is shown (h:min). Multidimensional images were constructed using Imaris blend filter. Bar = 10 µm.
(0.82 MB MOV)
Multiplication of L. major-DsRed2 promastigote within chimeric PV. Time-lapse recording of macrophages previously infected with L. amazonensis-WT for 48 hours and then superinfected with metacyclic-enriched L. major-DsRed2 promastigotes. Image acquisition started 6 days after L. major-DsRed2 promastigote addition. (A) Phase contrast (Ph2); (B) DsRed2 channel; (C) Lysotracker channel (green) merged with L. major-DsRed2 channel (red); (D) all channels merged. Time after promastigote addition is shown (d:h:min:sec). Bar = 10 µm.
(1.60 MB MOV)