Abstract
In acute myocardial infarction increased plasma levels of chromogranin A is correlated with decreased survival. At the human chromogranin A gene locus there are two naturally occurring amino acid substitution variants within the catestatin region, i.e. Gly364 Ser and Pro370Leu, displaying differential potencies towards inhibition of nicotinic cholinergic agonist-evoked catecholamine secretion from sympathochromaffin cells and different degrees of processing from the prohormone. Here, we examine whether two of the variants and the wild type catestatin may affect the development of infarct size during ischemic reperfusion in the Langendorff rat heart model. The hearts were subjected to regional ischemia followed by reperfusion in the presence or absence of synthetic variants of human catestatin. Compared to the Gly364Ser variant both the wild type and the Pro370Leu variant increased infarct size while decreasing the cardiac levels of phosphorylated Akt and two of its downstream targets, FoxO1 and BAD. In conclusion, these findings suggest that, in contrast to the Gly364Ser variant, the wild type catestatin and the Pro370Leu variant (allele frequency ~0.3%) increased myocardial infarct size via a mechanism involving dephosphorylation of Akt and the two downstream targets during ischemic reperfusion in the isolated rat heart.
Keywords: infarction, reperfusion, ischemia, catestatin, chromogranin A
INTRODUCTION
Ischemia/reperfusion (I/R) injury causes an inflammatory response as a consequence of oxidative damage, which then triggers stress-signaling processes resulting in death of cardiac myocytes and increasing the susceptibility to cardiac dysfunction [1]. In the clinical situation an acute myocardial infarction (MI) results from a regional occlusion of the coronary supply of oxygenated blood. It is well established that post-ischemic reperfusion may worsen the myocardial injury if the heart is not preconditioned or post-conditioned by brief periods of coronary occlusions before or after the ischemic insult [2]. However, as the onset of infarction is usually unpredictable, ischemic preconditioning is of little practical use. A large component of the ischemic damage to the myocardium takes place during the first minutes of reperfusion when the oxygen overflow amplifies the injury and/or cause additional damage [3]. Cardioprotection after MI is best achieved by pharmacological interventions during the post-ischemic reperfusion [4]. Accordingly, a period of global reperfusion after a regional ischemic insult offers an important window for cardioprotective therapy in the clinical setting. In this context, the regionally ischemic and reperfused ex vivo rat heart is a well-established model for evaluation of pharmacological interventions during post-ischemic reperfusion of relevance for the clinical situation [5, 6, 7]. Taking into account that plasma chromogranin A (CGA) is significantly elevated in patients after MI [8] and that after acute MI, CGA is predictive of mortality [8-12], a question arises whether elevated plasma levels of CGA are beneficial or detrimental to the ailing heart. At the human CHGA gene locus there are 2 naturally occurring amino acid substituted variants within the Cts region: Gly364 Ser (GS-Cts) and Pro370 Leu (PL-Cts) in addition to one outside this region (Arg374 Gln) [13, 14]. As these variants also display differential potencies towards inhibition of the basal cardiac performance and isoproterenol induced inotropism and lucitropism [15], we hypothesize that these Cts isoforms may also differ in protective potencies against the post-ischemic injury of the myocardium during reperfusion after a period of regional ischemia in the ex vivo rat heart model.
The human chromogranin A (CGA1-439) was originally identified in the adrenal medulla where it is co-stored and exocytotically co-released with catecholamines [16-18]. CGA is not only expressed throughout the neuroendocrine system [19, 20], but is also expressed in rat and human heart tissue [21-23]. Moreover, in human heart CGA is elevated in biopsies taken from patients with dilated or hypertrophic cardiomyopathy [23]. CGA is proteolytically processed to give rise to several peptides of biological importance, including the dysglycemic hormone pancreastatin [24], the vasodilator N-terminal vasostatin-I (CGA 1-76) [25] and the catecholamine release inhibitory peptide catestatin (Cts, human CGA352-372, bovine CGA344-364) [26]. Four N-terminal CGA peptides have been extracted from the rat heart: i.e. CGA4-113, CGA1-119, CGA1-124 and CGA1-135 [22]. Cts is a novel cardiosuppressive peptide in the isolated rat and frog hearts [15, 27] and mediates this action via activation of endothelial nitric oxide production including Akt dephosphorylation and subsequent inhibition of isoproterenol and endothelin signaling. We and others [5, 6, 7, 28] have shown a correlation between Akt phosphorylation and increased cytoprotection induced by pharmacological intervention at early ischemic reperfusion. The Akt signaling pathway is already a therapeutic target against I/R injury due to its pivotal role in cell survival [29]. Akt functions as a “survival” kinase by phosphorylating a number of apoptosis regulatory molecules acting in parallel, such as the forkhead transcription factor (FoxO1) [30-33] and the cytosolic protein BAD [34, 35]. When phosphorylated by Akt these proteins are retained in the cytoplasm through interaction with 14-3-3 proteins and thus become functionally inactivated. If Akt does not inactivate forkheads and BAD in this manner, the forkheads translocate to the nucleus and initiate transcription of pro-apoptotic proteins [30], while BAD translocates to the mitochondria with subsequent heterodimerization with Bcl-xl or Bcl-2 to promote cell death [34,35].
Given that CGA is expressed in the heart, that circulating levels of CGA are increased following CHF and MI in humans , and that these sources of CGA are processed into Cts variants to a variable degree [36-38], we have reasoned that Cts isoforms might also differ in their influence on the pathophysiology of cardiac I/R injury. When these isoforms are present during the post-ischemic reperfusion period following a regional ischemic injury in the ex vivo rat heart model, they may modulate the Akt-regulated phosphorylation of BAD [34, 35] and possibly also FoxO1 [30-33].
MATERIALS AND METHODS
All experiments were approved by the Norwegian State Commission for Laboratory Animals and carried out in accordance with the European Communities Council Directive of 1986 (86/609/EEC).
The ex vivo Langendorff perfused heart model
Male Wistar rats (250-350 g) fed with a standard diet were heparinized (200 IU) and anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). The hearts were excised and rapidly mounted onto a Langendorff perfusion system as described elsewhere [5, 39]. A water-filled latex balloon, connected to a hydrostatic physiological pressure transducer (SP844, Memscap, Norway) and coupled to a high performance data acquisition system (PowerLab 8/30, Chart Pro software-MLS250), was inserted into the left ventricle (LV) through an incision in the left atrium and inflated to set an end diastolic pressure (LVDP) of 5-10 mm Hg-[5, 39].
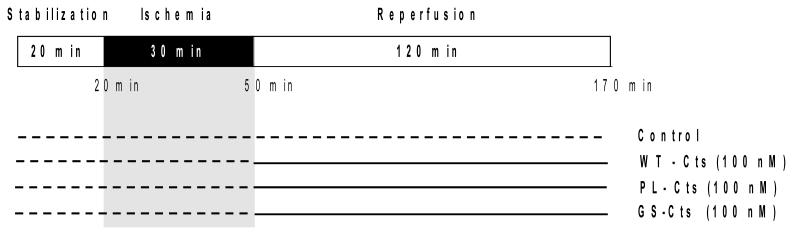
Coronary flow (CF) was measured by timed collection of effluent over 1 min at each sampling point. A 3-0 silk suture was passed around the main branch of the left coronary artery, and the ends were threaded through a small vinyl tube to form a snare. Regional ischemia was achieved by pulling the snare and was confirmed by a substantial fall in both LVDP and CF. All hearts underwent 20 min of stabilization [5, 39, 40], 30 min of regional ischemia (RI), and then 120 min of reperfusion during which Cts isoforms were present for comparison of effects upon reoxygenation (Fig. 1).
Figure 1. Experimental protocol for the Langendorff perfused rat heart.
The isolated heart was subjected to 20 min of stabilization, 30 min of regional ischemia and 120 min of reperfusion. Cts peptides were administrated to the hearts at onset of reperfusion.
Four groups of hearts were included: 1) control (in Krebs-Henseleit buffer, KHB), 2) GS-Cts, 3) PL-Cts or 4) WT-Cts (all in 100 nM in KHB from the onset of reperfusion). The control group was exposed to the identical protocol as for the hearts treated with the Cts-isoforms, both in regards to infarct size and protein status. The ligation was retied at the end of the experimental protocol, and Evans blue dye (EBD) 0.2% (w/v) was infused to demarcate the risk zone (RZ). The hearts were frozen at −20°C and thereafter cut into 2-mm tick slices from the apex to the atrioventricular groove. The slices were then stained with 1% triphenyltetrazolium chloride (TTC) in phosphate buffer (pH 7.4) at 37 °C for 20 min, before fixation in 10% (v/v) formalin solution to enhance the contrast of the stain. The slices were then compressed to a uniform thickness by placing them between two glass plates separated by a 2-mm spacer. The area of the left ventricle, the infarcted area (TTC negative) and the risk zone (blue) were traced on an acetate transparency, and the infarct size was determined using a computerized planimetry program. The infarct size/risk ratio (%) was determined by expressing the infarcted (TTC positive) area in percent of the risk zone (RZ; EBD negative). The measurement of ischemic risk zone and infarct size was performed in a blinded fashion.
Preparation of neonatal mouse cardiomyocyte cultures
The isolation procedure for cardiac myocytes from hearts of C57BL/6 mice was based on the method of Simpson [41] with additional modifications [42] using sequential digestion in collagenase type II (Worthington Biochem Inc., Lakewood, NJ). The cardiomyocyte cell suspension was transferred to 24-well, (1-cm diameter) 2% (wt/vol) gelatin-coated plates at a density of 105 cells/well for protein extraction. After 24 h the cell medium was replaced with DMEM supplemented with 1% (vol/vol) fetal bovine serum for an additional 24 h. Within 2 d a confluent monolayer of spontaneously beating myocytes was formed.
Preparation of adult mouse cardiomyocyte cultures
Adult mouse ventricular cardiomyocytes were prepared by excising hearts from three-month-old mice (anesthetized with 0.25% (wt/vol) Avertin and anticoagulated with heparin 250 U/mouse i.p.) and placed in ice-cold DMEM containing 4% (vol/vol) fetal bovine serum (FBS). Then the hearts were mounted onto a modified Langendorff apparatus and perfused at a rate of 3.5 ml/min for 1 min with prewarmed 37°C Ca2+-free Joklik's medium supplemented with 10 mM HEPES, 30 mM taurine, 2 mM DL-carnitine, and 2 mM creatine (pH 7.36–7.4) (Sigma). The hearts were then perfused and digested with 0.75 mg/ml collagenase type 2 in 0.1% (wt/vol) BSA for 9–15 min (20 μM CaCl2) in supplemented Joklik's media. The hearts were excised and digested for a further 3–6 min in the collagenase solution and washed thoroughly in 70 μm2 nylon mesh with supplemented Joklik's medium containing 10% (w/v) BSA and 20 μM CaCl2 (wash solution). CaCl2 was added gradually until a concentration of 2 mM was reached. The cardiomyocytes were then plated in DMEM medium (Gibco BRL, Grand Island, NY) containing 4% (vol/vol) FBS at a density of 1 × 104 cells/well on 10 μg/ml prelaminated (Sigma) coated plates for 1 h (6 cm2). The media were replaced with DMEM (serum free) before experimentation.
Peptides
The human WT-Cts; CGA352-372 (S352SMKLSFRARGYGFRGPGPQL372), or its two variants Gly364Ser (SSMKLSFRARAYS364FRGPGPQL) and Pro370Leu (SSMKLSFRARAYGFRGPGL370QL), were synthesized by the solid-phase method and purified as previously described [36, 43-45].
Signalling analyses
Rat heart tissue was isolated and snap-frozen. Later the tissue were thawed and homogenized in 1 ml of ice cold heart lysis buffer: 0.2 M sucrose, 10 mM Tris maleate (pH 7.0) buffer, 2 mM EDTA (pH 8.0), 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 1 mM henylmethylsulfonyl fluoride, 10 μg/ml leupeptin and 10 μg/ml aprotinin. Cytosolic fractions were isolated and the protein content determined by the Bradford colorimetric assay (BIO-RAD, CA, USA). Cytosolic protein (25 μg) was subjected to immunoblot analysis for phosphorylated Akt-Ser473 (P-Akt-Ser473), Akt-Threonine308 (P-Akt-Thr308), total Akt (T-Akt) and P-FoxO1-Ser256 using an antibody that detects a doublet including P-FoxO1-Ser256 (all from Cell Signaling Technology, CA, USA). P-FoxO1 levels were normalized to actin (Santa Cruz Biotechnology, CA, USA).
Immunoreactive Cts in the myocardium
Protein extracts were retained for immunoblot analysis of catestatin-containing CGA using a specific rabbit-polyclonal antibody that was commercially generated against the human catestatin domain (hCGA352-372) by Strategic Biosolutions, Windham, Maine, USA [46]. The antibody detects the Cts sequence within the full-length and processed fragments of CGA and cross-reacts with mouse and human Cts and CGA. The primary antibody and dilutions were routinely characterized for specificity towards intact CGA and free WT-Cts peptide (data not shown).
To investigate the expression of immunoreactive Cts, samples containing 40 μg of total cytosolic protein were taken from baseline control hearts, the non-ischemic area (NI) and risk zone (RZ) area of hearts subjected to I/R. Extracts from neonatal and adult mouse cardiac myocytes were prepared as described elsewhere [47]. Cytosolic protein from these cells were separated on NuPAGE® Novex 4-12% Bis-Tris Midi gels (Invitrogen, CA) using 2-(N-morpholino)ethanesulfonic acid (MES) buffer (Invitrogen, CA) using the XCell SureLock® Mini-Cell. After separation, the proteins were transferred on to 0.2 μm nitrocellulose membranes and XCell II™ Blot Module Kit (Invitrogen, CA) and the membranes were immunoblotted with a 1:5000 dilution of the primary anti-human Cts antibody. The apparent molecular weight of CGA in heart extracts was compared to CGA in 5 μg of mouse adrenal extract protein. Membranes were then washed in phosphate buffer saline (PBS) supplemented with 0.05% Tween-20 and incubated with 1:2000 dilution of peroxidase conjugated anti- rabbit IgG (Bio-Rad Laboratories, Hercules, CA) followed by detection using an enhanced chemiluminescence kit (Amersham Pharmacia Biotech). Additional immunoblots were conducted on adult and neonatal cell extracts. To clearly identify the molecular weight forms of Cts-immunoreactive proteins in heart vs. the adrenal, 20 and 40 μg aliquots of heart extract were run for comparison with 5 or 40 μg samples of adrenal extract.
P-BAD immunoprecipitation
Protein (100 μg) from cell lysate was pre-cleared by incubation with 20 μl of a 50% slurry of protein G plus (Santa Cruz) with agitation (20 min, 4 °C). Following centrifugation (10,000 rpm, 10 min, 4 °C), the cell lysates were incubated with agitation (18 h, 4 °C) with an anti-Phospho-BAD-Ser136 (P-BAD-Ser136) antibody (1:100). Subsequently, 20 μl of a 50% slurry of protein G plus was incubated with the lysate for 3 h, with agitation, at 4 °C. P-Bad immunoreactive proteins were pelleted by centrifugation (10,000 rpm, 10 min, 4 °C) and washed three times with ice-cold heart lysis buffer. Thirty μl of 2X Laemmli sample buffer was added to the pellet which was then heated and subjected to SDS-PAGE. BAD levels were determined by immunoblot analysis using a P-BAD-Ser136 specific antibody [5].
Statistics
Values are presented as mean ± SEM. For protein analysis, the volumes of total and phosphorylated proteins were quantified using Quantity One (Bio-Rad), normalized to actin or total protein. Fold increase was calculated from the control of non-ischemic hearts. Comparisons of coronary flow (CF), heart rate (HR) and left ventricular developed pressure (LVDP) between groups were performed with repeated-measures by the general linear model and within-group differences were tested by the paired Student's t-test. Infarct size, immunoblots and hemodynamic results were tested for group differences by ANOVA (one-way analysis of variance) combined with Fisher's post-hoc test. *p ≤ 0.05 was considered statistically significant.
RESULTS
Endogenous cardiac CGA is affected by ischemia-reperfusion
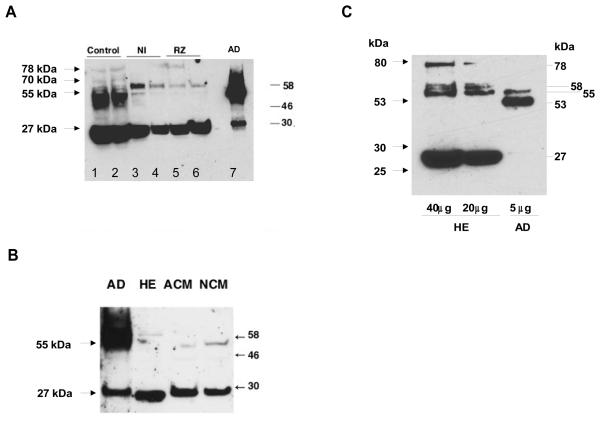
Using an antibody directed against human Cts [46], two major immunoreactive, Cts-containing forms were detected in the control hearts with apparent molecular weights of ~55 kDa and ~27 kDa, respectively (Fig. 2A). Higher molecular weight bands, including a faint band of 78 kDa, were detected in the control hearts. In the NI and RZ samples one form of ~ 58 kDa was observed in addition to the predominance of the ~27 kDa form (Fig. 2A). In the mouse adrenal gland used as a positive control the ~55 kDa form dominated over the ~58 kDa and the ~27 kDa forms (Fig. 2A, right lane and Fig. 2B, left lane). On the other hand, in the ex vivo rat heart tissue (HE) and in the isolated adult (ACM) and neonatal mouse cardiac myocytes (NCM), the ~27 kDa form of Cts-immunoreactivity dominated (Fig. 2B). In HE, five forms of Cts-immunoreactive peptides were observed (78, 58, 55, 53 and 27 kDa, respectively). In the adrenal extract at 1/8 dilution (5 μg protein, Fig.2C), only two major forms of Cts positive fragments of 55 and 53 kDa were detected. At this dilution the 27 kDa band was no longer visible.
Figure 2. Expression and processing of CGA during the I/R experiment.
A) Two Cts-containing CGA size forms of ~55 kDa and ~27 kDa, respectively, were apparent in the non-perfused hearts (control, left lanes 1 and 2). Both forms were also expressed in the mouse adrenal (AD, 40μg) as positive control (lane 7) Tissue from the non-ischemic area (NI, lanes 3 and 4) and risk zone (RZ, lanes 5 and 6) revealing markedly diminished levels of the ~55 kDa form. B) Both the ~55 and ~27 kDa Cts immunoreactive peptides were found in mouse adrenal (AD, 40μg) while in the isolated rat heart (HE) and in isolated adult (ACM) and neonatal mouse cardiac myocytes (NCM) the ~27 kDa form accounted for the predominant Cts immunoreactivity. C) To clearly demonstrate the MW forms of Cts-immunoreactive peptides in heart extract (HE) and adrenal (AD) we separated 40 and 20 μg of HE against 5μg of adrenal extract (AD). In HE, five forms of Cts-immunoreactive peptides were observed (78, 58, 55, 53 and 27 kDa). In adrenal, two major forms of 55 and 53 kDa were detected with 1 μg of protein. Positions of molecular weight markers (in kDa) are indicated at right sides of A) and C) and at the left side of B).
Effect of Cts variants on infarct size and hemodynamic variables
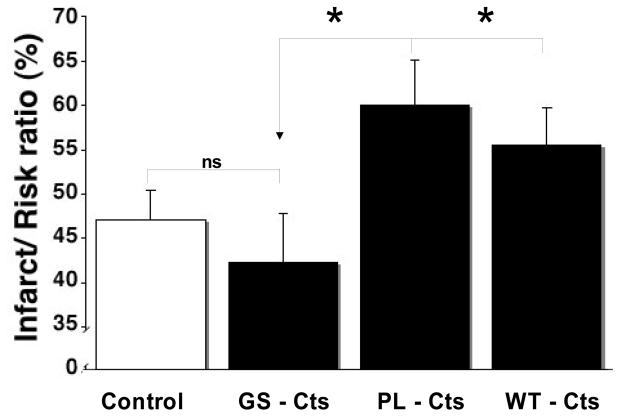
The marked declines in the ~55 kDa Cts-immunoreactive band in the perfused hearts suggested a considerable degree of CGA processing, presumably generating Cts-containing fragments also smaller than the predominant ~27 kDa form. We therefore examined whether WT-Cts and the two human variants could modulate infarct size following simulated I/R stress. Administration of WT-Cts, PL-Cts or GS-Cts from the onset of reperfusion resulted in comparable infarct sizes for the control versus the GS-Cts groups and for the WT-Cts versus the PL-Cts groups, respectively (Fig. 3), however, with significantly higher degrees of infarct size for the latter than for the former. The average area at risk (RZ)/left ventricular mass ratio was 50% for all groups. A significant reduction in LVDP and CF after 5 min of regional ischemia (RI, Table 1) confirmed that all groups obtained similar and expected degrees of ischemia relative to the baseline values at stabilization. On the other hand, there was no difference between groups with in regard to LVDP, CF and HR at 20 min of stabilization or at 60 and 120 min of reperfusion (Table 1). However, there was a significant fall in both LVDP and CF in all groups as compared to the corresponding stabilization value at both 60 and 120 min of ischemic reperfusion.
Figure 3. Effect of reperfusion treatment with Cts variants on infarct size after I/R.
Rat hearts were subject to ischemia and perfused with Cts peptides (100 nM) or control at reperfusion. The infarct size given as the infarct/risk ration in % are shown for the control (n=5), GS-Cts, (n=10), PL-Cts (n=6) or WT-Cts (n=5). Bars represent means ± SEM. * p≤ 0.05 for differences from CS-Cts.
Table 1.
Cardiac hemodynamics in the isolated rat heart during ischemic-reperfusion.
| GROUP | Stabilization 20 min |
RI 5 min |
Reperfusion 60 min |
Reperfusion 120 min |
|
|---|---|---|---|---|---|
|
LVDP (mmHg) |
Control | 128 ± 24 | 62 ± 16 * | 70 ± 8 * | 65 ± 6 * |
| WT-Cts | 133 ± 10 | 67 ± 20 * | 80 ± 6 * | 66 ± 8 * | |
| PL-Cts | 131 ± 14 | 68 ± 8 * | 74 ± 9 * | 71 ± 9 * | |
| GS-Cts | 137 ± 7 | 71 ± 11 * | 69 ± 5* | 60 ± 5 * | |
|
CF (ml/min) |
Control | 14 ± 1 | 8 ± 1 * | 9 ± 1 * | 7 ± 1 * |
| WT-Cts | 13 ± 1 | 7 ± 1* | 10 ± 1* | 9 ± 2 * | |
| PL-Cts | 13 ± 1 | 8 ± 1 * | 9 ± 1 * | 7 ± 1 * | |
| GS-Cts | 12 ± 1 | 8 ± 1 * | 8 ± 1 * | 7 ± 1 * | |
|
HR (beats/min) |
Control | 300 ± 14 | 259 ± 22 | 261 ± 21 | 254 ± 22 |
| WT-Cts | 295 ± 9 | 248 ± 43 | 285 ± 45 | 265 ± 16 | |
| PL-Cts | 270 ± 12 | 226 ± 22 | 252 ± 16 | 242 ± 18 | |
| GS-Cts | 271 ± 8 | 233 ± 20 | 254 ± 8 | 245 ± 9 |
RI - regional Ischemia; LVDP - Left Ventricular Developed Pressure; CF - Coronary Flow; HR - heart rate. Values represent mean ± SEM. Control (Krebs-Heinsleit buffer, KHB) and peptides (100nM) in KHB. n≥5.
p<0.05 vs. corresponding stabilization value.
Effect of reperfusion treatment with Cts on Akt-meditated signaling
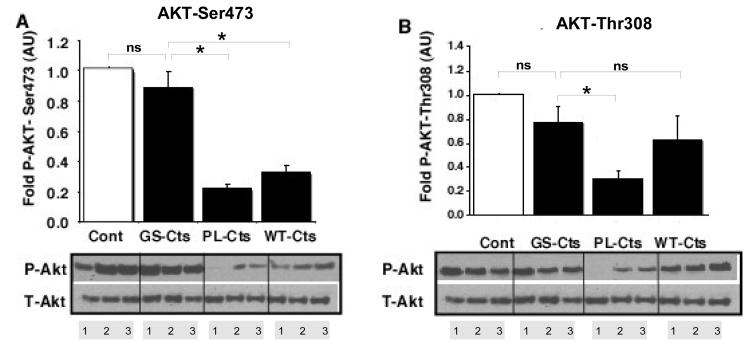
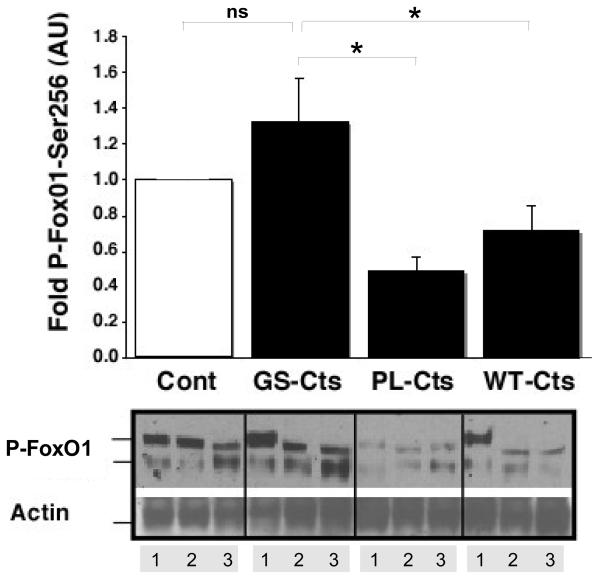
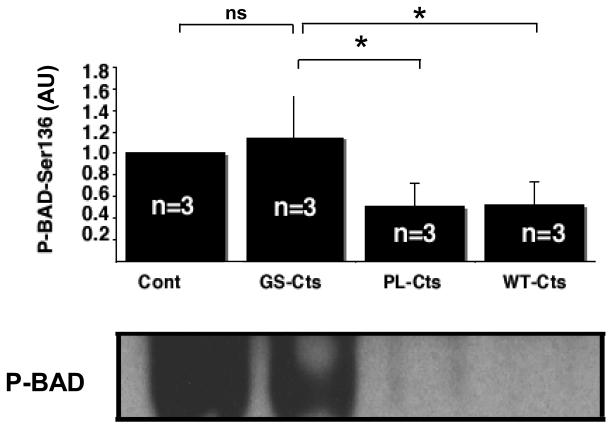
To probe the mechanism by which WT-Cts and PL-Cts increased infarct size as compared to GS-Cts, signaling analysis were conducted on cytosolic fractions of heart tissue. Both WT-Cts and PL-Cts significantly decreased the levels of phosphorylated the Akt-Ser473 isoform compared to the effects of GS-Cts and control hearts (Fig. 4A). The diminished phosphorylation of this isoform of Akt corresponded to the increased infarct size in the same groups relative to the GS-Cts treated and control groups. In addition, phosphorylated levels of the other Akt isoform, Akt-Thr308, was also significantly decreased in the PL-Cts hearts (Fig. 4B). Consistent with decreased levels of P-Akt, we also observed modifications in the phosphorylation of P-FoxO1-Ser236 (Fig. 5) and P-BAD (Fig. 6). However, there was no difference between the control and the GS-Cts group in folds of P-FoxO1 and P-BAD, while the PL-Cts and WT-Cts groups were significantly lower than the GS-Cts group in this respect.
Figure 4. Effects of Cts variants on Akt phosphorylation after I/R.
The phosphorylation status of A) Akt-Ser473 and B) Akt-Thr308 were normalized to total Akt. Densitometric scans are represented in arbitrary units (AU) relative to the control (Cont = 1). The bars represent mean ± SEM of 3 hearts (lanes 1,2 and 3). *p≤ 0.05 for differences between PL-Cts and WT-Cts versus GS-Cts.
Figure 5. Effects of Cts variants on signaling downstream from Akt.
Levels of protein phosphorylation were normalized to actin and expressed in arbitrary units (AU) relative to control hearts (Cont = 1). The double bands of immunoreactive FoxO1 are indicated. The bars represent the sum of immunostaining in both bands as means ± SEM of three hearts (lanes 1-3). *p≤ 0.05 for differences between PL-Cts and WT-Cts versus GS-Cts.
Figure 6. Effects of Cts variants on BAD phosphosphorylation.
Levels of BAD phosphorylation expressed in arbitrary units (AU) relative to control hearts (Cont = 1). The bars represent mean ± SEM of 3 hearts. *p≤ 0.05 for differences between PL-Cts and WT-Cts versus GS-Cts.
Correlation between infarct size and reduction of Akt phosphorylation
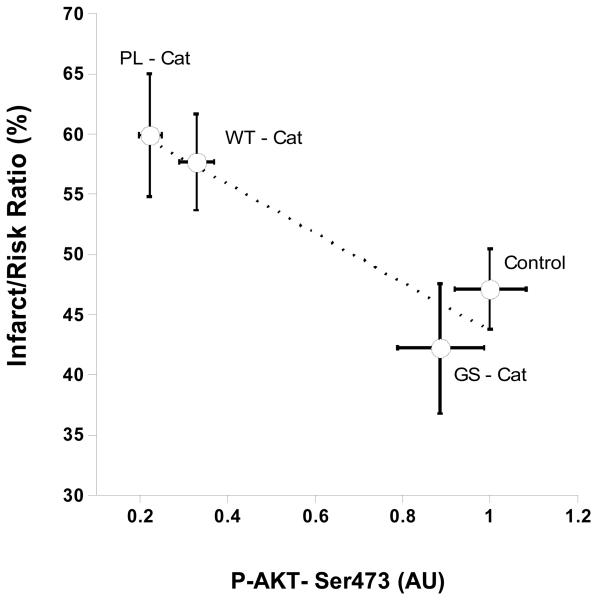
A 2-D plot was drawn to compare the effects of the different Cts- variant peptides with the % infarct size (Fig. 3) and Akt phosphorylation at Ser473 (Fig. 4). As shown in Fig. 7, the greatest % increase of infarct size correlated with the most marked decrease in Akt-Ser473 phosphorylation, as evident for the PL-Cts and WT-Cts peptides.
Figure 7. Correlation between infarct size (%) and Akt-Ser473 phosphorylation.
A 2-D plot was constructed using the Kalidograph® programme for the data for AktSer473 phosphorylation (Fig.4) versus the % infarct size (Fig.3). The correlation value (r2=0.877) indicates that the infarct size is inversely correlated with Akt-Ser473 phosphorylation for the Cts peptides examined.
DISCUSSION
Cts-containing fragments from cardiac CgA in the rat heart are indeed generated in response to regional ischemia. Atrial myocardial secretory granules are established as a source of CGA and CGB in the rat [21], while both CGA [23] and CGB [48] are produced by the human and rat ventricular myocytes, respectively. Putative candidates for proteolytic enzymes are members of the prohormone converting enzyme reported to be differentially expressed in heart [37] and assumed to be involved in processing of atrial CGA to Cts peptides. Cathepsin L, co-localized with CGA in chromaffin granules, has also been reported to generate Cts and other active peptides [38]. If present within the atrial granules, this enzyme might also contribute to processing of cardiac CGA into Cts-containing fragments. In the extracellular space the plasmin system is a likely candidate for processing of CGA into to Cts [36]. The potential of these and other enzymes in processing of CGA in the I/R hearts of the rat remains a challenge for future studies.
It is well established that I/R injury culminates with hypercontracture of the cardiomyocytes, causing sarcolemmal rupture and cell death [49]. Opening of the mitochondrial permeability transition pore (MPTP) and the calcium overload are crucial players in the final stage of this detrimental process [50]. Most of the damage takes place during the first minutes of reperfusion and this period is therefore critical for cardioprotection. Inhibition of MPTP is mediated via the reperfusion injury salvation kinase (RISK) pathway, involving NO-dependent and –independent cascades activated by G protein coupled receptors. [6]. Here, Akt is pivotal for three subsequent downstream cascades terminating on inhibition of MPTP opening. While the e-NOS – cGMP-PKC cascade leads cardioprotection via opening of the mitochondrial potassium ATP channel and subsequent activation of reactive oxygen species [51], two other NO-independent cascades are also activated by Akt, namely by down regulation of BAD [5, 6] and by phosphorylation of GSK-3β [52] .
In the normoxic rat heart ex vivo Cts isoforms have been shown to vary in their activation of the endothelial NO cascade [15]. The present study has therefore aimed at an evaluation of the cardioprotective potentials of the Cts isoforms when present throughout the reperfusion following the ischemic insult. Unexpectedly, the human WT-Cts and its naturally occurring variant PL-Cts significantly enhance infarct size in the ex vivo rat heart when present from onset of reperfusion. In contrast, the most frequent variant, GS-Cts, was without effect on infarct size relative to control. Moreover, the enhanced infarct sizes in the WT-Cts and PL-Cts groups correlate with blunted Akt phosphorylation, implicating minimal cardioprotective potentials of these two isoforms in the ex vivo rat heart.
Detection of Cts-containing CGA forms during I/R in the rat heart
Using the antibody specifically directed towards full length human WT-Cts [46], we show that immunoreactive Cts is present in rat as a ~55 kDa form which predominates in the control hearts. However, this form decreased markedly relative to the ~27 kDa form in response to treated hearts. This suggests a pronounced degree of proteolytic processing of the larger form of immunoreactive Cts, presumably leading to release of Cts-containing peptides also smaller sized than the ~27 kDa form. In a previous study N-terminal cleavage of normal rat heart CGA into betagranin-like peptides (CGA4-113, CGA1-119, CGA1-124, and CGA1-135) has been demonstrated [22], consequently resulting in a range of betagranin-free forms of variable sizes. The present detection of the ~ 55 kDa bands as the predominant immunoreactive Cts in control hearts may correspond to such a partly processed, betagranin-free rat CGA while the ~ 58 kDa form in the NI and RZ samples may be another, probably induced by the ischemic stress. It is likely that the slight differences in apparent molecular weight of the Cts containing bands between adrenal and heart tissues represent variations in post-translational modifications in these two tissues.
Effect of Cts variants on infarct size during ischemia-reperfusion
The concentration of Cts peptides (100 nM) was selected on the basis of the IC50 value for WT-Cts inhibition of the nicotinic cholinergic receptor-mediated catecholamine release in bovine adrenal chromaffin cells [26] and previous studies in the isolated rat [15] and frog heart [27]. In the present study the local concentration of Cts in the coronary effluent was not determined, either under basal conditions or in the reperfusion period following ischemia. Of note, circulating concentration of CGA in patients 3 days after a MI reached 36 ng/ml or ~0.78 nM [16], i.e. close to the normal value. On the other hand, in CHF patients in the New York Heart failure class IV plasma CGA may reach 11 nM [9]. Thus, in the present experiments the rat hearts were perfused with 10-100 fold higher concentrations of Cts peptide than the concentrations of the parent CGA in plasma of CHF or MI patients [8-10].
The allele frequency for the variant GS-Cts accounts for no more that 3-4% of the total, predicting a ~6-8% population prevalence of heterozygotes, while that of the PL-Cts variant the frequency (~0.3%) is much lower [45]. Our present data shows that the infarct size with the GS-Cts peptide was not different from the control (Fig. 3). Hence, the rank order for increase in infarct size was PL-Cts = WT-Cts>> GS-Cts = control, i.e. similar to the rank order for decrease in Akt phosphorylation (Figs. 4A and 6). Analogously, the rank order of potency for the inhibition of nicotinic stimulated catecholamine secretion from chromaffin cells is PL-Cts>WT-Cts>GS-Cts [14]. Although a receptor for Cts in the heart has yet to be identified, the inotropic and lusitropic negative effects of WT-Cts under normoxic conditions in the rat heart involved an endothelial Gi/o protein → nitric oxide → cGMP signaling pathway and a non-competitive activation of beta-2-adrenergic receptors [15]. Importantly, the eNOS-NO-cGMP-PKG cascade mediates specific cardioprotection via the RISK pathway [6]. Stimulation of beta-2-adrenergic receptors also acts through Akt to phosphorylate eNOS to generate NO [15]. Furthermore, these authors suggest that the negative inotropism and lusitropism found after infusion of 110 nM of WT-Cts is caused by reduced P-PLN (phospholamban) levels that can influence basal Ca2+ handling and contractility via the SR Ca2+ pump (SERCA2a) and the SR Ca2+ release via ryanodine receptor 2a, resulting in altered Ca2+ transients with consequences for inotropy and lusitropy.
WT-Cts and PL-Cts suppress the pro-survival Akt signaling pathway in the I/R hearts
The PL-Cts and WT-Cts treated hearts had considerably larger infarct sizes (Fig. 3) and lower pro-survival P-Akt levels compared to the GS-Cts treated and control hearts (Fig. 4). Thus, the levels of phosphorylated Akt were inversely correlated to infarct size in the peptide treated groups (Fig. 7). The reduction in P-Akt with WT-Cts presently observed is consistent with recent findings of reduced phosphorylation of Akt in the normoxic rat heart [15]. The possibility that WT-Cts might induce cell death also in normoxic conditions was not supported using cultures of cardiac myocytes (data not shown). Relevant in this context is the important contributions of AKT and ERK ½ to the RISK-signaling pathway, conveying cardioprotection during ischemic-reperfusion [5, 6, 28]. Both in the RISK cascade [6] and in the β2-ARs-Gi/o protein-eNOS-NO-cGMP-PKG dependent cGMP signaling pathway, e-NOS is downstream to Akt [15]. If the β2-ARs-Gi/o protein-eNOS-NO-cGMP-PKG pathway stimulated by WT-Cts [15] also depended on Akt phosphorylation [15], one would have expected the total Akt phosphorylation from EC and the myocardium to be elevated in presence of WT-Cts. However, Cts infusion dephosphorylated both AKT and ERK ½ under basal conditions [15] and failed to alter ERK1/2 phosphorylation during ischemic-reperfusion in our study (data not shown). Hence, it is most likely that a Cts-induced dephosphorylation of Akt, independent of the NO-dependent pathway, may play the most important role under the present conditions.
Akt inhibition by WT-Cts and PL-Cts variants also inhibits downstream FoxO1 and BAD
Akt plays a critical role in controlling survival and apoptosis in a variety of cells and tissues [29-33]. Because WT-Cts and the human variant PL-Cts inhibited Akt activity as a result of ischemic reperfusion, these peptides would also be expected to inhibit the phosphorylation of downstream anti-apoptotic targets of this kinase. Our data show that both WT-Cts and PL-Cts target to the FoxO1 forkhead transcription factor and the pro-apoptotic protein BAD. Thus, the inhibition of Akt, FoxO1 and BAD pathways by both PL-Cts and WT-Cts compared to GS-Cts may relate to the observed differences between these Cts peptides with respect to infarct size.
In the normoxic heart [15] the negative inotropy and lusitropy was associated with reduced P-PLN (phospholamban) levels that could influence basal Ca2+ handling and contractility via the SR Ca2+ pump (SERCA2a) and the SR Ca2+ release via ryanodine receptor 2a [15]. However, there is today no data suggesting that Cts works directly on any particular subcellular compartment. Therefore, the effects of Cts were examined in the cytosolic fraction only. On the other hand, the reduced phosphorylation of AKT in this fraction of neonatal and adult mice cardiomyocytes suggests that the enhanced infarct size in response to WT-Cts and PL-Cts in the rat heart most likely is linked to a reduced inhibition of MPTP as the final stage in the RISK cascade.
Blood pressure is lower in individuals with the GS-Cts variant than in those with WT-and PL-Cts [13, 45]. Also, GS-Cts heterozygotes display increased baroreceptor slope, increased cardiac parasympathetic index, and decreased cardiac sympathetic index [53]. Thus, the present findings suggests that in addition to having favorable blood pressure-lowering effects, persons who carry the most common variant, i.e. GS-Cts, may be at diminished risk during I/R injury in comparison to normal individuals or PL-Cts carriers due to marked differences in suppression of the Akt signaling pathway as presently demonstrated. Importantly, under basal conditions WT-Cts exerted maximal negative inotropic and lucitropic effects 5 min after administration, remaining stable for 15 min and then graduallydecreasing with time [15]. On the other hand, after ischemia the cardiac function of the isolated rat hearts with a RZ of 50% would remain depressed at the start of reperfusion. Moreover, during ischemic reperfusion LVDP and CF were not significantly different between the groups (Table 1). It is therefore unlikely that these factors could have contributed significantly towards the WT-Cts and PL-Cts mediated increases on infarct size (Fig. 3). Intriguingly, the deleterious response to WT-Cts in the ischemic rat heart is in contrast to the protective effect of another CGA peptide, the N-terminal vasostatin-I against the development of cardiac infarction when administered as a mimetic of ischemic preconditioning [54].
Concluding remarks
Consistent with previous findings in rat [22] and human [23] hearts, our study has demonstrated CGA in the rat heart and mouse cardiocytes. Importantly, our data makes it evident that the endogenous cardiac CGA was modified in response to the I/R injury, resulting in an enhanced processing into immunoreactive Cts-containing fragments such as the 27 kDa band prominent not only in the rat heart, but also in the mouse cardiomyocytes.
The cardiovascular actions previously demonstrated for the two CGA derived peptides vasostatin-I and Cts under normoxic conditions have now been extended to post-ischemic reperfusion injury. However, in contrast to beneficial effects of Vasostatin-I during preconditioning [54], the present data imply that WT-Ct may enhance the infarct size when present at high 100 nM concentration during the reperfusion phase following a period of regional ischemia. We have also demonstrated that three isoforms of the human Cts differently affect recovery from regional ischemic injury in the rat heart ex vivo. This fact points to significant differences in primary structure for activation of the signaling cascades implicit in the cardiac responses.
The negative inotropic effect of WT-Cts via activation of eNOS in the normoxic rat heart model is reported to be sensitive to inhibition of pertussis toxin, implicating a Gαi/o subunit in the signaling pathway [15]. In want of a surface receptor for Cts outside the sympathoadrenal system, receptor-independent cell activation via a Gαi/o subunit has been proposed for the cationic Cts in rat mast cells and cardiac tissue [55]. Alternatively, the cationic Cts may penetrate through pores in the cell membranes, as postulated for the antimicrobial properties [56] and conformation plasticity [57] of the biologically active cateslytin domain of the bovine WT-Cts (bCGA344-358). Of note, while PL-Cts accounts for a substitution of Pro370 for Leu, i.e. outside the cateslytin region, GS-Cts comprises a substitution of Gly364 to Ser within the cationic cluster of the active domain. This substitution site corresponds to Gly356 of the bovine Cts, previously shown to be one of the crucial sites for inhibition of desensitization of CA release [43]. Hence, it seems likely that the substitution Gly364Ser in human Cts has rendered this isoform inactive compared to WT-Cts and PL-Cts with respect to infarct size and Akt phosphorylation during I/R reperfusion.
The quantitative aspects of the cardiac synthesis and constitutive release of CGA have yet to be established. So far, our data have made it evident that when the human WT-Cts is present at a high 100 nM concentrations during reperfusion after the regional ischemic insult in the rat heart ex vivo, the infarct size was increased relative to the peptide-free control. Moreover, as the two human variants, PL-Cts and GS-Cts varied markedly with respect post-ischemic infarct size, and only PL-Cts caused reduced phosphorylation of the pro-survival Akt kinase and the downstream signaling mediator FoxO1 and BAD, these findings are consistent with a mechanistic link between infarct size and reduced phosphorylation of Akt also via the NO-independent, FoxO1 and BAD cascades. This aspect certainly calls for future studies.
A possible limitation of this study could be our use of regional rather than global ischemia prior to the post-ischemic reperfusion in presence of the Cts isoforms. In view of the reported vasodilatory actions of Cts in the normoxic rat heart [15] we had expected to see a vasodilatory action following 120 min reperfusion in presence of Cts, manifested by increased coronary flow and potentially decreased infarct size. However, as both WT-Cts and PL-Cts were deleterious, this is unlikely to be due to vasodilator properties of these two isoforms, in contrast to GS-Cts containing a substitution in the biologically active cateslytin domain of this peptide [43, 56]. In the vast majority of clinical events with MI the ischemic injury is regional and not global. Therefore, our findings suggest that ischemic reperfusion injury following regional ischemia in the ex vivo rat model is not alleviated by the presence of free Cts isoforms.
Elevated plasma CGA levels in the acute and subacute phase after MI and later stages of CHF signify a poor prognosis in humans [8-10]. Assuming an increased degradation also of the circulating CGA during phases of regional ischemic in humans, as presently demonstrated in the ex vivo rat model, the GS-Cts variant may be of considerable advantage during the recovery phase after an ischemic insult.
ACKNOWLEDGEMENTS
This work was supported by a grant awarded to AKJ and BKB by the University of Bergen. AKJ is also supported by the University of Bergen Heart Foundation and the L. Meltzer Foundation and The Norwegian Heart Foundation. KBH is supported by a grant from the Tordis and Fritz Rieber Legacy in Bergen. Support from grants from the Department of Veterans Affairs and the National Institutes of Health (R01 DA011311 and P01 HL58120 to Sushil K. Mahata for salary for B.K.B) is also gratefully acknowledged.
Abbreviations
- Akt
a phosphatidylinositol 3′-kinase dependent Ser/Thr kinase
- AU
arbitrary units
- BAD-Bcl-2
protein
- Cts
human CgA352-372
- CGA
chromogranin A
- CGB
chromogranin B
- CF
coronary flow
- CHF
chronic heart failure
- CHGA
chromogranin A gene locus
- EBD
Evans blue dye
- FoxO1
forkhead transcription factor
- GS-Cts
Gly364Ser-catestatin
- HR
heart rate
- IA
infarct myocardial area
- I/R
ischemia/reperfusion
- KHB
Krebs-Henseleit buffer
- LVDP
left ventricular developed pressure
- MI
myocardial infaction
- PKB
protein kinaseB
- PL-Cts
Pro370Leu-catestatin
- P-Akt-Ser473
phosphorylated Akt-Ser473
- P-Akt-Thr308
Akt-Threonine308
- P-FoxO1-Ser256
Phospho-FoxO1-Ser256
- RI
regional ischemia
- NI
non-infarct myocardial zone
- RZ
risk zone
- T-Akt
total-Akt
- TTC
triphenyltetrazolium chloride
- WT-Cts
hCGA352-372
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: none declared.
REFERENCES
- 1.Rodríguez-Sinovas A, Abdallah Y, Piper HM, Garcia-Dorado D. Reperfusion injury as a therapeutic challenge in patients with acute MI. Heart Fail Rev. 2007;12:207–216. doi: 10.1007/s10741-007-9039-9. [DOI] [PubMed] [Google Scholar]
- 2.Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. J Cell Mol Med. 2008;12:435–458. doi: 10.1111/j.1582-4934.2007.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Penna C, Perrelli MG, Raimondo S, Tullio F, Merlino A, Moro F, Geuna S, Mancardi D, Pagliaro P. Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim Biophys Acta. 2009;1787:794–801. doi: 10.1016/j.bbabio.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Jonassen AK, Sack MN, Mjøs OD, Yellon DM. Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res. 2001;89:1191–1198. doi: 10.1161/hh2401.101385. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 7.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R. Postconditioning and protection from reperfusion injury: where do we stand?: Position Paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010 Jun 12; doi: 10.1093/cvr/cvq129. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Omland T, Dickstein K, Syversen U. Association between plasma chromogranin A concentration and long-term mortality after MI. Am J Med. 2003;114:25–30. doi: 10.1016/s0002-9343(02)01425-0. [DOI] [PubMed] [Google Scholar]
- 9.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 10.Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J. 2009;30:25–32. doi: 10.1093/eurheartj/ehn513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulze PC. Chromogranin A: friend or foe of the failing myocardium? Eur Heart J. 2007;28:1052–1053. doi: 10.1093/eurheartj/ehm047. [DOI] [PubMed] [Google Scholar]
- 12.Corti A, Ferrari R, Ceconi C. Chromogranin A and tumor necrosis factor-alpha [TNF] in chronic heart failure. Adv Exp Med Biol. 2000;482:351–359. doi: 10.1007/0-306-46837-9_28. [DOI] [PubMed] [Google Scholar]
- 13.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O'Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT. The catecholamine release-inhibitory “catestatin” fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 15.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC. The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takiyyuddin MA, Brown MR, Dinh TQ, Cervenka JH, Braun SD, Parmer RJ, Kennedy B, O'Connor DT. Sympatho-adrenal secretion in humans: factors governing catecholamine and storage vesicle peptide co-release. Autonom Pharmacol. 1994;14:187–200. doi: 10.1111/j.1474-8673.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 17.Banks P, Helle K. The release of protein from the stimulated adrenal medulla. Biochem J. 1965;97:40C–41C. doi: 10.1042/bj0970040c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD. Secretion of a chromaffin granule protein, chromogranin, from the adrenal gland after splanchnic stimulation. Nature. 1967;215:58–59. doi: 10.1038/215058a0. [DOI] [PubMed] [Google Scholar]
- 19.Huttner WB, Gerdes HH, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- 20.Winkler H, Fischer-Colbrie R. The chromogranins A and B: the first 25 years and future perspectives. Neuroscience. 1992;49:497–528. doi: 10.1016/0306-4522(92)90222-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner HJ, Weiler R, Ludescher C, Schmid KW, Winkler H. Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J Histochem Cytochem. 1990;38:845–850. doi: 10.1177/38.6.2139887. [DOI] [PubMed] [Google Scholar]
- 22.Glattard E, Angelone T, Strub JM, Corti A, Aunis D, Tota B, Metz-Boutigue MH, Goumon Y. Characterization of natural vasostatin-containing peptides in rat heart. FEBS J. 2006;273:3311–3321. doi: 10.1111/j.1742-4658.2006.05334.x. [DOI] [PubMed] [Google Scholar]
- 23.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 24.Tatemoto K, Efendić S, Mutt V, Makk G, Feistner GJ, Barchas JD. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986;324:476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- 25.Aardal S, Helle KB, Elsayed S, Reed RK, Serck-Hanssen G. Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol. 1993;5:405–412. doi: 10.1111/j.1365-2826.1993.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 26.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazza R, Gattuso A, Mannarino C, Brar BK, Barbieri SF, Tota B, Mahata SK. Catestatin (chromogranin A344-364) is a novel cardiosuppressive agent: Inhibition of isoproterenol and endothelin signaling in the frog heart. Am J Physiol Heart Circ Physiol. 2008;295:H113–122. doi: 10.1152/ajpheart.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell RM, Yellon DM. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol. 2003;35:185–193. doi: 10.1016/s0022-2828(02)00310-3. [DOI] [PubMed] [Google Scholar]
- 29.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest. Surg. 2007;20:195–203. doi: 10.1080/08941930701366471. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Tindall DJ. FOXO factors: a matter of life and death. Future Oncol. 2006;2:83–89. doi: 10.2217/14796694.2.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Juhasz B, Thirunavukkarasu M, Pant R, Zhan L, Penumathsa SV, Secor ER, Jr, Srivastava S, Raychaudhuri U, Menon VP, Otani H, Thrall RS, Maulik N. Bromelain induces cardioprotection against ischemia-reperfusion injury through Akt/FOXO pathway in rat myocardium. Am J Physiol Heart Circ Physiol. 2008;294:H1365–1370. doi: 10.1152/ajpheart.01005.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 33.Li XL, Man K, Ng KT, Sun CK, Lo CM, Fan ST. The influence of phosphatidylinositol 3-kinase/Akt pathway on the ischemic injury during rat liver graft preservation. Am J Transpl. 2005;5:1264–1275. doi: 10.1111/j.1600-6143.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 34.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 35.Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 36.Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, Torpey JW, O'Connor DT, Mahata SK. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology. 2008;149:749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scamuffa N, Calvo F, Chrétien M, Seidah NG, Khatib AM. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 38.Biswas N, Rodriguez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O'Connor DT, Mahata SK. Cathepsin L colocalizes with chromogranin a in chromaffin vesicles to generate active peptides. Endocrinology. 2009;150:3547–3557. doi: 10.1210/en.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol. 2007;102:529–541. doi: 10.1007/s00395-007-0672-1. [DOI] [PubMed] [Google Scholar]
- 40.Stensløkken KO, Rutkovskiy A, Kaljusto ML, Hafstad AD, Larsen TS, Vaage J. Inadvertent phosphorylation of survival kinases in isolated perfused hearts: a word of caution. Basic Res Cardiol. 2009;104:412–423. doi: 10.1007/s00395-009-0780-1. [DOI] [PubMed] [Google Scholar]
- 41.Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 1982;50:101–16. doi: 10.1161/01.res.50.1.101. [DOI] [PubMed] [Google Scholar]
- 42.Brar BK, Jonassen AK, Egorina EM, Chen A, Negro A, Perrin MH, Mjøs OD, Latchman DS, Lee KF, Vale W. Urocortin-II and urocortin-III are cardioprotective against ischemia reperfusion injury: an essential endogenous cardioprotective role for corticotropin releasing factor receptor type 2 in the murine heart. Endocrinology. 2004;145:24–35. doi: 10.1210/en.2003-0689. [DOI] [PubMed] [Google Scholar]
- 43.Mahata SK, Mahata M, Wakade AR, O'Connor DT. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A(344-364)): identification of amino acid residues crucial for activity. Mol Endocrinol. 2000;14:1525–1535. doi: 10.1210/mend.14.10.0531. [DOI] [PubMed] [Google Scholar]
- 44.Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release: The novel catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999;274:2920–2928. doi: 10.1074/jbc.274.5.2920. [DOI] [PubMed] [Google Scholar]
- 45.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O'Connor DT. Catecholamine release-inhibitory peptide catestatin (chromogranin A352-372): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 46.Vaingankar SM, Li Y, Corti A, Biswas N, Gayen J, O'Connor DT, Mahata SK. Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic “rescue” of disease phenotypes in the mouse Chga knockout. Physiol Genomics. 2010;41:91–101. doi: 10.1152/physiolgenomics.00086.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negro A, Brar BK, Gu Y, Peterson KL, Vale W, Lee KF. erbB2 is required for G protein-coupled receptor signaling in the heart. Proc Natl Acad Sci USA. 2006;103:15889–15893. doi: 10.1073/pnas.0607499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heidrich FM, Zhang K, Estrada M, Huang Y, Giordano FJ, Ehrlich BE. Chromogranin B regulates calcium signaling, nuclear factor kappaB activity and brain natriuretic peptide production in cardiomyocytes. Circ Res. 2008;102:1230–1238. doi: 10.1161/CIRCRESAHA.107.166033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrabés JA, Garcia-Dorado D, Ruiz-Meana M, Piper HM, Solares J, González MA, Oliveras J, Herrejón MP, Soler Soler J. Myocardial segment shrinkage during coronary reperfusion in situ. Relation to hypercontracture and myocardial necrosis. Pflugers Arch. 1996;431:519–526. doi: 10.1007/BF02191898. [DOI] [PubMed] [Google Scholar]
- 50.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- 52.Fang NX, Yao YT, Shi CX, Li LH. Attenuation of ischemia-reperfusion injury by sevoflurane postconditioning involves protein kinase B and glycogen synthase kinase 3 beta activation in isolated rat hearts. Mol Biol Rep. 2010 Mar 10; doi: 10.1007/s11033-010-0030-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Mahapatra NR. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc Res. 2008;80:330–338. doi: 10.1093/cvr/cvn155. [DOI] [PubMed] [Google Scholar]
- 54.Cappello S, Angelone T, Tota B, Pagliaro P, Penna C, Rastaldo R, Corti A, Losano G, Cerra MC. Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signaling mechanism. Am J Physiol Heart Circ Physiol. 2007;293:H719–727. doi: 10.1152/ajpheart.01352.2006. [DOI] [PubMed] [Google Scholar]
- 55.Helle KB. The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res. 2010;85:9–16. doi: 10.1093/cvr/cvp266. [DOI] [PubMed] [Google Scholar]
- 56.Briolat J, Wu SD, Mahata SK, Gonthier B, Bagnard D, Chasserot-Golaz S, Helle KB, Aunis D, Metz-Boutigue MH. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62:377–385. doi: 10.1007/s00018-004-4461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jean-François F, Khemtémourian L, Odaert B, Castano S, Grélard A, Manigand C, Bathany K, Metz-Boutigue MH, Dufourc EJ. Variability in secondary structure of the antimicrobial peptide Cateslytin in powder, solution, DPC micelles and at the air-water interface. Eur Biophys J. 2007;36:1019–1027. doi: 10.1007/s00249-007-0169-8. [DOI] [PubMed] [Google Scholar]