Abstract
Bone marrow, or cells selected from bone marrow, were reported recently to give rise to cells with a neural phenotype after in vitro treatment with neural-inducing factors or after delivery into the brain. However, we showed previously that untreated bone marrow cells express products of the neural myelin basic protein gene, and we demonstrate here that a subset of ex vivo bone marrow cells expresses the neurogenic transcription factor Pax-6 as well as neuronal genes encoding neurofilament H, NeuN (neuronal nuclear protein), HuC/HuD (Hu-antigen C/Hu-antigen D), and GAD65 (glutamic acid decarboxylase 65), as well as the oligodendroglial gene encoding CNPase (2′,3′ cyclic nucleotide 3′-phosphohydrolase). In contrast, astroglial glial fibrillary acidic protein (GFAP) was not detected. These cells also were CD34+, a marker of hematopoietic stem cells. Cultures of these highly proliferative CD34+ cells, derived from adult mouse bone marrow, uniformly displayed a phenotype comparable with that of hematopoietic progenitor cells (CD45+, CD34+, Sca-1+, AA4.1+, cKit+, GATA-2+, and LMO-2+). The neuronal and oligodendroglial genes expressed in ex vivo bone marrow also were expressed in all cultured CD34+ cells, and GFAP was not observed. After CD34+ cell transplantation into adult brain, neuronal or oligodendroglial markers segregated into distinct nonoverlapping cell populations, whereas astroglial GFAP appeared, in the absence of other neural markers, in a separate set of implanted cells. Thus, neuronal and oligodendroglial gene products are present in a subset of bone marrow cells, and the expression of these genes can be regulated in brain. The fact that these CD34+ cells also express transcription factors (Rex-1 and Oct-4) that are found in early development elicits the hypothesis that they may be pluripotent embryonic-like stem cells.
Adult bone marrow contains stem cells that replenish the hematopoietic system at a high turnover rate by generating cells of the myeloid and lymphoid lineages. Because bone marrow cells are accessible and readily available, the hypothesis arose that bone marrow may be a source of stem cells for tissues other than the hematopoietic system. The consequence of this rationale is that several laboratories are attempting to develop strategies to use bone marrow cells for brain cell-replacement therapy. They have used ex vivo bone marrow cells, either an unselected (1–4) or a selected (5, 6) subpopulation, or cells cultured from bone marrow (7–11). When injected into recipient animals, bone marrow cells were found in the brain expressing neural markers in most cases. Previously, the gene encoding myelin basic protein (MBP) was found to be expressed in bone marrow in vivo (12). This finding raised the possibility that some in vivo bone marrow cells express other neural genes. Indeed, we report here that neural and oligodendroglial genes are expressed in a subset of ex vivo bone marrow cells that are CD34+. A culture system was developed to generate pure populations of highly proliferative cells from adult bone marrow that express both neural and hematopoietic stem cell markers in addition to CD34. On transplantation into adult mouse brain, the cultured CD34+ cells survive for 14 months, the longest time tested; differentiate morphologically into cells that resemble neurons, astrocytes, and oligodendrocytes; and express distinct markers specific to each of these cell types.
Materials and Methods
Bone Marrow CD34+ Stem Cell Cultures. Bone marrow was collected aseptically from the femurs of 16 C57BL/6J, 4 SJL/J, 4 C3H, and 2 FVB-129 adult mice. Cells from one adult mouse femur were suspended in 10 ml of DMEM (GIBCO) containing 10% FBS and in 10 ml of hybridoma cell-defined serum-free medium (GIBCO) and distributed into two T75 tissue-culture flasks. Both media were supplemented with mouse IL-3 (R & D Systems), mouse IL-6 (R & D Systems), mouse stem cell factor (R & D Systems), and 2-mercaptoethanol to a final concentration of 5 ng/ml IL-3/10 ng/ml IL-6/10 ng/ml stem cell factor and a 1:1,000 dilution of 10 μl 2-mercaptoethanol in 2.9 ml of HOH. No matrix, substrate, or feeder cells were added to the liquid medium in the tissue-culture flasks. Cells were grown at 37°C in humidified 10% CO2/90% air. Cells were observed and fed or passaged as needed two times per week. Cells were fed by the addition of 5 ml of fresh medium to each flask. When the cell culture was dense enough to subculture, only the floating cells were collected, leaving behind the cells attached to the culture flask. These attached cells were bone marrow stromal cells, endothelial cells, and macrophages, etc. Floating cells were subcultured in 50% conditioned medium from the previous culture and 50% fresh medium at 2 × 106 cells per 10 ml. After 3–4 weeks, the cultures contained only dividing floating cells and the cells no longer differentiated into macrophages and other cells that attached to the flask.
RT-PCR. RNA was obtained from adult mouse bone marrow, from CD34+ cells cultured from 6 weeks to 4 months, and from postnatal day 2 mouse brain, and RT-PCR was performed by standard methodology by using the following DNA primers: GATA-2, 5′-ATGGAGGTGGCGCCTGAGCAGCCT-3′ (forward) and CTGCCGCCTTCCATCTTCATGCTC-3′ (reverse); LMO-2, 5′-ATGTCCTCGGCCATCGAAAGGAAG-3′ (forward) and 5′-GATGATCCCATTGATCTTGGTCCA-3′ (reverse); Rex-1, 5′-CACCATCCGGGATGAAAGTGAGAT-3′ (forward) and 5′-ACCAGAAAATGTCGCTTTAGTTTC-3′ (reverse); Oct-4, 5′-CCGTGAAGTTGGAGAAGGTG (forward) and 5′-TGATTGGCGATGTGATGTAT (reverse); Flk-2, 5′-CGTACCGAATGGTGCGAGGATCCC-3′ (forward) and 5′-CATGGTTCACATGGATGGCCTTAC-3′ (reverse); TAL-1, 5′-GATGACGGAGCGGCCGCCGAGCGAGGCG-3′ (forward) and 5′-CGCACTACTTTGGTGTGAGGACCA-3′ (reverse); CD34, 5′-CAGTAT T TCCACT TCAGAGATGAC-3′ (forward) and 5′-GTGTAATAAGGGTCTTCACCCAGC-3′ (reverse); neurofilament H, 5′-ATTGGCTTTGGTCCGAGTCC (forward) and 5′-GGGGGTTCTTTGGCTTTTAC (reverse); neurofilament M, 5′-CTTTCCTGCGGCGATATCAC (forward) and 5′-TCCTCAACCTTTCCCTCAAT (reverse); and neurofilament L, 5′-GCAGAACGCCGAAGAGTGGT (forward) and 5′-CGAGCAGACATCAAGTAGGA (reverse). PCR products were separated by base pair size on gels by using standard protocols.
Immunocytochemistry. For ex vivo studies, in situ bone marrow cells were removed and treated immediately with 4% paraformaldehyde. In vitro bone marrow cells from 6-, 21-, 28-, 48-, 56-, and 110-day cultures were incubated in 4% paraformaldehyde at 4°C for 15 min, washed three times in Dulbecco's PBS, applied to microscope slides by cytocentrifuge, and used immediately or stored at –80°C until use. Cells were then treated with 0.25% Tween 20 for 3 min at 21°C, washed three times in PBS, and analyzed by standard immunocytochemistry methodology with the following antibodies: primary antibodies CD34 (553731, Pharmingen), Sca-1 (557403, Pharmingen), AA4.1 (559158, Pharmingen), cKit (CBL1359, Cymbus Biotechnology, Chandlers Ford, England), H-2K (553567, Pharmingen), CD45 (553076, Pharmingen), F4/80 (MCAP497, Serotec), Pax-6 (sc-11357, Santa Cruz Biotechnology), Oct-4 (sc-9081, Santa Cruz Biotechnology), Hu-antigen C/Hu-antigen D (HuC/HuD; A-21275, Molecular Probes), neurofilament H (SMI 312, Sternberger Monoclonals, Lutherville, MD; AB1989, Chemicon), neuronal nuclear protein (NeuN; MAB377, Chemicon), glutamic acid decarboxylase 65 (GAD65; AB5082, Chemicon), M2 muscarinic acetylcholine receptor (AB166–50UL, Chemicon), glial fibrillary acidic protein (GFAP; MAB3402, AB5040, and AB5804, Chemicon), 2′,3′ cyclic nucleotide 3′-phosphohydrolase (CNPase; MAB326, Chemicon), myelin oligodendrocyte-specific protein (MOSP; MAB328, Chemicon), NG2 chondroitin sulfate proteoglycan (A B5320, Chemicon), galactocerebroside (AB142, Chemicon), oligodendrocyte-marker antibody O4 (MAB345, Chemicon), MAG (myelin-associated glycoprotein; MAB1567, Chemicon), and proteolipid protein (PLP; MAB388, Chemicon). Secondary antibodies were FITC-labeled F(ab′)2 donkey anti-rabbit (711-096-152, Jackson ImmunoResearch), tetramethylrhodamine B isothiocyanate-labeled F(ab′)2 donkey anti-rat (712-026-150, Jackson ImmunoResearch), isothiocyanate-labeled F(ab′)2 goat anti-mouse IgG + IgM (115-026-044, Jackson ImmunoResearch), isothiocyanate-labeled F(ab′)2 rabbit anti-mouse (315-026-045, Jackson ImmunoResearch), FITC-conjugated goat anti-mouse IgG1 Fcγ fragment-specific antibody (115-095-008, Jackson ImmunoResearch), Cy5-labeled F(ab′)2 donkey anti-rabbit (711-176-152, Jackson ImmunoResearch), horseradish peroxidase-conjugated goat F(ab′)2 anti-rabbit IgG (heavy and light chains) (L4300-7, Caltag, South San Francisco, CA), and Fab fragment goat anti-mouse IgG (115-007-003, Jackson ImmunoResearch). In the cases of mouse monoclonal IgG1 antibody binding to ex vivo mouse bone marrow cells, the standard protocol was modified to expose fixed permeablized cells for 1 h at room temperature to 5% normal goat serum in PBS, followed by six washes with PBS. Cells were then exposed for 1 h to 20 μg/ml Affinipure Fab fragment goat anti-mouse IgG1 (115-007-003, Jackson ImmunoResearch), exposed for 1 h to primary mouse monoclonal antibody IgG1 to the antigens of interest, washed six times in PBS, and finally exposed for 1 h to secondary FITC-goat anti-mouse IgG1 Fcγ fragment-specific antibody, washed six times with PBS. The following two controls were used: a lack of primary antibody and primary mouse monoclonal IgG1 anti-GFAP.
Western Blot Analysis. Proteins from cultured CD34+ cells were separated by 10%, 12%, and 4–20% gradient polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes as reported (12), and analyzed for specific proteins by using the antibodies listed above.
Vital Dye Labeling of CD34+ Cells. CD34+ cells were labeled by fluorescent dye 5-(and 6)-{[(4-chloromethyl)benzoyl]amino}-tetramethylrhodamine (Cell Tracker Orange, CTO; CMTMF; Molecular Probes) as follows. CD34+ cells (2 × 108) were incubated in a final concentration of 25 μM CTO from a ×400 stock of 10 mM dye in DMSO. Cells were incubated in 5 ml of dye containing DMEM10 at 37°C for 15 min, pelleted by centrifugation, washed in 15 ml of DMEM10, incubated for 30 min at 37°C, pelleted again, washed again in 15 ml of DMEM10 at 37°C for 15 min, pelleted again, and resuspended in DMEM10 at 104 cells per μl.
Stereotactic Injection of CD34+ Cells into Adult Mouse Brain. Thirtyfour anesthetized adult C57BL/6J mice were injected stereotactically with 104 C57BL/6J CTO-labeled CD34+ cells in 1 μl of DMEM10 into the hippocampus and striatum of each brain. Injected animals were grown for 1–14 months and then killed and perfused with PBS, followed by 4% paraformaldehyde. Brains were removed, equilibrated in 30% sucrose, embedded in cryo-embedding compound, frozen, cut into 30-μm thick cross sections, prepared for immunohistochemistry by using standard methods, and counterstained with 25 ng/ml 4′-diamidino-2-phenylindole (DAPI). Implanted CD34+ cells were observed and images were captured by conventional fluorescence and laser confocal microscopy with rhodamine, fluorescein, Cy5, and DAPI optics.
Results and Discussion
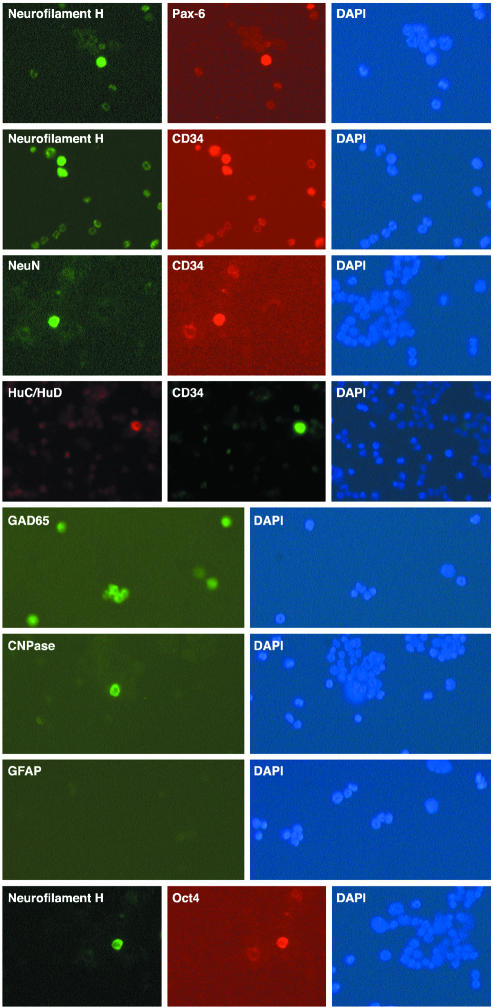
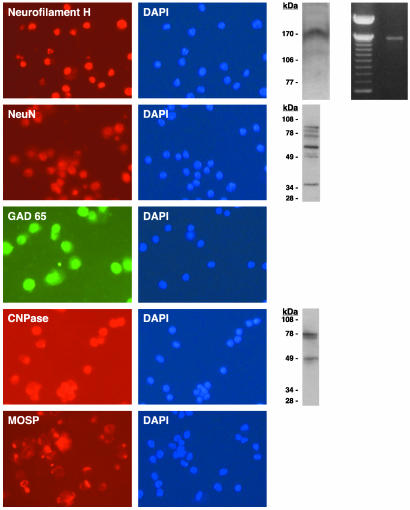
Neural Antigens Present in a Subset of ex Vivo Bone Marrow Cells. Previous studies observed that different bone marrow cell preparations can express neural molecules after transplantation into brain. However, it has not been established whether the neural molecules are the consequence of transplantation or are already present in the bone marrow, as shown formerly for products of the gene encoding myelin basic protein (MBP) (12). The expression of neural markers in noncultured ex vivo bone marrow, therefore, was investigated (Fig. 1). The neurogenic transcription factor (Pax-6) and the four neuronal proteins that were examined (neurofilament H, NeuN, HuC/HuD, and GAD65) were present in 1.5% of adult bone marrow cells (see Table 2). Double immunocytochemistry labeling demonstrated that Pax-6 and neurofilament H were present in the same cells. In addition, whereas the oligodendroglial protein CNPase also was discovered in some bone marrow cells, no labeling was detected with antibody to astroglial GFAP.
Fig. 1.
Immunocytochemical detection of neural gene expression in a subset of adult mouse whole ex vivo bone marrow (see Materials and Methods). Double immunocytochemical detection of neurofilament H and Pax-6 was performed in the same subset of bone marrow cells, and expression of neuronal neurofilament H, NeuN, and HuC/HuD was present in a subset of CD34+ bone marrow cells. GAD65, an enzyme responsible for synthesis of a major neurotransmitter, also was present in a subset of bone marrow cells. Oligodendroglial CNPase was present in a subset of bone marrow cells, whereas the astroglial marker GFAP was not detected on ex vivo bone marrow. Neurofilament H and Oct-4 were found in the same subset of ex vivo bone marrow cells. DAPI stains of the nuclei of all cells are shown.
Table 2. Neural cell markers on ex vivo and cultured CD34+ cells.
| % positive cells
|
|||
|---|---|---|---|
| Marker | Ex vivo | Day 21 | Days 56 and 110 |
| Neural transcription factors | |||
| Pax-6 | 1.5 | 92 | 100 |
| Oct-4 | 1.5 | 92 | 100 |
| Neurons | |||
| HuC/HuD | 1.5 | 92 | 100 |
| Neurofilament H | 1.5 | 92 | 100*† |
| NeuN | 1.5 | 91 | 100* |
| Glutamic acid decarboxylase | 1.5 | ND | 100 |
| GAD65 | |||
| Tyrosine hydroxylase | ND | ND | 0 |
| M2 muscarinic acetylcholine | ND | ND | 0 |
| receptor | |||
| Glial astrocytes | |||
| GFAP | 0 | 0 | 0*† |
| Oligodendrocytes | |||
| CNPase | 1.5 | 92 | 100*† |
| MOSP | ND | ND | 100* |
| HMBP/MBP2 | 100 | ND | 100*† |
| Galactocerebroside | ND | ND | 100* |
| NG2 chondroitin sulfate | ND | ND | 100* |
| proteoglycan | |||
| O4 | 0 | 0 | 0 |
HMBP/MBP2, hemopoietic myelin basic protein and myelin basic protein 2 (12). ND, not determined.
Determined by Western blot analysis.
Determined by PCR analysis.
To determine whether the bone marrow cells, which express neural antigens, represent hematopoietic stem cells, double immunocytochemistry was carried out with neural markers and CD34, a marker of bone marrow stem cells. Strong labeling with antibodies to neurofilament H, NeuN, GAD65, HuC/HuD, Pax-6, and CNPase was present in only a subset (≈20%) of ex vivo CD34+ cells (Fig. 1).
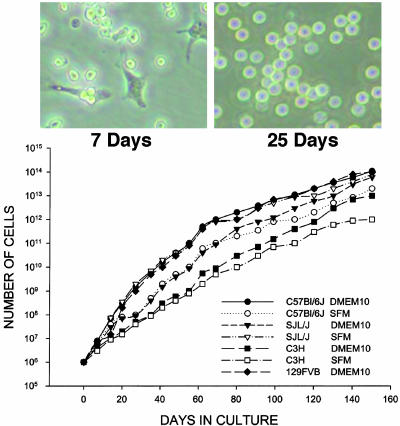
Generation of Highly Proliferating Hematopoietic Progenitors. Because neural antigens were present in a subset of bone marrow cells bearing CD34, an antigen that can be found on hematopoietic progenitors, a method was developed to generate cultures of highly proliferative CD34+ cells. Bone marrow of four strains of mice was harvested from 26 adult femurs and cultured individually in liquid medium containing the hematopoietic stem cell growth factors IL-3, IL-6, stem cell factor, and 2-mercaptoethanol. Only nonadhering floating cells were subcultured continuously over 4 months as described above. With time in culture, the percentage of adherent cells decreased to zero by 3–4 weeks (Fig. 2). These floating cells, which grow over 30 generations, show a high proliferative capacity. Indeed, over a 4-month period of culture, 1 × 1014 cells were generated from 1 × 106 bone marrow cells that were obtained from one mouse femur. A 3-μl pellet of bone marrow cells can be expanded into a 300-liter pellet of pure CD34+ cells, as evidenced by PCR and immunocytochemistry. Similar proliferation rates were observed in all cultures regardless of whether they were in serum-containing or serum-free medium (Fig. 2).
Fig. 2.
Long-term cultures of CD34+ cells from adult mouse bone marrow. (Upper) Photomicrographs of bone marrow cells at days 7 and 25. (Lower) Growth curves of cells from adult bone marrow of C57BL/6J, C3H, SJL/J, and FVB-129 mice in serum-containing and serum-free media. Growth curves of cells from the four strains of mice in serum-containing and serum-free media were comparable.
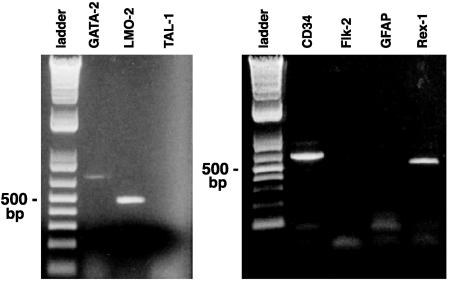
The cells were assayed for hematopoietic markers at various time points in culture. After 4–5 weeks, all cells were highly CD34+ and CD45+, a general marker of all hematopoietic cells. In contrast, macrophage F4/80; endothelial cell factor 8; erythroblast TER119; B and T lymphocyte markers CD19, CD4, and CD8; and B and T lymphocyte transcription factor TAL-1 were not detected (Fig. 4 and Table 1). These results indicate that the CD34+ cells were not expressing hematopoietic differentiation markers and, therefore, suggest that they might correspond to stem cells. The cells then were analyzed for additional hematopoietic stem cell markers and found to be Sca-l+, AA4.1+, and cKit+ (Table 1 and Fig. 3). Thus, these cells had a cell-surface phenotype that was comparable with that found in hematopoietic stem cells. Furthermore, they expressed transcriptional factors GATA-2 and LMO-2, known to be present in hematopoietic progenitors (Fig. 4). In addition, the absence of FLK-2 receptor kinase suggests that these cells are analogous to hematopoietic stem cells, which are capable of long-term multilineage reconstruction.
Fig. 4.
Detection of RT-PCR products of mRNA from a pure population of CD34+ cells derived from adult C57BL/6J bone marrow after 6 weeks in culture. The first lane of each panel shows a 500-bp DNA ladder. (Left) GATA-2 and LMO-2 are hematopoietic stem cell transcription factors. TAL-1 is a lymphocyte transcription factor. (Right) CD34 is a marker of hematopoietic stem cells. FLK-2 is a tyrosine kinase receptor present in hematopoietic stem cells with short-term multilineage reconstitution potential but not long-term potential. GFAP is a marker of differentiated astroglial cells. Rex-1 is a transcription factor in early embryonic stem cells. Equal quantities of RNA were used for each lane from a common pool of CD34+ cells in culture. CD34 was used as a positive control.
Table 1. Hematopoietic markers in C57B1/6J mouse bone marrow cells cultured in IL-3, IL-6, and stem cell factor.
| % positive cells
|
|||
|---|---|---|---|
| Marker | Week 3 | Week 4 | Week 16 |
| Hematopoietic stem cells | |||
| CD34 | 95-99 | 100* | 100 |
| Sca-1 | 95-99 | 100 | 100 |
| AA4.1 | 95-99 | 100 | 100 |
| cKit | 95-99 | 100 | 100 |
| All hematopoietic cells | |||
| CD45 | 100 | 100 | 100 |
| HMBP | 100 | 100 | 100 |
| Macrophages | |||
| F4/80 | 1-3 | 0† | 0 |
| Endothelial cells | |||
| Factor 8 | 0 | 0 | ND |
| B cells | |||
| CD19 | 0 | 0 | ND |
| T cells | |||
| CD4 | 0 | 0 | ND |
| CD8 | 0 | 0 | ND |
ND, not determined.
All of the analyzed cells were positive.
None of the analyzed cells were positive.
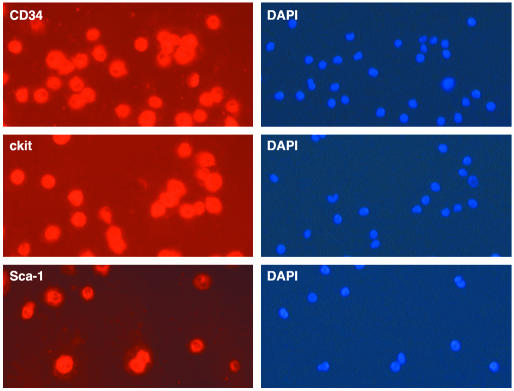
Fig. 3.
Immunocytochemical detection of CD34, cKit, and Sca-1 on all cells in 6-week cultures of adult C57BL/6J bone marrow. All cells in CD34+ cultures also expressed cKit and Sca-1.
Neural Markers in Hematopoietic Progenitors Cultured from Bone Marrow. Neural genes were found to be expressed in a minor subset of ex vivo CD34+ bone marrow cells. Therefore, their presence was examined in the highly proliferative cultures of hematopoietic progenitors at 3 weeks and at later times when all cells were CD34+. Both neural transcription factors and markers of differentiated neurons, astroglia, and oligodendrocytes were investigated. When all cells were CD34+, all cells were positive also for the neurogenic transcription factor Pax-6 and neuronal RNA-binding protein HuC/HuD. Then, the pure population of CD34+ cells was assessed for expression of general neuronal markers and neurotransmitters (Fig. 5). Cells probed for neurofilaments H, M, and L by RT-PCR were found to express neurofilament H but not neurofilaments M and L, whereas the same primers used to probe the CD34+ cells gave the expected products in postnatal day 2 mouse brain (data not shown). Immunocytochemistry also revealed that all cultured CD34+ cells expressed neurofilament H but not neurofilaments M and L. Additionally, Western blot analysis showed neurofilament H at 170 kDa but did not show bands for neurofilaments M and L. Immunocytochemistry and Western blot analyses of cultured CD34+ cells showed that NeuN was abundant in all cells and was expressed at the expected molecular masses of 66, 48, and 46 kDa. Because general markers of neurons were present in the CD34+ cultures, markers of neuronal function were investigated also. Indeed, GAD65, the enzyme responsible for GABA synthesis, was detected in all cells examined, whereas tyrosine hydroxylase and the M2 muscarinic acetylcholine receptor were not detected (Table 2). The next step was to determine the presence of molecules that are considered to be markers of glial cells (i.e., astrocytes and oligodendrocytes). The intermediate filament of astrocytes, GFAP, was not detected at the mRNA or protein level at any stage in the culture of CD34+ cells (Fig. 4B). In contrast, oligodendrocyte markers CNPase, MOSP (Fig. 5), galactocerebroside, and NG2 chondroitin sulfate proteoglycan were present (Table 2), whereas O4 was not detected (data not shown). These data indicate that early transcription factors, as well as markers of differentiated cells of the nervous system, are present in the bone marrow-derived CD34+ cell cultures.
Fig. 5.
Neural gene expression in adult mouse bone marrow cells cultured for 6–10 weeks. The three neuronal genes were detected as follows: neurofilament H was detected by immunocytochemistry, Western blot analysis, and RT-PCR; NeuN was detected by immunocytochemistry and Western blot; and GAD65 was detected by immunocytochemistry. The two oligodendroglial genes were detected as follows: CNPase was detected by immunocytochemistry and Western blot, and MOSP was detected by immunocytochemistry. Except for MOSP, which is a cell-surface molecule expressed in caps or rings in the plasma membrane, all markers are located inside the cells. DAPI stains of the nuclei of all cells are shown.
Early Embryonic Cell Markers in CD34+ Cell Cultures. The most plausible origin of the CD34+ cell cultures that express neural genes is the amplification of a small percentage of CD34+ cells present in ex vivo bone marrow, which also express neural genes. It may be that these CD34+ cells derive from pluripotent bone marrow cells, somewhat similar to embryonic stem cells. Therefore, the cultured CD34+ cells were screened for early general transcription factors Rex-1 and Oct-4 by PCR and found to be positive (Rex-1, Fig. 4; Oct-4, data not shown). Immunocytochemistry indicated that a small subset of ex vivo bone marrow cells was positive for Oct-4 (Fig. 1) as were 100% of the cultured CD34+ cells (data not shown). This finding suggests that, indeed, the cultured CD34+ cells may be stem cells with a greater potential than merely hematopoietic stem cells.
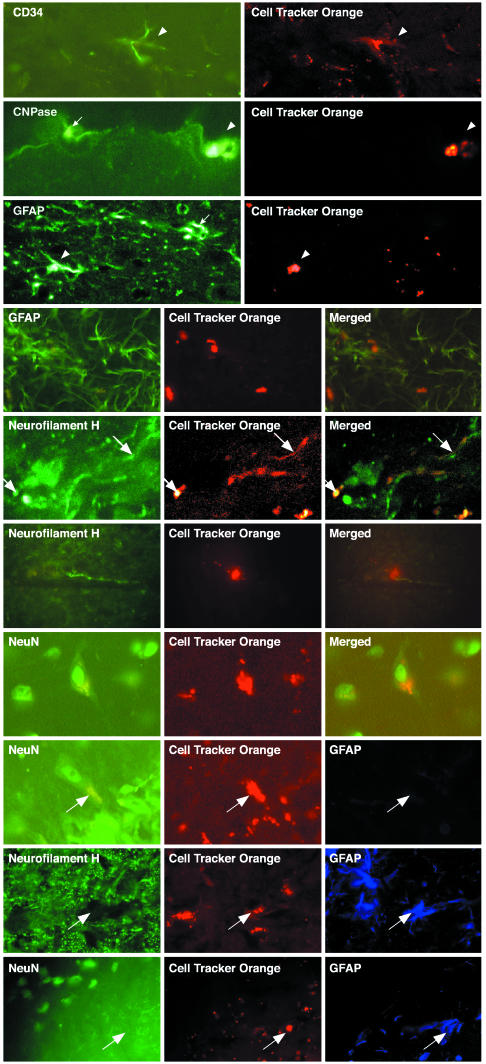
Transplantation of Cultured CD34+ Cells into Brain. Because these cells express molecules compatible with a neural phenotype, we thought it was reasonable to transplant them into adult mouse brain without any further treatment. CD34+ cells, cultured for from 6 weeks to 3 months, were labeled with CTO and injected stereotactically into the brain striatum and hippocampus of 34 adult mice. Brains were processed for immunohistochemistry and fluorescence microscopy at 1–14 months after transplantation. The transplanted CTO-labeled cells were found to survive in high numbers in both striatum and hippocampus (≈40% of injected cells) for 14 months, the longest time tested, without any obvious alteration in the behavior of the animals. This high percentage of survival of implanted cells in brain is in contrast to the findings of other laboratories that injected cells into the circulating blood of sublethally or lethally irradiated mice and into the peritoneum of newborn PU.1 mice (1–3). In addition, the CD34+ cells injected into the brain migrated from the injection site throughout the striatum and hippocampus and beyond. Some cells remained spherical in shape, whereas other cells extended short processes and continued to express CD34 at 1–2 months after implantation (Fig. 6, top row); at 6 months they exhibited morphologies reminiscent of neurons, astroglia, and oligodendrocytes. The implanted brain sections were immunolabeled for markers of neurons (neurofilament H and NeuN), astroglia (GFAP), and oligodendrocytes (CNPase). A striking finding was that, whereas at the time of injection into brain all CD34+ cells expressed neurofilament H, NeuN, and CNPase, at 6 months and 1 year after transplantation only 40% of implanted cells expressed neurofilament H and/or NeuN, and 30% of implanted cells expressed CNPase (Fig. 6 and Table 3). In addition, whereas no CD34+ cells in culture expressed GFAP, after implantation into the brain 30% of CD34+ cells in culture did express GFAP. Double labeling demonstrated that cells expressing neurofilament H or NeuN did not express CNPase or GFAP (Fig. 6). Similarly, GFAP was not detected in cells that expressed CNPase (data not shown). Thus, neurofilament H, NeuN, and CNPase immunoactivity is lost in 60–70% of the implanted CD34+ cells, whereas GFAP appeared in 30% of implanted CD34+ cells. Therefore, these data indicate that there are two stages of expression of neural markers in the CD34+ cells reported here. Whereas all cells in the CD34+ cultures express neurofilament H, NeuN, and CNPase in vitro, in transplanted cells, neuronal and oligodendrocyte markers in sharp contrast segregated into distinct populations by suppressing either the neuronal gene expression or oligodendrocyte gene expression, or both, in cells that became GFAP+ after transplantation. These data indicate that GFAP, neurofilament H, and CNPase expression are regulated under the environmental control of the brain. The plasticity of these CD34+ cells in brain to become neurons or glia is reminiscent of earlier reports of the capacity of glial cells to become neurons in vivo (13–16).
Fig. 6.
Immunohistochemical analysis by laser confocal microscopy of gene expression by CTO-labeled cultured CD34+, Sca-1+, AA4.1+, and cKit+ adult C57BL/6J mouse bone marrow cells transplanted into adult C57BL/6J mouse brain hippocampus and striatum. First row, CTO-labeled cells continue to express CD34 6 weeks after implantation into adult brain, and host brain cells fail to express CD34. Second row, oligodendroglial CNPase (transplanted cell, arrowhead; host cell, arrow). Third and fourth rows, astroglial GFAP (transplanted cell, arrowhead; host cell, arrow). Fifth and sixth rows, neuronal neurofilament H. Seventh row, NeuN expression in CTO-labeled adult mouse bone marrow cells 1 year after implantation into adult mouse brain. The last three rows show that transplanted CTO-labeled cells express either astroglial (GFAP) or neuronal (neurofilament H and NeuN) proteins but not both; arrows show the same cell in each row triple-labeled 1 year after implantation.
Table 3. CD34+ stem cells implanted in adult mouse brain selectively express neural markers.
| Protein | No. of positive cells | % positive |
|---|---|---|
| Neurofilament H | 815 | 42 |
| NeuN | 795 | 42 |
| GFAP | 490 | 25 |
| CNPase | 580 | 30 |
The demonstration that a minor population of ex vivo bone marrow cells expresses neural antigens as well as an hematopoietic stem cell marker may lead to a new interpretation of data from other laboratories that reported expression of neural antigens in bone marrow cells transplanted into brain. Indeed, these other studies have suggested that it is the environment of the brain that leads to the transdifferentiation of bone marrow cells for the acquisition of neural antigens (1, 2). In contrast, it has been reported that selected bone marrow cells that are CD34– failed to express neural antigens when transplanted into brain (6). Because cells expressing neural antigens are only a minor population of the bone marrow, these divergent findings may be accounted for by the fact that different laboratories may be implanting distinct populations of bone marrow cells, which may or may not include the minor population expressing neural antigens.
In summary, a major finding of this study is that ex vivo bone marrow cells with a hematopoietic stem cell or progenitor cell phenotype do express molecules associated with the nervous system, indicating that adult hematopoietic stem cells, which classically are thought to be of mesodermal origin, express neural genes, which are regarded as restricted to cells derived from ectoderm. The presence of neural transcription factors and neural differentiation antigens in ex vivo CD34+ bone marrow cells may indicate that these cells are permissive or predisposed to differentiate into neural cells when placed in the milieu of the brain.
This work has focused on the neural aspects of these CD34+ hematopoietic progenitor cells. It must be determined whether they are multipotent beyond the nervous system or indeed totipotent, as the presence of Rex-1 and Oct-4 suggests. Stem cells from bone marrow are the only known source of stem cells that circulate in the blood and have access to all tissues of the body, with the exception of the brain, unless the blood–brain barrier is compromised. If these cells were multipotent, they might provide a source for seeding stem cells in other tissues of the body.
If similar CD34+ cell culture methods were adapted for humans, a patient's own bone marrow would be an ideal source for therapeutic cell replacement in neurodegeneration by obviating the problems of immunohistocompatibility and pathogen transfer from donor to host, as well as the limited source of human embryonic stem cells and human fetal brain cells.
Acknowledgments
We thank Seth Crawford for composition of the figures in this article. This work was funded by the Department of Veterans Affairs Research Enhancement Award Program (P.S.F.), the National Multiple Sclerosis Society (S.D.-J.), the Department of Veterans Affairs Multiple Sclerosis Center of Excellence (S.D.-J., C.T.B., and D.T.), the Department of Veterans Affairs Merit Review (C.T.B. and D.T.), and the Fondation Jérôme Lejeune (B.P.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CTO, Cell Tracker Orange; CNPase, 2′,3′ cyclic nucleotide 3′-phosphohydrolase; DAPI, 4′-diamidino-2-phenylindole; GAD65, glutamic acid decarboxylase 65; GFAP, glial fibrillary acidic protein; HuC/HuD, Hu-antigen C/Hu-antigen D; MOSP, myelin oligodendrocyte-specific protein; NeuN, neuronal nuclear protein.
References
- 1.Brazelton, T. R., Rossi, F. M., Keshet, G. I. & Blau, H. M. (2000) Science 290, 1775–1779. [DOI] [PubMed] [Google Scholar]
- 2.Mezey, E., Chandross, K. J., Harta, G., Maki, R. A. & McKercher, S. R. (2000) Science 290, 1779–1782. [DOI] [PubMed] [Google Scholar]
- 3.Makar, T. K., Wilt, S., Dong, Z., Fishman, P., Mouradian, M. M. & Dhib-Jalbut, S. (2002) J. Interferon Cytokine Res. 22, 783–791. [DOI] [PubMed] [Google Scholar]
- 4.Hess, D. C., Hill, W. D., Martin-Studdard, A., Carroll, J., Brailer, J. & Carothers, J. (2002) Stroke (Dallas) 33, 1362–1368. [DOI] [PubMed] [Google Scholar]
- 5.Bonilla, S., Alaroon, P., Villaverdi, R. Aparicio, P., Silva, A. & Martinez, S. (2002) Eur. J. Neurosci. 15, 575–582. [DOI] [PubMed] [Google Scholar]
- 6.Castro, R. F., Jackson, K. A., Goodell, M. A., Robertson, C. S., Liu, H. & Shine, H. D. (2002) Science 297, 1299. [DOI] [PubMed] [Google Scholar]
- 7.Azizi, S. A., Stokes, D., Augelli, B. J., DiGirolamo, C. & Prockop, D. J. (1998) Proc. Natl. Acad. Sci. USA 95, 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kopen, G. C., Prockop, D. J. & Phinney, D. G. (1999) Proc. Natl. Acad. Sci. USA 96, 10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodbury, D., Schwarz, E. J., Prockop, D. J. & Black, I. B. (2000) J. Neurosci. Res. 61, 364–370. [DOI] [PubMed] [Google Scholar]
- 10.Kabos, P., Ehtesham, M., Kabosova, A., Black, K. L. & Yu, J. S. (2002) Exp. Neurol. 178, 288–293. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., Schwartz, R. E., Keene, C. D., Ortiz-Gonzalez, X. R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M., et al. (2002) Nature 418, 41–49. [DOI] [PubMed] [Google Scholar]
- 12.Marty, M. C., Alliot, F., Rutin, J., Fritz, R., Trisler, D. & Pessac, B. (2002) Proc. Natl. Acad. Sci. USA 99, 8856–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laywell, E. D., Rakic, P., Kukekov, V. G., Holland, E. C. & Steindler, D. A. (2000) Proc. Natl. Acad. Sci. USA 97, 13883–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, A. J. & Rey, T. A. (2001) Nat. Neurosci. 4, 247–252. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, A. J., McGuire, C. R., Dierks, B. D. & Rey, T. A. (2002) J. Neurosci. 22, 9387–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malatesta, P., Hack, M. A., Hartfuss, E., Kettenmann, H., Klinkert, W., Kirchhoff, F. & Götz, M. (2003) Neuron 37, 751–764. [DOI] [PubMed] [Google Scholar]