Abstract
Cbp, a C-terminal Src kinase (Csk)-binding protein, is a transmembrane phosphoprotein that has been implicated in the regulation of the Src family kinase (SFK) through recruiting Csk, a negative regulator of SFK, to a membrane microdomain of lipid rafts. To examine the contribution of Cbp to cell adhesion signaling mediated by SFK, we investigated the kinase responsible for phosphorylating Cbp and the mode of phosphorylation during the cell adhesion process. The results obtained by using mutant mice or cells that lack Csk and/or a member of SFK, Fyn, reveal that Cbp is phosphorylated predominantly by raft-localized Fyn in vivo. Upon cell adhesion onto fibronectin, Cbp becomes transiently phosphorylated (consistent with SFK activation) and recruits Csk to lipid rafts. These events are completed before the full activation of focal adhesion kinase, indicating that the transient activation and down-regulation of SFK in lipid rafts are earlier events in cell adhesion signaling. In Csk-deficient cells, continuous hyperactivation of SFK leads to continuous hyperphosphorylation of Cbp, accompanied by impaired cell spreading and migration. Silencing of Cbp by RNA interference also induced impaired cell spreading. These findings suggest that Cbp could serve as a sensor of SFK activity in early stages of cell adhesion signaling, and that Csk-mediated down-regulation of SFK is essential to allow dynamic cellular events involved in the regulation of cell spreading and migration.
Keywords: Fyn, C-terminal Src kinase, integrin
The Src family kinases (SFK) is a large family of nonreceptor tyrosine kinases that serves as a molecular switch involved in the initiation of a variety of cellular signaling processes in animals (1). The most characteristic feature of SFK is that its activity is strictly regulated by phosphorylation of two tyrosine residues. Autophosphorylation at a tyrosine residue located in the kinase domain is required for full activity of these kinases, and phosphorylation at another tyrosine in the C-terminal region leads to inactivation of SFK activity. The structure of SFK is ordinarily in equilibrium between an inactive conformation in which the phosphorylated C-terminal tyrosine binds intramolecularly to its own Src homology 2 (SH2) domain, and a “primed” conformation in which the C-terminal tyrosine is dephosphorylated (2, 3). In response to an extracellular signal, the primed SFK is functionally activated through accumulation of SFK itself and/or with other components that bind to SH2 or Src homology 3 domains of SFK. It is thus suggested that the function of SFK is strictly controlled by a balance between the activities of a kinase and a phosphatase that target the C-terminal regulatory tyrosine of SFK.
The phosphorylation of the C-terminal tyrosine is catalyzed by another protein tyrosine kinase, the C-terminal Src kinase (Csk) (4, 5). In contrast to SFK that is associated with the plasma membrane through the fatty acylated N terminus, Csk is basically a cytoplasmic enzyme. However, a line of evidence suggested the possible existence of a membrane factor that can recruit Csk to the membrane where SFK is localized (6, 7). Cbp (Csk-binding protein), which is sometimes called PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains), is a transmembrane phosphoprotein localized in membrane microdomain lipid rafts. Cbp was recently identified as one of the anchor proteins that recruits Csk to the plasma membrane (8, 9). When Cbp/PAG (hereafter referred to as Cbp) is phosphorylated at a specific tyrosine residue (e.g., Y314 in rat), Csk binds to Cbp through its SH2 domain. The formation of the Csk–Cbp complex could boost the kinase activity of Csk (10). Because the Csk binding site could be phosphorylated by SFK, it has been suggested that there exists a feedback regulatory loop of SFK function (11). The role of Cbp in the negative regulation of SFK has so far been studied in immune systems (9, 12, 13). However, the physiological role of this regulatory system in other cell types is only poorly understood.
Cell adhesion to the extracellular matrix mediated by integrins has been regarded as one of the primary stages of SFK function (14–16). It has been shown that integrin engagement can activate Src (17), Src can localize to focal adhesion sites (18), and SFK associates with focal adhesion proteins, such as focal adhesion kinase (FAK) and paxillin, through SH2 and Src homology 3 domains (19, 20). Previous observations that FAK directly interacts with the β-subunit of integrin and autophosphorylated FAK binds to the SH2 domain of SFK to boost their activities have placed FAK upstream of SFK in integrin signaling (21). Based on these findings, a sequential pathway from FAK activation leading to mitogen-activated protein kinase activation has been proposed (22, 23). It was also shown that SFK activated by FAK phosphorylates focal adhesion proteins to promote cell spreading or migration (21, 24). However, others have pointed out that activation of Src is transient upon cell adhesion, whereas phosphorylation of FAK remains stable even after the completion of cell spreading (17). Moreover, activation of mitogen-activated protein kinase on cell plating occurs before and independent of FAK activation in fibroblasts (25, 26), and there is only low-level FAK phosphorylation in Src/Fyn/Yes triple mutant cells (27). These findings suggest that FAK is downstream of SFK under certain conditions, although more definitive study should be undertaken to clarify the actual pathway.
It is now widely accepted that the membrane microdomain lipid rafts, in which a variety of signaling molecules including SFK are localized (28, 29), could serve as a platform for intracellular signaling. Recently, it was reported that functions of several integrins are influenced by the cholesterol or glycosphingolipid content of the plasma membrane (30–32), and that cell attachment to fibronectin induces recruitment of β1 integrin to the raft fraction and is regulated by a tyrosine phosphatase, SHP-2 (33). These findings suggest a role for lipid rafts in the regulation of cell adhesion signaling mediated by integrins, although the precise mechanism remains unknown. Taken together, these findings, along with the fact that some SFK members and Cbp are stably localized in lipid rafts, support the hypothesis that SFK signaling and integrin signaling could be integrated in lipid rafts.
To address the above possibility, we examined the role of SFK, Csk, and Cbp in cell adhesion signaling by focusing on lipid rafts. In this study, we first investigated the kinase responsible for phosphorylating Cbp in lipid rafts and then examined the activity changes in SFK and the mode of Cbp phosphorylation during the cell adhesion process. We show that raft-localized Fyn is a predominant kinase for Cbp in vivo and propose the possibility that Cbp serves as a sensor of SFK activity in cell adhesion signaling, and that Csk-mediated feedback regulation of SFK is essential to allow dynamic cellular events involved in the regulation of cell spreading and migration.
Materials and Methods
Antibodies. Polyclonal rabbit antibodies against the GST-Cbp (residues 53–208) fusion protein were generated and purified by the GST-Cbp-coupled resin. Anti-Csk, anti-Fyn, anti-Lyn, and anti-FAK antibodies were obtained from Santa Cruz Biotechnology. Anti-Src antibody was from Oncogene Science. Anti-phosphotyrosine (4G10) was from Upstate Biotechnology (Lake Placid, NY). Anti-Yes and anti-paxillin antibodies were from Transduction Laboratories (Lexington, KY). Anti-Src (pY418), anti-Src (pY529), anti-FAK (pY397), and anti-FAK (pY576) antibodies were from BioSource International (Camarillo, CA).
Mice and Cells. The Fyn-deficient mice were kindly provided by Dr. Yagi Takeshi (Institute for Molecular and Cellular Biology, Osaka University, Osaka) (34). Mouse embryonic fibroblasts were kind gifts from Dr. Imamoto Akira (Ben May Institute for Cancer Research and Center for Molecular Oncology, University of Chicago, Chicago) (35). All cells were cultured in DMEM supplemented with 10% FBS. For expression of Csk protein in Csk-deficient cells, semiconfluent cells were transiently transfected with Lipofectamine 2000 (Invitrogen).
Cell Adhesion and Invasion Assays. Cells were grown to 90–95% confluence and starved overnight in serum-free DMEM, then harvested by limited trypsin-EDTA treatment (0.25% trypsin/0.02% EDTA in PBS). Trypsin was inactivated by soybean trypsin inhibitor (0.25 mg/ml), and cells were collected by centrifugation, resuspended in serum-free DMEM containing 0.2% BSA, and held in suspension at 37°C for 1 h. Cell suspensions were centrifuged and resuspended in serum-free DMEM, one-fifth of the cell suspension was recentrifuged and lysed as nonattached cells, and four-fifths were replated on dishes coated with 4 μg/ml fibronectin and incubated for various periods at 37°C. After removing the medium, the attached cells were observed under a microscope for cell morphology and lysed for preparation of raft fractions. For invasion assays, suspended cells were added to the upper wells of Matrigel invasion chambers (Becton Dickinson), and the chamber was incubated at 37°C for 22 h. The migrated cells on the lower side were stained with Diff-Quik staining solution (International Reagents, Kobe, Japan) and counted.
Preparation of Raft Fraction. Raft fractions from neonatal mouse brains were prepared essentially as described in ref. 8. Tissues were lysed with homogenization buffer A (50 mM Tris-HCl, pH 7.4/150 mM NaCl/1 mM EDTA/10 μg/ml aprotinin/10 μg/ml leupeptin/1 mM PMSF/1 mM sodium orthovanadate/50 mM NaF/5 mM 2-mercaptoethanol) containing 1% Triton X-100, and the raft fractions were separated by ultracentrifugation on a discontinuous sucrose density gradient (40% to 35% to 5%). A part of raft fraction was solubilized with ODG buffer (buffer A plus 2% octyl-d-glucoside and 1% Nonidet P-40) and subjected to an immunoprecipitation assay. For preparation of raft fractions from embryonic fibroblasts, cells were lysed with homogenization buffer A supplemented with 0.25% Triton X-100 and subjected to separation on a sucrose density gradient (4 ml). Immunoprecipitation, SDS/PAGE, and immunoblotting were performed as described in ref. 36.
Silencing of Cbp by RNA Interference (RNAi). The 21-bp small interfering RNA sequence that could efficiently target mouse Cbp was from positions 1160–1180 (5′-AAGCCATACAGACTCTAAACA-3′). The nucleotide was inserted into pSilencer 1.0-U6 small interfering RNA vector (Ambion, Austin, TX), and the vector was cotransfected with a puromycin resistance plasmid into wild-type mouse embryonic fibroblasts by using Lipofectamine 2000. After 24 h, cells were replated onto fibronectin-coated dishes, and the transfected cells were selected with 2 μg/ml puromycin for 2 days and further grown in the presence of 1 μg/ml puromycin.
Results
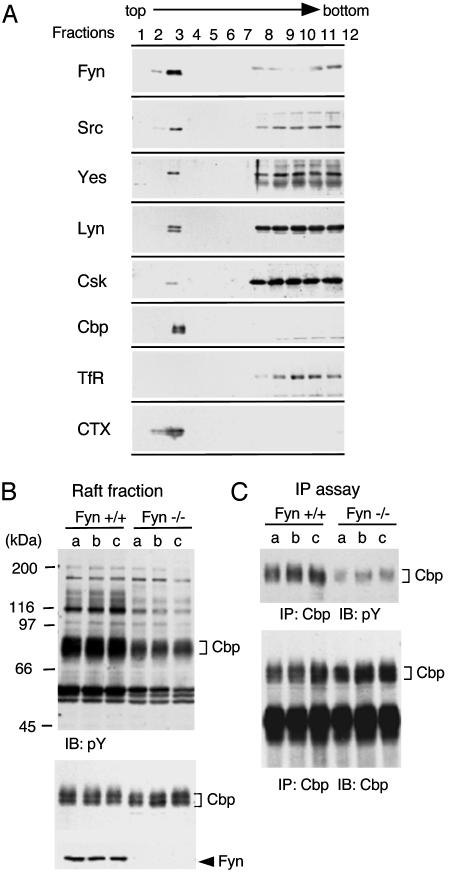
Localization of SFK in Lipid Rafts and Cbp Phosphorylation in Fyn-Deficient Mouse Brain. We first analyzed the subcellular localization of several members of SFK, Csk, and Cbp in neonatal mouse brains, where these proteins are expressed at high levels. As shown in Fig. 1A, all of the SFK members tested (Fyn, Src, Yes, and Lyn) were localized in the raft fraction, but Fyn was preferentially concentrated in the raft fraction. A small part of Csk was recovered in the raft fraction, and Cbp was localized exclusively to the raft fraction. It was already shown that Cbp can be a target of SFK (8–10), but it remained unclear whether there are specificities among the SFK members. Previous analysis showed that phosphorylation of Cbp is substantially down-regulated in the immune system of Fyn-deficient mice (37), suggesting the potential role of Fyn in Cbp phosphorylation. To further verify this finding, we analyzed the raft fraction in neonatal brains of Fyn-deficient mice. In the total raft fraction of Fyn-deficient mice, tyrosine phosphorylation levels of several proteins including Cbp were evidently reduced compared with those in wild-type mice (Fig. 1B). Cbp was then immunoprecipitated and analyzed for tyrosine phosphorylation. As shown in Fig. 1C, Cbp phosphorylation was greatly decreased in the brains of Fyn-deficient mice, suggesting that Fyn is one of the in vivo kinases for Cbp.
Fig. 1.
(A) Localization of SFK-related proteins in lipid rafts. Neonatal mouse brains were homogenized in a buffer containing 1% Triton X-100, and the raft fractions were separated on a discontinuous sucrose gradient. The fractions (1 ml) were collected from the top of the gradient and subjected to immunoblotting with antibodies against indicated proteins. Transferrin receptor and B-subunit of cholera toxin-reactive ganglioside GM1 were detected as a marker of non-raft membrane protein and a marker of lipid rafts, respectively. (B) Tyrosine phosphorylation of proteins in lipid rafts from wild-type and Fyn-deficient mouse brain. Lipid raft fractions were prepared from three littermates (a, b, and c) of wild-type (fyn+/+) and Fyn-deficient (fyn–/–) mice and immunoblotted with anti-phosphotyrosine (pY) (Top), anti-Cbp (Middle) and anti-Fyn (Lower) antibodies. Positions of molecular mass markers are shown on the left of the blots. (C) Tyrosine phosphorylation of Cbp from wild-type and Fyn-deficient mouse brain. Cbp was immunoprecipitated (IP) from lipid raft fractions, and the immunoprecipitates were immunoblotted with anti-pY (Upper) and anti-Cbp (Lower) antibodies.
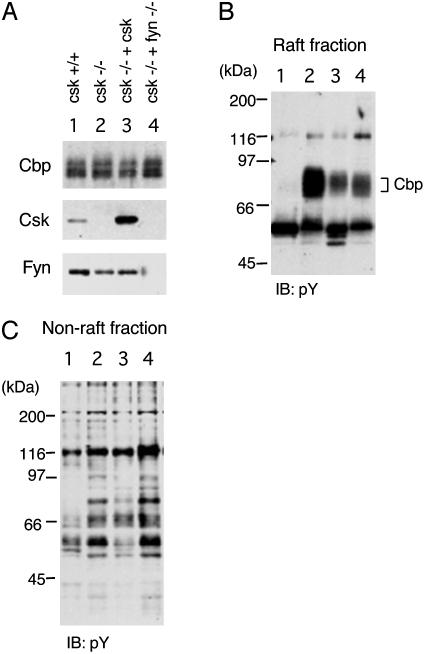
Cbp Phosphorylation in Csk-Deficient Cells. We next examined the phosphorylation of Cbp in Csk-deficient cells in which SFK is hyperactive (35, 38) (Fig. 2). In wild-type cells, phosphorylation of Cbp was barely detected. However, Cbp was hyperphosphorylated in Csk-deficient cells, and a transient expression of Csk could suppress the phosphorylation of Cbp. Furthermore, a loss of Fyn in the Csk (–/–) background greatly suppressed the phosphorylation of Cbp, demonstrating that Fyn is responsible for Cbp phosphorylation (Fig. 2B). In the non-raft fraction, there was a correlation between loss of Csk and hyperphosphorylation of some cellular proteins, but the loss of Fyn did not greatly affect the phosphorylation of non-raft proteins (Fig. 2C). These findings further support the contribution of raft-localized Fyn to Cbp phosphorylation in vivo.
Fig. 2.
Phosphorylation of Cbp in Csk-deficient embryonic fibroblasts. (A) Raft fractions were prepared from wild-type cells (lane 1), Csk-deficient cells (lane 2), Csk-deficient cells transiently transfected with Csk cDNA (lane 3), and double mutants for Csk and Fyn (lane 4), and immunoblotted with anti-Cbp (Top), anti-Csk (Middle), and anti-Fyn (Lower) antibodies. (B) The raft fractions were probed with an anti-phosphotyrosine (pY) antibody. Positions of molecular mass markers are shown on the left of the blots. (C) Non-raft fractions were probed with an anti-pY antibody.
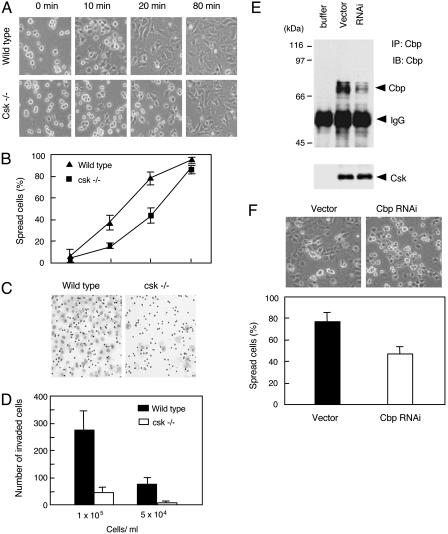
Cbp Phosphorylation in Response to Cell Adhesion. To examine whether Csk is involved in the regulation of cell adhesion signaling, we first observed cell adhesion and spreading processes in wild-type and Csk-deficient cells (Fig. 3). As depicted in Fig. 3A, there was a striking disparity between the rates of spreading in wild-type and Csk-deficient cells. Both types of cells attached to fibronectin within 10 min; however, wild-type cells were entirely spread within 40 min after plating, whereas Csk-deficient cells took 80 min to spread (Fig. 3 A and B). In addition, we observed that the ability of migration is greatly impaired in Csk-deficient cells (Fig. 3 C and D). The contribution of Cbp to these processes was assessed by RNAi of endogenous Cbp. When the expression of Cbp was down-regulated up to ≈20%, the cell spreading was significantly delayed (Fig. 3 E and F) as observed in Csk-deficient cells (Fig. 3A). These findings suggest that loss of Csk-mediated SFK regulation affects the cellular events that control cell adhesion and spreading.
Fig. 3.
Essential role of Csk in cell spreading and migration. (A) Serum-starved wild-type and Csk-deficient cells were trypsinized, resuspended in 0.2% BSA/DMEM for 1 h, and then plated onto fibronectin-coated dishes. After 10, 20, 40, and 80 min, cell shapes were observed by phase-contrast microscopy. (B) The numbers of cells having a round shape or a non-round shape were counted, and percentages of spread cells at each time point were plotted. Approximately 250 cells were counted for each point. (C) Migration activity of the cells was determined in Matrigel invasion chambers. Suspended cells at concentrations of 1 × 105 and 5 × 104 were added to the upper wells, and migrated cells on the lower side of the filter were stained with Diff-Quick staining solution. (D) The numbers of migrated cells per field ± SE. (E) Silencing of endogenous Cbp by RNAi. Wild-type cells were transfected with empty pSilencer vector or the vector carrying small interfering sequence (Cbp RNAi), and the protein expression of Cbp was detected by immunoprecipitation followed by immunoblotting with anti-Cbp antibody. Csk was detected as an internal control of protein amount in the lysates. (F) Serum-starved transfected cells were replated onto fibronectin-coated dishes, and cell shapes were observed after 30 min. The averaged percentages of spread cells ± SE calculated from five independent experiments are shown.
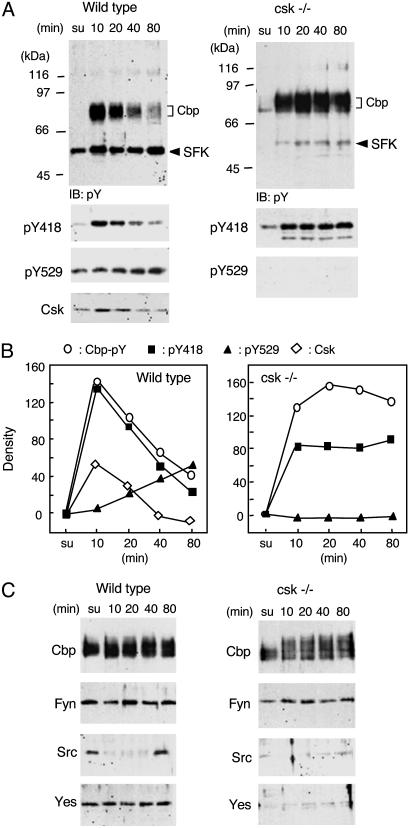
We then examined SFK activity and Cbp phosphorylation in raft fractions of wild-type and Csk-deficient cells (Fig. 4). In this study, activity status of SFK was assessed by immunoblotting with anti-Src (pY418) and anti-Src (pY529) antibodies that recognize an autophosphorylated tyrosine of multiple SFK members (Src, Fyn, Yes, and Lyn) and a phosphorylated tyrosine at the negative regulatory site, respectively. Because it is known that SFK autophosphorylation is directly associated with its activity (39), we defined the functional activity of SFK as the phosphorylation levels at pY418. In wild-type cells, activation of SFK was detected within 10 min after plating (Fig. 4A). Simultaneously, increases in tyrosine phosphorylation of Cbp and recruitment of Csk to the raft fraction were observed. During cell spreading, SFKs were inactivated, accompanied by an increase in phosphorylation at pY529, and Cbp phosphorylation was substantially decreased (Fig. 4 A and B Left). In Csk-deficient cells, we found activation of SFK and a dramatic increase in Cbp phosphorylation upon cell adhesion, but the activity of SFK and Cbp hyperphosphorylation were never down-regulated and stabilized during the cell spreading process (Fig. 4 A and B Right). This coincidence between SFK activity and Cbp phosphorylation suggests that Cbp would be one of the sensitive targets of SFK in lipid rafts.
Fig. 4.
Changes in tyrosine phosphorylation of raft proteins in response to cell adhesion. (A) Wild-type and Csk-deficient cells were trypsinized, resuspended in 0.2% BSA/DMEM, and plated onto fibronectin-coated dishes. Cells in suspension (su) and plated onto fibronectin-coated dishes for the indicated times were lysed with homogenization buffer containing 0.25% Triton X-100, and the raft fractions were prepared as described in Materials and Methods. The raft fractions were immunoblotted with anti-phosphotyrosine (pY), anti-Src (pY418), anti-Src (pY529), and anti-Csk antibodies. Positions of molecular mass markers are shown on the left side of the blots. (B) The densities of each signal shown in A were quantified by nih image software. The values in suspended cells were adjusted to 1, and the relative values were plotted. Representative data of three independent experiments are shown. (C) The raft fractions were immunoblotted with anti-Cbp, -Fyn, -Src, and -Yes antibodies.
It has been shown that there is a change in subcellular distribution of Src in response to cell adhesion (17) and that Fyn might be a key kinase for cell adhesion signaling (40). To segregate the roles of SFK members in cell adhesion signaling, we examined the amount of SFK protein in lipid rafts after cell adhesion (Fig. 4C). In either wild-type or Csk-deficient cells, Fyn was constitutively localized in lipid rafts during the cell adhesion process. In contrast, although Src was found in lipid rafts of the cells in suspension, it disappeared from there at early stages of cell adhesion and then relocated when cell spreading was complete (Fig. 4C Left). Furthermore, in the Csk-deficient cells, Src protein was markedly down-regulated potentially through ubiquitination as reported (5, 41, 42) (Fig. 4C Right). Like Fyn, Yes protein was also stably localized in lipid rafts of wild-type cells but was substantially degraded in Csk-deficient cells (Fig. 4C). These findings demonstrate that only Fyn is tightly associated with Cbp phosphorylation, supporting its preferential role in Cbp phosphorylation.
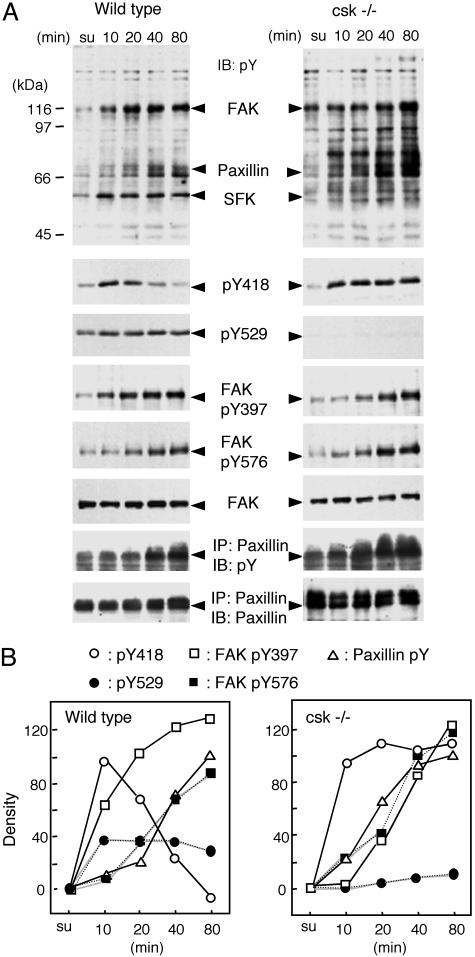
Phosphorylation of Non-Raft Proteins in Response to Cell Adhesion. We next investigated the phosphorylation of proteins present in non-raft fractions. Non-raft samples were prepared from wild-type and Csk-deficient cells during cell adhesion processes as described above and subjected to immunoblotting (Fig. 5). As a whole, tyrosine phosphorylation levels were increased in Csk-deficient cells. Elevated phosphorylation of several proteins, such as an 80-kDa protein, were detected in Csk-deficient cells (Fig. 5A Upper Right). In wild-type cells, tyrosine phosphorylation of proteins ≈120 and 65–75 kDa, corresponding to FAK and paxillin, respectively, were prominently enhanced in response to adhesion. A transient activation of SFK was detected in a manner similar to that in lipid raft fractions. However, autophosphorylation of FAK at Y397 and phosphorylation by SFK at Y576 were induced gradually and did not necessarily correspond to the change in SFK activity. Paxillin was also phosphorylated gradually in the same manner as that of FAK (Fig. 5). Even when the SFK activity declined to basal levels, the phosphorylation of FAK and paxillin was still increasing, suggesting that FAK activation is not correlated with SFK activation (Fig. 5B Left). In Csk-deficient cells, phosphorylation of FAK and paxillin also proceeded in a time course similar to that in wild-type cells, although the levels of phosphorylation were increased to some extent. By comparisons with the time course of SFK activation and Cbp phosphorylation, it appears that activation and regulation of SFK take place before the activation of FAK.
Fig. 5.
Changes in tyrosine phosphorylation of non-raft proteins in response to cell adhesion. (A) Non-raft proteins were prepared from wild-type and Csk-deficient cells as described in Fig. 4 and immunoblotted with the indicated antibodies. Tyrosine phosphorylation of paxillin was detected with antiphosphotyrosine antibody for the immunoprecipitated paxillin. (B) The densities of each signal shown in A were quantified by nih image, the values in suspended cells were adjusted to 1, and the relative values were plotted. Representative data of three independent experiments are shown.
Discussion
We here show that (i) raft-localized Fyn is a predominant kinase for Cbp, (ii) Cbp can serve as a sensitive sensor of SFK activated in an initial stage of cell adhesion signaling, and (iii) Csk-mediated regulation of SFK is essential for dynamic cellular events such as cell spreading and migration. These findings highlight a critical role of the regulatory system of SFK in cell adhesion signaling.
In this study, we found that Fyn was constantly present in lipid rafts during the cell adhesion process, whereas Src was transiently relocated to the non-raft fraction. This may reflect the differential roles of SFKs potentially because of the differential modifications at their unique amino termini (1). It is observed in Csk-deficient cells that constitutive activation of SFK induces their own ubiquitin-mediated degradation (42). Indeed, protein contents of Src and Yes were substantially reduced in our Csk-deficient cells. However, only Fyn was stably expressed even in these cells. Furthermore, it has been shown that the kinase activity of Fyn is relatively resistant to suppression by Csk compared with those of other SFKs (5, 35). These findings demonstrate that Fyn has unique features as an SFK and plays specific roles particularly in lipid rafts. We here showed that Fyn is a kinase responsible for the raft-specific transmembrane protein Cbp, and that Cbp phosphorylation depends on cell adhesion, implicating a role of raft-localized Fyn in a transduction of cell adhesion signaling. Upon cell adhesion, there was indeed a quick and transient activation of SFK, especially Fyn, in lipid rafts, followed by a gradual activation of FAK. This suggests that Fyn plays triggering roles in cell adhesion signaling as proposed (27, 43, 44). However, how Fyn in lipid rafts is activated in response to integrin activation still remains unclear. To address this at molecular levels, potential components that can functionally interact with Fyn and/or integrin in lipid rafts are now under investigation.
In normal fibroblasts, Cbp is ordinarily unphosphorylated but was dramatically phosphorylated in response to cell adhesion and then rapidly dephosphorylated. This time course coincided perfectly with that of activity of SFK, suggesting that phosphorylation status of Cbp could reflect the activity status of SFK. Thus, it seems that Cbp could serve as a sensitive sensor of SFK activity to close the negative feedback loop by recruiting Csk. An RNAi study revealed that down-regulation of Cbp could induce impaired cell spreading, supporting an important role of Cbp/Csk-mediated regulation of SFK. However, because it has been known that Csk can interact with some additional SFK targets, including paxillin and FAK, the possibility that there is an alternative or compensating system in the regulation of Csk cannot be excluded. It has been shown that Cbp is already phosphorylated in resting T cells, and that T cell antigen receptor stimulation induces dephosphorylation of Cbp and the dissociation of Csk (9, 45). These observations suggest that the status of Cbp phosphorylation is substantially different among cell types. Ordinarily, SFK is at equilibrium between inactive and primed conditions. In lymphoid cells, the equilibrium may be shifted to the primed condition potentially because of high activity of tyrosine phosphatase such as CD45. In adhered fibroblasts, the equilibrium is shifted to the inactive condition; therefore, the phosphorylation of Cbp is very low. Once the fibroblasts are detached, SFK becomes primed as a result of the activation of certain tyrosine phosphatases; therefore, the transient phosphorylation of Cbp could be observed upon cell adhesion. Thus, it would be interesting to hypothesize that the equilibrium conditions of SFK can generally define the sensitivity of the cells to extracellular signals.
Regardless of the pathway, it is well established that SFK-mediated phosphorylation of FAK and paxillin is important for the formation and maintenance of focal contacts (46). Because SYF triple mutant cells can form focal contacts normally, it is suggested that SFK activity is not essential for focal contact formation itself (27). However, the rate of focal contact formation in such mutant cells is greatly impaired, resulting in delayed cell spreading and migration. In contrast, Csk-deficient cells, where SFK is hyperactive, show an increased number of focal contacts and impaired formation of actin stress fibers (35, 38), which also cause delayed cell spreading and migration. These findings demonstrate that SFK acts to promote focal contact formation, but that its activity should be dynamically regulated to allow normal cell spreading and migration. We here observed there is a transient activation and down-regulation of SFK through feedback regulation mediated by Csk. This regulation may dynamically occur at local sites in migrating cells, such as focal contacts where formation and degradation of the structure are actively taking place. To directly relate the regulated SFK activity to cellular events, we are now investigating an initial and transient target of SFK that enables the turnover of focal contacts and/or its cytoskeletal support. In this sense, Cbp could be one of the candidate targets. Because Cbp has multiple phosphorylatable tyrosine residues other than Csk binding site, it is possible that Cbp plays some additional roles by recruiting adapter proteins having SH2 or phosphotyrosine-binding domain, such as Shc and Nck, both of which have been implicated in cell adhesion signaling (40, 47). Contributions of these potential targets are now under investigation.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas from Ministry of Education, Culture, Sports, Science and Technology of Japan.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SH2, Src homology 2; Csk, C-terminal Src kinase; SFK, Src family kinase; FAK, focal adhesion kinase; RNAi, RNA interference.
References
- 1.Thomas, S. M. & Brugge, J. S. (1997) Annu. Rev. Cell Dev. Biol. 13, 513–609. [DOI] [PubMed] [Google Scholar]
- 2.Xu, W., Harrison, S. C. & Eck, M. J. (1997) Nature 385, 595–602. [DOI] [PubMed] [Google Scholar]
- 3.Young, M. A., Gonfloni, S., Superti-Furga, G., Roux, B. & Kuriyan, J. (2001) Cell 105, 115–126. [DOI] [PubMed] [Google Scholar]
- 4.Nada, S., Okada, M., MacAuley, A., Cooper, J. A. & Nakagawa, H. (1991) Nature 351, 69–72. [DOI] [PubMed] [Google Scholar]
- 5.Nada, S., Yagi, T., Takeda, H., Tokunaga, T., Nakagawa, H., Ikawa, Y., Okada, M. & Aizawa, S. (1993) Cell 73, 1125–1135. [DOI] [PubMed] [Google Scholar]
- 6.Howell, B. W. & Cooper, J. A. (1994) Mol. Cell. Biol. 14, 5402–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow, L. M., Fournel, M., Davidson, D. & Veillette, A. (1993) Nature 365, 156–160. [DOI] [PubMed] [Google Scholar]
- 8.Kawabuchi, M., Satomi, Y., Takao, T., Shimonishi, Y., Nada, S., Nagai, K., Tarakhovsky, A. & Okada, M. (2000) Nature 404, 999–1003. [DOI] [PubMed] [Google Scholar]
- 9.Brdicka, T., Pavlistova, D., Leo, A., Bruyns, E., Korinek, V., Angelisova, P., Scherer, J., Shevchenko, A., Hilgert, I., Cerny, J., et al. (2000) J. Exp. Med. 191, 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi, S., Takayama, Y., Ogawa, A., Tamura, K. & Okada, M. (2000) J. Biol. Chem. 275, 29183–29186. [DOI] [PubMed] [Google Scholar]
- 11.Cary, L. A. & Cooper, J. A. (2000) Nature 404, 945–947. [DOI] [PubMed] [Google Scholar]
- 12.Davidson, D., Bakinowski, M., Thomas, M. L., Horejsi, V. & Veillette, A. (2003) Mol. Cell. Biol. 23, 2017–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindquist, J. A., Simeoni, L. & Schraven, B. (2003) Immunol. Rev. 191, 165–182. [DOI] [PubMed] [Google Scholar]
- 14.Hirst, R., Horwitz, A., Buck, C. & Rohrschneider, L. (1986) Proc. Natl. Acad. Sci. USA 83, 6470–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glenney, J. R., Jr., & Zokas, L. (1989) J. Cell Biol. 108, 2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanner, S. B., Reynolds, A. B., Vines, R. R. & Parsons, J. T. (1990) Proc. Natl. Acad. Sci. USA 87, 3328–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan, K. B., Swedlow, J. R., Morgan, D. O. & Varmus, H. E. (1995) Genes Dev. 9, 1505–1517. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan, K. B., Bibbins, K. B., Swedlow, J. R., Arnaud, M., Morgan, D. O. & Varmus, H. E. (1994) EMBO J. 13, 4745–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobb, B. S., Schaller, M. D., Leu, T. H. & Parsons, J. T. (1994) Mol. Cell. Biol. 14, 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown, M. T. & Cooper, J. A. (1996) Biochim. Biophys. Acta 1287, 121–149. [DOI] [PubMed] [Google Scholar]
- 21.Hanks, S. K. & Polte, T. R. (1997) BioEssays 19, 137–145. [DOI] [PubMed] [Google Scholar]
- 22.Schaller, M. D., Hildebrand, J. D., Shannon, J. D., Fox, J. W., Vines, R. R. & Parsons, J. T. (1994) Mol. Cell. Biol. 14, 1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer, D. D., Hanks, S. K., Hunter, T. & van der Geer, P. (1994) Nature 372, 786–791. [DOI] [PubMed] [Google Scholar]
- 24.Parsons, J. T. & Parsons, S. J. (1997) Curr. Opin. Cell Biol. 9, 187–192. [DOI] [PubMed] [Google Scholar]
- 25.Lin, T. H., Aplin, A. E., Shen, Y., Chen, Q., Schaller, M., Romer, L., Aukhil, I. & Juliano, R. L. (1997) J. Cell Biol. 136, 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barberis, L., Wary, K. K., Fiucci, G., Liu, F., Hirsch, E., Brancaccio, M., Altruda, F., Tarone, G. & Giancotti, F. G. (2000) J. Biol. Chem. 275, 36532–36540. [DOI] [PubMed] [Google Scholar]
- 27.Cary, L. A., Klinghoffer, R. A., Sachsenmaier, C. & Cooper, J. A. (2002) Mol. Cell. Biol. 22, 2427–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurzchalia, T. V. & Parton, R. G. (1999) Curr. Opin. Cell Biol. 11, 424–431. [DOI] [PubMed] [Google Scholar]
- 29.Galbiati, F., Razani, B. & Lisanti, M. P. (2001) Cell 106, 403–411. [DOI] [PubMed] [Google Scholar]
- 30.Pande, G. (2000) Curr. Opin. Cell Biol. 12, 569–574. [DOI] [PubMed] [Google Scholar]
- 31.Gopalakrishna, P., Chaubey, S. K., Manogaran, P. S. & Pande, G. (2000) J. Cell Biochem. 77, 517–528. [PubMed] [Google Scholar]
- 32.Zheng, M., Fang, H., Tsuruoka, T., Tsuji, T., Sasaki, T. & Hakomori, S. (1993) J. Biol. Chem. 268, 2217–2222. [PubMed] [Google Scholar]
- 33.Lacalle, R. A., Mira, E., Gomez-Mouton, C., Jimenez-Baranda, S., Martinez-A, C. & Manes, S. (2002) J. Cell Biol. 157, 277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yagi, T., Aizawa, S., Tokunaga, T., Shigetani, Y., Takeda, N. & Ikawa, Y. (1993) Nature 366, 742–745. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, S. M., Soriano, P. & Imamoto, A. (1995) Nature 376, 267–271. [DOI] [PubMed] [Google Scholar]
- 36.Shima, T., Okumura, N., Takao, T., Satomi, Y., Yagi, T., Okada, M. & Nagai, K. (2001) J. Biol. Chem. 276, 42233–42240. [DOI] [PubMed] [Google Scholar]
- 37.Yasuda, K., Nagafuku, M., Shima, T., Okada, M., Yagi, T., Yamada, T., Minaki, Y., Kato, A., Tani-Ichi, S., Hamaoka, T. & Kosugi, A. (2002) J. Immunol. 169, 2813–2817. [DOI] [PubMed] [Google Scholar]
- 38.Nada, S., Okada, M., Aizawa, S. & Nakagawa, H. (1994) Oncogene 9, 3571–3578. [PubMed] [Google Scholar]
- 39.Sabe, H., Okada, M., Nakagawa, H. & Hanafusa, H. (1992) Mol. Cell. Biol. 12, 4706–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wary, K. K., Mariotti, A., Zurzolo, C. & Giancotti, F. G. (1998) Cell 94, 625–634. [DOI] [PubMed] [Google Scholar]
- 41.Imamoto, A. & Soriano, P. (1993) Cell 73, 1117–1124. [DOI] [PubMed] [Google Scholar]
- 42.Harris, K. F., Shoji, I., Cooper, E. M., Kumar, S., Oda, H. & Howley, P. M. (1999) Proc. Natl. Acad. Sci. USA 96, 13738–13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A. & Soriano, P. (1999) EMBO J. 18, 2459–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlaepfer, D. D., Broome, M. A. & Hunter, T. (1997) Mol. Cell. Biol. 17, 1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torgersen, K. M., Vang, T., Abrahamsen, H., Yaqub, S., Horejsi, V., Schraven, B., Rolstad, B., Mustelin, T. & Tasken, K. (2001) J. Biol. Chem. 276, 29313–29318. [DOI] [PubMed] [Google Scholar]
- 46.Ilic, D., Furuta, Y., Kanazawa, S., Takeda, N., Sobue, K., Nakatsuji, N., Nomura, S., Fujimoto, J., Okada, M., Yamamoto, T. & Aizawa, S. (1995) Nature 377, 539–544. [DOI] [PubMed] [Google Scholar]
- 47.Buday, L., Wunderlich, L. & Tamas, P. (2002) Cell Signalling 14, 723–731. [DOI] [PubMed] [Google Scholar]