Abstract
Anxious temperament (AT) in human and non-human primates is a trait-like phenotype evident early in life that is characterized by increased behavioural and physiological reactivity to mildly threatening stimuli 1–4. Studies in children demonstrate that AT is an important risk factor for the later development of anxiety disorders, depression, and comorbid substance abuse 5. Despite its importance as an early predictor of psychopathology, little is known about the factors that predispose vulnerable children to develop AT and the brain systems that underlie its expression. To characterize the neural circuitry associated with AT and the extent to which the function of this circuit is heritable, we performed a study in a large sample of rhesus monkeys phenotyped for AT. Using 238 young monkeys from a multigenerational single-family pedigree, we simultaneously assessed brain metabolic activity and AT while monkeys were exposed to the relevant ethological condition that elicits the phenotype. High-resolution 18F-deoxyglucose positron emission tomography (FDG-PET) was selected as the imaging modality since it provides semi-quantitative indices of absolute glucose metabolic rate, allows for simultaneous measurement of behaviour and brain activity, and has a time course suited to assess temperament-associated sustained brain responses. Results demonstrated that the central nucleus region of amygdala and the anterior hippocampus are key components of the neural circuit predictive of AT. Quantitative genetic analysis demonstrated significant heritability of the AT phenotype. Additionally, a voxelwise analysis revealed significant heritability of metabolic activity in AT-associated hippocampal regions. However, activity in the amygdala region predictive of AT was not significantly heritable. Furthermore, the heritabilities of the hippocampal and amygdala regions significantly differed from each other. Even though these structures are closely linked, the results suggest differential influences of genes and environment on how these brain regions mediate AT and the ongoing risk to develop anxiety and depression.
Anxiety disorders are among the most common forms of psychopathology 6, and frequently begin during childhood and adolescence. While all children experience acute anxiety, children with AT display extreme behavioural and physiological reactivity to novel stimuli and in the presence of strangers inhibit their locomotor activity and vocalizations 3. Furthermore, children with AT are maladaptively shy and chronically suffer from worry and apprehension. Some children with AT also exhibit increased pituitary-adrenal and autonomic activity 4. Identifying neural intermediate phenotypes of AT is a critical step in elaborating how environmental and genetic factors influence the development of anxiety and affect-related psychopathology. While AT is assumed to be partially heritable, the extent to which genetic variation influences metabolic activity in the neural circuit that underlies AT remains to be determined. We previously validated a nonhuman primate model of AT 7 and demonstrated that brain activity assessed across stressful and non-stressful contexts predicted AT, revealing the stable trait-like characteristics of brain metabolic activity associated with this disposition 2.
To characterize the extent to which individual differences in AT-related brain activity are heritable, we concomitantly assessed AT and regional brain metabolism in 238 young rhesus monkeys (male = 116, female = 122, mean age = 2.4 years, range = 0.74 – 4.2 years) belonging to a multigenerational single-family pedigree of more than 1500 individuals. The statistical power of an extended pedigree approach to quantitative genetic analysis is derived from the presence of substantial numbers of closely related, more distantly related, and unrelated pairs that all contribute information concerning the effects of kinship (shared genes) on phenotypic similarity (see supplementary information).
Similar to AT in children, monkey AT was assessed using measures of threat-induced freezing behaviour and inhibited vocalizations, as well as plasma cortisol concentrations (see supplementary information). AT and brain metabolism were assessed when monkeys freely behaved in a test cage by themselves for 30 minutes in a potentially threatening situation in which a human “intruder” entered the room and stood 2.5 meters from the cage 8. During this time the intruder presented his profile to the monkey ensuring that he avoided eye contact with the animal (No Eye Contact; NEC). Animals with the greatest AT froze longer, vocalized less, and had elevated plasma cortisol levels. The rationale underlying the use of the NEC paradigm is: 1) it optimally elicits the behaviours associated with the AT phenotype 8, 2) increased amygdala metabolism occurs during NEC 2, and 3) selective dorsal amygdala lesions attenuate NEC-induced behavioural and physiological responses 9.
To further understand the relation between regional brain activity and AT, monkeys were injected with FDG immediately prior to NEC. Following NEC exposure, blood was collected for cortisol assessment and monkeys were anesthetized and placed in a high-resolution microPET scanner to measure the FDG uptake that occurred during the NEC challenge. FDG is glucose analog with a half-life of 110 minutes that is trapped by metabolically active cells. Since the time course of FDG uptake reflects brain activity over an approximate 30-minute period, and remains stably detectable in the brain, it is an ideal radiotracer to simultaneously study behaviour and brain activity elicited by exposure to ethologically relevant situations. Furthermore, FDG-PET allows for measurement of brain activity within a single condition, and unlike fMRI does not require a contrast with a baseline signal. These features make FDG-PET particularly useful in understanding the sustained brain responses associated with temperament, which by definition is a persistent and relatively context-independent disposition.
The results demonstrated highly significant correlations between individual differences in AT and glucose metabolism within several large clusters (left and right anterior temporal lobe, midline parietal cortex, left and right visual cortex, right posterior thalamus and right temporal parietooccipital area; see Table 1). The anterior temporal lobe clusters (Fig 1) are of particular interest since they contain the amygdala, extended amygdala and anterior hippocampus, regions consistently implicated in mediating emotional behaviour, fear-related responses, and anxiety and depressive disorders 1,10–14. Mean glucose metabolism in the left and right anterior temporal lobe clusters (residualized for age, sex and mean gray matter probability) accounted for 24.4% and 27.5% of the variance in AT, respectively. Because these clusters contain anatomically distinct brain structures, we sought to specify the regions within the anterior temporal lobe clusters that most strongly predicted AT (i.e., the global maxima of the right and left clusters). The voxels most strongly predictive of AT were located in the lateral portion of the right dorsal amygdala (r=0.44, p=2.38e-13; Fig 1A and Fig 2A), which contains the central nucleus of the amygdala (CeA) and the amygdalostriatal transition zone (ASTZ), and in the left hippocampus (r=0.45, p=8.3e-13; Fig 1E and Fig 2B). No effects of sex or laterality were observed in any of the reported correlations (all p’s > 0.10), nor were there any significant main effects of sex on the components of AT (all p > 0.10).
Table 1. Regions where regression analyses revealed that AT was significantly correlated with brain metabolism during the No-Eye-Contact challenge.
Data are presented with the direction of the correlation, hemisphere, brain regions involved, and the volume of each AT-correlated cluster. The local maxima for each anatomical region within the statistical cluster with its corresponding t-value (p=0.05, corrected) and location (in millimeters relative to the anterior commissure) are also reported. CeA; amygdala central nucleus, ASTZ; amygdalostriatal transition zone.
| Clusters correlated with Anxious Temperment | Local maxima within clusters | Location relative to anterior commisure (in mm) |
||||||
|---|---|---|---|---|---|---|---|---|
| direction of correlation |
Hemisphere | Regions within cluster | cluster volume in mm3) |
Area | Max t-value |
x | y | z |
| positive | R | amygdala/temporal cortex/ | 850.8 | dorsal amygdala (CeA/ASTZ region) | 7.68 | 12.500 | −0.625 | −8.750 |
| anterior hippocampus | ventral putamen | 7.40 | 14.375 | −5.625 | −8.125 | |||
| superior temporal sulcus (anterior) | 7.17 | 18.750 | −0.625 | −11.250 | ||||
| temporopolar cortex | 6.68 | 19.375 | 5.000 | −8.125 | ||||
| L | amygdala/temporal cortex/ | 675.1 | anterior hippocampus | 7.61 | −12.500 | −3.750 | −9.375 | |
| anterior hippocampus | ventral putamen/posterior amygdala | 7.38 | −11.250 | −2.500 | −8.125 | |||
| mid hippocampus | 6.71 | −15.625 | −7.500 | −10.625 | ||||
| claustrum | 6.33 | −14.375 | 3.125 | −10.000 | ||||
| superior temporal sulcus | 6.08 | −18.750 | 0.625 | −8.125 | ||||
| hypothalamus | 5.75 | −1.875 | −4.375 | −6.875 | ||||
| R | posterior thalamus | 11.5 | pulvinar | 5.75 | 10.625 | −15.625 | 1.250 | |
| negative | crosses | parietal cortex | 493.6 | intraparietal sulcus (right) | −6.85 | 4.375 | −27.500 | 16.875 |
| midline | precuneus (left) | −6.69 | −3.125 | −29.375 | 15.000 | |||
| V3 (right) | −5.68 | 6.250 | −33.750 | 9.375 | ||||
| L | visual cortex | 455.8 | V2 | −6.71 | −10.000 | −28.125 | −3.750 | |
| parietooccipital sulcus | −5.96 | −3.750 | −36.875 | 1.875 | ||||
| V1 | −5.57 | −13.750 | −31.250 | −1.875 | ||||
| R | visual cortex | 287.1 | V2 | −7.35 | 6.875 | −30.625 | −2.500 | |
| V3 | −5.56 | 11.250 | −31.875 | −6.875 | ||||
| R | primary visual cortex | 45.9 | V1 | −5.98 | 3.125 | −43.750 | −3.750 | |
| R | temporal parietooccipital area | 8.3 | superior temporal sulcus (posterior) | −5.78 | 15.625 | −25.000 | 8.750 | |
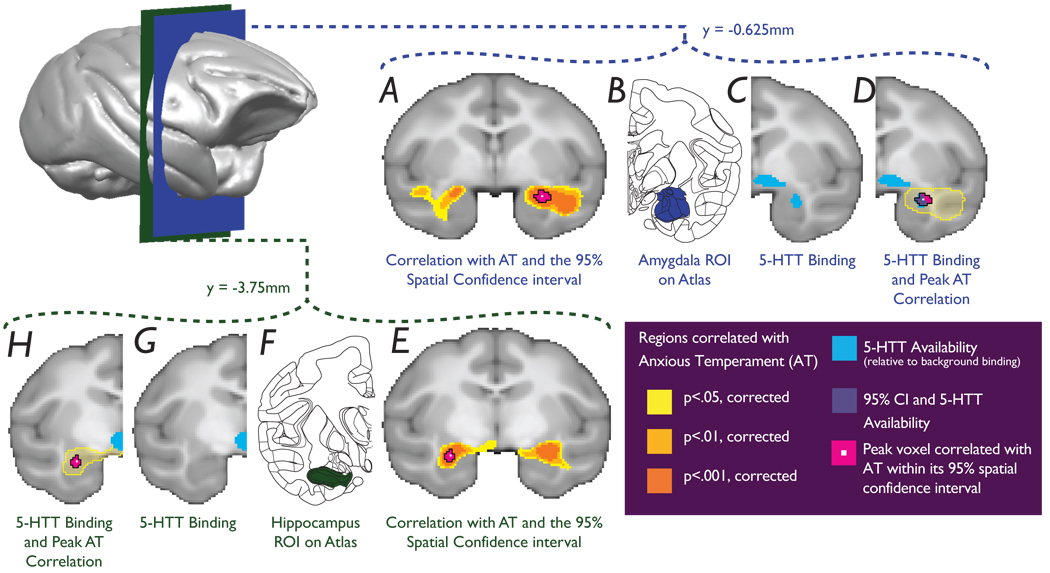
Figure 1. Glucose metabolism in the anterior temporal lobes is predictive of AT.
a, amygdala and e, hippocampus (significance of correlations - yellow: p < 0.05, light orange: p < 0.01, dark orange: p < 0.001, corrected). Pink areas represent 95% spatial confidence intervals of the peak correlations. b and f, corresponding slices adapted from The Rhesus Monkey Brain in Stereotaxic Coordinates (2009). c, g, 5-HTT binding differentiates the CeA region from the anterior hippocampus. d, The CeA, defined by the 5-HTT map, encompasses the amygdala peak. h, The hippocampal peak is distinct and does not overlap with the 5-HTT map.
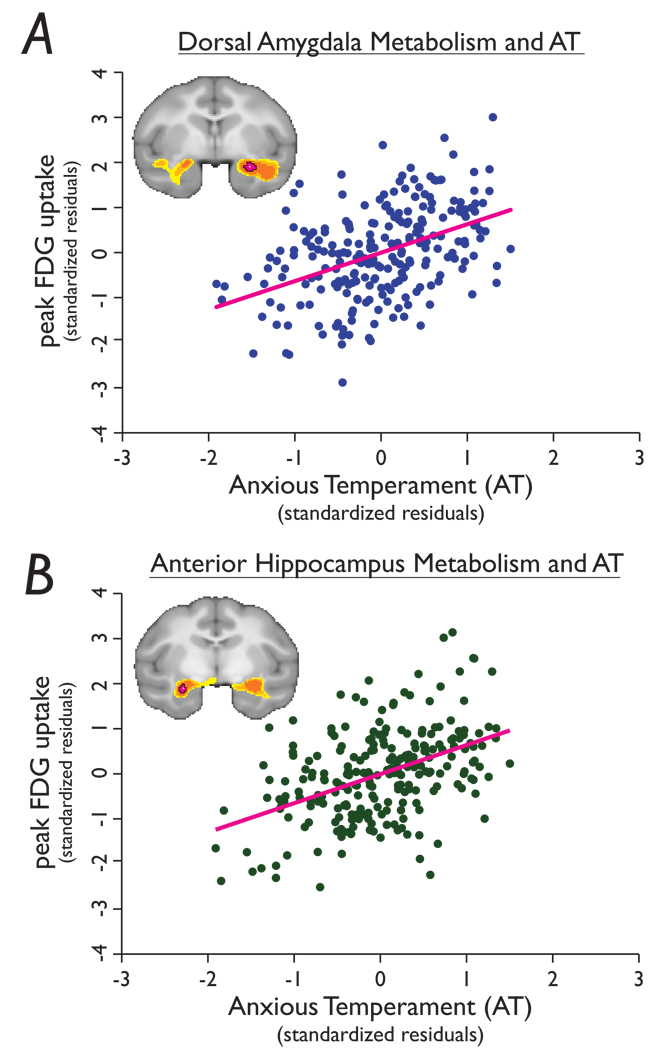
Figure 2. Peak correlations between AT and anterior temporal lobe glucose metabolism.
a, Voxel within the amygdala (see Fig 1a) reflecting the peak correlation between metabolism and AT (r=0.44, p=2.38e-13) and b, voxel within the hippocampus (see Fig 1e) reflecting the peak correlation between metabolism and AT (r=0.45, p=8.3e-13). 18F-deoxyglucose values were extracted from each animal, residualized for the effects of age, sex and gray matter probability, and plotted against individual differences in AT.
The amygdala and hippocampus work in concert in mediating emotion-modulated memory 15, however, it is important to emphasize that these structures uniquely contribute to other aspects of emotional processing. For example, hippocampal lesions in rats and rhesus monkeys produce alterations in anxious behavior that are distinct from the effects of amygdala lesions 16–18. As the dorsal amygdala and anterior hippocampus are adjacent structures, calculating the spatial confidence intervals around the voxels containing the maximum t-values further delineated the locations of these peaks. The confidence intervals represent volumes that with 95% certainty contain the peak correlations between metabolic activity and AT (see supplementary information). To further demarcate the location of these peaks the volumes contained within these confidence intervals were superimposed on a voxelwise map of [11C- DASB] serotonin transporter (5-HTT) binding created from an independent sample of rhesus monkeys (see supplementary information). This 5-HTT map, thresholded at 250× background binding, can be used to precisely localize the CeA and differentiate it from the anterior hippocampus, since compared to surrounding regions the lateral division of the CeA has the highest density of 5-HTT binding 19. As can be seen in Fig. 1A–D, the right dorsal amygdala 95% confidence interval encompasses the lateral region of the right CeA and the laterally adjacent ASTZ. Anatomical studies show that the ASTZ shares many similarities with the CeA, and may represent a posterior division of the ventral striatum/extended amygdala 13. The left hippocampal 95% confidence interval does not overlap with the lateral CeA region as defined by 5-HTT binding, further confirming the spatial dissociation between the dorsal amygdala and anterior hippocampal regions predictive of AT (Fig 1E–H).
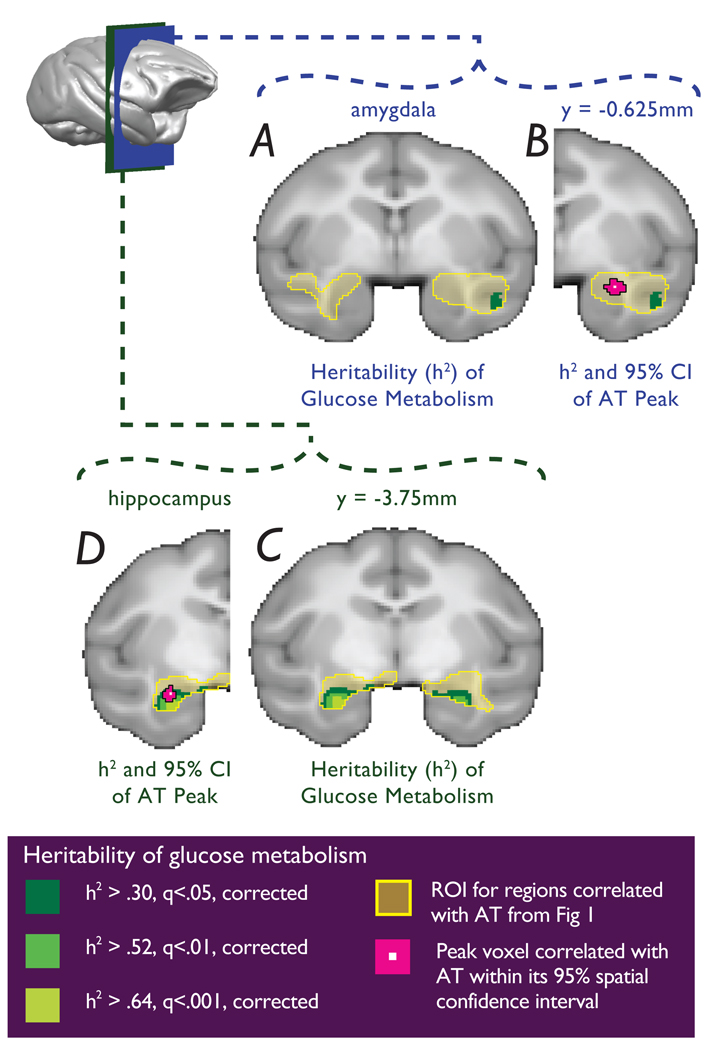
To estimate the heritability of AT, the pedigree relationships and individual phenotype measures were used in maximum likelihood variance components analyses computed using SOLAR (see supplementary information). Consistent with prior findings in rhesus monkeys 20, as well as studies of human anxiety 21, AT was significantly heritable (h2=0.36, p=0.015). A voxelwise heritability analysis for brain activity was performed using SOLAR controlling for the potential confounds of age, age2 and sex. Analyses were limited to those voxels within the FDG clusters that were significantly predictive of AT. Since previous studies demonstrated that heritable individual differences in brain structure relate to stress reactivity 22, gray matter probability was also included as a voxelwise covariate. Glucose metabolic activity was significantly heritable in voxels in the anterior temporal lobe clusters (Fig 3) as well as in the other clusters predictive of AT (see supplementary information). Within the anterior temporal lobe clusters, significantly heritable regions were found in bilateral hippocampus (right sided maximally significant heritable voxel: h2=0.65, p=3.83e-05; left sided maximally significant heritable voxel: h2=0.76, p=3.4e-06) and in the right superior temporal sulcus (maximally significant heritable voxel: h2=0.46, p=5.92e-03). No significantly heritable voxels were observed in the amygdala regions predictive of AT (see Fig 3). As with any null finding, the possibility exists that significant heritability of amygdala metabolic activity could be detected with a larger sample.
Figure 3. Overlap between regional metabolic activity predictive of AT with regions that are significantly heritable.
a and b, No significantly heritable voxels were observed in the dorsal amygdala region, although within the same slice significant heritability was detected in the superior temporal sulcus. c, Glucose metabolism was significantly heritable in both the right hippocampus and left hippocampus, d, where it overlaps with the left anterior hippocampal region that correlated with AT. (yellow = regions predictive of AT from Fig 1; dark green to light green: FDR: q < 0.05, q < 0.01, q < 0.001).
The heritability estimates for the peak AT-predictive hippocampal and amygdala voxels were: h2=0.52 (p=0.001) and h2=0.02 (p=0.454), respectively. To test whether the heritability of metabolic activity in these hippocampal and amygdala AT-predictive voxels significantly differed from each other, a model that allowed the two heritability estimates of these voxels to vary independently was compared with a model that constrained the heritability estimates to be equal. The observed difference in heritability between the hippocampal and amygdala peak voxels was 0.518 (95% CI: 0.238 – 0.799). Results confirmed that the heritability of metabolic activity in the anterior hippocampal voxel was significantly greater than that in the dorsal amygdala voxel (χ2=6.08, df=1, p<0.0137). A similar difference in heritability was found for the amygdala and hippocampal regions defined by the 95% spatial confidence intervals of the most AT-predictive peaks (χ2=6.24, df=1, p<0.0125). For these regions the observed difference in heritability was 0.508 (95% CI: 0.218 – 0.798). These results suggest that the heritable risk to develop AT is more likely to be related to hippocampal, and not amygdala, metabolic activity when assessed during the NEC condition. Given that the amygdala and anterior hippocampus are anatomically linked, and are both highly predictive of AT, we did not expect the heritability of these regions to be dissociable. Demonstrating differential heritability between these two closely related structures is highly valuable as it provides new insight into the neural circuits underlying AT. Additionally, it establishes a model system that can be used to further investigate the genetic and environmental mechanisms that may differentially affect amygdala and hippocampal function relevant to the development of anxiety-related psychopathology. Since heritability estimates are influenced by context, environmental variation, and population characteristics including age 23,24, it is possible that greater heritability of amygdala function would be detected when examining different phenotypes, other developmental stages, or when using different paradigms to understand other amygdala-dependent functions.
The lack of significant additive genetic effects (heritability) observed in the amygdala region may appear to be inconsistent with the numerous reported effects of single genes, most notably the repeat length polymorphism in the promoter region of the serotonin transporter gene (5HTTLPR), on emotion-related amygdala reactivity 25–28. The majority of these single gene effects are context-dependent, revealed by comparing acute changes in amygdala reactivity to a baseline state. In a previous study, with a considerably smaller sample of monkeys, we demonstrated such context-dependent effects of the 5HTTLPR on amygdala metabolic activity by comparing an activated state to a baseline condition 29. To further understand similarities between the current paradigm and those demonstrating influences of single genes on amygdala reactivity, animals were genotyped for the 5HTTLPR and measured genotype analyses, sensitive to effects of single genes, were performed. Results demonstrated no significant effect of the 5HTTLPR on either AT (p = 0.271) or metabolism in the amygdala and hippocampal regions predictive of AT (FDR q > 0.05). These data are consistent with a recent large-sample human study that failed to demonstrate a relation between the 5HTTLPR and resting amygdala activity as measured with perfusion MRI, which like FDG-PET does not require a comparison condition 30. These findings emphasize the importance of distinguishing between studies assessing sustained trait-like brain responses associated with AT from those investigating acute reactivity of the amygdala in relation to adult anxiety.
While the amygdala and hippocampus have been recognized as important in emotion and psychopathology, little data exist regarding the role of these regions in the development of temperamental dispositions such as AT. Recent theories have implicated the amygdala in mediating acute fear and vigilance 11,12, whereas the hippocampus has primarily been linked to mechanisms underlying declarative memory 15. Of interest, earlier theorists emphasized the septo-hippocampal system as being central to anxiety and specifically involved in threat-related behavioural inhibition 10. The current findings provide support for an important role of the anterior hippocampus in the development of anxious dispositions 16,17, and suggest that the highly interconnected regions of the hippocampus and amygdala are differentially influenced by genetic and environmental factors. These data support a new model combining measures of metabolic brain activity with ethologically relevant behavioural challenges to discover genes that mediate the endophenotype underlying the risk to develop anxiety and depression.
METHODS SUMMARY
Functional and structural (MRI) brain data for each animal were co-registered to a standard space based on an age-appropriate rhesus monkey brain template. Whole-brain linear regression analyses examined the relations between FDG uptake and AT. To account for potential confounds, age and sex were included in the regression model as covariates. Gray matter probability was also included as a voxelwise covariate to account for the possibility that structural differences affected the relation between brain metabolic activity and AT. The resulting 3D t-map was corrected for multiple comparisons using the Šidák equation (1 − (1 − α)1 / n), which is similar to the Bonferroni method and determined the statistical threshold of p=0.05, corrected (t > 5.47). To estimate the heritability of AT, phenotypic covariance/correlation amongst pairs of relatives was modeled as a function of expected pairwise kinship values to estimate the magnitude of additive genetic variance relative to that of the observed phenotypic variance. Age, age2 and sex were included in the mean effects model as covariates to control for these potential confounds. The resulting heritability data were corrected for multiple comparisons based on the total volume of all the clusters correlated with AT using a False Discovery Rate (FDR; q-value = 0.05). Measured genotype analyses use the same variance components approach as the heritability analyses, and were implemented using SOLAR. Measured genotype analyses simultaneously estimate the effect of specific genotypic differences among animals and the overall effect of pair-wise kinship, thus testing for the effect of a single polymorphism while accounting for background allele sharing across the genome due to genealogical relatedness. Full details on methods and any associated references are presented in the Supplemental Information.
Supplementary Material
Acknowledgements
This work has been supported by NIH grants MH046729 (N.H.K.), MH081884 (N.H.K. and J.R.), MH084051 (R.J.D and N.H.K.), and the HealthEmotions Research Institute. The SOLAR statistical genetics computer package is supported by NIH grant MH059490 (J.B.). The supercomputing facilities used for this work were supported in part by a gift from the AT&T Foundation. We thank the staff at the Wisconsin National Primate Center, the Harlow Center for Biological Psychology, the HealthEmotions Research Institute, the Waisman Laboratory for Brain Imaging and Behavior, Brad Christian, Pat Roseboom, Helen van Valkenberg, Kyle Myer, Liz Larsen, Irina Monosov and Reuven Stone. We also thank Roy Garcia (SFBR) for assistance in genotyping the 5HTTLPR polymorphism.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions N.H.K., S.E.S., J.R. and A.S.F. designed the study. S.E.S oversaw data collection. J.A.O., A.S.F., and N.H.K. analyzed the imaging data. T.R.O., A.S.F. and J.B. developed analytical tools. R.J.D. provided theoretical assistance. J.R. and W.S. performed the genotyping and maintained the pedigree record. J.R., W.S., T.D.D. and J.B. performed the genetic analyses. J.A.O., N.H.K., A.S.F., J.R. J.B. and R.J.D wrote the paper.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Schwartz CE, et al. Inhibited and uninhibited infants "grown up": adult amygdalar response to novelty. Science. 2003;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- 2.Fox AS, et al. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 4.Fox NA, et al. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, et al. Further evidence of association between behavioral inhibition and social anxiety in children. Am J Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 8.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 9.Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2nd ed. New York: Oxford University Press; 2000. [Google Scholar]
- 11.LeDoux Joseph E. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 12.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 13.Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JL, Drevets WC. Neurocircuitry of Mood Disorders. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 16.McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behavioral Neuroscience. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- 17.Chudasama Y, Izquierdo A, Murray EA. Distinct contributions of the amygdala and hippocampus to fear expression. Eur J Neurosci. 2009;30:2327–2337. doi: 10.1111/j.1460-9568.2009.07012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Rourke H, Fudge JL. Distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry. 2006;60:479–490. doi: 10.1016/j.biopsych.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers J, et al. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 2008;7:463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 22.Lyons DM, et al. Early life stress and inherited variation in monkey hippocampal volumes. Arch Gen Psychiatry. 2001;58:1145–1151. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- 23.Davis OS, Haworth CM, Plomin R. Dramatic increase in heritability of cognitive development from early to middle childhood: an 8-year longitudinal study of 8,700 pairs of twins. Psychol Sci. 2009;20:1301–1308. doi: 10.1111/j.1467-9280.2009.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visscher PM, Hill WG, Wray NR. Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- 25.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 26.Zubieta JK, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer-Lindenberg A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalin NH, et al. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viviani R, et al. Baseline brain perfusion and the serotonin transporter promoter polymorphism. Biol Psychiatry. 67:317–322. doi: 10.1016/j.biopsych.2009.08.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.