Abstract
Neurodegenerative insults and glial activation during glaucomatous neurodegeneration initiate an immune response to restore tissue homeostasis and facilitate tissue cleaning and healing. However, increasing risk factors over a chronic and cumulative period may lead to a failure in the regulation of innate and adaptive immune response pathways and represent a route for conversion of the beneficial immunity into a neuroinflammatory degenerative process contributing to disease progression. Oxidative stress developing through the pathogenic cellular processes of glaucoma, along with the aging-related component of oxidative stress, likely plays a critical role in shifting the physiological equilibrium. This review aims to provide a perspective on the complex interplay of cellular events during glaucomatous neurodegeneration by proposing a unifying scheme that integrates oxidative stress-related risk factors with the altered regulation of immune response in glaucoma.
Keywords: glaucoma, retinal ganglion cell, glia, oxidative stress, immune system, aging
It is today’s common view that glaucoma is a multifactorial disease and many of the proposed mechanisms traditionally linked to elevated intraocular pressure (IOP)-related factors may facilitate disease progression independently from IOP elevation. Elevated IOP-related factors are well recognized to trigger initial neuronal damage through biomechanical and ischemic injury processes. However, a complex interplay of cellular events triggered by IOP-related or -unrelated stimuli may also amplify the primary injury process and contribute to disease progression. IOP-dependent versus IOP-independent components of the glaucomatous injury are commonly thought to be determined by individual susceptibility factors related with various genetic and epigenetic parameters yet to be further identified (Weinreb, Khaw 2004; Quigley 2005). Keeping the big picture of glaucoma in view, in addition to endogenous signals triggered in retinal ganglion cells (RGCs), environmental influences, particularly including neuron-glia interactions, are equally important for neuronal cell death or survival decisions. Complex cellular interactions determining the RGC fate in response to glaucomatous stress also exhibit important links to different components of the immune system (Tezel 2009).
The pioneering work of Rosario Hernandez on optic nerve head astrocytes has provided important impacts in the field of glaucoma research (Hernandez, Ye 1993; Hernandez, Pena 1997; Hernandez et al. 2008) and inspired many other studies illuminating different aspects of neuron-glia interactions in glaucomatous neurodegeneration and immune response.
Glial Activity Response During Glaucomatous Neurodegeneration
Besides intrinsic signals triggered in different subcellular compartments of RGCs, signals arisen from the microenvironment are also critically important for neuronal cell fate decisions during glaucomatous neurodegeneration. Macroglial cells, including retina and optic nerve astrocytes and retinal Müller cells, constitute the major cell type exhibiting important homeostatic interactions with RGCs. Another glial cell type, also having important impacts in glaucomatous neurodegeneration, is microglia, specialized tissue macrophages. Progressive degeneration of optic nerve axons and RGCs in human glaucoma is accompanied by chronic alterations in structural and functional characteristics of glial cells in the optic nerve head (Hernandez, Pena 1997; Hernandez et al. 2008) and retina (Wang et al. 2002; Tezel et al. 2003). Elevation of IOP in experimental animal models similarly results in a prominent activation response of both macroglial and microglial cells (Wang et al. 2000; Naskar et al. 2002; Lam et al. 2003; Woldemussie et al. 2004; Ju et al. 2006; Inman, Horner 2007).
Rosario Hernandez was the front-runner of experimental studies focused on the glial response to glaucomatous conditions. Over the past two decades, experimental studies using in vitro and in vivo models, along with the studies of human donor eyes, have substantially contributed to current understanding of the diverse roles of glial cells in glaucoma. Findings of these studies show that the high level of plasticity of glial cells allows them to rapidly respond to any homeostatic imbalance by exhibiting a phenotype commonly referred to as activated on the basis of changes in cell morphology and expression of cell markers (Hernandez, Pena 1997; Tezel et al. 2003; Hernandez et al. 2008). Another widely accepted outcome of these studies is that after transformation into an activated phenotype, glial cells may exhibit insufficiency or dysfunction in their normal neurosupportive abilities, thereby leading to increased vulnerability of RGCs and their axons to injury (Morgan 2000; Neufeld, Liu 2003; Hernandez et al. 2008; Johnson, Morrison 2009; Tezel 2009).
Altered glial functions in glaucoma are supported by dramatic alterations in gene expression involved in signal transduction, cell proliferation, cell-cell interaction, cell adhesion, extracellular matrix synthesis, and immune response (Johnson et al. 2000; Hernandez et al. 2002; Miyahara et al. 2003; Ahmed et al. 2004; Steele et al. 2006; Kompass et al. 2008). Despite progress in understanding of glial responses, little is known about their distinct states or temporal sequences during the course of glaucomatous neurodegeneration (Nickells 2007). For example, a prominent increase in astrocyte reactivity has been detected early in the neurodegeneration process in different animal models of glaucoma. In contrast, the activation of microglia and the loss of oligodendrocytes have occurred late after large-scale neuronal loss (Son et al. 2010). In a more recent study of experimental rat glaucoma, astrocytic dedifferentiation and proliferation, rather than reactive hypertrophy, have characterized early response in the glaucomatous optic nerve head (Johnson et al. 2010). These early responses of astrocytes may be important for their compromised ability to provide axonal support in early disease stages. In addition to temporal and spatial alterations in glial responses, evidence also supports distinct profiles of gene expression in optic nerve head astrocyte cultures derived from human donor eyes (Miao et al. 2008). The population-based differences in glial responses may be related to differential susceptibility for the development and/or progression of glaucomatous neurodegeneration.
With an emphasis on the relative protection of glial cells from glaucomatous injury, chronic activity response of stressed glial cells may increase their own survival (Tezel, Yang 2005) but result in a lessening of their neurosupport functions. For example, the ability of glial cells in buffering extracellular glutamate may diminish in glaucoma (Martin et al. 2002; Moreno et al. 2005). Besides such a passive neurodestructive influence, glial cells may also be actively damaging through a range of cellular processes that promote neuronal injury.
Owing to the increased production of extracellular matrix and adhesion molecules, glial cells play important roles in remodeling of the optic nerve head in glaucoma (Hernandez 2000). Alterations in the biomechanical environment of the optic nerve head through tissue remodeling events may generate additional stress on axons (Downs et al. 2005) and inhibit axonal regeneration (Silver, Miller 2004). A complex range of glial responses in human glaucoma also includes an increased production of cell death mediators, such as TNF-α (Yan et al. 2000; Tezel et al. 2001) and nitric oxide (Liu, Neufeld 2000). Experimental evidence supports that these mediators produced by glia can be directly injurious to RGCs (Neufeld 1999a; Yuan, Neufeld 2000; Nakazawa et al. 2006; Tezel 2008).
Among diverse roles of glial cells during glaucomatous neurodegeneration as neurosupportive or neurodestructive, one is linked to their immunoregulatory functions. Given their roles in phagocytosis, glial cells, mainly including microglia, are important components of immune surveillance involved in clearing and shielding of the injured tissue (Nimmerjahn et al. 2005). Although they act as standby cells in service of both the immune system and the neuronal tissue (Schwartz 2003a; Ransohoff, Perry 2009), microglial cells have also been associated with chronic neurodegenerative diseases (Streit 1996; Stoll, Jander 1999). It remains unclear whether there is an insufficiency in the glial removal of cell debris that may create an antigenic stimulus for abnormal immune response. However, recent studies increasingly implicate glial cells as participants of neuronal injury. It is clear that the glial pro-inflammatory cytokines produced at increased amounts are capable of promoting RGC death signals (Tezel, Wax 2000b), besides signaling to immune system cells and regulating their function. With respect to their increased production of cytokines (Tezel et al. 2001), immunomediators (Luo et al. 2010b) and complement components (Kuehn et al. 2006; Tezel et al. 2010), reactive glial cells appear to be well equipped candidates to participate in innate neuronal injury in glaucoma.
In addition to innate immune activities, glial cells are also thought to play important roles in initiation of an adaptive immune response in glaucoma (Tezel et al. 2007b). Similar to CNS glia, retina and optic nerve head glial cells express MHC molecules and function as resident antigen-presenting cells. Not only microglial cells (Matsubara et al. 1999), but also astrocytes express HLA-DR in human glaucoma (Yang et al. 2001b). Recent proteomic and immunohistochemical findings also support an up-regulation of glial toll-like receptors (TLRs) in the glaucomatous human retina (Luo et al. 2010b). Besides serving in the host defense against microbial components, specific signaling through TLRs exhibits a sensitive detection system involved in the immune response to intrinsic danger signals. In vitro studies of rat retinal microglia and astrocytes have shown that components of the glaucomatous tissue stress, including up-regulated heat shock proteins (HSPs) and oxidative stress (Tezel et al. 2000; Tezel et al. 2007a), can similarly stimulate innate and adaptive immune responses through the glial TLR signaling as evident by increased cytokine production and MHC class II expression of glial cells, and increased proliferation and cytokine production of co-cultured T cells (Luo et al. 2010b). These findings support that as being local players of the immune system in the retina and optic nerve, glial cells are able to rapidly sense the tissue stress and initiate an immune response.
Another immunoregulatory function of glial cells is linked to maintaining the perivascular barrier integrity (Ransom et al. 2003; Nimmerjahn et al. 2005). This glial function is important for securing the immune privileged status of retina and optic nerve head to protect neurons against any vision-threatening systemic effects. Cytokines produced by perivascular microglia or astrocytes (Zlokovic 2008; Banks, Erickson 2010; Nishioku et al. 2010) and various other glia-related alterations in the milieu may potentially contribute to a perivascular barrier dysfunction in glaucoma. For example, increased expression of matrix metalloproteinases by optic nerve head astrocytes in human glaucoma (Yan et al. 2000; Agapova et al. 2001) may lead to degradation of the basement membrane and glia limitans consisting from astrocytic and microglial end feet. Splinter hemorrhages at the border of the optic nerve head that are commonly detected in glaucoma patients may best exemplify the weakened perivascular barrier integrity in glaucoma (Grieshaber, Flammer 2007). In addition to perivascular barriers, peripapillary chorioretinal atrophy zones, also commonly detected in glaucomatous eyes, exhibit another site in which the blood-retina barrier is broken. Activation and redistribution of microglia in the peripapillary chorioretinal regions in glaucomatous eyes further support the gatekeeper functions of these cells in controlling the blood-brain barrier (Neufeld 1999b).
Taken together, glial cells profoundly respond to glaucomatous stress by exhibiting a chronic activation response. As discussed below, glial reactivation is commonly accepted as the hallmark of neuroinflammation in the CNS. Under normal physiological conditions, glial immunoregulatory functions do not cause an immunological imbalance and their activity may promote immune privilege rather than neurodegenerative immunity. However, in the presence of accumulating risk factors, chronic glial activation may result in a dysfunction of regulatory mechanisms, thereby leading to innate and adaptive cytotoxicity (Ransohoff, Perry 2009). It is hoped that therapeutic manipulation may help modify the outcome of glial response for the gain of RGCs and optic nerve axons in glaucoma (Tezel 2009). An improved understanding of glial responses and functional alterations at the molecular level may offer innovative treatment options targeting glial cells so that neurodestructive consequences of the glial activation response may be selectively inhibited without compromising glial neurosupportive and homeostastic functions.
Immune Activity and Diverse Consequences
Evidence obtained from clinical and experimental studies over the past decade strongly supports diverse roles of the immune system in glaucoma as beneficial or destructive. An immune response is initially beneficial and necessary to restore tissue homeostasis and promote tissue cleaning, healing, and functionality without causing an autoimmune disease. As opposed to the concept that autoimmunity is a deleterious phenomenon, the intriguing view of protective autoimmunity accepts that boosting the immune activity by immunization (with the appropriate antigen, at specific timing and predetermined optimal dosing) can provide neuroprotection (Schwartz, Kipnis 2002; Schwartz 2003b). Despite seemingly conflicting aspects of the immune system involvement in glaucoma, it is now commonly recognized that if there is a defect in immune response pathways due to accumulating risk factors, the physiological equilibrium may be impaired, thereby switching the beneficial immunity into an autoimmune neurodestructive process (Schwartz, Ziv 2008; Tezel 2009). In view of the current knowledge summarized herein, there is an increased risk of failure in immune regulation under glaucomatous stress conditions.
Increasing number of evidence provided by clinical studies support an abnormal activity of the immune system in glaucoma patients (Tezel, Wax 2007). Many patients with glaucoma exhibit increased prevalence of monoclonal gammopathy (Wax et al. 1994; Hammam et al. 2008) and abnormal T cell subsets (Yang et al. 2001a) similar to detected in autoimmune diseases. In addition, a recent study has detected significant alterations in Th1 and Th2 cytokine levels in the glaucomatous patient serum (Huang et al. 2010). Ongoing studies have consistently detected increased titers of serum antibodies to various retina and optic nerve antigens which commonly include HSPs (Tezel et al. 1998; Wax et al. 1998b; Maruyama et al. 2000; Joachim et al. 2007; Tezel, Wax 2007; Grus et al. 2008). Following applications of the mass spectrometry-based techniques have resulted in an increasing list of antigens that are recognized by serum autoantibodies in glaucoma patients (Joachim et al. 2008; Reichelt et al. 2008; Thornton et al. 2010). It is important to clarify that clinical studies of immune activity have initially focused on patients with normal-pressure glaucoma due to the lack of IOP-related pathogenic factors. However, not only is the distinction of such glaucoma subgroups arbitrary, but many glaucoma patients with elevated IOP may also exhibit similar findings of aberrant immune activity. In addition to primary open-angle glaucoma, a more recent study using protein macroarrays has characterized increased serum antibodies in patients with pseudoexfoliation glaucoma (Dervan et al. 2010).
In consistence with clinical observations, growing evidence obtained from histopathological and proteomic studies of human donor tissues supports the activation of glial immunoregulation and antigen presentation functions as discussed above in the previous section. Expression of MHC class II molecules (Yang et al. 2001b), TLRs (Luo et al. 2010b), and different complement components (Kuehn et al. 2006; Stasi et al. 2006; Tezel et al. 2010) is up-regulated in the retina and optic nerve head of glaucomatous human eyes. There is also evidence of immunoglobulin deposition in the glaucomatous human retina (Wax et al. 1998a). As discussed above, peripapillary chorioretinal atrophy areas (Wax et al. 1998a) and splinter hemorrhages (Grieshaber, Flammer 2007), both commonly detected in glaucomatous eyes, may provide a window of opportunity to contact with the systemic immune system and facilitate the access of serum autoantibodies into the retina and optic nerve head in these patients.
Findings of experimental glaucoma are also consistent with the findings of human glaucoma. Experimental studies using microarray analysis techniques have revealed a prominent up-regulation of gene expression mediating immune response in different animal models of elevated IOP-induced glaucoma (Steele et al. 2006; Johnson et al. 2007; Hernandez et al. 2008; Kompass et al. 2008; Panagis et al. 2010). In addition to increased glial production of immunomediators and complement molecules, these animals with experimental glaucoma also exhibit increased proliferative activity of T cells (Tezel et al. 2008) and increased titers of serum antibodies to retina and optic nerve proteins (Joachim et al. 2009). Similar findings in different experimental paradigms suggest that the immune activity reflects a native response to tissue stress and injury.
Neurons and glia normally produce immunosuppressive signals to prevent immune-mediated injury to CNS (Neumann 2001). Similarly, retina and optic nerve head have an actively regulated immune privilege mechanism (Caspi 2006; Forrester 2009) to protect these vital tissues against damaging effects of an inflammatory immune response. Among many factors controlling the immune privilege, elimination of infiltrating reactive T cells by apoptosis is an important common pathway in preventing the development of neurodegenerative autoimmune diseases, because these cells can recruit and activate other inflammatory cells and initiate an autoimmune injury process (Bauer et al. 1998; Pender, Rist 2001). Despite the immune privileged status and the immunological self-tolerance to CNS, the immune system is not fully ignorant of the nervous tissue. Parallel to profound alterations in the systemic immune repertoire, there is a shift in the local milieu of the brain from immune-hostile to immune-friendly during neurodegenerative processes (Wekerle et al. 1987; Odoardi et al. 2007). Many factors present in glaucomatous tissues may similarly create a proper environment to form an immunological synapse with T cells for antigen presentation. Glial antigen-presenting ability is increased in human glaucoma as supported by up-regulation of MHC class II molecules (Yang et al. 2001b) and increased expression of immunomediators (Luo et al. 2010b) and cytokines (Tezel et al. 2001). Meanwhile, reactive oxygen species evident in human glaucoma (Tezel et al. 2007a; Tezel et al. 2010) can function as co-stimulatory molecules during antigen presentation (Tezel et al. 2007b). Based on observations in the brain, loss of RGCs and their physiological activity during glaucomatous neurodegeneration may be particularly important in rendering these neurons recognizable by the immune system cells (Neumann 2001). Many other factors summarized below may also contribute to an aberrant immune activity in glaucoma.
Not only do numerous proteins exhibit increased expression in glaucoma, but also antigen exposure is increased due to neuronal injury. Obviously, dead cells represent an important source of autoantigens that can initiate an autoimmune response. The cell death process during the course of glaucomatous neurodegeneration may similarly cause tissue specific systemic autoimmunity, since apoptotic RGCs with self-antigens are phagocytosed by microglia, where they may be processed and presented to the systemic immune system. It seems critically important that besides increased protein expression and exposure, various proteins exhibit post-translational modifications in glaucoma, such as oxidation (Tezel et al. 2005), phosphorylation (Yang et al. 2008), or citrullination (Bhattacharya 2009). By changing antigenic features of proteins, these protein modifications may also facilitate an autoimmune response. Recent observations using immunoproteomic analysis techniques support the possibility of altered antigenity in glaucomatous tissues. For example, glaucomatous patient sera react more with the glaucomatous human retinal proteins as opposed to retinal proteins obtained from non-glaucomatous control donors (Thornton et al. 2010).
Among numerous proteins exhibiting up-regulated expression in glaucoma, HSPs (Tezel et al. 2000; Luo et al. 2010b) are known to be highly antigenic and have been associated with various autoimmune diseases, including neurodegenerative diseases (Young 1992; van Noort et al. 1995). Besides intrinsic neuroprotective abilities of intracellular HSPs, membrane-bound or extracellular HSPs after active secretion or passive release from dying neurons may serve as immunostimulatory signals. HSPs may induce cytokine production, deliver antigenic peptides to MHC class I and class II, and activate TLR signaling, thereby playing important roles in the activation of innate and adaptive immunity implicated in autoimmune diseases (van Eden et al. 2003; Calderwood et al. 2007). Recent experimental findings support that glial TLRs can initiate immunostimulatory signaling upon binding by glaucomatous stress-related intrinsic ligands, including HSPs (Luo et al. 2010b). In addition to TLRs, other groups of pattern recognition receptors may also be involved in the immunostimulatory signaling in glaucoma. For example, mannose-binding lectins, C-type lectins (Tezel et al. 2010), and perhaps also C-reactive proteins (Leibovitch et al. 2005) although controversial (Su et al. 2007), may promote innate immune activity with or without activating the complement system.
Taken together, various cellular events that are triggered in response to glaucomatous stress stimuli may serve as endogenous danger/alarm signals to the immune system, thereby stimulating an immune response. However, it is currently unclear whether the immune activity evident in glaucoma patients is merely an epiphenomenon of the disease reflecting an intrinsic effort to facilitate tissue cleaning and healing, or whether it has any pathogenic impact on neuronal injury. For example, autoantibodies and complement components may serve to opsonize the toxic cell debris, thereby facilitating their phagocytic removal as a necessary step towards promoting axon regeneration (Vargas, Barres 2007). Nevertheless, growing evidence obtained using in vitro and in vivo models strongly suggests that any immune activity, if uncontrolled, may have destructive consequences contributing to the progression of neurodegenerative injury. In addition to innate cytotoxicity, cellular and humoral components of the adaptive immunity may also adversely affect neuronal survival. For example, autoreactive T cells may induce RGC apoptosis in culture mainly through Fas/FasL signaling (Wax et al. 2008). Regarding humoral immunity, HSP antibodies, when exogenously applied at concentrations similar to detected in the glaucomatous patient serum, may be internalized by retinal neurons, co-localize with the native protein, and facilitate neuronal cell death in the isolated human retina (Tezel, Wax 2000a). Moreover, complement-mediated synapse elimination may become aberrantly reactivated in mouse glaucoma (Stevens et al. 2007). Formation of the terminal membrane attack complex in human glaucoma (Kuehn et al. 2006; Tezel et al. 2010) may also support the possibility of collateral cell lysis by uncontrolled complement activation.
As an effort to better determine the pathogenic importance of immune system activity in glaucoma, ongoing research aims to produce animal models. Initial findings of these studies support that the immunization of rats with HSPs can induce RGC loss in a topographically-specific pattern resembling human glaucoma (Wax et al. 2008). Immunogenic neuronal injury may also be induced by adoptive transfer using a rat model of experimental glaucoma (Tezel et al. 2008). An interesting common observation of these studies using HSP immunization (Wax et al. 2008) or adoptive transfer (Tezel et al. 2008) is an early and transient T cell infiltration into the retina and optic nerve head.
Seemingly contrary to observations in animal models, virtually no T cell invasion is evident in the glaucomatous human retina or optic nerve. Although previous studies have not specifically focused on T cell infiltration using the necessary labeling techniques, it should be emphasized that the lack of observations supporting a parenchymal T cell invasion in glaucomatous tissues is not a good argument against a pathogenic role of immune activity. This is because T cell invasion is a temporary event and depending on the nature of T cells, functional status of antigen-presenting cells, and the existing set of cytokines, chemokines and co-stimulatory molecules, even a small number of T cells can be sufficient to induce a massive cascade of events leading to neurodegenerative injury (Wekerle et al. 1987; Odoardi et al. 2007). An immune-mediated component of the neurodegenerative injury in glaucoma is supported by a recent study that using immune deficient Rag1 knockout mice which lack mature B- and T-lymphocytes has provided robust neuroprotection when compared to wild-type controls in a mouse model of experimental glaucoma (McKinnon et al. 2010).
It is also important to highlight that despite the lack of clear evidence supporting classical hallmarks of inflammation in human glaucoma, continuous glial activation in the glaucomatous retina and optic nerve head may be sufficient to indicate an ongoing neuroinflammatory process. Such an adaptive response to tissue stress has been referred to as “para-inflammation” and implicated to contribute to the initiation and progression of aging-related neurodegenerative diseases (Medzhitov 2008; Xu et al. 2009). This type of inflammatory response that is intermediate between the basal homeostatic state and a classic inflammatory response is likely to be more common but of lower magnitude. While the physiological purpose of para-inflammation is to restore tissue homeostasis and functionality, it may become chronic or turn into overt destructive inflammation if tissue stress or cellular dysfunction persists for a sustained period (Medzhitov 2008; Xu et al. 2009).
Thus, despite limited evidence to support a definite pathogenic role for autoimmunity in glaucoma, current knowledge agrees with the neurodegenerative potential of uncontrolled immune activity. Present evidence collectively supports that innate immune cells, autoreactive T cells, autoantibodies, and excess complement attack all are potent stimuli to harm RGC somas, axons, and synapses (Tezel, Wax 2004;2007; Wax, Tezel 2009). The risk of developing autoimmune injury seemingly depends on co-existing risk factors, which include aging, chronic tissue stress and injury, efficiency of tissue cleaning, activity status of resident and systemic immune cells, and the ability to inhibit or activate T cell entry and activation. With an emphasis on the present evidence of such risk factors in glaucoma, a neurodegenerative process may possibly be stimulated through bystander autoreactive T cell activation as opposed to induction of regulatory T cells and protective immunity. As discussed in the next section, oxidative stress appears to be particularly important for fine tuning of the homoeostatic balance in glaucoma. Once the regulatory mechanisms of immune activity in glaucoma are better understood, immunomodulatory treatment strategies can be designed to manipulate the immune response towards enhanced tissue repair and function, while avoiding an autoimmune neurodegenerative injury.
Glaucoma-Related and Aging-Related Oxidative Stress
A number of cellular events triggered during glaucomatous neurodegeneration may result in conditions of oxidative stress when amplified production of reactive oxygen species exceeds antioxidant defenses (Ko et al. 2005). Oxidative stress, due to mitochondrial dysfunction (Tezel, Yang 2004; Osborne 2010) and perhaps also other mechanisms, has long been linked to pathogenic mechanisms by which elevated IOP-related or -unrelated factors result in the neurodegenerative injury to RGCs and their axons in glaucoma (Levin 1999; Osborne 2008; Kong et al. 2009). Oxidative stress is intimately linked with an integrated series of cellular phenomena, which all seem to contribute to further progression of glaucomatous neurodegeneration. For example, oxidative stress leads to oxidative damage to cellular macromolecules, such as DNA, proteins and lipids, and impairs the cellular redox balance (Tezel et al. 2005). Oxidative stress may also trigger intracellular signaling pathways by acting as a second messenger and modulating protein function by redox modifications of downstream effectors (Tezel 2006). Ongoing studies focused on the immunogenic aspects of glaucoma support that oxidative stress is also linked to immunostimulatory signaling as discussed in the next section (Tezel 2009).
Although neurons are e prone to build up oxidative stress as implicated in many neurodegenerative diseases, there is also an aging-related component of the oxidative stress. While oxidative stress increases in aging brain, the intrinsic ability of cells to respond to oxidative damage declines with increasing age. Consequently, increased accumulation of reactive oxygen and nitrogen species produced from endogenous metabolic pathways is the main cause for the reduced ability of neurons to cope with stressful conditions in the elderly. Similarly, aging is a well recognized risk factor for the initiation and progression of glaucomatous neurodegeneration (Gordon et al. 2002; Broman et al. 2008). In addition to an aging-related decline in optic nerve axon counts and function (Mikelberg et al. 1989; Repka, Quigley 1989; Jonas et al. 1992), experimental studies using animal models of glaucoma also demonstrate increased susceptibility to neuronal injury with aging (Wang et al. 2007). Thus, oxidative stress developing through the pathogenic cellular processes of glaucoma appears to be amplified by the aging-related oxidative stress. Enhanced accumulation of advanced glycation end-products (AGEs) in the glaucomatous retina and optic nerve head supports an accelerated aging process in glaucomatous eyes (Tezel et al. 2007a).
Parallel to aging-related loss of neurons, glial cells exhibit remarkable alterations with increasing age. For example, aging-related alterations in glial extracellular matrix production (Hernandez et al. 1989) and biomechanics (Burgoyne, Downs 2008) have been proposed to account for the clinical behavior and increased susceptibility of the aged optic nerve head to glaucomatous damage. Existing evidence also supports the aging-related deterioration of microglia with implications for neurodegenerative diseases. Besides aging-related alterations in cytokine and chemokine profiles, growth factor production, and complement activation, the aging process also adversely affects the cellular viability and self-renewal capacity resulting in the generation of dysfunctional microglia (Streit 2006).
Aging can also cause a decline in immunocompetence and impair functional interactions that occur between the brain and the immune system. Aging of the immune system, referred to as immunosenescence, appears to be closely related to chronic stress-related factors, mainly including the oxidative stress. The immune system cells show an increase in oxidant and inflammatory compounds and a parallel decrease in antioxidant defenses with aging (Bauer et al. 2009; Maue et al. 2009; Panda et al. 2009). Oxidative stress and accumulation of oxidation products are considered to be major factors serving as local triggers for retinal para-inflammatory responses with aging and have been associated with the pathogenesis of aging-related retinal diseases (Xu et al. 2009). Paradoxically to this low-grade chronic inflammation, there is an aging-related decline in immune functions as characterized by a decrease in cell-mediated and humoral immune functions (Bauer et al. 2009; Maue et al. 2009; Panda et al. 2009). This aging-related impairment is thought to cause increased vulnerability to infection, cancer, and autoimmune diseases with aging. One of the key roles of oxidative stress in cell aging mechanisms is dampening the telomerase activity leading to telomere shortening. Since the adaptive immune response relies on the ability of lymphocytes to undergo periodic massive expansion, aging-related decline in immune functions has been linked to critical roles of telomeres and telomerase in T cell differentiation and function (Kaszubowska 2008). Despite seemingly contradicting aspects of the aging-related alterations in immune response, such as para-inflammation versus impaired immune functions, cumulative deterioration of regulatory mechanisms and functional interactions between the neurons, glia, and immune system cells during aging appear to be critically important. Potential mechanisms that contribute to increased autoimmune and/or inflammatory responses with aging include epigenetic alterations, especially DNA methylation and histone acetylation (Yung, Julius 2008; Agrawal et al. 2010).
Thus, oxidative stress besides cellular senescence is a major factor affecting normal cellular processes in the elderly, thereby leading to a cumulative deterioration with aging. Standing the hypothesis that oxidative stress favors immune processes inducing autoimmune and/or inflammatory diseases, glaucomatous tissue stress along with the aging-related factors may lead to the loss of physiological homeostasis and create an environment that is permissive to immunogenic neurodegenerative injury.
Oxidative Stress and Altered Regulation of Immune Response in Glaucoma
Experimental studies aiming to illuminate immunogenic aspects of glaucoma support that besides inducing neuronal injury, oxidative stress-related events may also alter the regulation of immune response in many different ways. One of these events is oxidative protein modifications as detected by proteomic analysis of the retina in experimental glaucoma (Tezel et al. 2005). Oxidation may change the antigenic features of these proteins, thereby serving as an immunostimulatory signal during glaucomatous neurodegeneration. Rosario Hernandez’s work has indicated that during the transition of quiescent astrocytes to reactive phenotype altered astrocyte homeostatic functions include the release of antioxidant enzymes to counteract the cytotoxic effects of oxidative stress (Malone, Hernandez 2007; Hernandez et al. 2008). Despite the activation of antioxidant mechanisms; however, not only RGCs, but also glial cells exhibit the evidence of protein and lipid oxidation in experimental glaucoma (Tezel et al. 2005; Govindarajan et al. 2009). Thus, in addition to increasing antigenity, oxidative modifications may also affect the neurosupportive and immunoregulatory functions of glial cells.
Oxidized proteins, lipids, and DNA become para-inflammatory stimuli and signal to resident immune cells, mainly including microglia, to initiate an innate immune response (Xu et al. 2009). With enhanced scavenger functions, microglial cells are able to remove oxidation products by phagocytosis. In the meantime, they may release growth factors and cytokines to promote tissue healing (Schwartz 2003a; Ransohoff, Perry 2009). This is in the same notion proposed for regulatory T cells (Schwartz, Kipnis 2002). However, if oxidative stress reaches to a certain level, the physiological homeostasis may be impaired, thereby evolving into an injury process. In this case, initial glial response expands and leads to increased production of pro-inflammatory cytokines. Relevant to human glaucoma, TNF-α is a major pro-inflammatory cytokine regulated by a redox-sensitive transcription factor, nuclear factor-kappa-B (Tezel et al. 2001). Another consequence of this cascade of events is the induction of inflammatory enzymes, such as inducible nitric oxide synthase and cyclooxygenase-2, also evident in human glaucoma (Neufeld et al. 1997; Liu, Neufeld 2000).
Complement activation constitutes another important component of the innate immune activities detected in glaucomatous human donor eyes (Kuehn et al. 2006; Stasi et al. 2006; Tezel et al. 2010). Although complement activation serves as a tissue cleaning process, uncontrolled complement activity may also accelerate the neurodegenerative injury through cell lysis. Interestingly, oxidative stress has recently been shown to also modulate complement regulation by down-regulating an important complement regulatory molecule (Tezel et al. 2010).
Other consequences of oxidative stress facilitating an aberrant immune activity in glaucoma include the augmented generation of AGEs through oxidative stress-dependent processes (Tezel et al. 2007a). AGEs may act as persistent antigenic stimulus and also be immunostimulatory through a specific receptor (RAGE)-mediated signaling that leads to pro-inflammatory cytokine production (Lin 2006). Finally, oxidative stress that provides a common trigger for many downstream pathways compromising the perivascular barrier function (Pun et al. 2009) may similarly affect blood vessels in human glaucoma (Feilchenfeld et al. 2008).
Regarding the role of oxidative stress in adaptive immune responses, a recent in vitro study has revealed that oxidative stress stimulates antigen presentation to T cells by up-regulating glial MHC class II expression and cytokine production. Findings of this experimental study also support that reactive oxygen species act as co-stimulatory molecules during antigen presentation (Tezel et al. 2007b). In addition, up-regulated expression of glial TLRs and specific signaling molecules in the glaucomatous human retina supports their involvement in innate and adaptive immune responses in glaucoma. Based on in vitro findings, oxidation products similar to stress proteins may function as intrinsic ligands of the TLR signaling in glaucoma, thereby leading to T cell stimulation (Luo et al. 2010b). Ongoing experimental studies provide supportive evidence that the immunoreactivity of the glaucomatous patient sera against oxidatively stressed retinal cell culture proteins is greater than the immunoreactivity of the same sera to control retinal proteins (Luo et al. 2010a).
Concluding Remarks
There is no doubt that the immune system functions for neuronal maintenance and repair. But, alterations in the immune system regulation due to accumulating risk factors may shift the physiological equilibrium and switch the protective immunity into a neuroinflammatory degenerative process over a chronic and cumulative period (Figure 1). Oxidative stress developing through the pathogenic cellular processes of glaucoma, along with the aging-related oxidative stress, appears to contribute to altered regulation of immune response in glaucoma.
Figure 1.
Elevated intraocular pressure(IOP)-related factors play an important role in initiation and progression of glaucomatous neurodegeneration. The immune system responds to glaucomatous tissue stress and injury as an intrinsic effort to facilitate tissue cleaning and healing. However, if there is a failure in the regulation of immune response due to accumulation of risk factors, the protective immunity may turn into a neurodegenerative process contributing to disease progression.
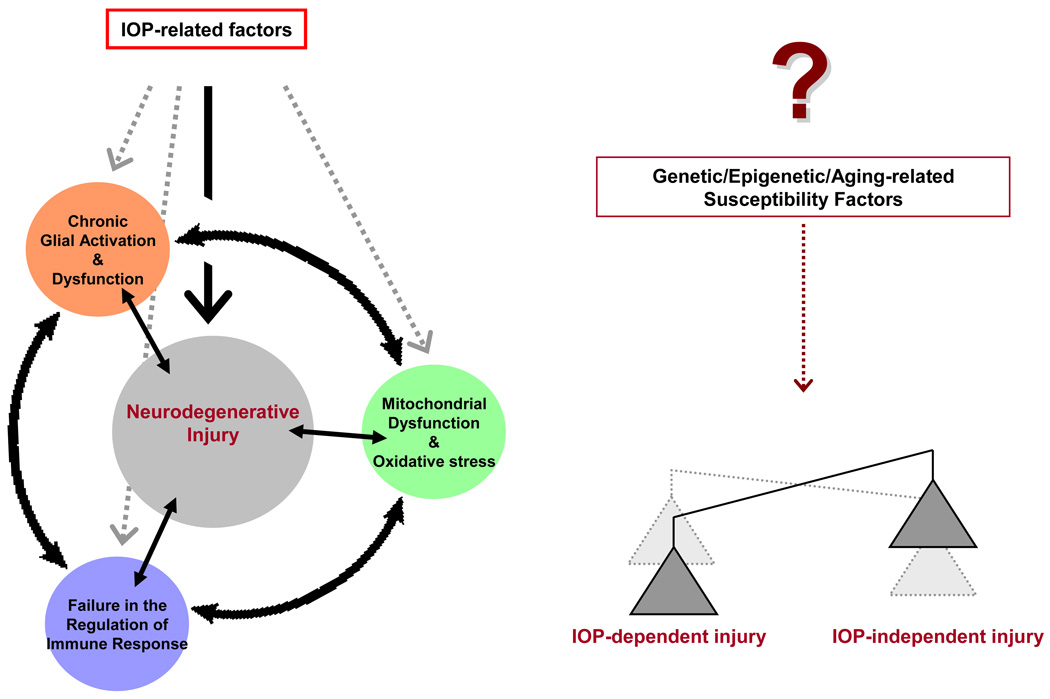
Thus, elevated IOP-related factors can initiate neurodegenerative injury in glaucomatous eyes, but seems to be only a small aspect of the big picture. A complex interplay of cellular events, including glial activation/dysfunction, mitochondrial dysfunction/oxidative stress, and immune response, may also amplify the primary injury process and contribute to disease progression. Oxidative stress is a major component of this cycle of cellular events. IOP-dependent versus IOP-independent components of these injurious events is likely determined by a number of individual susceptibility factors (Figure 2). Future studies should determine whether therapeutic inhibition of oxidative stress may act to break the cycle of events leading to neurodegenerative injury in glaucoma.
Figure 2.
Elevated intraocular pressure(IOP)-related factors can initiate neurodegenerative injury in glaucoma, but also trigger various cellular events. A complex interplay of these events, which exhibits important links to oxidative stress, may amplify the primary injury process and contribute to the progression of neurodegeneration. IOP-dependent versus IOP-independent components of the neurodegenerative injury is determined by individual susceptibility factors yet to be fully identified.
In summary, the proposed unifying scheme of multiple cellular processes integrates different risk factors, including neurodegenerative insults, glial activity response, aging, and oxidative stress, with the altered regulation of immune response in glaucoma. Based on the view that alterations in the regulation of immune response have important impacts in glaucomatous neurodegeneration, an improved understanding of the regulatory mechanisms can help therapeutic modulation of the immune response to restore tissue homeostasis in the retina and optic nerve. Ongoing studies with the advent of refined experimental models and emerging sophisticated analysis techniques promise to expand the current knowledge and offer new treatment possibilities for glaucoma patients.
Acknowledgements
This study was supported in part by National Eye Institute (2R01 EY013813, 1R01 EY017131, R24 EY015636), Bethesda, MD; an unrestricted grant to University of Louisville Department of Ophthalmology & Visual Sciences from Research to Prevent Blindness Inc., New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agapova OA, Ricard CS, Salvador-Silva M, Hernandez MR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia. 2001;33:205–216. doi: 10.1002/1098-1136(200103)33:3<205::aid-glia1019>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Tay J, Yang GE, Agrawal S, Gupta S. Age-associated epigenetic modifications in human DNA increase its immunogenicity. Aging. 2010;2:93–100. doi: 10.18632/aging.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Bauer J, Bradl M, Hickley WF, Forss-Petter S, Breitschopf H, Linington C, Wekerle H, Lassmann H. T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition. Am J Pathol. 1998;153:715–724. doi: 10.1016/s0002-9440(10)65615-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Ann N Y Acad Sci. 2009;1153:139–152. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK. Retinal deimination in aging and disease. IUBMB Life. 2009;61:504–509. doi: 10.1002/iub.184. [DOI] [PubMed] [Google Scholar]
- Broman AT, Quigley HA, West SK, Katz J, Munoz B, Bandeen-Roche K, Tielsch JM, Friedman DS, Crowston J, Taylor HR, Varma R, Leske MC, Bengtsson B, Heijl A, He M, Foster PJ. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne CF, Downs JC. Premise and prediction-how optic nerve head biomechanics underlies the susceptibility and clinical behavior of the aged optic nerve head. J Glaucoma. 2008;17:318–328. doi: 10.1097/IJG.0b013e31815a343b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann N Y Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- Caspi RR. Ocular autoimmunity: the price of privilege? Immunol Rev. 2006;213:23–35. doi: 10.1111/j.1600-065X.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Dervan EW, Chen H, Ho SL, Brummel N, Schmid J, Toomey D, Haralambova M, Gould E, Wallace DM, Prehn JH, O'Brien CJ, Murphy D. Protein macroarray profiling of serum autoantibodies in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2968–2975. doi: 10.1167/iovs.09-4898. [DOI] [PubMed] [Google Scholar]
- Downs JC, Suh JK, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005;46:540–546. doi: 10.1167/iovs.04-0114. [DOI] [PubMed] [Google Scholar]
- Feilchenfeld Z, Yucel YH, Gupta N. Oxidative injury to blood vessels and glia of the pre-laminar optic nerve head in human glaucoma. Exp Eye Res. 2008;87:409–414. doi: 10.1016/j.exer.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Forrester JV. Privilege revisited: an evaluation of the eye's defence mechanisms. Eye. 2009;23:756–766. doi: 10.1038/eye.2008.259. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Junk A, Algeciras M, Salomon RG, Bhattacharya SK. Increased isolevuglandin-modified proteins in glaucomatous astrocytes. Mol Vis. 2009;15:1079–1091. [PMC free article] [PubMed] [Google Scholar]
- Grieshaber MC, Flammer J. Does the blood-brain barrier play a role in glaucoma? Surv Ophthalmol. 2007;52 Suppl 2:S115–S121. doi: 10.1016/j.survophthal.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Grus FH, Joachim SC, Wuenschig D, Rieck J, Pfeiffer N. Autoimmunity and glaucoma. J Glaucoma. 2008;17:79–84. doi: 10.1097/IJG.0b013e318156a592. [DOI] [PubMed] [Google Scholar]
- Hammam T, Montgomery D, Morris D, Imrie F. Prevalence of serum autoantibodies and paraproteins in patients with glaucoma. Eye. 2008;22:349–353. doi: 10.1038/sj.eye.6702613. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Luo XX, Andrzejewska W, Neufeld AH. Age-related changes in the extracellular matrix of the human optic nerve head. Am J Ophthalmol. 1989;107:476–484. doi: 10.1016/0002-9394(89)90491-1. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Ye H. Glaucoma: changes in extracellular matrix in the optic nerve head. Ann Med. 1993;25:309–315. doi: 10.3109/07853899309147290. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD. The optic nerve head in glaucomatous optic neuropathy. Arch Ophthalmol. 1997;115:389–395. doi: 10.1001/archopht.1997.01100150391013. [DOI] [PubMed] [Google Scholar]
- Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res. 2000;19:297–321. doi: 10.1016/s1350-9462(99)00017-8. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia. 2002;38:45–64. doi: 10.1002/glia.10051. [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Miao H, Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 2008;173:353–373. doi: 10.1016/S0079-6123(08)01125-4. [DOI] [PubMed] [Google Scholar]
- Huang P, Qi Y, Xu YS, Liu J, Liao D, Zhang SS, Zhang C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma. 2010;19:324–330. doi: 10.1097/IJG.0b013e3181b4cac7. [DOI] [PubMed] [Google Scholar]
- Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr Eye Res. 2007;32:501–509. doi: 10.1080/02713680701375183. [DOI] [PubMed] [Google Scholar]
- Joachim SC, Reichelt J, Berneiser S, Pfeiffer N, Grus FH. Sera of glaucoma patients show autoantibodies against myelin basic protein and complex autoantibody profiles against human optic nerve antigens. Graefes Arch Clin Exp Ophthalmol. 2008;246:573–580. doi: 10.1007/s00417-007-0737-8. [DOI] [PubMed] [Google Scholar]
- Joachim SC, Grus FH, Kraft D, White-Farrar K, Barnes G, Barbeck M, Ghanaati S, Cao S, Li B, Wax MB. Complex antibody profile changes in an experimental autoimmune glaucoma animal model. Invest Ophthalmol Vis Sci. 2009;50:4734–4742. doi: 10.1167/iovs.08-3144. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Deppmeier LM, Wentzien SK, Hsu I, Morrison JC. Chronology of optic nerve head and retinal responses to elevated intraocular pressure. Invest Ophthalmol Vis Sci. 2000;41:431–442. [PubMed] [Google Scholar]
- Johnson EC, Jia L, Cepurna WO, Doser TA, Morrison JC. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Morrison JC. Friend or foe? Resolving the impact of glial responses in glaucoma. J Glaucoma. 2009;18:341–353. doi: 10.1097/IJG.0b013e31818c6ef6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Doser TA, Dyck JA, Guo Y, Lambert WS, Cepurna WO, Morrison JC. Early optic nerve head astrocytic reaction to elevated intraocular pressure is characterized by dedifferentiation and proliferation. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 5215. Available at www.iovs.org. [Google Scholar]
- Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–2018. [PubMed] [Google Scholar]
- Ju KR, Kim HS, Kim JH, Lee NY, Park CK. Retinal glial cell responses and Fas/FasL activation in rats with chronic ocular hypertension. Brain Res. 2006;1122:209–221. doi: 10.1016/j.brainres.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 2008;59 Suppl 9:169–186. [PubMed] [Google Scholar]
- Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39:365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Kompass KS, Agapova OA, Li W, Kaufman PL, Rasmussen CA, Hernandez MR. Bioinformatic and statistical analysis of the optic nerve head in a primate model of ocular hypertension. BMC Neurosci. 2008;9:93. doi: 10.1186/1471-2202-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- Kuehn MH, Kim CY, Ostojic J, Bellin M, Alward WL, Stone EM, Sakaguchi DS, Grozdanic SD, Kwon YH. Retinal synthesis and deposition of complement components induced by ocular hypertension. Exp Eye Res. 2006;83:620–628. doi: 10.1016/j.exer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Lam TT, Kwong JM, Tso MO. Early glial responses after acute elevated intraocular pressure in rats. Invest Ophthalmol Vis Sci. 2003;44:638–645. doi: 10.1167/iovs.02-0255. [DOI] [PubMed] [Google Scholar]
- Leibovitch I, Kurtz S, Kesler A, Feithliher N, Shemesh G, Sela BA. C-reactive protein levels in normal tension glaucoma. J Glaucoma. 2005;14:384–386. doi: 10.1097/01.ijg.0000176932.06606.6e. [DOI] [PubMed] [Google Scholar]
- Levin LA. Direct and indirect approaches to neuroprotective therapy of glaucomatous optic neuropathy. Surv Ophthalmol. 1999;43 Suppl 1:S98–S101. doi: 10.1016/s0039-6257(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Lin L. RAGE on the Toll Road? Cell Mol Immunol. 2006;3:351–358. [PubMed] [Google Scholar]
- Liu B, Neufeld AH. Expression of nitric oxide synthase-2 in reactive astrocytes of the human glaucomatous optic nerve head. Glia. 2000;30:178–186. doi: 10.1002/(sici)1098-1136(200004)30:2<178::aid-glia7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Luo C, Thornton I, Yang X, Soltau JB, Tezel G. Serum biomarkers in glaucoma patients: Oxidized proteins. Invest Ophthalmol Vis Sci. 2010a;51 E-Abstract 2670. Available at www.iovs.org. [Google Scholar]
- Luo C, Yang X, Kain A, Powell D, Kuehn MH, Tezel G. Glaucomatous tissue stress and the regulation of immune response through the glial toll-like receptor signaling. Invest Ophthalmol Vis Sci. 2010b doi: 10.1167/iovs.10-5407. (In press, Epub Date: 2010/06/12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone PE, Hernandez MR. 4-Hydroxynonenal, a product of oxidative stress, leads to an antioxidant response in optic nerve head astrocytes. Exp Eye Res. 2007;84:444–454. doi: 10.1016/j.exer.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KR, Levkovitch-Verbin H, Valenta D, Baumrind L, Pease ME, Quigley HA. Retinal glutamate transporter changes in experimental glaucoma and after optic nerve transection in the rat. Invest Ophthalmol Vis Sci. 2002;43:2236–2243. [PubMed] [Google Scholar]
- Maruyama I, Ohguro H, Ikeda Y. Retinal ganglion cells recognized by serum autoantibody against gamma- enolase found in glaucoma patients. Invest Ophthalmol Vis Sci. 2000;41:1657–1665. [PubMed] [Google Scholar]
- Matsubara T, Pararajasegaram G, Wu GS, Rao NA. Retinal microglia differentially express phenotypic markers of antigen-presenting cells in vitro. Invest Ophthalmol Vis Sci. 1999;40:3186–3193. [PubMed] [Google Scholar]
- Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon SJ, Kasmala LT, Dixon AL. Severe B- and T-lymphocyte immunodeficiency caused by Rag1 knockout prevents optic nerve axon loss in a mouse glaucoma model. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 2523. Available at www.iovs.org. [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miao H, Chen L, Riordan SM, Li W, Juarez S, Crabb AM, Lukas TJ, Du P, Lin SM, Wise A, Agapova OA, Yang P, Gu CC, Hernandez MR. Gene expression and functional studies of the optic nerve head astrocyte transcriptome from normal African Americans and Caucasian Americans donors. PLoS One. 2008;3:e2847. doi: 10.1371/journal.pone.0002847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikelberg FS, Drance SM, Schulzer M, Yidegiligne HM, Weis MM. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989;96:1325–1328. doi: 10.1016/s0161-6420(89)32718-7. [DOI] [PubMed] [Google Scholar]
- Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 2003;44:4347–4356. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- Moreno MC, Sande P, Marcos HA, de Zavalia N, Keller Sarmiento MI, Rosenstein RE. Effect of glaucoma on the retinal glutamate/glutamine cycle activity. FASEB J. 2005;19:1161–1162. doi: 10.1096/fj.04-3313fje. [DOI] [PubMed] [Google Scholar]
- Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 2000;14(Pt 3B):437–444. doi: 10.1038/eye.2000.128. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, Noda K, Hisatomi T, She H, Michaud N, Hafezi-Moghadam A, Miller JW, Benowitz LI. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006;26:12633–12641. doi: 10.1523/JNEUROSCI.2801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962–2968. [PubMed] [Google Scholar]
- Neufeld AH, Hernandez MR, Gonzalez M, Geller A. Cyclooxygenase-1 and cyclooxygenase-2 in the human optic nerve head. Exp Eye Res. 1997;65:739–745. doi: 10.1006/exer.1997.0394. [DOI] [PubMed] [Google Scholar]
- Neufeld AH. Nitric oxide: a potential mediator of retinal ganglion cell damage in glaucoma. Surv Ophthalmol. 1999a;43 Suppl 1:S129–S135. doi: 10.1016/s0039-6257(99)00010-7. [DOI] [PubMed] [Google Scholar]
- Neufeld AH. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. 1999b;117:1050–1056. doi: 10.1001/archopht.117.8.1050. [DOI] [PubMed] [Google Scholar]
- Neufeld AH, Liu B. Glaucomatous optic neuropathy: When glia misbehave. Neuroscientist. 2003;9:485–495. doi: 10.1177/1073858403253460. [DOI] [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287. [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishioku T, Matsumoto J, Dohgu S, Sumi N, Miyao K, Takata F, Shuto H, Yamauchi A, Kataoka Y. Tumor necrosis factor-alpha mediates the blood-brain barrier dysfunction induced by activated microglia in mouse brain microvascular endothelial cells. J Pharmacol Sci. 2010;112:251–254. doi: 10.1254/jphs.09292sc. [DOI] [PubMed] [Google Scholar]
- Odoardi F, Kawakami N, Klinkert WE, Wekerle H, Flugel A. Blood-borne soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease. Proc Natl Acad Sci U S A. 2007;104:18625–18630. doi: 10.1073/pnas.0705033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN. Pathogenesis of ganglion "cell death" in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008;173:339–352. doi: 10.1016/S0079-6123(08)01124-2. [DOI] [PubMed] [Google Scholar]
- Osborne NN. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. doi: 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Panagis L, Zhao X, Ge Y, Ren L, Mittag TW, Danias J. Gene expression changes in areas of focal loss of retinal ganglion cells in the retina of DBA/2J mice. Invest Ophthalmol Vis Sci. 2010;51:2024–2034. doi: 10.1167/iovs.09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender MP, Rist MJ. Apoptosis of inflammatory cells in immune control of the nervous system: role of glia. Glia. 2001;36:137–144. doi: 10.1002/glia.1103. [DOI] [PubMed] [Google Scholar]
- Pun PB, Lu J, Moochhala S. Involvement of ROS in BBB dysfunction. Free Radic Res. 2009;43:348–364. doi: 10.1080/10715760902751902. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Glaucoma: macrocosm to microcosm the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2005;46:2662–2670. doi: 10.1167/iovs.04-1070. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last) Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Reichelt J, Joachim SC, Pfeiffer N, Grus FH. Analysis of autoantibodies against human retinal antigens in sera of patients with glaucoma and ocular hypertension. Curr Eye Res. 2008;33:253–261. doi: 10.1080/02713680701871157. [DOI] [PubMed] [Google Scholar]
- Repka MX, Quigley HA. The effect of age on normal human optic nerve fiber number and diameter. Ophthalmology. 1989;96:26–32. doi: 10.1016/s0161-6420(89)32928-9. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J. Autoimmunity on alert: naturally occurring regulatory CD4(+)CD25(+) T cells as part of the evolutionary compromise between a 'need' and a 'risk'. Trends Immunol. 2002;23:530–534. doi: 10.1016/s1471-4906(02)02322-0. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003a;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Neurodegeneration and neuroprotection in glaucoma: development of a therapeutic neuroprotective vaccine: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2003b;44:1407–1411. doi: 10.1167/iovs.02-0594. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Ziv Y. Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol. 2008;29:211–219. doi: 10.1016/j.it.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Son JL, Soto I, Oglesby E, Lopez-Roca T, Pease ME, Quigley HA, Marsh-Armstrong N. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780–789. doi: 10.1002/glia.20962. [DOI] [PubMed] [Google Scholar]
- Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, Mittag T, Danias J. Complement component 1Q upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2006;47:1024–1029. doi: 10.1167/iovs.05-0830. [DOI] [PubMed] [Google Scholar]
- Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in brain injury. Neurotoxicology. 1996;17:671–678. [PubMed] [Google Scholar]
- Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Su WW, Ho WJ, Cheng ST, Chang SH, Wu SC. Systemic high-sensitivity C-reactive protein levels in normal-tension glaucoma and primary open-angle glaucoma. J Glaucoma. 2007;16:320–323. doi: 10.1097/IJG.0b013e3180391a83. [DOI] [PubMed] [Google Scholar]
- Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2277–2287. [PubMed] [Google Scholar]
- Tezel G, Hernandez MR, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol. 2000;118:511–518. doi: 10.1001/archopht.118.4.511. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The mechanisms of hsp27 antibody-mediated apoptosis in retinal neuronal cells. J Neurosci. 2000a;20:3552–3562. doi: 10.1523/JNEUROSCI.20-10-03552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J Neurosci. 2000b;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2001;42:1787–1794. [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest Ophthalmol Vis Sci. 2003;44:3025–3033. doi: 10.1167/iovs.02-1136. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. The immune system and glaucoma. Curr Opin Ophthalmol. 2004;15:80–84. doi: 10.1097/00055735-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X. Caspase-independent component of retinal ganglion cell death, in vitro. Invest Ophthalmol Vis Sci. 2004;45:4049–4059. doi: 10.1167/iovs.04-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X. Comparative gene array analysis of TNF-alpha-induced MAPK and NF-kappaB signaling pathways between retinal ganglion cells and glial cells. Exp Eye Res. 2005;81:207–217. doi: 10.1016/j.exer.2005.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Cai J. Proteomic identification of oxidatively modified retinal proteins in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2005;46:3177–3187. doi: 10.1167/iovs.05-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog Retin Eye Res. 2006;25:490–513. doi: 10.1016/j.preteyeres.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Luo C, Yang X. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007a;48:1201–1211. doi: 10.1167/iovs.06-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Glaucoma. Chem Immunol Allergy. 2007;92:221–227. doi: 10.1159/000099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest Ophthalmol Vis Sci. 2007b;48:705–714. doi: 10.1167/iovs.06-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog Brain Res. 2008;173:409–421. doi: 10.1016/S0079-6123(08)01128-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun D, Kaplan HJ. T cell-mediated autoimmune component of glaucomatous neurodegeneration in a chronic pressure-induced rat model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49 E-Abstract 3699. Available at www.iovs.org. [Google Scholar]
- Tezel G. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. 2009;50:1001–1012. doi: 10.1167/iovs.08-2717. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Kain A, Powell D, Kuehn MH, Kaplan HJ. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5289. (In press, Epub Date: 2010/05/21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton I, Luo C, Yang X, Soltau JB, Tezel G. Immunoproteomic analysis of glaucomatous patient serum and aqueous humor antibodies: Differential immunoreactivity against glaucomatous versus non-glaucomatous retinal proteins. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 2673. Available at www.iovs.org. [Google Scholar]
- van Eden W, Koets A, van Kooten P, Prakken B, van der Zee R. Immunopotentiating heat shock proteins: negotiators between innate danger and control of autoimmunity. Vaccine. 2003;21:897–901. doi: 10.1016/s0264-410x(02)00538-8. [DOI] [PubMed] [Google Scholar]
- van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, Lassmann H, Ravid R. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature. 1995;375:798–801. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Wang AL, Yuan M, Neufeld AH. Age-related changes in neuronal susceptibility to damage: comparison of the retinal ganglion cells of young and old mice before and after optic nerve crush. Ann N Y Acad Sci. 2007;1097:64–66. doi: 10.1196/annals.1379.027. [DOI] [PubMed] [Google Scholar]
- Wang L, Cioffi GA, Cull G, Dong J, Fortune B. Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci. 2002;43:1088–1094. [PubMed] [Google Scholar]
- Wang X, Tay SS, Ng YK. An immunohistochemical study of neuronal and glial cell reactions in retinae of rats with experimental glaucoma. Exp Brain Res. 2000;132:476–484. doi: 10.1007/s002210000360. [DOI] [PubMed] [Google Scholar]
- Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1994;117:561–568. doi: 10.1016/s0002-9394(14)70059-5. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Edward PD. Clinical and ocular histopathological findings in a patient with normal-pressure glaucoma. Arch Ophthalmol. 1998a;116:993–1001. doi: 10.1001/archopht.116.8.993. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Saito I, Gupta RS, Harley JB, Li Z, Romano C. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol. 1998b;125:145–157. doi: 10.1016/s0002-9394(99)80084-1. [DOI] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Yang J, Peng G, Patil RV, Agarwal N, Sappington RM, Calkins DJ. Induced autoimmunity to heat shock proteins elicits glaucomatous loss of retinal ganglion cell neurons via activated T-cell-derived fas-ligand. J Neurosci. 2008;28:12085–12096. doi: 10.1523/JNEUROSCI.3200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax MB, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009;88:825–830. doi: 10.1016/j.exer.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- Wekerle H, Sun D, Oropeza-Wekerle RL, Meyermann R. Immune reactivity in the nervous system: modulation of T-lymphocyte activation by glial cells. J Exp Biol. 1987;132:43–57. doi: 10.1242/jeb.132.1.43. [DOI] [PubMed] [Google Scholar]
- Woldemussie E, Wijono M, Ruiz G. Muller cell response to laser-induced increase in intraocular pressure in rats. Glia. 2004;47:109–119. doi: 10.1002/glia.20000. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch Ophthalmol. 2000;118:666–673. doi: 10.1001/archopht.118.5.666. [DOI] [PubMed] [Google Scholar]
- Yang J, Patil RV, Yu H, Gordon M, Wax MB. T cell subsets and sIL-2R/IL-2 levels in patients with glaucoma. Am J Ophthalmol. 2001a;131:421–426. doi: 10.1016/s0002-9394(00)00862-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Yang P, Tezel G, Patil RV, Hernandez MR, Wax MB. Induction of HLA-DR expression in human lamina cribrosa astrocytes by cytokines and simulated ischemia. Invest Ophthalmol Vis Sci. 2001b;42:365–371. [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Pierce WM, Tezel G. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008;49:2483–2494. doi: 10.1167/iovs.07-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DB. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992;4:396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. [PubMed] [Google Scholar]
- Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008;41:329–335. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]