The study of long-lived C. elegans mutants suggests that mitochondrial oxidants can actually help reduce aging by acting as stress signals, rather than acting solely as toxic molecules.
Abstract
The nuo-6 and isp-1 genes of C. elegans encode, respectively, subunits of complex I and III of the mitochondrial respiratory chain. Partial loss-of-function mutations in these genes decrease electron transport and greatly increase the longevity of C. elegans by a mechanism that is distinct from that induced by reducing their level of expression by RNAi. Electron transport is a major source of the superoxide anion (O⋅ –), which in turn generates several types of toxic reactive oxygen species (ROS), and aging is accompanied by increased oxidative stress, which is an imbalance between the generation and detoxification of ROS. These observations have suggested that the longevity of such mitochondrial mutants might result from a reduction in ROS generation, which would be consistent with the mitochondrial oxidative stress theory of aging. It is difficult to measure ROS directly in living animals, and this has held back progress in determining their function in aging. Here we have adapted a technique of flow cytometry to directly measure ROS levels in isolated mitochondria to show that the generation of superoxide is elevated in the nuo-6 and isp-1 mitochondrial mutants, although overall ROS levels are not, and oxidative stress is low. Furthermore, we show that this elevation is necessary and sufficient to increase longevity, as it is abolished by the antioxidants NAC and vitamin C, and phenocopied by mild treatment with the prooxidant paraquat. Furthermore, the absence of effect of NAC and the additivity of the effect of paraquat on a variety of long- and short-lived mutants suggest that the pathway triggered by mitochondrial superoxide is distinct from previously studied mechanisms, including insulin signaling, dietary restriction, ubiquinone deficiency, the hypoxic response, and hormesis. These findings are not consistent with the mitochondrial oxidative stress theory of aging. Instead they show that increased superoxide generation acts as a signal in young mutant animals to trigger changes of gene expression that prevent or attenuate the effects of subsequent aging. We propose that superoxide is generated as a protective signal in response to molecular damage sustained during wild-type aging as well. This model provides a new explanation for the well-documented correlation between ROS and the aged phenotype as a gradual increase of molecular damage during aging would trigger a gradually stronger ROS response.
Author Summary
An unequivocal demonstration that mitochondria are important for lifespan comes from studies with the nematode Caenorhabditis elegans. Mutations in mitochondrial proteins such as ISP-1 and NUO-6, which function directly in mitochondrial electron transport, lead to a dramatic increase in the lifespan of this organism. One theory proposes that toxicity of mitochondrial reactive oxygen species (ROS) is the cause of aging and predicts that the generation of the ROS superoxide should be low in these mutants. Here we have measured superoxide generation in these mutants and found that it is in fact elevated, rather than reduced. Furthermore, we found that this elevation is necessary and sufficient for longevity, as it is abolished by antioxidants and induced by mild treatment with oxidants. This suggests that superoxide can act as a signal triggering cellular changes that attenuate the effects of aging. This idea suggests a new model for the well-documented correlation between ROS and the aged phenotype. We propose that a gradual increase of molecular damage during aging triggers a concurrent, gradually intensifying, protective superoxide response.
Introduction
Mitochondrial function has been linked to the aging process in a number of ways [1]. In particular, mitochondria are crucial in energy metabolism and as such have been implicated in the aging process by one of the very first theories of aging [2], the rate-of-living theory of aging [3], which suggested that the rate of aging is proportional to the rate of energy metabolism (reviewed in [4]). Mitochondrial function in animals is also known to decline with age [5],[6], which, together with the finding that mitochondria are an important source of toxic reactive oxygen species (ROS), has led to the oxidative stress (or free radical) theory of aging [7],[8].
Two types of mutations that affect mitochondrial function have been found to affect the rate of aging in C. elegans, mutations that shorten lifespan, such as mev-1 [9] and gas-1 [10], and mutations that lengthen lifespan, such as clk-1 [11], isp-1 [12], lrs-2 [13], and nuo-6 [14]. lrs-2 encodes a mitochondrial leucyl-tRNA-synthetase, and its effect on the function of mitochondrial electron transport is likely relatively indirect, via partial impairment of mitochondrial translation. However, clk-1 encodes an enzyme necessary for the biosynthesis of ubiquinone, a lipid antioxidant and an electron transporter of the respiratory chain [15], and mev-1, gas-1, isp-1, and nuo-6 all encode subunits of mitochondrial respiratory complexes. On the strength of the oxidative stress theory of aging it has been suggested, and supported by a number of observations (reviewed in [16],[17]), that the mev-1 and gas-1 mutations reduce lifespan by increasing mitochondrial oxidative stress, and clk-1, isp-1, and nuo-6 increase lifespan by reducing it.
In addition to genomic mutations that affect mitochondrial proteins, it has been found that knockdown by RNA interference of C. elegans genes that encode subunits of mitochondrial complexes, including isp-1 and nuo-6, also prolongs lifespan [13],[18],[19]. Although the effect of RNAi on ETC subunits, which is conserved in Drosophila [20], was initially believed to be similar to that of the mutations [21],[22],[23], it was recently found that it is in fact distinct and separable [14].
A recent study analyzed patterns of gene expression in isp-1 mutants together with those in clk-1 and cyc-1(RNAi) [23] and suggested that the overlap between these patterns could define the biochemical processes that underlie the effect of all interventions that impact mitochondria. However, our recent findings that isp-1(qm150) and isp-1(RNAi) trigger fully separable mechanisms suggests that the overlapping gene expression changes identified by Cristina et al. [23] might not be sufficient to prolong lifespan. Rather some of the gene expression changes that are specific to each type of intervention are necessary for their effect on lifespan and can act additively.
isp-1 mutants show a trend toward low levels of oxidative damage to proteins, increased expression of the cytoplasmic Cu/Zn superoxide dismutase (SOD-1) and of the mitochondrial Mn superoxide dismutase (SOD-2) [24], and increased resistance to acute treatment with the prooxidant paraquat [14]. However, although knocking down the genes encoding the major superoxide dismutase by RNAi results in normal or elevated levels of oxidative damage, it had no effect on the lifespan of the mutants [24], suggesting that the reduced oxidative damage found in isp-1 mutants is not responsible for their longevity. Furthermore, the notion that mitochondrial oxidative stress could be the cause of aging has recently been challenged by a number of studies in C. elegans [24],[25],[26],[27],[28], in Drosophila [29], and in mice (reviewed in [30]).
ROS are not just toxic metabolites that lead to oxidative stress but are also signaling molecules that are believed to be involved in a mitochondria-to-nucleus signaling pathway that could impact aging [1],[31],[32],[33]. Interfering with mitochondrial function has the potential to alter the rate and/or the pattern of production of ROS by mitochondria, including in counter-intuitive ways. For example, reducing oxygen concentration increases ROS production by mitochondrial complex III in vertebrate cells [34],[35], and the knockout of sod-2 in C. elegans can lead to normal [25] or increased lifespan in spite of increased oxidative damage [26]. Here we examined ROS production by mitochondrial mutants and found that isp-1 and nuo-6 mutants have increased generation of the superoxide anion but not increased levels of other ROS and that this increase is necessary and sufficient for longevity, suggesting that superoxide triggers mechanisms that slow down aging, presumably at the level of gene expression.
Results
isp-1, nuo-6, and daf-2 Mutant Mitochondria Display Elevated Generation of Superoxide But Not of Overall ROS
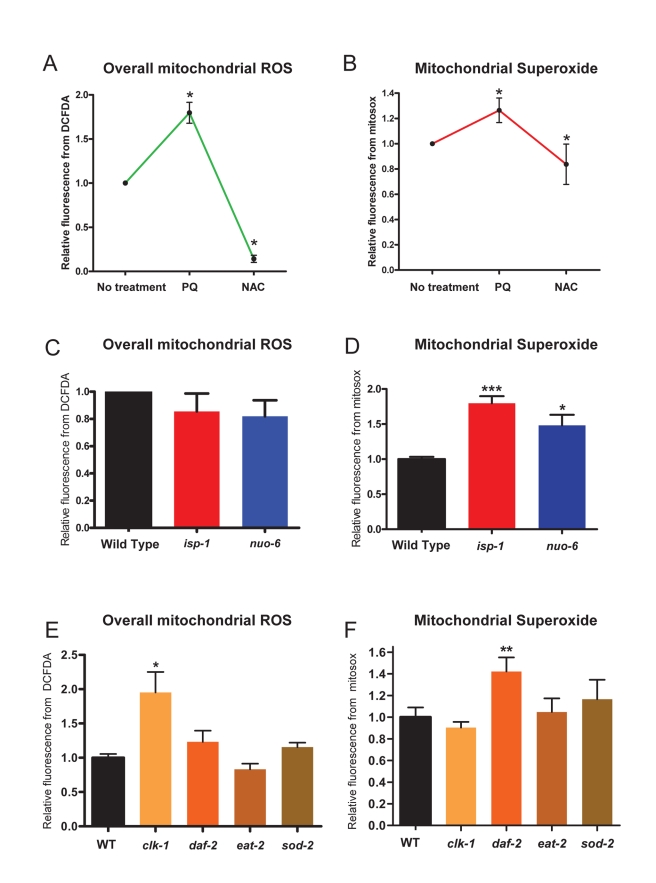
To measure changes in mitochondrial ROS generation that could affect signaling, it is not adequate to measure the level of ROS damage, as a change in ROS damage levels can be brought about by changes in detoxification of ROS, in protein turnover, or in damage repair. However, it is notoriously difficult to directly visualize or measure ROS generation and ROS levels in intact organisms including in living worms. To overcome this difficulty we have adapted a technique originally developed for vertebrates that uses flow cytometry to sort isolated intact mitochondria and measure ROS levels with indicator dyes (Figure S1) [37]. Mitochondria were extracted from worms by standard techniques and loaded with either one of two fluorescent indicator dyes, H2DCFDA, a dye that is sensitive to a variety of ROS but rather insensitive to superoxide [38],[39], and MitoSox, a dye that is exclusively sensitive to superoxide [40]. The prooxidant paraquat (PQ) induces mitochondrial superoxide generation [41], and the antioxidant N-acetyl-cysteine (NAC) has an antioxidant effect on all types of ROS [42],[43]. As expected, when purified mitochondria were treated with PQ, the fluorescence of both H2DCFDA and MitoSox increased, and the fluorescence of both decreased when treated with NAC (Figures 1A, 1B, and S1B). One limitation of this technique is the need for a rather large amount of mitochondria. For example, a sufficient amount of worms is not readily obtained from worms treated by RNAi, and we have therefore focused on long-lived mutants only.
Figure 1. Reactive oxygen species (ROS) in isolated mitochondria of long-lived mutants and in response to paraquat (PQ) and N-acetyl-cysteine (NAC) treatment.
Global ROS levels were measured by quantifying the fluorescence of the reporter dye H2DCFDA, and superoxide with the dye MitoSox, in FACS-sorted mitochondria. Values are normalized to the value of the untreated sample or the wild type. PQ and NAC, respectively, increase and decrease the levels of both global ROS (A) and superoxide (B). Mitochondria isolated from isp-1(qm150) and nuo-6(qm200) mutants show slightly decreased global ROS generation (C) but significantly increased superoxide generation (D). Mitochondria from clk-1 mutants, but not from daf-2(e1370), eat-2(ad1116), and sod-2(ok1030) mutants, show significantly increased global ROS levels (E). Mitochondria from daf-2 mutants, but not from clk-1, eat-2, and sod-2 mutants, show increased superoxide levels (F). * p<0.05, ** p<0.001, *** p<0.0001, by the paired t test.
We used the cytometry technique to determine the generation of mitochondrial superoxide and of overall mitochondrial ROS in a number of long-lived mutants. Both isp-1 and nuo-6 mutations did not affect H2DCFDA fluorescence (overall ROS) significantly, but both showed elevated MitoSox fluorescence (superoxide) (Figure 1C and 1D). Mutants of four other genes (clk-1(qm30), eat-2(ad1116), daf-2(e1370), and sod-2(ok1030)) were also tested (Figure 1E and 1F). clk-1 mutants showed an elevation of overall ROS-associated fluorescence but not of superoxide-associated fluorescence. daf-2 mutants were most similar to the mitochondrial respiratory chain mutants with an elevation of superoxide-associated fluorescence but no significant elevation in overall ROS-associated fluorescence. Finally, eat-2 and sod-2 mutants showed no significant elevation in either signal but only a trend for low overall ROS in the case of eat-2 mutants and a trend for increased superoxide in the case of sod-2 mutants.
The elevated MitoSox signal in isp-1, nuo-6, and daf-2 corresponds mostly to increased superoxide generation, as all three mutants are known for elevated levels of the mitochondrial SOD-2 and SOD-3 [12],[14],[24],[44], whose activity would prevent the accumulation of superoxide. Elevated superoxide detoxification, however, should not prevent measuring increased superoxide generation as superoxide is generated at prosthetic electron carriers such as ubiquinone in complex III [45],[46] and FMN in complex I [47],[48], which are at least partially buried in the complexes. Thus a small molecular weight dye that has access to these sites can trap the superoxide before it has the opportunity to diffuse toward the SOD-2 and SOD-3 proteins. There is no increase in the H2DCFDA signal in these mutants likely because this dye is not particularly sensitive to superoxide [49]. It appears therefore that in the presence of efficient detoxification the level of overall ROS is not significantly increased by the increased superoxide generation that we observe. This is consistent with the finding that these mutants do not have increased oxidative damage [14],[24].
sod-2 deletion mutants do not show a significant increase in the MitoSox signal (Figure 1E and 1F), indicating that decreased detoxification does not lead to an easily measurable increase in this signal in purified mitochondria. The signal from H2DCFDA, a dye which has very broad sensitivity but is not very sensitive to superoxide [49], is also unchanged, suggesting that, at least in isolated worm mitochondria, electron transport is not the main source of the type of ROS to which H2DCFDA dye is significantly sensitive. The level of superoxide generation in these mutants might also be kept moderately low because of their reduced electron transport [26], although low electron transport could in principle also result in elevated superoxide as we have observed in isp-1 and nuo-6 mutants.
clk-1 mutants have only a small deficit in electron transport [24],[50],[51], in spite of a strongly altered content in quinones [51],[52],[53],[54]. Indeed, while wild-type animals contain endogenously synthesized UQ9 as well as a small amount of dietary bacterial UQ8, clk-1 mutants contain only the dietary ubiquinone and no UQ9. Here we found that clk-1 mutants have normal superoxide generation but enhanced overall ROS levels, which suggests that the antioxidant function of UQ9 is a crucial sink for mitochondrial ROS, whose absence appears to lead to an increase of overall ROS even in the absence of increase superoxide generation. eat-2 mutants are long-lived because of reduced food intake (dietary restriction) [55]. Although dietary restriction has been found to impinge on mitochondrial function in other systems, no changes in mitochondrial superoxide and overall ROS signals were observed.
Elevated Superoxide Promotes Longevity
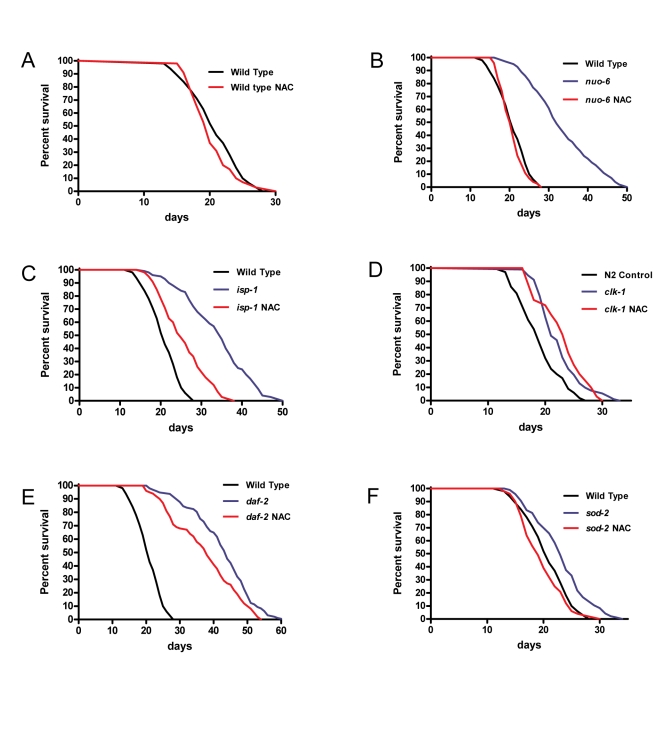
To determine how the elevated superoxide affects the lifespan of mutants, we treated worms with 10 mM of NAC and scored their survival (Figure 2 and Table 1). The treatment had no effect on the survival of the wild type (Figure 2A), which shows that it is not toxic for lifespan at the concentration used. However, NAC treatment fully abolished the increased longevity of nuo-6 and severely limited that of isp-1 (Figure 2B and 2C). The lesser effect on isp-1 is consistent with the larger increase of superoxide in these mutants (Figure 1D), given that the effect of NAC is gradual (1 mM has less effect than 8 mM, which has less than 10 mM; Table S1). At high concentration (>10–15 mM) NAC can be deleterious even on the wild type, but at the concentration used (10 mM) NAC had no effect on the apparent health of the mutants, whose overall aspect after treatment was indistinguishable from that of the untreated worms (Figure S2A). We have also quantified several phenotypes, including defecation, swimming, brood size, and post-embryonic development, after NAC treatment of the wild type and of nuo-6, which is the mutant that is most sensitive to NAC (10 mM NAC completely abolishes its increased longevity). Treatment with 1 mM vitamin C also significantly shortened the lifespan of both isp-1 and nuo-6 mutants without affecting the wild type (Table S1). Most effects of NAC were quite small (Figure S2B–E), except on the post-embryonic development of the wild type (Figure S2C). Furthermore, for defecation, brood size, and post-embryonic development, the effect of NAC on the mutant produced a change in the same direction as on the wild type but of a lesser extent. Only for swimming is the effect greater on the mutant. But the effect consists of swimming faster after NAC treatment and thus bringing the mutant phenotype closer to the wild-type. We conclude that there is little evidence of an indirect deleterious effect of NAC.
Figure 2. Lifespans of wild-type animals and mutants treated with 10 mM NAC.
The treatment has no effect on wild type (A) but dramatically suppresses the lifespan extension of the respiratory chain subunit mutants nuo-6 (B) and isp-1 (C). In contrast, it has no lifespan-shortening effects on the long-lived clk-1 mutants (D). NAC treatment has only a moderate effect on the very long-lived daf-2 mutants (E) but also completely abolished the extended longevity of sod-2 mutants (F). See Tables 1 and S1 for details of genotype, sample size, and statistical analysis.
Table 1. Longevity after paraquat and NAC treatment.
| Control | PQ | NAC | ||||||
| Mean ± SD Sample Size | Maximum Lifespan | Mean ± SD Sample Size | Maximum Lifespan | % Change p Value | Mean ± SD Sample Size | Maximum Lifespan | % Change p Value | |
| Wild Type (N2) | 18.4±3.5 | 29 | 29.0±5.1 | 43 | +58% | 20.2±3.2 | 30 | +10% |
| (n = 400) | (n = 200) | p<0.0001 | (n = 100) | 0.1655 | ||||
| nuo-6(qm200) | 33.4±7.6 | 49 | 36.1±8.3 | 54 | +8% | 20.7±3.0 | 28 | −38% |
| (n = 200) | (n = 200) | p = 0.0125 | (n = 73) | <0.0001 | ||||
| isp-1(qm150) | 33.9±7.9 | 53 | 35.0±7.3 | 50 | +3% | 25.6±5.6 | 38 | −24% |
| (n = 200) | (n = 150) | p = 0.0961 | (n = 200) | <0.0001 | ||||
| clk-1(qm30) | 20.7±3.9 | 32 | 36.8±7.4 | 58 | +78% | 23.0±3.9 | 30 | +11% |
| (n = 150) | (n = 200) | p<0.0001 | (n = 100) | <0.0001 | ||||
| daf-2(e1370) | 43.8±9.0 | 67 | 47.9±8.8 | 74 | +9% | 37.3±9.8 | 54 | −15% |
| (n = 150) | (n = 150) | p = 0.0002 | (n = 100) | 0.0006 | ||||
| eat-2(ad1116) | 29.4±7.1 | 44 | 39.4±6.3 | 58 | +34% | ND | ND | ND |
| (n = 100) | (n = 150) | p<0.0001 | ||||||
| daf-16(m26) | 16.7±2.3 | 21 | 22.6±3.6 | 28 | +35% | ND | ND | ND |
| (n = 100) | (n = 100) | p<0.0001 | ||||||
| aak-2(ok524) | 17.3±2.5 | 23 | 22.4±4.2 | 30 | +29% | ND | ND | ND |
| (n = 100) | (n = 100) | p<0.0001 | ||||||
| jnk-1(gk7) | 18.1±3.0 | 25 | 29.0±6.0 | 39 | +60% | ND | ND | ND |
| (n = 100) | (n = 100) | p<0.0001 | ||||||
| wwp-1(ok1102) | 21.9±2.9 | 28 | 29.3±5.3* | 37 | +34% | ND | ND | ND |
| (n = 100) | (n = 100) | p<0.0001 | ||||||
| skn-1(zn67) | 17.6±2.3 | 22 | 22.8± 2.8* | 25 | +30% | ND | ND | ND |
| (n = 50) | (n = 50) | p<0.0001 | ||||||
| hsf-1(y441) | 14.9±3.1 | 22 | 22.5±5.7 | 37 | +51% | ND | ND | ND |
| (n = 100) | (n = 100) | p<0.0001 | ||||||
| hif-1(ia4) | 23.4±4.1 | 31 | 28.7±6.8 | 42 | +23% | ND | ND | ND |
| (n = 50) | (n = 50) | p<0.0001 | ||||||
*For these genotypes paraquat was only applied after the worms reached adulthood because of lethal effects during development. They should be compared to the wild type (N2) treated only during adulthood (not shown in the table), whose lifespan is 25.8±4.8, which is a 39% increase compared to non-treated worms.
NAC had only a moderate effect on the lifespan of the insulin-signaling daf-2 mutants (Figure 2E), suggesting that only a small part of the increased longevity of these mutants requires elevated mitochondrial superoxide. However, NAC fully abolished the increased lifespan of sod-2 mutants (Figure 2F), suggesting that, although increased generation of superoxide and other ROS as detected by our techniques were not significantly altered in these mutants, their increased lifespan depends on an elevation of superoxide or some other ROS. NAC did not shorten the lifespan of clk-1 mutants at 10 mM (Figure 2D), or even at 15 mM (Table S1), indicating that ROS metabolism is relatively irrelevant to the aging phenotype of these mutants. The effect of NAC on the lifespan of eat-2 could not be scored because NAC treatment rendered the animals unable to lay their eggs and they died from internal hatching at a young age. The origin of this effect is unknown. We also could not score the effect of NAC on RNAi-treated worms because 10 mM NAC was excessively damaging to the dsRNA-producing bacterial strain (HT115).
Treatment With PQ at Low Concentration (0.1 mM) Increases Lifespan
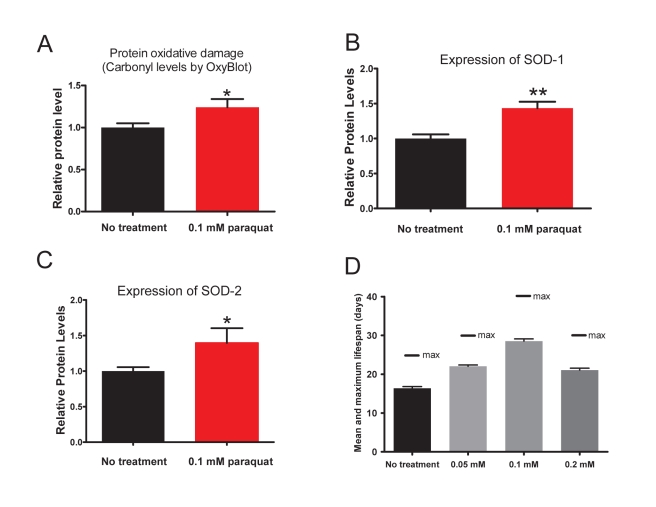
To determine whether an elevation in mitochondrial superoxide generation is sufficient to increase lifespan, we used the superoxide generator PQ. Treatment of C. elegans with high concentration of PQ (>0.2 mM) is severely deleterious. We thus first tested the ability of PQ to increase ROS damage in the animals at a very low concentration (0.1 mM). We found that this treatment indeed measurably increased the level of oxidative damage to proteins at the young adult stage as assessed by determination of protein carbonylation (Figure 3A) and increased the expression of both the main cytoplasmic (SOD-1) and the main mitochondrial (SOD-2) superoxide dismutases (Figure 3B and 3C). We then tested whether PQ could increase the lifespan of the wild type at three different concentrations (0.05, 0.1, and 0.2 mM) and found that at all three concentrations both the mean and maximum lifespan were increased, with a maximal effect at 0.1 mM (Figures 3D and 4A, and Tables 1 and S1). The effect of 0.2 mM was less pronounced than that of 0.1 mM and similar to that of 0.05 mM, likely because at 0.2 mM a toxic effect starts to balance the pro-longevity effect. The effect does not depend on the exact chemical structure of paraquat, as benzyl-viologen, a compound with similar activity as PQ but structurally different, also increases lifespan (Table S1). A small effect of the prooxidant juglone under different conditions has also been documented previously [56]. The effect did not depend on an effect of PQ on the E. coli (OP50) food source, as the effect was also observed with heat-killed cells (Table S1). Finally, the effect was not confined to development or adulthood as PQ prolongs lifespan whether provided only during adult lifespan or only during development (Table S1).
Figure 3. Treatment with 0.1 mM of paraquat (PQ) increases protein oxidative damage, superoxide dismutase expression, and lifespan.
(A) Young wild type adults treated with 0.1 mM PQ have higher protein oxidative damage compared to untreated control. (B) Young wild type adults treated with 0.1 mM PQ since hatching express significantly more SOD-1 protein than untreated animals. (C) Young wild type adults treated with 0.1 mM paraquat since hatching express significantly more SOD-2 protein compared to untreated wild type worms. (D) Treatment with 0.05, 0.1, or 0.2 mM PQ increases mean and maximum lifespan significantly (see also Tables 1 and S1). *<0.05, ** p<0.001.
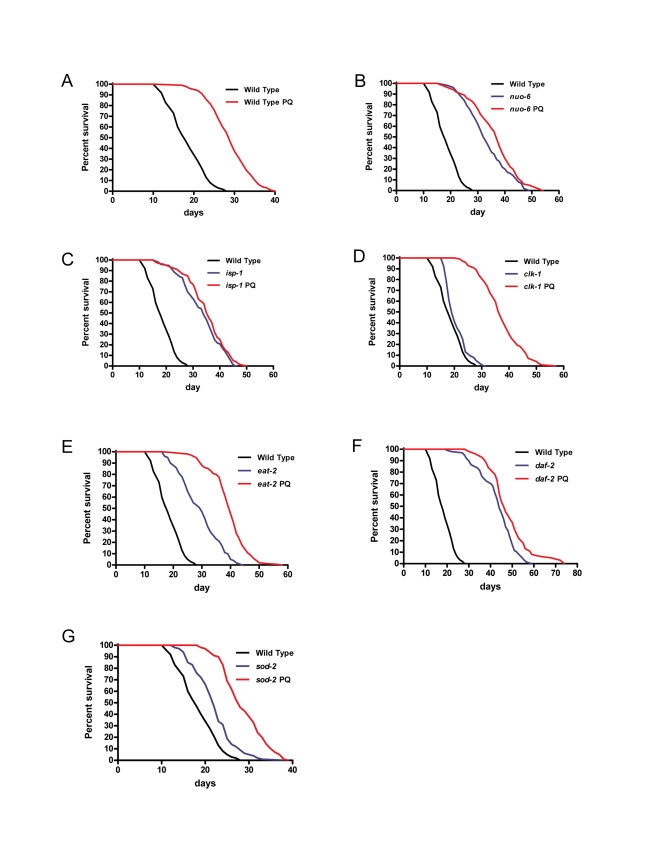
Figure 4. Lifespans of wild-type animals and mutants treated with 0.1 mM paraquat (PQ).
The treatment had a dramatic lifespan-lengthening effect on the wild type (A) but no effect on the nuo-6 (B) and isp-1 (C) respiratory chain subunit mutants. In contrast, the treatment had a dramatic additive effect on the long-lived clk-1 (D) and eat-2 (E) mutants. The treatment has only a very moderate effect on daf-2 mutants (F) but a strong effect on sod-2 mutants (G). See Tables 1 and S1 for details of genotype, sample size, and statistical analysis.
PQ treatment failed to significantly prolong the lifespan on nuo-6 and isp-1 mutants (Figure 4B and 4C, and Tables 1 and S1). This experiment is equivalent to genetic epistasis analysis and suggests that nuo-6, isp-1, and PQ increase lifespan by the same mechanism. It also suggests that the maximum level of lifespan extension that can be obtained by increasing mitochondrial superoxide generation is already reached in these two mutants and further increase of superoxide generation through PQ treatment cannot increase lifespan further. This was not due to a resistance of these mutants to PQ as 0.2 mM PQ shortened the lifespan of the two mutants (Table S1). sod-2 mutants, whose longevity is suppressed by NAC, are not as long-lived when untreated as wild type animals that are treated with PQ. However, treatment with PQ makes the sod-2 mutants live as long as wild type animals treated with PQ (Figure 4G). This absence of additivity suggests that the longevity of sod-2 mutants is indeed due to a small increase in superoxide, as expected from the function of SOD-2, and the suppressing effect of NAC on the mutant lifespan. In contrast to what we observed with nuo-6, isp-1, and sod-2, PQ treatment dramatically enhanced the lifespan of clk-1 and eat-2 mutants, significantly beyond the longevity increases induced by the mutations alone or by PQ applied to the wild type (Figure 4D and 4E, and Tables 1 and S1). This indicates that the effects of these mutations and the effect of superoxide are mechanistically distinct and additive, as expected from the finding that clk-1 and eat-2 mutants did not show increased mitochondrial superoxide levels (Figure 1F) and that the lifespan of clk-1 mutants could not be shortened by NAC treatment (Figure 2D). PQ treatment had only a small lifespan-lengthening effect on daf-2 (Figure 4F, and Tables 1 and S1), which is consistent with the finding that daf-2 mutants already show a substantial increase in superoxide generation.
NAC and PQ Treatments Do Not Affect Other Mitochondrial Parameters in a Manner That Could Predict Their Effect on Lifespan
We sought to determine whether the mutations and the PQ treatment had other common effects on mitochondrial function that could be responsible for the increased lifespans, besides elevation of superoxide levels. Work in other systems has suggested that increased mitochondrial biogenesis could impact lifespan positively [57],[58],[59], and mitochondrial defects in C. elegans have been found to stimulate mitochondrial biogenesis, resulting in a denser mitochondrial network [13]. We have examined mitochondrial density in the two mitochondrial mutants and in PQ-treated worms with Mitotracker Red, which is specific to mitochondria in mammalian cells [60],[61], which stains worms uniformly, and whose staining fully overlaps with that of mitochondrially targeted green fluorescent protein (GFP) (Figure S3). We found that isp-1 and nuo-6 display a denser mitochondrial network, as expected (Figure 5). However, this was not observed in wild type worms treated with PQ (Figure 5), indicating that the mechanism by which the superoxide triggers longevity does not require increased mitochondrial biogenesis. We also tested the effects of PQ and NAC treatment on oxygen consumption and ATP levels in the wild type and in the two mitochondrial mutants (Figure S4). NAC treatment increased oxygen consumption in the wild type and in the mutants. This result uncouples oxygen consumption from lifespan as NAC has no effect on the lifespan of the wild type, and its effect on the oxygen consumption of isp-1 mutants is larger than on that of nuo-6 mutants, although its effect on aging is smaller. PQ had an effect only on nuo-6, and it was small. Thus the effect of PQ on oxygen consumption also did not mirror its effect on lifespan. For ATP levels the only effect observed was a reduction by PQ of the elevated ATP levels that are observed in nuo-6 mutants.
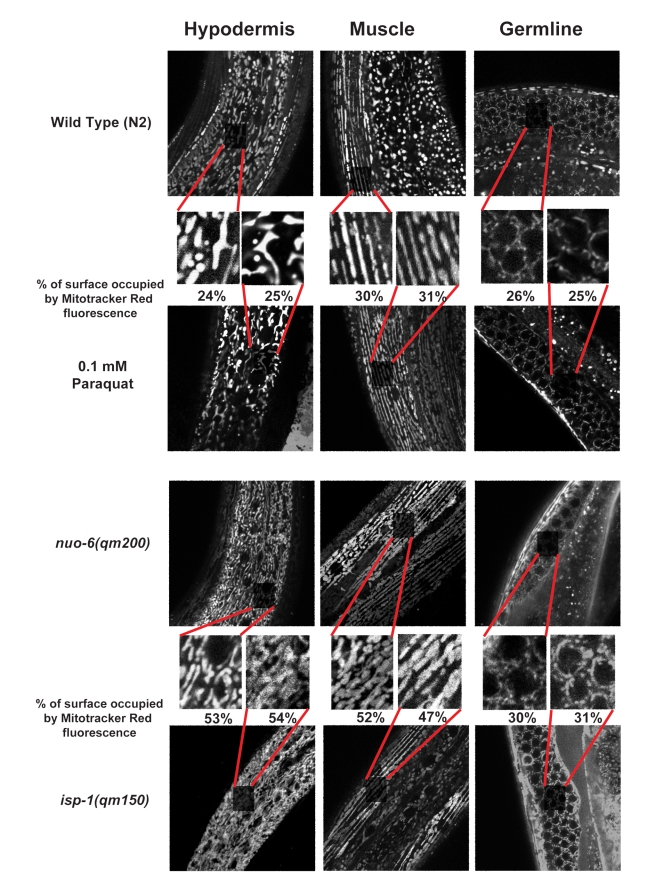
Figure 5. Treatment with 0.1 mM PQ does not affect mitochondrial abundance.
Worms were treated with Mitotracker Red at 50 nM (final concentration) in M9 buffer for 20 min. All pictures were taken by confocal microscopy at 400×. A scale bar of 20 µm is shown in the upper right corner of the figure. For each genotype/treatment three tissues (hypodermis, muscle, and germline) were selected, and for each tissue at least five pictures from different worms were taken. An equal section of each picture was enlarged for quantitative comparisons. The percentage of surface occupied by mitochondria as stained by Mitotracker Red was measured and related to the total area of the selected region. A representative example for each tissue and condition is shown in the figure. The quantification for each sample is also shown in the figure below the enlarged areas. The sample size for the hypodermis was >15, and it was 5 for muscles and the germline. In muscles and in the hypodermis, the difference between the ETC mutants and the wild type was significant (p<0.001), while the difference between PQ treatment and untreated wild type worms was not. Thus, the nuo-6 and isp-1 mutations, but not treatment with 0.1 mM PQ, affect mitochondrial abundance.
PQ Is Able to Considerably Lengthen the Lifespan of a Variety of Mutants That Define Genetic Pathways of Aging
daf-2 mutants have elevated superoxide levels, and they are sensitive to NAC (lifespan shortening by 15%). However, the level of superoxide in daf-2 appears not to be sufficient for a maximal effect as these mutants remain somewhat sensitive to PQ (lifespan lengthening by 9%). To further study how superoxide plays a role in the lifespan of daf-2 we studied genes that function downstream of daf-2. At least three genes are known to be required for the full lifespan extension of daf-2, that is, daf-16, aak-2, and hsf-1 [62],[63],[64]. If one of these genes were necessary for an activity that mediates the small effect of PQ on daf-2 mutants, PQ should not be able to prolong the lifespan of mutants of such a gene. In fact, however, we found that PQ prolonged the lifespan of all three mutants tested (Table 1). The lifespan increase upon PQ treatment of daf-16 (35% increase) and aak-2 (29% increase) is not as large as upon treatment of the wild type (58% increase). This suggests that part but not all of the lifespan increase determined by superoxide requires daf-16 and aak-2. These findings are consistent with the observations that the lifespan extension provided by nuo-6 and daf-2(e1370) are only partially additive (Table S1), similarly to what was found previously for isp-1 and daf-2 [12], and that elimination of daf-16 partially shortens the lifespan of isp-1 [12].
We also tested the sensitivity to PQ of mutants that are diagnostic of a variety of pathways of aging. In particular mutants of genes that, based on their known functions in C. elegans or that of their homologues in other systems, might encode the targets of superoxide signaling or be otherwise necessary for implementing superoxide signaling. The c-Jun N-terminal kinase 1 (JNK-1) is involved in stress responses in vertebrate cells and is a positive regulator of DAF-16 that acts in parallel to the effect of daf-2 on daf-16 [65]. We treated jnk-1(gk7) mutants with PQ and obtained a particularly large lifespan increase (Table 1). Although it is not clear what activities lie upstream of jnk-1 in C. elegans nor whether it has other targets than daf-16, its activity does not appear necessary for the effect of superoxide. The transcription factor SKN-1 defends against oxidative stress by mobilizing the conserved phase II detoxification response and can delay aging independently of DAF-16 [66]. Although PQ induces oxidative stress and induces enzymes that protect from oxidative stress (Figure 3), it was still able to prolong the lifespan of skn-1(zn67) mutants (Table 1), indicating that skn-1 does not act downstream of superoxide. wwp-1 encodes a conserved E3 ubiquitin ligase that is necessary for lifespan extension by dietary restriction [67]. Treatment of wwp-1(ok1102) with PQ prolonged lifespan of these mutants, which is consistent with our finding that PQ can considerably extend the lifespan of eat-2 mutants (Figure 4E). This confirmed that the lifespan increase produced by the superoxide increase in mitochondrial mutants is distinct from the mechanisms that support the lifespan effects of dietary restriction [14]. hif-1 encodes a worm homologue of the vertebrate hypoxia inducible factor 1α (HIF-1α), a transcription factor involved in a number of protective mechanisms. In C. elegans hif-1 is necessary for a lifespan pathway that involves proteolysis and that is distinct from insulin signaling [68] and has also been involved in the dietary restriction pathway [69]. In vertebrates HIF-1α is positively regulated by mitochondrial ROS [34],[35], which would make it an interesting candidate to mediate the effects of superoxide. However, PQ was fully capable of increasing the lifespan of the hif-1 mutants (Table 1).
Several of the genes whose mutants remain sensitive to PQ, including daf-16, have been involved in stress responses, including oxidative stress, yet they do not seem necessary for the effect of PQ. Similarly we have shown previously that although the expression of SOD-1 and SOD-2 are elevated in isp-1(qm150) mutants, the elevation is not necessary for the extended lifespan of these mutants [24]. nuo-6(qm200) mutants also show elevated SOD-1 and SOD-2 expression [14], but this too is unnecessary for the longevity of the mutants, as RNAi against sod-1 an sod-2, which we have shown to be efficient in reducing enzyme levels [24], does not shorten the lifespan of nuo-6 mutants (Figure S5). We conclude that the mitochondrial mutants protect from an aspect of the aging process that has not yet been studied through mutants that affect stress. In addition, our observations suggest that the lifespan effect we observed is not hormetic, as neither superoxide-detoxifying enzymes, nor the regulatory factors that are involved in protection from oxidative stress, are crucially implicated.
Discussion
We have shown previously that mutations in isp-1 and nuo-6 prolong lifespan by a common mechanism [14]. Using direct measurement of ROS and superoxide we find here that this mechanism involves an increase in mitochondrial superoxide generation that is necessary and sufficient for the longevity of these mutants. As ROS, including superoxide [70],[71],[72], are known to be intracellular messengers, the increased superoxide might trigger a signal transduction pathway that ultimately results in changes in nuclear gene expression [23]. Superoxide is highly reactive and could trigger such a signal by modifying proteins in the mitochondria or in the nearby cytosol after having escaped from the mitochondria through an appropriate channel [73],[74]. Although no superoxide sensor has yet been identified, a similar type of mechanism, in which a highly reactive, quickly diffusing, molecule modifies a signal transduction protein, has been evidenced for nitric oxide (NO), which covalently and permanently modifies guanylyl cyclases. Similarly, hydrogen peroxide (H2O2), the product of superoxide dismutation, can inactivate phosphatases involved in signal transduction. Future work will aim at using forward and reverse genetic screens in C. elegans to uncover the molecular machinery that reacts to the superoxide signal, as well as the transcription factors that are needed to regulate nuclear gene expression in response to the pathway's activation. In addition, the pattern of gene expression that results in increased lifespan in these mutants could be defined very specifically by identifying changes in the gene expression patterns that are common to isp-1, nuo-6, and PQ treatment and that are suppressed by treatment with NAC.
A number of studies in C. elegans have explored hormesis by treating animals with sub-lethal but clearly deleterious treatments for a short period of time and observing subsequent prolongation of lifespan [75]. These hormetic effects are different from what we have observed and describe here, as both the genetic mutations and the very low level PQ are present throughout life and as only a part of the effect we observe might require the insulin signaling pathway. Furthermore, although in nuo-6 and isp-1 mutants the expression levels of the superoxide dismutases SOD-1 and SOD-2 are elevated, likely in response to the elevated superoxide generation, and as one expects in the hormetic response, these elevations are not necessary for the lifespan prolongation of nuo-6 (Figure S5) or isp-1 [24].
CLK-1 is a mitochondrial protein that is required for ubiquinone biosynthesis and its absence affects mitochondrial function [50], although it could potentially affect many other processes as ubiquinone is found in all membranes. Furthermore, ubiquinone is both a prooxidant as co-factor in the respiratory chain and an anti-oxidant. Interestingly, the mechanism of lifespan prolongation induced by clk-1 appears to be entirely distinct from, but particularly synergistic with, that induced by elevated superoxide. Indeed, clk-1 mutants do not show elevated superoxide generation and are not affected by NAC. Furthermore, although double mutant combinations of clk-1 with nuo-6 and isp-1 are not viable (unpublished data) the lifespans of clk-1 mutants treated with PQ (Figure 4D), or of sod-2;clk-1 mutants [26], or of clk-1;daf-2 mutants [76] are much greater than expected from simple additivity of the effects of individual mutations or treatments.
Studies in yeast [77] and in worms [78] have suggested that an increase in ROS from mitochondria might also be important in triggering the lifespan extension produced by glucose restriction. However, our results here with an eat-2 mutation, one of the ways in which global dietary restriction can be produced in worms, as well as with a wwp-1 and hif-1, which may function downstream of dietary restriction, did not reveal an involvement of superoxide signaling, providing further evidence for a distinction between the mechanisms of glucose restriction and dietary restriction. It remains possible, however, that DR could lead to superoxide or ROS production when it is induced by other methods than the use of an eat-2 mutant, as it is well documented that different types of DR induce different molecular mechanisms [79].
One question that our current experiments do not address is whether the mitochondrial dysfunction in the mutants, or the effect of PQ, is necessary in every tissue in order to increase longevity. There are indications for both the insulin signaling pathway mutants [80],[81] and dietary restriction [67],[82] that the entire effect might be mediated by action in particular cells that influence the physiology of the whole organism. Similarly, the presence or absence of the germline is sufficient for a dramatic effect on lifespan [83]. For mitochondrial dysfunction the question could be addressed in the future by mosaic analysis and by purifying and analyzing mitochondria from specific tissues using our flow cytometry technique to purify mitochondria expressing GFP in a tissue-specific manner.
The oxidative stress theory of aging has been one of the most acknowledged theories of aging for the simple reason of the strikingly good correlation between the levels of oxidative stress and the aged phenotype [8]. A number of recent results in worms and in mice, however, have suggested that oxidative stress cannot be the cause of aging [24],[25],[26],[30]. Our findings suggest a conceptual framework for why oxidative stress and the aged phenotype are so tightly correlated [31]. In this model mitochondria, like the rest of the cell, sustain a variety of age-dependent insults (not only and not even principally from oxidative stress) that trigger an increase in superoxide, which acts as a signal that induces general protective and repair mechanisms. However, aging in most animals is clearly irreversible, indicating that the protective mechanisms, which must have evolved to control damage in young organisms, are unable to fully prevent the accumulation of age-related damage. Thus, as superoxide is a reactive molecule as well as a signal, and as age-dependent damage cannot be fully reversed, it is possible that at high ages the chronically elevated superoxide will participate in creating some of the damage itself. This could explain the strong tendency for aged animals to have high oxidative stress and high oxidative damage, although it does not imply that ROS cause aging or even that they are a major source of age-dependent damage. In this model, the nuo-6 and isp-1 mutations lead to increased longevity because they turn on the stress signal prematurely and thus slow down the entire process.
Materials and Methods
Lifespan Scoring
Eggs were placed on plates at 20°C and left for 1 h to hatch. Larvae that had hatched during that period were placed onto fresh plates and monitored once daily until death. The animals were transferred once daily while producing eggs to keep them separate from their progeny. Animals were scored as dead when they no longer responded with movement to light prodding on the head and tail. Missing worms and worms that have died because of internal hatching (bagging) were replaced from a backup group. Survival was scored every day.
Drug Treatment
Drugs were added into NGM media from a high concentration stock solution (500 mM for NAC, 1 M for PQ, and 500 mM for vitamin C) before pouring of the plates. Plates were made fresh each week. Gravid adult worms were transferred from normal NGM plates to drug plates and left to lay eggs for 3 h. With each transfer of worms a substantial amount of bacteria was also transferred onto the new plates. The progeny was then scored for different phenotypes.
Staining and Confocal Imaging
All dyes except MitoSox were diluted in DMSO at high concentration (all at 5 mM except H2DCFDA, which is at 10 mM) and frozen at −20°C as a stock. MitoSox was prepared fresh at 5 mM for each use. Before staining stocks were diluted in M9 buffer at a 1∶1000 dilution. Young adult worms were transferred into staining solution and stained for 20 min. Worms were mounted on a thick layer of half-dried agar pad on microscopic glass slides and then subjected to confocal microscopy (Zeiss LSM 510 Meta). Pictures were taken by Zeiss LSM Imaging software and analyzed by Volocity V4.0 software.
Oxygen Consumption
Five young adult worms (1st day of adulthood) were placed into 0.25 µl M9 buffer in a 0.5 µl sealed chamber at 22°C. A fiber optical oxygen sensor (AL300 FOXY probe from Ocean Optics) was inserted into this chamber and oxygen partial pressure was monitored for 15 to 30 min. Oxygen consumption measured in this way was normalized to body volume. For this worms were photographed at each measurement day under a binocular microscope and their cross-section was calculated with ImageJ software. Worm volume was determined by the formula: volume (nl) = 1.849 • 10–7 (nl/µm3) • area 1.5 (µm3) [84].
Expression Levels of Superoxide Dismutases (SODs)
After RNAi treatment, 100 young adult worms of each genotype were picked, lysed in 2× loading buffer, and subjected to electrophoresis in 12% SDS–polyacrylamide gels (SDS–PAGE), and then blotted onto nitrocellulose membrane (Bio-Rad). After applying primary antibody (1∶1000, rabbit polyclonal antibody against worm SOD-1 or SOD-2) and secondary antibody (1∶10,000 mouse anti-rabbit IgG, Invitrogen), the membranes were incubated with the ECL plus detection reagent (Amersham Biosciences) and scanned using a Typhoon trio plus scanner. Band densities were analyzed by ImageQuant TL V2003.03.
Fluorescence Activated Cell Sorting
For fluorescence activated cell sorting [37], adult worms grown on large NGM plates were collected and washed 3 times with M9 buffer. Worms were then suspended in 5× isolation buffer (200 mM mannitol; 120 mM sucrose; 10 mM Tris; 1 mM EGTA; pH 7.4) and set on ice. Worms were broken up with a 5 ml glass-glass homogenizer and centrifuged at 600 g for 10 min, the supernatant was collected and re-centrifuged at 7,800 g for 10 min, and the pellet was washed once with isolation buffer and then suspended in isolation buffer and kept on ice. Different dyes were added from stocks into the analysis buffer (250 mM sucrose; 20 mM MOPS; 100 uM KPi; 0.5 mM MgCl2; 1 uM CsA pH 7.0) at a 1∶1000 dilution before staining. 100 µl of mitochondria was added to 900 µl of analysis buffer with dye and substrate and incubated for 1 h at room temperature. Mitochondria were recollected by 7,800 g centrifugation and then suspended in 500 µl analysis buffer. A FACSCalibur instrument equipped with a 488 nm Argon laser and a 635 nm red diode laser (Becton Dickinson) was used. Data from the experiments were analyzed using the CellQuest software (Becton Dickinson). To exclude debris, samples were gated based on light-scattering properties in the SSC (side scatter) and FSC (forward scatter) modes, and 20,000 events per sample were collected, using the “low” setting for sample flow rate (Figure S1).
ATP Content
200 age-synchronized young adult worms were collected in M9 buffer and washed three times. Worm pellets were treated with three freeze/thaw cycles and boiled for 15 min to release ATP and destroy ATPase activity, and then spun at 4°C and 11,000 g for 10 min. ATP contents were measured with a kit (Invitrogen, Carlsbad, California, USA; Cat: A22066). The ATP content value was then normalized to the soluble protein level of the same preparation, measured with the protein assay from Bio-Rad.
Dyes Used for Staining and FACS
Mitotracker green (Invitrogen M7514) stock concentration 5 mM; H2DCFDA (Invitrogen D399) stock concentration 10 mM; Mitotracker red (Invitrogen M7512) stock concentration 5 mM.
Supporting Information
Selection of isolated mitochondria and ROS-sensitive dye analysis. Mitochondria were prepared as described in Materials and Methods. (A) A FACSCalibur flow cytometry cell sorter from Becton Dickinson equipped with a 488 nm Argon laser and a 635 nm red diode laser was used. Data from the experiments were analyzed using the CellQuest software (Becton Dickinson). To exclude debris, samples were gated based on light-scattering properties in the SSC (side scatter) and FSC (forward scatter) modes, and 20,000 events per sample within the region (gate) delimitated by a square in (A) were collected, using the “low” setting for sample flow rate. 99% of the particles in that region successfully stained with the mitochondria-specific dye Mitotracker Green. (B) Isolated mitochondria were incubated with analysis buffer containing substrate (see Materials and Methods) and MitoSox (1 µM) at room temperature for 1 h and then sorted and the fluorescence of mitochondria in the gate measured. The purple area represents un-stained control. Paraquat (red line) was able to increase ROS generation over the untreated control (green line), while NAC (blue line) decreased the superoxide signal. Note that the x-axis shows a log scale. (C) Isolated mitochondria (see A) from wild-type worms were stained with both H2DCF-DA (the signal plotted on FL1-H; 530±15 nm channel) and Mitotracker Red (the signal plotted on FL3-H; ≥670 nm channel). When particles were stained by both dyes (upper-right region), the signals were strongly correlated. Furthermore, 89.6%±2.4% (n = 4) of the particles stained by H2DCF-DA were also stained by Mitotracker Red.
(0.11 MB PDF)
Absence of deleterious effects of N-acetyl-cysteine. (A) NAC (N-acetyl-cysteine) had no effect on the apparent health of isp-1 or nuo-6 mutants. Mutant animals were treated or not with 10 mM NAC throughout their lives and all pictures in the panel were taken on day 23 of their lifespan, when less than 25% of untreated mutants but more than 75% of NAC-treated mutants had already died. NAC-treated isp-1 and nuo-6 mutants did not show any visible ill effects from the treatment. All worms are shown at the same magnification; scale bar is 0.5 mm. Phenotypes possibly resulting from NAC treatment of nuo-6(qm200) mutants were also quantified. We chose to examine nuo-6 mutants because their longevity was the most sensitive to NAC (completely suppressed at 10 mM). Adult worms were allowed to lay eggs on NAC plates and phenotypes of the resulting F1 progeny were scored. (B) NAC significantly decreased defecation cycle length of the wild type (p = 0.0104), while it has no significant effect on that of nuo-6(qm200) mutant (n = 15). (C) NAC significantly increased post-embryonic development length of both the wild type and nuo-6(qm200) mutants (n = 100). (D) NAC has no significant effect on brood size of both the wild type and nuo-6(qm200) mutants (n = 50). (E) NAC has no significant effect on the swimming rate (frequency of thrashing) of the wild type, but it significantly increased that of nuo-6(qm200) mutants (p = 0.0024) (n = 15).
(0.06 MB PDF)
Co-localization of Mitotracker Red and GFP signals in C. elegans mitochondria. We used Mitotracker Red to stain worms carrying the transgene qmIs16[Pclk-1::clk-1::gfp], which expresses the mitochondrial protein CLK-1 fused to GFP [50]. Staining was as described in Materials and Methods. Worms were mounted on agar pads on slides and subjected to confocal microscopical analysis. (A) Mitotracker Red expression in hypodermal tissue. (B) The same region as in (A) expressing the mitochondrial GFP fusion. (C) The merged images of (A) and (B). The Mitotracker Red and GFP signals are co-localized.
(0.10 MB PDF)
Effect of paraquat (PQ) and N-acetylcysteine (NAC) on energy metabolism. Untreated wild type controls and animals treated with 0.1 mM PQ or 10 mM NAC since hatching were collected at the first day of adulthood for both experiments. (A) Animals in groups of 5 (n≥3) were transferred in 0.25 µl M9 buffer into a 0.5 µl chamber where oxygen concentration was measured with a fiber optic oxygen sensor (AL300 FOXY probe from Ocean Optics) for 15–30 min. The body volume of animals was calculated from pictures of the same worms and used for normalization. PQ had a small but significant consumption-increasing effect only on nuo-6 mutants. NAC increased oxygen consumption in all three genotypes, with the largest effect on isp-1 mutants. (B) The ATP content from 200 worms was normalized to the amount of soluble protein from the same sample (n≥6). Both PQ and NAC treatment had no effect on ATP content with the exception of PQ-treated nuo-6mutants, in which the treatment suppressed the high ATP content that is observed in the untreated animals. For all statistic analyses we used the Student's t test.* p<0.05, ** p<0.01 and *** p<0.001.
(0.06 MB PDF)
SOD-1 and SOD-2 are not necessary for the longevity of nuo-6(qm200) . Knocking down sod-1 (red) or sod-2 (blue) does not shorten the long lifespan of nuo-6(qm200) mutant. In fact silencing these two genes slightly increases the lifespan of nuo-6 mutants. Mean lifespan of control (empty vector) is 33 d (green), mean lifespan after sod-1 RNAi treatment is 35 d, and mean lifespan after sod-2 RNAi treatment is 36.5 d; p<0.05 for both RNAi experiments compared to control, analyzed by curve comparison using the log-rank test.
(0.02 MB PDF)
Individual aging experiments and statistics.
(0.14 MB PDF)
Acknowledgments
We thank Jérôme Lapointe and Robyn Branicky for carefully reading and commenting on the manuscript.
Abbreviations
- GFP
green fluorescent protein
- HIF
hypoxia-inducible factor
- JNK-1
c-Jun N-terminal kinase 1
- NAC
N-acetyl-cysteine
- PQ
paraquat
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
The authors have declared that no competing interests exist.
The work was supported in part by a grant from the Canadian Institutes of Health Research to SH (grant #216377) and by McGill University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Balaban R. S, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Rubner M. Munich: Oldenberg; 1908. Das Problem det Lebensdaur und seiner beziehunger zum Wachstum und Ernarnhung (The problem of longevity and its relation to growth and nutrition). [Google Scholar]
- 3.Pearl R. London: University of London Press; 1928. The rate of living. [Google Scholar]
- 4.Speakman J. R. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 6.Shigenaga M. K, Hagen T. M, Ames B. N. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Beckman K. B, Ames B. N. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 9.Ishii N, Fujii M, Hartman P. S, Tsuda M, Yasuda K, et al. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 10.Kayser E. B, Morgan P. G, Hoppel C. L, Sedensky M. M. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J Biol Chem. 2001;276:20551–20558. doi: 10.1074/jbc.M011066200. [DOI] [PubMed] [Google Scholar]
- 11.Wong A, Boutis P, Hekimi S. Mutations in the clk-1 gene of Caenorhabditis elegans affect developmental and behavioral timing. Genetics. 1995;139:1247–1259. doi: 10.1093/genetics/139.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Bussiere F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell. 2001;1:633–644. doi: 10.1016/s1534-5807(01)00071-5. [DOI] [PubMed] [Google Scholar]
- 13.Lee S. S, Lee R. Y, Fraser A. G, Kamath R. S, Ahringer J, et al. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet. 2003;33:40–48. doi: 10.1038/ng1056. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Hekimi S. Aging Cell In Press; 2010. Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. [DOI] [PubMed] [Google Scholar]
- 15.Ewbank J. J, Barnes T. M, Lakowski B, Lussier M, Bussey H, et al. Structural and functional conservation of the Caenorhabditis elegans timing gene clk-1. Science. 1997;275:980–983. doi: 10.1126/science.275.5302.980. [DOI] [PubMed] [Google Scholar]
- 16.Hekimi S, Guarente L. Genetics and the specificity of the aging process. Science. 2003;299:1351–1354. doi: 10.1126/science.1082358. [DOI] [PubMed] [Google Scholar]
- 17.Van Raamsdonk J, Hekimi S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid Redox Signal Comprehensive invited review 2010 doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 18.Dillin A, Hsu A. L, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, et al. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton B, Dong Y, Shindo M, Liu W, Odell I, et al. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev. 2005;19:1544–1555. doi: 10.1101/gad.1308205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copeland J. M, Cho J, Lo T, Jr, Hur J. H, Bahadorani S, et al. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol. 2009;19:1591–1598. doi: 10.1016/j.cub.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Rea S. L, Ventura N, Johnson T. E. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durieux J, Dillin A. Mitochondria and aging: dilution is the solution. Cell Metab. 2007;6:427–429. doi: 10.1016/j.cmet.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Cristina D, Cary M, Lunceford A, Clarke C, Kenyon C. A regulated response to impaired respiration slows behavioral rates and increases lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000450. doi: 10.1371/journal.pgen.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Li J, Hekimi S. A Measurable increase in oxidative damage due to reduction in superoxide detoxification fails to shorten the life span of long-lived mitochondrial mutants of Caenorhabditis elegans. Genetics. 2007;177:2063–2074. doi: 10.1534/genetics.107.080788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doonan R, McElwee J. J, Matthijssens F, Walker G. A, Houthoofd K, et al. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Raamsdonk J. M, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Raamsdonk J, Meng Y, Camp D, Yang W, Jia X, et al. Genetics in press; 2010. Decreased energy metabolism extends lifespan in Caenorhabditis elegans without reducing oxidative damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keaney M, Matthijssens F, Sharpe M, Vanfleteren J, Gems D. Superoxide dismutase mimetics elevate superoxide dismutase activity in vivo but do not retard aging in the nematode Caenorhabditis elegans. Free Radic Biol Med. 2004;37:239–250. doi: 10.1016/j.freeradbiomed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Magwere T, West M, Riyahi K, Murphy M. P, Smith R. A, et al. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127:356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Lapointe J, Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storz P. Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Sci STKE. 2006;2006:re3. doi: 10.1126/stke.3322006re3. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg F, Chandel N. S. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- 34.Brunelle J. K, Bell E. L, Quesada N. M, Vercauteren K, Tiranti V, et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Guzy R. D, Hoyos B, Robin E, Chen H, Liu L, et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Van Raamsdonk J. M, Meng Y, Camp D, Yang W, Jia X, et al. Decreased Energy Metabolism Extends Lifespan in Caenorhabditis elegans Without Reducing Oxidative Damage. Genetics. 2010 doi: 10.1534/genetics.110.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattiasson G. Analysis of mitochondrial generation and release of reactive oxygen species. Cytometry A. 2004;62:89–96. doi: 10.1002/cyto.a.20089. [DOI] [PubMed] [Google Scholar]
- 38.Myhre O, Andersen J. M, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65:1575–1582. doi: 10.1016/s0006-2952(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 39.LeBel C. P, Ischiropoulos H, Bondy S. C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 40.Robinson K. M, Janes M. S, Pehar M, Monette J. S, Ross M. F, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drechsel D. A, Patel M. Differential contribution of the mitochondrial respiratory chain complexes to reactive oxygen species production by redox cycling agents implicated in parkinsonism. Toxicol Sci. 2009;112:427–434. doi: 10.1093/toxsci/kfp223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benrahmoune M, Therond P, Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic Biol Med. 2000;29:775–782. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 43.Aruoma O. I, Halliwell B, Hoey B. M, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 44.Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- 45.Cadenas E, Boveris A, Ragan C. I, Stoppani A. O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys. 1977;180:248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 46.Turrens J. F, Alexandre A, Lehninger A. L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 47.Kudin A. P, Bimpong-Buta N. Y, Vielhaber S, Elger C. E, Kunz W. S. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 48.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haughland R. Eugene, OR: Molecular Probes Inc; 2009. Molecular probes. Handbook of fluorescent probes and research chemicals. 10th Edition ed.862 [Google Scholar]
- 50.Felkai S, Ewbank J. J, Lemieux J, Labbe J. C, Brown G. G, et al. CLK-1 controls respiration, behavior and aging in the nematode Caenorhabditis elegans. Embo J. 1999;18:1783–1792. doi: 10.1093/emboj/18.7.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyadera H, Amino H, Hiraishi A, Taka H, Murayama K, et al. Altered quinone biosynthesis in the long-lived clk-1 mutants of Caenorhabditis elegans. J Biol Chem. 2001;276:7713–7716. doi: 10.1074/jbc.C000889200. [DOI] [PubMed] [Google Scholar]
- 52.Miyadera H, Kano K, Miyoshi H, Ishii N, Hekimi S, et al. Quinones in long-lived clk-1 mutants of Caenorhabditis elegans. FEBS Lett. 2002;512:33–37. doi: 10.1016/s0014-5793(02)02282-2. [DOI] [PubMed] [Google Scholar]
- 53.Jonassen T, Larsen P. L, Clarke C. F. A dietary source of coenzyme Q is essential for growth of long-lived Caenorhabditis elegans clk-1 mutants. Proc Natl Acad Sci U S A. 2001;98:421–426. doi: 10.1073/pnas.021337498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Branicky R, Nguyen P. A, Hekimi S. Uncoupling the pleiotropic phenotypes of clk-1 with tRNA missense suppressors in Caenorhabditis elegans. Mol Cell Biol. 2006;26:3976–3985. doi: 10.1128/MCB.26.10.3976-3985.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heidler T, Hartwig K, Daniel H, Wenzel U. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology. 11:183–195. doi: 10.1007/s10522-009-9239-x. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, et al. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Reznick R. M, Zong H, Li J, Morino K, Moore I. K, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hailey D. W, Rambold A. S, Satpute-Krishnan P, Mitra K, Sougrat R, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hattori F, Chen H, Yamashita H, Tohyama S, Satoh Y. S, et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods. 2010;7:61–66. doi: 10.1038/nmeth.1403. [DOI] [PubMed] [Google Scholar]
- 62.Hsu A. L, Murphy C. T, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 63.Curtis R, O'Connor G, DiStefano P. S. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 64.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 65.Oh S. W, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis R. J, et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tullet J. M, Hertweck M, An J. H, Baker J, Hwang J. Y, et al. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrano A. C, Liu Z, Dillin A, Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009 doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehta R, Steinkraus K. A, Sutphin G. L, Ramos F. J, Shamieh L. S, et al. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science. 2009;324:1196–1198. doi: 10.1126/science.1173507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen D, Thomas E. L, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W. C, Chio C. C, Chi K. H, Wu H. M, Lin W. W. Superoxide anion-dependent Raf/MEK/ERK activation by peroxisome proliferator activated receptor gamma agonists 15-deoxy-delta(12,14)-prostaglandin J(2), ciglitazone, and GW1929. Exp Cell Res. 2002;277:192–200. doi: 10.1006/excr.2002.5546. [DOI] [PubMed] [Google Scholar]
- 71.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol. 2001;155:1003–1015. doi: 10.1083/jcb.200105057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madesh M, Hawkins B. J, Milovanova T, Bhanumathy C. D, Joseph S. K, et al. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Budzinska M, Galganska H, Karachitos A, Wojtkowska M, Kmita H. The TOM complex is involved in the release of superoxide anion from mitochondria. J Bioenerg Biomembr. 2009;41:361–367. doi: 10.1007/s10863-009-9231-9. [DOI] [PubMed] [Google Scholar]
- 74.Piskernik C, Haindl S, Behling T, Gerald Z, Kehrer I, et al. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim Biophys Acta. 2008;1782:280–285. doi: 10.1016/j.bbadis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Cypser J. R, Tedesco P, Johnson T. E. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakowski B, Hekimi S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 1996;272:1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- 77.Lin S. J, Kaeberlein M, Andalis A. A, Sturtz L. A, Defossez P. A, et al. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- 78.Schulz T. J, Zarse K, Voigt A, Urban N, Birringer M, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Greer E. L, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Libina N, Berman J. R, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 81.Wolkow C. A, Kimura K. D, Lee M. S, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 82.Bishop N. A, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 83.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 84.Suda H, Shouyama T, Yasuda K, Ishii N. Direct measurement of oxygen consumption rate on the nematode Caenorhabditis elegans by using an optical technique. Biochem Biophys Res Commun. 2005;330:839–843. doi: 10.1016/j.bbrc.2005.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection of isolated mitochondria and ROS-sensitive dye analysis. Mitochondria were prepared as described in Materials and Methods. (A) A FACSCalibur flow cytometry cell sorter from Becton Dickinson equipped with a 488 nm Argon laser and a 635 nm red diode laser was used. Data from the experiments were analyzed using the CellQuest software (Becton Dickinson). To exclude debris, samples were gated based on light-scattering properties in the SSC (side scatter) and FSC (forward scatter) modes, and 20,000 events per sample within the region (gate) delimitated by a square in (A) were collected, using the “low” setting for sample flow rate. 99% of the particles in that region successfully stained with the mitochondria-specific dye Mitotracker Green. (B) Isolated mitochondria were incubated with analysis buffer containing substrate (see Materials and Methods) and MitoSox (1 µM) at room temperature for 1 h and then sorted and the fluorescence of mitochondria in the gate measured. The purple area represents un-stained control. Paraquat (red line) was able to increase ROS generation over the untreated control (green line), while NAC (blue line) decreased the superoxide signal. Note that the x-axis shows a log scale. (C) Isolated mitochondria (see A) from wild-type worms were stained with both H2DCF-DA (the signal plotted on FL1-H; 530±15 nm channel) and Mitotracker Red (the signal plotted on FL3-H; ≥670 nm channel). When particles were stained by both dyes (upper-right region), the signals were strongly correlated. Furthermore, 89.6%±2.4% (n = 4) of the particles stained by H2DCF-DA were also stained by Mitotracker Red.
(0.11 MB PDF)
Absence of deleterious effects of N-acetyl-cysteine. (A) NAC (N-acetyl-cysteine) had no effect on the apparent health of isp-1 or nuo-6 mutants. Mutant animals were treated or not with 10 mM NAC throughout their lives and all pictures in the panel were taken on day 23 of their lifespan, when less than 25% of untreated mutants but more than 75% of NAC-treated mutants had already died. NAC-treated isp-1 and nuo-6 mutants did not show any visible ill effects from the treatment. All worms are shown at the same magnification; scale bar is 0.5 mm. Phenotypes possibly resulting from NAC treatment of nuo-6(qm200) mutants were also quantified. We chose to examine nuo-6 mutants because their longevity was the most sensitive to NAC (completely suppressed at 10 mM). Adult worms were allowed to lay eggs on NAC plates and phenotypes of the resulting F1 progeny were scored. (B) NAC significantly decreased defecation cycle length of the wild type (p = 0.0104), while it has no significant effect on that of nuo-6(qm200) mutant (n = 15). (C) NAC significantly increased post-embryonic development length of both the wild type and nuo-6(qm200) mutants (n = 100). (D) NAC has no significant effect on brood size of both the wild type and nuo-6(qm200) mutants (n = 50). (E) NAC has no significant effect on the swimming rate (frequency of thrashing) of the wild type, but it significantly increased that of nuo-6(qm200) mutants (p = 0.0024) (n = 15).
(0.06 MB PDF)
Co-localization of Mitotracker Red and GFP signals in C. elegans mitochondria. We used Mitotracker Red to stain worms carrying the transgene qmIs16[Pclk-1::clk-1::gfp], which expresses the mitochondrial protein CLK-1 fused to GFP [50]. Staining was as described in Materials and Methods. Worms were mounted on agar pads on slides and subjected to confocal microscopical analysis. (A) Mitotracker Red expression in hypodermal tissue. (B) The same region as in (A) expressing the mitochondrial GFP fusion. (C) The merged images of (A) and (B). The Mitotracker Red and GFP signals are co-localized.
(0.10 MB PDF)
Effect of paraquat (PQ) and N-acetylcysteine (NAC) on energy metabolism. Untreated wild type controls and animals treated with 0.1 mM PQ or 10 mM NAC since hatching were collected at the first day of adulthood for both experiments. (A) Animals in groups of 5 (n≥3) were transferred in 0.25 µl M9 buffer into a 0.5 µl chamber where oxygen concentration was measured with a fiber optic oxygen sensor (AL300 FOXY probe from Ocean Optics) for 15–30 min. The body volume of animals was calculated from pictures of the same worms and used for normalization. PQ had a small but significant consumption-increasing effect only on nuo-6 mutants. NAC increased oxygen consumption in all three genotypes, with the largest effect on isp-1 mutants. (B) The ATP content from 200 worms was normalized to the amount of soluble protein from the same sample (n≥6). Both PQ and NAC treatment had no effect on ATP content with the exception of PQ-treated nuo-6mutants, in which the treatment suppressed the high ATP content that is observed in the untreated animals. For all statistic analyses we used the Student's t test.* p<0.05, ** p<0.01 and *** p<0.001.
(0.06 MB PDF)
SOD-1 and SOD-2 are not necessary for the longevity of nuo-6(qm200) . Knocking down sod-1 (red) or sod-2 (blue) does not shorten the long lifespan of nuo-6(qm200) mutant. In fact silencing these two genes slightly increases the lifespan of nuo-6 mutants. Mean lifespan of control (empty vector) is 33 d (green), mean lifespan after sod-1 RNAi treatment is 35 d, and mean lifespan after sod-2 RNAi treatment is 36.5 d; p<0.05 for both RNAi experiments compared to control, analyzed by curve comparison using the log-rank test.
(0.02 MB PDF)
Individual aging experiments and statistics.
(0.14 MB PDF)