Abstract
Human tryptophanyl-tRNA synthetase (TrpRS) is active in translation and angiogenesis. In particular, an N-terminally truncated fragment, T2-TrpRS, that is closely related to a natural splice variant is a potent antagonist of vascular endothelial growth factor-induced angiogenesis in several in vivo models. In contrast, full-length native TrpRS is inactive in the same models. However, vascular endothelial growth factor stimulation is only one of many physiological and pathophysiological stimuli to which the vascular endothelium responds. To investigate more broadly the role of T2-TrpRS in vascular homeostasis and pathophysiology, the effect of T2-TrpRS on well characterized endothelial cell (EC) responses to flow-induced fluid shear stress was studied. T2-TrpRS inhibited activation by flow of protein kinase B (Akt), extracellular signal-regulated kinase 1/2, and EC NO synthase and prevented transcription of several shear stress-responsive genes. In addition, T2-TrpRS interfered with the unique ability of ECs to align in the direction of fluid flow. In all of these assays, native TrpRS was inactive, demonstrating that angiogenesis-related activity requires fragment production. These results demonstrate that T2-TrpRS can regulate extracellular signal-activated protein kinase, Akt, and EC NO synthase activation pathways that are associated with angiogenesis, cytoskeletal reorganization, and shear stress-responsive gene expression. Thus, this biological fragment of TrpRS may have a role in the maintenance of vascular homeostasis.
Keywords: tryptophanyl-tRNA synthetase, signaling
Aminoacyl-tRNA synthetases have a broad repertoire of functions beyond translation, including transcriptional and translational regulation as well as cell signaling (1). In particular, mammalian tyrosyl-tRNA synthetase (TyrRS) and tryptophanyl-tRNA synthetase (TrpRS) have been shown to regulate angiogenesis (2–7). The cell-signaling activity by human synthetases arose during evolution through individual sequence adaptations and domain acquisitions. For example, embedded in mammalian TyrRS is a tripeptide Glu-Leu-Arg motif essential for cell signaling (2, 4). Introduction of this mammalian motif into yeast TyrRS conferred cell-signaling activity on the otherwise inactive yeast tRNA synthetase (8). Furthermore, mammalian TyrRSs contain an appended cytokine domain not found in TyrRSs from lower organisms such as Caenorhabditis elegans and yeast (8). As a stand-alone domain, the TyrRS-appended domain stimulated mononuclear phagocyte chemotaxis and tumor necrosis factor-α production in a manner similar to the cytokine endothelial and monocyte-activating polypeptide II (2). These discoveries demonstrated a link between translation and cell signaling.
Human TrpRS, a close homologue of TyrRS, was subsequently established to participate in cell-signaling pathways (5). Human cells contain two distinct TrpRS isoforms, TrpRS (471 aa) and mini-TrpRS (424 aa), that arise naturally by alternative mRNA splicing (9, 10). Additionally, the expression of TrpRS and mini-TrpRS is strongly induced by the antiproliferative cytokine IFN-γ (8–10). Although both alternative splicing and IFN-γ induction have been known for several years, the significance of these observations was not understood until it was demonstrated that the alternatively spliced mini-TrpRS had antiangiogenic activities in a variety of in vitro and in vivo assays. For example, mini-TrpRS blocked vascular endothelial growth factor-induced migration of human umbilical vein endothelial cells. In addition, mini-TrpRS and a closely related proteolytic variant, T2-TrpRS, blocked vascular endothelial growth factor-stimulated angiogenesis in both chick cell adhesion molecule and mouse matrigel assays in vivo (5, 6). In contrast, full-length TrpRS had no effect (5, 6). As an antagonist of vascular endothelial growth factor-stimulated angiogenesis, T2-TrpRS had an IC50 of 1.7 nM (5, 6). T2-TrpRS was also a potent inhibitor of retinal angiogenesis in the neonatal mouse, where it localized to retinal blood vessels. Together, these results suggest endothelial cells (ECs) as a direct target of T2-TrpRS.
ECs of the vasculature are constantly subjected to mechanical forces resulting from pulsatile blood flow. These hemodynamic forces, including shear stress and pressure, have profound effects on EC biology and thus play a major role in vascular homeostasis and pathophysiology. The principal mechanical stimulus for ECs is fluid shear stress, which regulates a variety of EC functions, including migration, proliferation, and survival, which are key components of angiogenesis. Shear stress regulates other endothelial functions, such as production of vasoactive mediators and expression of adhesion molecules. It is thus the driving force for the regulation of vessel architecture (11). Shear stress is also critical for determining the distribution of atherosclerotic plaques; lesions appear almost exclusively at regions of the vasculature where flow is disturbed due to curvature and at the proximal region of branching vessels.
Angiogenesis is the formation of new capillaries from preexisting vasculature by migration and proliferation of ECs. It has a fundamental role in the growth, survival, and function of normal and pathological tissues (12, 13). The process of angiogenesis requires loosening of intercellular junctions and degradation of the extracellular matrix by ECs, migration of ECs toward the angiogenic stimulus, sprout formation, formation of a lumen, and the joining of sprouts to form a capillary bed (13). Angiogenesis may be beneficial in some clinical circumstances, such as in tissue damage after reperfusion of ischemic tissue or cardiac failure, but maladaptive in other situations, such as cancer and intraplaque formation (13, 14). Because increased or decreased blood flow is linked with growth or regression of the vessels, shear stress has a role in angiogenesis (15–17). In addition, high blood flow occurs concomitantly with capillary growth in physiological conditions such as exercise or exposure to high altitude, and restriction of blood flow has been used to cause regression of tumors (18). More recently, physiological shear stress was demonstrated to enhance wound closure in ECs via the action of EC spreading and migration (19) and EC angiogenic activity (20).
To explore more broadly the roles of T2-TrpRS in vascular biology, we tested whether T2-TrpRS might affect mechanosensitive cell signaling. Our results show that T2-TrpRS affected activation of well known mechanosensitive signaling proteins [extra cellular signal-regulated kinase (ERK), Akt, endothelial cell NO synthase (eNOS)], shear stress-dependent gene expression, and the morphology of bovine aortic endothelial cells (BAECs) that were subjected to shear stress.
Materials and Methods
Cell Culture and Shear Stress. T2-TrpRS and TrpRS were prepared as described (6). BAECs were maintained in DMEM with 10% FBS/1% penicillin/streptomycin/2 mM l-glutamine (GIBCO/BRL) in a humidified 5% CO2/95% air incubator at 37°C. BAECs on 38 × 76-mm slides at confluence were subjected to shear stress at 12 dynes/cm2 (1 dyne = 10 μN) in a parallel plate flow chamber (21, 22).
ERK, Akt, and eNOS Assays. Cells were lysed in ice-cold 50 mM Tris, pH 7.2/0.5% Nonidet P-40/50 mM NaCl/10 μg/ml each leupeptin and aprotinin/1 mM PMSF/1 mM sodium orthovanadate. Lysates were centrifuged at 13,000 × g at 4°C for 10 min, and supernatants were mixed with Laemmli buffer. ERK activation was detected by Western blotting by using a monoclonal antibody against phospho-p44/42 mitogen-activated protein kinase (Thr-202/Tyr-204) (Cell Signaling Technology, Beverly, MA); total ERK was detected by using a polyclonal antibody against p44/42 mitogen-activated protein kinase (Cell Signaling Technology). Akt activation was detected by using a monoclonal phospho-Akt (Ser-473), and total Akt was detected by using a polyclonal anti-Akt (Cell Signaling Technology). Activated eNOS was detected by using a polyclonal antiphospho-eNOS (Ser-1172) (Cell Signaling Technology), and total eNOS was detected by using a monoclonal anti-eNOS (BD Biosciences).
Fluorescence Microscopy. Cells were fixed for 30 min in 2% formaldehyde in PBS, permeabilized in 0.2% Triton X-100/PBS, and rinsed twice with PBS. Nonspecific sites were blocked with 10% goat serum, incubated with tetramethylrhodamine B isothiocyanate-phalloidin (Sigma), and mounted in immunofluorescence mounting medium (ICN). Images of fixed cells were acquired by using a Bio-Rad 1024 confocal microscope.
Luciferase Assays. HIV(LTR)-Luc is a luciferase reporter driven by the HIV long-terminal repeat that contains two binding sites for NF-κB (23). Platelet-derived growth factor A (PDGF-A)/shear stress response element (SSRE) contains the firefly luciferase regulated by the early-growth response factor 1 binding site of the PDGF-A promoter, which contains the SSRE. HIV-(LTR)-Luc and PDGF-A/SSRE (1.0 μg) were cotransfected into BAECs for the luciferase induction assay by using Effectene according to the manufacturer's instructions (Qiagen, Valencia, CA). The pSVRenilla plasmid was also cotransfected to monitor the transfection efficiency. After 10 h in growth medium, cells were starved overnight in 0.5% serum before shear stress. Cells were then lysed in buffer containing 1% Triton X-100. ATP and luciferin were added to the lysate in a luminometer for measuring the total light output. The normalized luciferase activities represent the firefly luciferase activity corrected for transfection efficiency.
Results
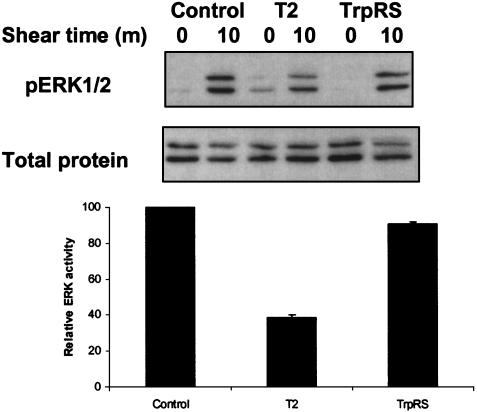
Effects of T2-TrpRS on Shear Stress-Dependent Signaling. Shear stress can influence a variety of EC functions, including activation of the mitogen-activated protein kinases (24–27). To investigate whether T2-TrpRS inhibited shear-dependent activation of ERK, cells were pretreated with T2-TrpRS or TrpRS before being subjected to shear stress. As expected, untreated BAECs showed stimulation of ERK phosphorylation after 10 min of shear stress. Cells treated with TrpRS behaved similarly to untreated cells (Fig. 1). In contrast, ERK activation in response to shear stress was inhibited by 60% in the cells that had been preincubated with T2-TrpRS (Fig. 1).
Fig. 1.
T2-TrpRS inhibits shear-dependent activation of ERK. BAECs were incubated with T2-TrpRS or TrpRS for 15 min at 37°C. Cells were then subjected to static control or shear stress (12 dyn/cm2 for 10 min). Cell lysates were analyzed by Western blots with antibodies specific to total ERK1/2 or phospho-ERK1/2 (pERK1/2). Values are means ± SE (n = 3) obtained from densitometric quantitation of pERK1 bands at 10 min after shear stress.
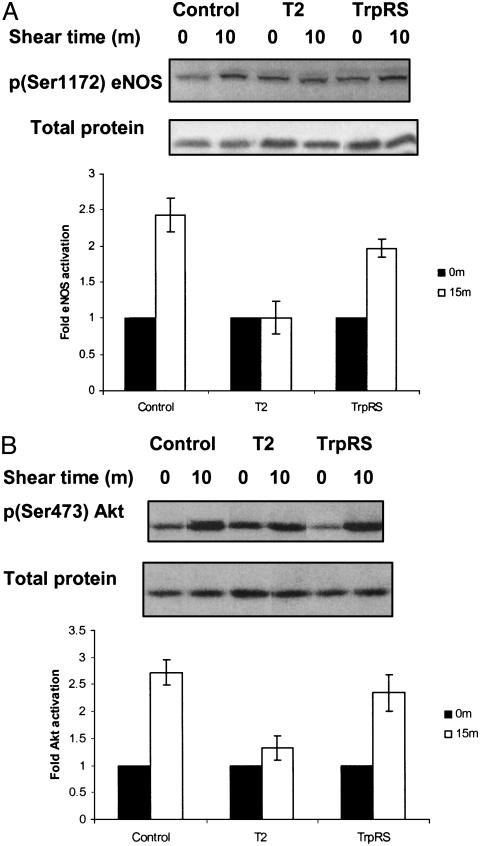
The exposure of ECs to shear stress up-regulates eNOS activity and triggers nitric oxide (NO) release. Physiologically, the most important stimulus for the continuous formation of NO is shear stress (28, 29). Because it regulates systemic blood pressure, vascular remodeling, and angiogenesis (30), endothelium-derived NO has a crucial role in the local regulation of vascular homeostasis. The bioavailability of NO is characteristically decreased in patients with coronary artery disease (31); that, in turn, aggravates the development of atherosclerotic lesions (32). As phosphorylation of eNOS-Ser-1179 is associated with increased activity of the enzyme (33, 34), we tested the effect of T2-TrpRS on the shear stress-induced phosphorylation of eNOS-Ser-1179. Shear stress increased eNOS activity by >2-fold as assessed by phosphorylation of Ser-1179. Incubation with T2-TrpRS completely prevented shear-stress-induced eNOS phosphorylation, whereas TrpRS did not affect phosphorylation (Fig. 2A).
Fig. 2.
Effect of T2-TrpRS on phosphorylation of eNOS at Ser-1179 (A) and Akt activation (B) in response to shear stress. BAECs were incubated with T2-TrpRS or TrpRS for 15 min at 37°C. Cells were then subjected to static control or shear stress (12 dyn/cm2 for 10 min). Cell lysates were analyzed by Western blots with antibodies specific to total eNOS or phospho-eNOS [p(Ser1172) eNOS] and total Akt or phospho-Akt [p(Ser473) Akt]. Values are means ± SE (n = 3).
Stimulation of Akt by shear stress elicits phosphorylation of eNOS on Ser-1179 (33, 35, 36). We therefore examined the effect of T2-TrpRS on shear stress-dependent activation of Akt. As shown in Fig. 2B, exposure of BAECs to shear stress for 10 min elicited the rapid phosphorylation and activation of Akt. In contrast, preincubation of cells with T2-TrpRS abrogated the shear stress-dependent activation of Akt, whereas native TrpRS had no effect (Fig. 2B). Thus, T2-TrpRS may inhibit activation of eNOS through inhibition of Akt.
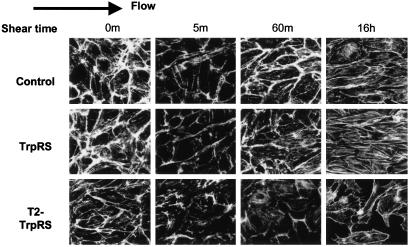
T2-TrpRS Inhibits Shear Stress-Induced Cell Alignment. Cells exposed to shear stress for longer periods have increased actin stress fibers, which elongate and align in the direction of flow (37). This adaptation to flow is believed to contribute to a reduction of shear gradients along the EC surface (38). The effect of native TrpRS and T2-TrpRS on stress fiber alignment in the direction of flow was therefore examined. BAECs exposed to shear stress showed an initial decrease in stress fibers at 5 min. This decrease is associated with a transient decrease in Rho activity (21) and is followed by a return of stress fibers and subsequently alignment in the direction of flow (39). Treated cells were subjected to shear stress for various times, and F-actin fibers were visualized using rhodamine–phalloidin (Fig. 3). Untreated control cells, as well as cells treated with TrpRS, initially showed a decrease in actin stress fibers followed by a recovery and partial alignment at 60 min (Fig. 3). After 16 h of shear stress, the restored stress fibers were aligned in the direction of flow. T2-TrpRS-treated cells showed a similar decrease in actin staining followed by incomplete recovery and random orientation of actin filaments. After 16 h, the orientation of actin stress fibers in cells treated with T2-TrpRS was significantly inhibited (Fig. 3). In fact, treatment with T2-TrpRS rendered cells sensitive to shearing forces, resulting in detachment of cells in response to shear. This effect may be a consequence of cytoskeletal organization. In contrast, unsheared cells remained firmly attached to their substratum despite the presence of T2-TrpRS.
Fig. 3.
T2-TrpRS blocks shear stress-induced cell alignment. BAECs were incubated with T2-TrpRS or TrpRS for 15 min at 37°C and subjected to shear stress for the indicated times or kept under static conditions. Cells were fixed and stained with rhodamine phalloidin. Direction of flow is indicated by arrow.
Sensitivity of ECs to detachment after shear stress could be due to defects in cytoskeletal attachments to the integrins and/or cell-substrate contacts (40, 41). In fact, the effect of T2-TrpRS is reminiscent of the effect of actin depolymerization reagents on shear stress responses. For example, Schnittler and colleagues reported that small disturbances in actin dynamics caused by clostridium botulinum C2 toxin inhibited shear stress-dependent alignment and caused intercellular gap formation and cell detachment under shear stress (42). Thus, T2-TrpRS may bind to a receptor on the surface of ECs and alter the response to shear stress in a way that renders the endothelial monolayer susceptible to shearing forces.
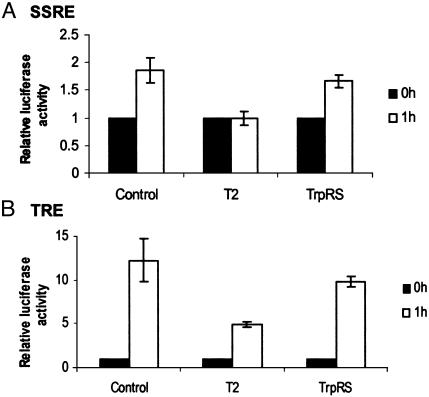
Regulation of Shear Stress-Induced Gene Expression by T2-TrpRS. Shear stress-mediated signaling into the endothelium also affects the transcription of many endothelial genes (43, 44). This transcriptional activity is mediated by positive or negative promoter elements such as the SSREs (45) and the phorbol ester 12-O-tetradecanoylphorbol 13-acetate-responsive elements (TRE) (46). To test the role of T2-TrpRS in shear stress-mediated transcription, BAECs were transfected with either a construct encoding the luciferase reporter gene regulated by a hybrid promoter containing PDGF-A/SSRE (47) or a luciferase construct containing the TRE consensus sequence of the monocyte chemotactic protein 1 gene. Renilla luciferase under the control of a constitutive promoter was included as an internal control. The transfected cells were then exposed to shear stress. In response to flow, expression of the SSRE-luciferase gene was induced by 2-fold in both control untreated cells and TrpRS pretreated cells but was completely blocked by T2-TrpRS (Fig. 4). Similarly, TRE-luciferase gene expression in control and TrpRS-treated cells was induced 12-fold in response to shear stress and was inhibited by ≈50% by T2-TrpRS (Fig. 4).
Fig. 4.
Transcriptional induction of SSRE- and TRE-luciferase vectors does not occur in the presence of T2-TrpRS. BAEC cultures were cotransfected with a vector containing the PDGF-A/SSRE regulating the expression of a firefly luciferase reporter gene and a Renilla luciferase regulated by a minimal promoter (A) or a vector containing the monocyte chemotactic protein 1/TRE regulating the expression of a firefly luciferase reporter gene and a Renilla luciferase regulated by a minimal promoter (B). The transfected cells were incubated under static conditions or exposed to laminar shear stress (12 dynes/cm2, 60 min), after being treated with TrpRS or T2-TrpRS, and the expression of the firefly luciferase was normalized to the expression of the Renilla luciferase. Statistical analysis was carried out on three independent experiments in which samples were in duplicate (P < 0.05).
Discussion
We show in this study that T2-TrpRS affects mechanical signaling of ECs by inhibiting ERK, Akt, and eNOS activation pathways to modulate cytoskeletal reorganization and gene expression. As shear stress occurs concomitantly with angiogenesis, perturbation of shear stress signaling by T2-TrpRS could explain its observed antiangiogenic activity. That is, by rendering cells unresponsive to the stimulatory effects of shear stress, T2-TrpRS inhibits formation and stabilization of new vessels. In addition to its role as an inhibitor of angiogenesis, the effects on shear stress signaling suggest a role for T2-TrpRS in vascular remodeling, regulation of blood pressure, and development of inflammation and atherosclerosis. For example, the results obtained in response to sudden onset of flow are likely to be relevant to locations of disturbed or turbulent shear in vivo, where ECs initiate inflammatory reactions that lead to atherosclerotic lesions. Regions of disturbed flow have elevated levels of adhesion molecules that attract monocytes and leukocytes and lead to increased EC motility (48, 49). Because shear stress provides a stimulus for the development of intimal hyperplasia (50), we could also envision a role for T2-TrpRS in inhibition of restenosis and endothelialization of intimal hyperplasia that occurs after bypass surgery, balloon angioplasty, and stent replacement.
The role of T2-TrpRS in signaling events that control morphology and gene expression of ECs is not surprising considering the similarity between TrpRS expression and expression of a number of other genes involved in signal transduction and cell adhesion pathways (7). Although the receptor mediating T2-TrpRS cell signaling is unknown, the fact that T2-TrpRS inhibited shear stress responses as well as vascular endothelial growth factor-stimulated signaling in cultured ECs suggests a more general mechanism in which T2-TrpRS specifically inhibits EC activation, which may explain the observed antiangiogenic activity in three separate in vivo angiogenesis models (5, 6).
Furthermore, T2-TrpRS administered during retinal angiogenesis inhibited vessel development and localized exclusively to preexisting retinal vessels (6). In this same retinal model, bone marrow-derived stem cells that produced T2-TrpRS in situ inhibited blood vessel development. As a control, non-T2-TrpRS producing bone marrow-derived stem cells integrated normally into developed blood vessels (6). This suggests that T2-TrpRS could act through an autocrine pathway in ECs to regulate angiogenesis. ECs (as well as several other cell types) strongly express TrpRS in response to the antiproliferative cytokine IFN-γ (8), which is known to up-regulate other antiangiogenic factors (51, 52).
Antiangiogenic fragments of TrpRS also inhibit the proangiogenic stimulation of mini TyrRS (4). Both synthetases require activation for signaling by removal of specific domains by either proteolysis or alternative splicing (2, 5), producing a closely related structural core that is active in cell signaling. It is noteworthy that this pair of synthetases coevolved cell signaling activities that balance each other and that the particular cell-signaling features found in TyrRS and TrpRS are conserved in organisms with developed vasculature (8). The opposing activities in two tRNA synthetases are analogous to the opposing activities seen in CXC chemokines to regulate angiogenesis. Imbalance of pro- and antiangiogenic CXC chemokines can alter net angiogenesis as seen, for example, in a study of patients with acute respiratory distress syndrome where expression favoring proangiogenic members was correlated with net angiogenesis (53). Together, the two synthetases could thus represent an early system for regulating blood vessel development or guidance.
Acknowledgments
We thank Dr. W. B. Kiosses for assistance with confocal images and helpful discussions. This work was funded by National Institutes of Health Grant CA92577, a fellowship from the National Foundation of Cancer Research, and U.S. Public Health Service Grant PO1 HL48728. E.T. is an American Heart Association Western States Fellow (032514Y), and J.S.R. is a Skaggs Institute for Chemical Biology Fellow.
Abbreviations: TrpRS, tryptophanyl-tRNA synthetase; TyrRS, tyrosyl-tRNA synthetase; EC, endothelial cell; ERK, extracellular signal-regulated kinase; BAEC, bovine aortic EC; PDGF-A, platelet-derived growth factor A; SSRE, shear stress response element; TRE, 12-O-tetradecanoylphorbol 13-acetate-responsive element; eNOS, EC NO synthase.
References
- 1.Martinis, S. A., Plateau, P., Cavarelli, J. & Florentz, C. (1999) EMBO J. 18, 4591–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wakasugi, K. & Schimmel, P. (1999) J. Biol. Chem. 274, 23155–23159. [DOI] [PubMed] [Google Scholar]
- 3.Wakasugi, K. & Schimmel, P. (1999) Science 284, 147–151. [DOI] [PubMed] [Google Scholar]
- 4.Wakasugi, K., Slike, B. M., Hood, J., Ewalt, K. L., Cheresh, D. A. & Schimmel, P. (2002) J. Biol. Chem. 277, 20124–20126. [DOI] [PubMed] [Google Scholar]
- 5.Wakasugi, K., Slike, B. M., Hood, J., Otani, A., Ewalt, K. L., Friedlander, M., Cheresh, D. A. & Schimmel, P. (2002) Proc. Natl. Acad. Sci. USA 99, 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otani, A., Slike, B. M., Dorrell, M. I., Hood, J., Kinder, K., Ewalt, K. L., Cheresh, D., Schimmel, P. & Friedlander, M. (2002) Proc. Natl. Acad. Sci. USA 99, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewalt, K. L. & Schimmel, P. (2002) Biochemistry 41, 13344–13349. [DOI] [PubMed] [Google Scholar]
- 8.Liu, J., Yang, X. L., Ewalt, K. L. & Schimmel, P. (2002) Biochemistry 41, 14232–14237. [DOI] [PubMed] [Google Scholar]
- 9.Tolstrup, A. B., Bejder, A., Fleckner, J. & Justesen, J. (1995) J. Biol. Chem. 270, 397–403. [DOI] [PubMed] [Google Scholar]
- 10.Turpaev, K. T., Zakhariev, V. M., Sokolova, I. V., Narovlyansky, A. N., Amchenkova, A. M., Justesen, J. & Frolova, L. Y. (1996) Eur. J. Biochem. 240, 732–737. [DOI] [PubMed] [Google Scholar]
- 11.Ishida, T., Takahashi, M., Corson, M. A. & Berk, B. C. (1997) Ann. N.Y. Acad. Sci. 811, 12–24. [DOI] [PubMed] [Google Scholar]
- 12.Nicosia, R. F. & Villaschi, S. (1999) Int. Rev. Cytol. 185, 1–43. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet, P. (2000) Nat. Med. 6, 1102–1103. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet, P. & Jain, R. K. (2000) Nature 407, 249–257. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, A. F. W. & Dann, L. (1941) Br. J. Exp. Pathol. 22, 9–14. [Google Scholar]
- 16.Ichioka, S., Shibata, M., Kosaki, K., Sato, Y., Harii, K. & Kamiya, A. (1997) J. Surg. Res. 72, 29–35. [DOI] [PubMed] [Google Scholar]
- 17.Milkiewicz, M., Brown, M. D., Egginton, S. & Hudlicka, O. (2001) Microcirculation 8, 229–241. [DOI] [PubMed] [Google Scholar]
- 18.Denekamp, J. (1993) Br. J. Radiol. 66, 181–196. [DOI] [PubMed] [Google Scholar]
- 19.Albuquerque, M. L., Waters, C. M., Savla, U., Schnaper, H. W. & Flozak, A. S. (2000) Am. J. Physiol. 279, H293–H302. [DOI] [PubMed] [Google Scholar]
- 20.Cullen, J. P., Sayeed, S., Sawai, R. S., Theodorakis, N. G., Cahill, P. A., Sitzmann, J. V. & Redmond, E. M. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 1610–1616. [DOI] [PubMed] [Google Scholar]
- 21.Tzima, E., del Pozo, M. A., Shattil, S. J., Chien, S. & Schwartz, M. A. (2001) EMBO J. 20, 4639–4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frangos, J. A., Eskin, S. G., McIntire, L. V. & Ives, C. L. (1985) Science 227, 1477–1479. [DOI] [PubMed] [Google Scholar]
- 23.Nabel, G. & Baltimore, D. (1987) Nature 326, 711–713. [DOI] [PubMed] [Google Scholar]
- 24.Tseng, H., Peterson, T. E. & Berk, B. C. (1995) Circ. Res. 77, 869–878. [DOI] [PubMed] [Google Scholar]
- 25.Traub, O., Monia, B. P., Dean, N. M. & Berk, B. C. (1997) J. Biol. Chem. 272, 31251–31257. [DOI] [PubMed] [Google Scholar]
- 26.Li, S., Kim, M., Hu, Y. L., Jalali, S., Schlaepfer, D. D., Hunter, T., Chien, S. & Shyy, J. Y. (1997) J. Biol. Chem. 272, 30455–30462. [DOI] [PubMed] [Google Scholar]
- 27.Jo, H., Sipos, K., Go, Y. M., Law, R., Rong, J. & McDonald, J. M. (1997) J. Biol. Chem. 272, 1395–1401. [DOI] [PubMed] [Google Scholar]
- 28.Davies, P. F. (1995) Physiol. Rev. 75, 519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busse, R. & Fleming, I. (1998) Kidney Blood Press. Res. 21, 264–266. [DOI] [PubMed] [Google Scholar]
- 30.Moncada, S. & Higgs, A. (1993) N. Engl. J. Med. 329, 2002–2012. [DOI] [PubMed] [Google Scholar]
- 31.Zeiher, A. M. (1996) Lancet 348, Suppl. 1, s10–s12. [DOI] [PubMed] [Google Scholar]
- 32.Moroi, M., Zhang, L., Yasuda, T., Virmani, R., Gold, H. K., Fishman, M. C. & Huang, P. L. (1998) J. Clin. Invest. 101, 1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallis, B., Corthals, G. L., Goodlett, D. R., Ueba, H., Kim, F., Presnell, S. R., Figeys, D., Harrison, D. G., Berk, B. C., Aebersold, R. & Corson, M. A. (1999) J. Biol. Chem. 274, 30101–30108. [DOI] [PubMed] [Google Scholar]
- 34.McCabe, T. J., Fulton, D., Roman, L. J. & Sessa, W. C. (2000) J. Biol. Chem. 275, 6123–6128. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R. & Zeiher, A. M. (1999) Nature 399, 601–605. [DOI] [PubMed] [Google Scholar]
- 36.Fisslthaler, B., Dimmeler, S., Hermann, C., Busse, R. & Fleming, I. (2000) Acta Physiol. Scand. 168, 81–88. [DOI] [PubMed] [Google Scholar]
- 37.Galbraith, C. G., Skalak, R. & Chien, S. (1998) Cell Motil. Cytoskeleton 40, 317–330. [DOI] [PubMed] [Google Scholar]
- 38.Barbee, K. A., Davies, P. F. & Lal, R. (1994) Circ. Res. 74, 163–171. [DOI] [PubMed] [Google Scholar]
- 39.Tzima, E., Del Pozo, M. A., Kiosses, W. B., Mohamed, S. A., Li, S., Chien, S. & Schwartz, M. A. (2002) EMBO J. 21, 6791–6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnittler, H. J., Franke, R. P., Akbay, U., Mrowietz, C. & Drenckhahn, D. (1993) Am. J. Physiol. 265, C289–C298. [DOI] [PubMed] [Google Scholar]
- 41.van Kooten, T. G., Schakenraad, J. M., van der Mei, H. C., Dekker, A., Kirkpatrick, C. J. & Busscher, H. J. (1994) Med. Eng. Phys. 16, 506–512. [DOI] [PubMed] [Google Scholar]
- 42.Schnittler, H. J., Schneider, S. W., Raifer, H., Luo, F., Dieterich, P., Just, I. & Aktories, K. (2001) Pflügers Arch. 442, 675–687. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Cardena, G., Anderson, K. R., Mauri, L. & Gimbrone, M. A., Jr. (2000) Ann. N.Y. Acad. Sci. 902, 294–297. [DOI] [PubMed] [Google Scholar]
- 44.Chen, B. P., Li, Y. S., Zhao, Y., Chen, K. D., Li, S., Lao, J., Yuan, S., Shyy, J. Y. & Chien, S. (2001) Physiol. Genomics 7, 55–63. [DOI] [PubMed] [Google Scholar]
- 45.Resnick, N., Collins, T., Atkinson, W., Bonthron, D. T., Dewey, C. F., Jr. & Gimbrone, M. A., Jr. (1993) Proc. Natl. Acad. Sci. USA 90, 4591–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shyy, J. Y., Lin, M. C., Han, J., Lu, Y., Petrime, M. & Chien, S. (1995) Proc. Natl. Acad. Sci. USA 92, 8069–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khachigian, L. M., Anderson, K. R., Halnon, N. J., Gimbrone, M. A., Jr., Resnick, N. & Collins, T. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 2280–2286. [DOI] [PubMed] [Google Scholar]
- 48.Traub, O. & Berk, B. C. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 677–685. [DOI] [PubMed] [Google Scholar]
- 49.Nagel, T., Resnick, N., Dewey, C. F., Jr. & Gimbrone, M. A., Jr. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1825–1834. [DOI] [PubMed] [Google Scholar]
- 50.Keynton, R. S., Evancho, M. M., Sims, R. L., Rodway, N. V., Gobin, A. & Rittgers, S. E. (2001) J. Biomech. Eng. 123, 464–473. [DOI] [PubMed] [Google Scholar]
- 51.Farber, J. M. (1993) Biochem. Biophys. Res. Commun. 192, 223–230. [DOI] [PubMed] [Google Scholar]
- 52.Strieter, R. M., Kunkel, S. L., Arenberg, D. A., Burdick, M. D. & Polverini, P. J. (1995) Biochem. Biophys. Res. Commun. 210, 51–57. [DOI] [PubMed] [Google Scholar]
- 53.Keane, M. P., Donnelly, S. C., Belperio, J. A., Goodman, R. B., Dy, M., Burdick, M. D., Fishbein, M. C. & Strieter, R. M. (2002) J. Immunol. 169, 6515–6521. [DOI] [PubMed] [Google Scholar]