Abstract
Encephalitozoon cuniculi (Phylum Microsporidia) infects a wide range of mammals, and replicates within resting macrophages. Activated macrophages, conversely, inhibit replication and destroy intracellular organisms. These studies were performed to assess mechanisms of innate immune responses expressed by macrophages to control E. cuniculi infection. Addition of reactive oxygen and nitrogen species inhibitors to activated murine peritoneal macrophages statistically significantly, rescued E. cuniculi infection ex vivo. Mice deficient in reactive oxygen species, reactive nitrogen species, or both survived ip inoculation of E. cuniculi, but carried significantly higher peritoneal parasite burdens than wild-type mice at 1 and 2 weeks post inoculation. Infected peritoneal macrophages could still be identified 4 weeks post inoculation in mice deficient in reactive nitrogen species. L-tryptophan supplementation of activated murine peritoneal macrophage cultures ex vivo failed to rescue microsporidia infection. Addition of ferric citrate to supplement iron, however, did significantly rescue E. cuniculi infection in activated macrophages and further increased parasite replication in non-activated macrophages over non-treated resting control macrophages. These results demonstrate the contribution of reactive oxygen and nitrogen species, as well as iron sequestration, to innate immune responses expressed by macrophages to control E. cuniculi infection.
Keywords: Microsporidia, parasites, parasite–host interactions, innate immunity, reactive nitrogen species, reactive oxygen species, peritoneal macrophages
1. Introduction
Encephalitozoon species of microsporidia infect a wide range of mammals and are known causes of opportunistic infections in persons with AIDS and other immune deficiencies [1]. Furthermore, these species were included on the NIAID/NIH Biodefense List of Category B pathogens that are a concern for food- and water-borne transmission [2]. Microsporidia are obligately intracellular fungal parasites. Members of the Encephalitozoon genus of microsporidia typically disseminate to cause systemic infections, virtually affecting any organ system [3; 4]. E. cuniculi is the best characterized species of this genus, and is a common laboratory animal contaminant that causes subclinical persistent infections in rodent and rabbit colonies [4; 5; 6]. In addition to epithelial cells, E. cuniculi infects macrophages and replicates within membrane-bound parasitophorous vacuoles by inhibiting acidification to avoid fusion with lysosomes [7]. Resistance to infection has been shown to depend upon IFNγ, and macrophages can be activated by IFNγ-dependent signals to destroy the intracellular microsporidia [8; 9; 10; 11; 12; 13]. Previous in vitro studies implicated a role for reactive nitrogen species (RNS) in the process of activated macrophage-mediated killing of E. cuniculi [14; 15]. In a subsequent study, however, inducible nitric oxide synthase (iNOS) knock-out mice were found to survive high-dose infection with E. cuniculi [8]. The purpose of this study was to clarify whether RNS, as well as reactive oxygen species (ROS) and nutrient deprivation, contribute to macrophage-mediated innate immune responses against E. cuniculi infection in vitro and in vivo.
2. Materials and methods
2.1. Reagents
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO USA) unless otherwise noted.
2.2 Organisms
E. cuniculiI organisms (originally isolated from rabbit; ATCC #50503) were grown in RK-13 rabbit kidney epithelial cells (ATCC #CCL-37) with complete medium comprised of RPMI 1640 supplemented with 5% fetal bovine serum, 2 mM L-glutamine, and antibiotics (100 unit penicillin/ml and100 μg streptomycin/ml). Medium was exchanged twice per week and culture supernatants were stored in sterile flasks at 4°C. To enrich for microsporidian spores (and remove host cell debris), collected culture supernatants were transferred to tubes, centrifuged (400 × g 10 min at 4° C), and washed sequentially by centrifugation in sterile solutions of dH2O, 0.3% Tween 20 in TBS, and TBS. The pellets were resuspended in TBS, mixed with an equal volume of 100% Percoll, and centrifuged for 45 min at 500 × g. The pellets containing spores were washed again with TBS and suspended to the desired concentration.
2.3. Mice
Female B6.129SF2/J (designated as wild-type; WT) mice and B6.129S6Cybbtm1Din/J mice that carry a null allele of the X-linked 91 kD subunit of oxidase cytochrome b and exhibit chronic granulomatous disease (designated as CGD or gp91phox-), were purchased from Jackson Laboratory (Bar Harbor, ME) at 5 weeks of age and allowed to acclimate at least 2 weeks prior to use. Mice were housed in sterile filter-topped cages on HEPA-filtered ventilator rack shelves and were fed sterile food and water ad libitum. Amino guanidine inhibits nitric oxide synthase, an enzyme required to generate nitric oxide from L-arginine. To generate mice deficient in nitric oxide, 2.5% aminoguanidine hemisulfate (AG) was added to the drinking water of WT or CGD mice as previously described [16], and no nitrite, a metabolite of nitric oxide, could be detected in plasma via Griess reagent test after one week of AG water treatment.
2.4. Macrophages and assessment of parasite infection
WT mice were inoculated ip with 2 ml of 3% (w/v) thioglycollate broth and peritoneal exudate cells (PEC) were recovered four days later. PEC were washed in complete RPMI 1640 medium, adjusted to 1 × 106 macrophages per ml medium, and plated at 0.4 ml per well into 8-well tissue culture chamber slides (Nunc; Naperville, IL USA). After incubation for 1 – 2 hours, non-adherent cells were washed off and 0.4 ml of complete medium containing 3 × 106 E. cuniculi spores per ml were added to the macrophages. This 3:1 ratio of microsporidia:macrophages results in 50 – 60% infection of the macrophages. Recombinant murine IFNγ (100 units/ml; eBioscience, Inc., San Diego, CA USA) and Escherichia coli serotype 0127:B8 LPS (10 ng/ml) were used to activate half of the macrophage cultures [14] and were added at the same time as the microsporidia along with RNS, ROS, and nutrient inhibitors or donors as indicated in the results. After specified intervals of time, the macrophage cultures were fixed with methanol for 10 min, stained for 5 min with Calcofluor White M2R (0.1% w/v in water adjusted to pH 8.0 with potassium hydroxide), rinsed with water, counterstained with Evan’s Blue (0.5% w/v in phosphate buffered saline), rinsed again in water, dried, and viewed under fluorescence microscopy at an excitation wavelength of 395 nm and at 600X magnification. Parasites in at least 500 macrophages (i.e. 5 counts of 100 macrophages) were counted per well and all treatments were replicated 4 times. The mean number of microsporidia per 100 macrophages was then calculated, and in some experiments, the data were presented as percent of medium-treated control macrophages (i.e. mean number of microsporidia per 100 medium-treated control macrophages = 100%). To determine the optimal range of concentrations for the treatments, experiments were first performed in replicates of four per treatment using the murine macrophage cell line, RAW264.7 γNO−/− (ATCC CRL-2278), because these macrophages were found to mimic murine peritoneal macrophages by requiring two signals (e.g. LPS and IFNγ) for activation and production of nitric oxide. Results presented here were from the repeated corroborating experiments using the murine PEC macrophages that were performed twice with 4 replicates per treatment. Results were presented as overall averages from all experiments.
2.5. Infection of mice with E. cuniculi
Four groups of five mice each were tested for susceptibility to E. cuniculi that included the WT control group, WT mice given AG in drinking water (RNS deficient), the CGD mice (ROS deficient) and the CGD mice given AG in drinking water (ROS and RNS deficient). Mice were inoculated intraperitoneally (ip) with 1 × 107 tissue culture-harvested and Percoll-purified E. cuniculi spores in one ml sterile saline.
2.6. Assessment of peritoneal parasite burden in infected mice
One, two, and four weeks after inoculation of the mice, peritoneal exudate cells (PEC) were obtained from anesthetized mice by ip injection of 3 ml warm sterile saline per mouse followed by aspiration of approximately 0.5 ml peritoneal cells suspension. Cytospin preparations of the PEC were fixed, stained with Calcofluor White and Evan’s blue, and viewed by microscopy to count percent of macrophages infected with microsporidia. At least 500 PECs per mouse were counted (i.e. 5 sets of 100 macrophages per mouse per time point), and an average was calculated per mouse. The means of each group of mice were then calculated, and the results were expressed as mean percent of infected PEC macrophages (per group) + standard deviation.
2.7. ELISA
To detect IgG responses in the inoculated mice against E. cuniculi, mice were bled from the tail vein 2 – 3x per week after week 1, and sera were assayed by ELISA as previously described [17].
2.8. Statistical analyses
Statistically significant differences were measured by two-tailed Student’s t Test when comparing two groups, or analysis of variance (ANOVA) when comparing more than two groups. Statistical analyses were performed using GraphPad Instat version 3 for Windows and graphs were generated using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com).
3. Results
3.1. Effect of RNS inhibitors and donors on microsporidia infection in macrophages in vitro
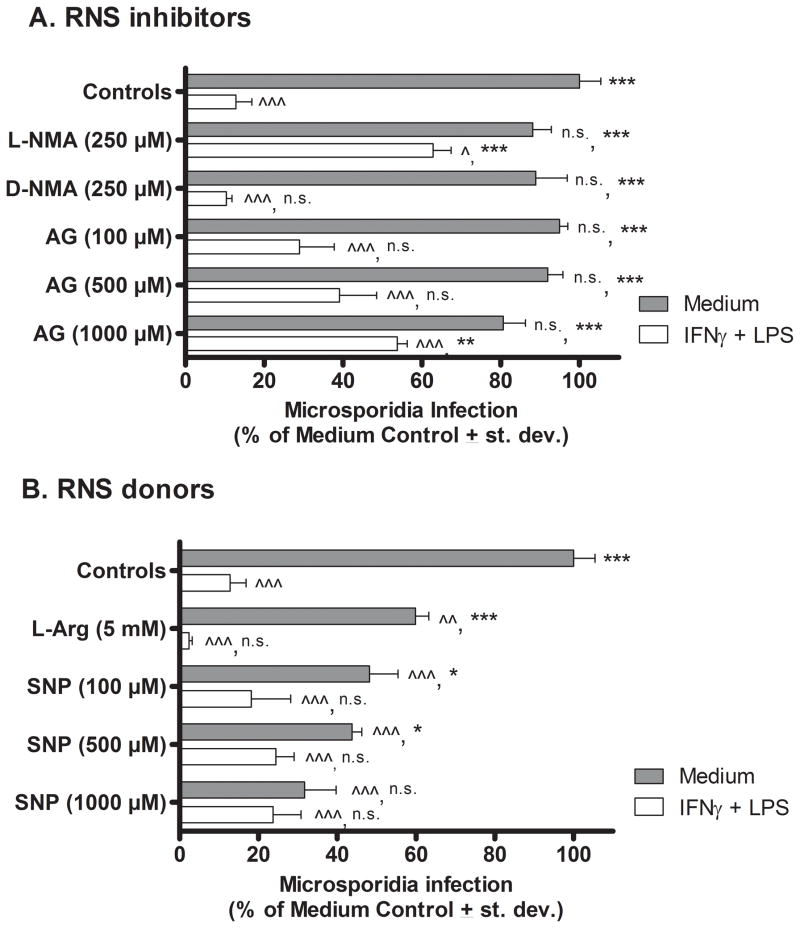
Sets of thioglycollate-induced PEC macrophages were treated with medium (i.e. resting) or IFNγ and LPS (activated) and both sets were inoculated with E. cuniculi. Experimental cultures were also treated with RNS inhibitors or donors that were added at the same time as infection with microsporidia. Three days (72 hrs) later, the cultures were fixed, stained, and viewed by fluorescent microscopy to count numbers of microsporidia per 100 macrophages. Results in Fig. 1A show that the activated peritoneal macrophages contained significantly fewer microsporidia (12.83 % +/− 9.1; P <0.001) compared to non-activated resting (medium-treated) macrophages (100% ± 12.2). Treatment of activated macrophages with the L-arginine analogue, NG-Methyl-L-arginine (L-NMA), that inhibits generation of nitric oxide, resulted in a significant rescue and increase in relative parasite infection (62.88 % ± 10.2; P < 0.001) compared with activated macrophages not treated with L-NMA or treated with the inactive L-arginine analogue, D-NMA (10.43 ± 3.04). Activated macrophages treated with the RNS inhibitor, AG, likewise reversed the microsporidia killing by activated macrophages at a dose of 1000 μM (53.8 % ± 5.67; P < 0.01) but this level of infection remained statistically significantly lower than the infection level in medium-treated resting macrophages suggesting that mechanisms in addition to RNI, contributed to macrophage killing of microsporidia ex vivo. Addition of AG or L-NMA to resting, non-activated macrophages did not significantly affect the relative microsporidia infection levels compared to infection levels in medium-only-treated macrophages.
Figure 1.
Effect of RNS inhibitors and donors on E. cuniculi replication in murine peritoneal macrophages in vitro. Cultures of WT mouse PEC macrophages were treated with medium (filled bars) or activated with IFNγ plus LPS (open bars), inoculated with E. cuniculi, and treated with RNI inhibitors (Panel A) or donors (Panel B). Three days later (72 hrs), the cultures were fixed and stained. Microsporidia were counted per 100 macrophages and results were calculated as percent of infected medium control (non-activated) macrophages (i.e. microsporidia per 100 medium-treated control macrophages = 100%). ANOVA was used to compare results of experimental groups against microsporidia infectivity in resting medium-treated control macrophages (denoted by ^ in the first position) and against activated (LPS + IFNγ-treated) control macrophages (denoted by * in the second position). Statistically significant differences were designated; ^ or * as P < 0.05; ^^ or ** as P < 0.01; ^^^ or *** as P < 0.001; n.s. = not significant.
Results in Fig. 1B corroborate a role for nitric oxide in controlling microspoidia infection in macrophages. L-arginine is a biological substrate donor for nitric oxide, and addition of L-arginine at 5 mM significantly reduced the microsporidia infection levels in non-activated macrophages (59.9 % ± 7.5; P < 0.01) compared with non-treated non-activated controls (100 % ± 12.83). Sodium nitroprusside (SNP), a vasodilator that is catabolized into nitric oxide, was added in increasing concentrations to the non-activated macrophages and also statistically significantly reduced parasite infection levels at all doses tested (10 μM – 1000 μM) compared with medium-treated (non-activated) control macrophages not treated with SNP. The strongest effect was observed at 1000 μM SNP (31.75% ± 17.85; P < 0.001). Furthermore, addition of 1000 μM SNP reduced parasite infection in the non-activated macrophages to levels that were not statistically significant different than those in the LPS + IFNγ-activated control macrophages (not treated with SNP)
3.2. Effect of ROS inhibitors on microsporidia infection in macrophages in vitro
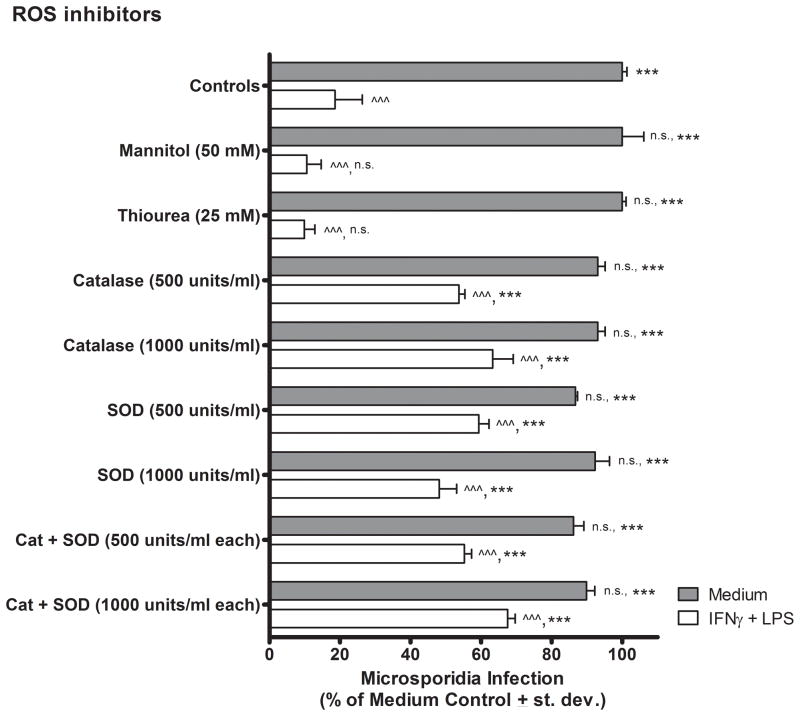
Mannitol and thiourea are two inhibitors of ROS that scavenge hydroxyl radicals. Neither of these hydroxyl scavengers statistically significantly reversed the killing of E. cuniculi by the activated (IFNγ plus LPS-treated) macrophages at the highest doses tested in comparison to the activated macrophages not treated with the inhibitors (Fig. 2). Superoxide dismutase (SOD) converts superoxide to hydrogen peroxide (H2O2) and treatment of activated macrophages at concentrations of 500 and 1000 units SOD / ml statistically significantly reversed killing of (i.e. rescued) E. cuniculi to 59.3 % ± 6.5 (P < 0.001) and 48.1 % ± 11.1 (P < 0.001) infectivity, respectively, from 18.6 % ± 7.14 infection in the IFNγ plus LPS-activated control macrophages. Catalase converts hydrogen peroxide to water and oxygen, and treatment of macrophages at doses of 500 and 1000 units catalase / ml, statistically significantly reversed killing of E. cuniculi to 53.7 % ± 3.8 (P < 0.001) and 63.3 % ± 12.9 (P < 0.001), respectively. The combination of SOD and catalase at doses of 500 and 1000 units/ml each, also reversed the killing of E. cuniculi by activated macrophages to 55.2 % ± 4.7 (P < 0.001) and 67.6 % ± 4.7 of controls, respectively. Treatment of activated PEC macrophage cultures with the combination of SOD and catalase, however, did not further reduce microsporidia infection beyond levels detected in activated macrophage cultures treated by either of these inhibitors added independently, indicating that these ROS inhibitors did not act in an additive or synergistic manner (i.e. inhibited at different stages of the same pathway).
Figure 2.
Effects of ROS inhibitors on E. cuniculi replication in murine peritoneal macrophages in vitro. Macrophage cultures were established as described in Fig. 1 except that ROS inhibitors were added in place of RNS donors and inhibitors at the same time as microsporidia inoculation and macrophage activation (or medium treatment). ANOVA was applied for statistical comparisons between each experimental group versus macrophage controls treated with medium only (filled bars) and versus IFNγ plus LPS only (open bars) as described in Fig. 1.
3.3 Effect of RNS and ROS deficiency on E. cuniculi infectivity in mice
To ascertain whether ROS and RNS affect microsporidia infection in macrophages in vivo, E. cuniculi-infected mice deficient in RNS (WT mice treated with AG), deficient in ROS (CGD mice with gp91phox−/−) and deficient in both RNS and ROS (CGD mice with gp91phox−/− given AG) were compared to infection in WT mice. Mice of all groups survived for at least 6 weeks after experimental infection. Body weights of the mice were monitored and the WT mice gained an average of 3.64 ± 1.0 grams during the first four weeks after inoculation compared with a weight change of 0.24 ± 1.4 (P < 0.001) in the WT mice treated with AG (RNS-deficient). The CGD (ROS-deficient) mice gained an average of 2.24 ±1.7 grams which was not significantly different than the weight gained by the WT mice, while the CGD mice treated with AG (RNS- and ROS-deficient) exhibited a weight change of −0.08 ± 1.04 grams (P < 0.001 and P < 0.05) that was significantly less than that of the WT and CGD mice, respectively. The time until peak expression of IgG antibodies to E. cuniculi as measured by ELISA, was delayed by approximately one day in the RNS- and ROS-deficient mice compared to the other groups, but this was not statistically significantly different from the WT controls. The mean peak ELISA antibody titers of the four groups of mice, also did not differ significantly between groups (data not shown).
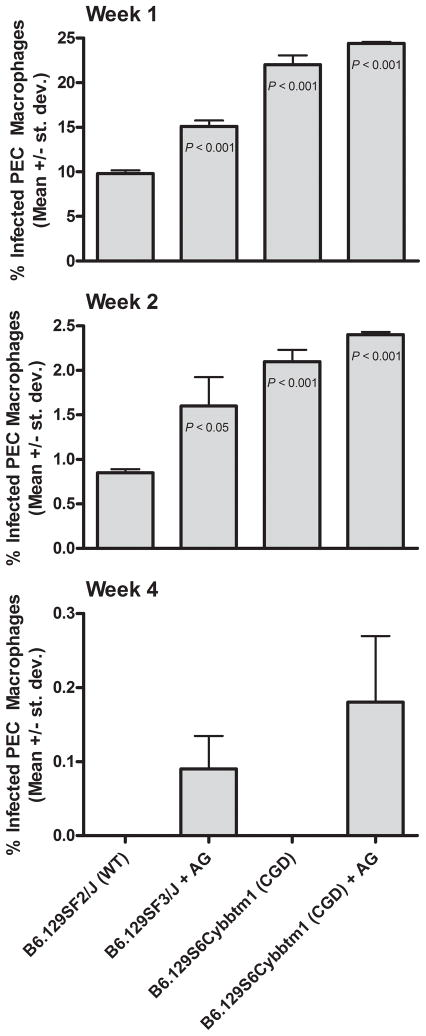
The results in Fig. 3 demonstrate the percent of infected PEC macrophages 1, 2 and 4 weeks after inoculation of mice with microsporidia. After 1 and 2 weeks, the percent of infected PEC macrophages was statistically significantly higher in the mice deficient in production of RNS, ROS, or both in comparison to that of WT mice. Mice deficient in both ROS and RNS exhibited the highest infection at both time points. Four weeks after inoculation, no microsporidia-infected macrophages were detected in any of the WT and CGD mice, but a few infected PEC macrophages were still observed in each of the RNS-deficient and RNS + ROS deficient mice. Six weeks after inoculation, no infected macrophages were detected in any of the mice by histochemistry staining with Calcofluor White. Necropsy and histopathology (Gram stain) were performed on the mice six weeks after inoculation with E. cuniculi, and liver was observed to be the most affected organ. Due to the wide variations in numbers of parasite-associated lesions per cross section, the differences between groups of mice were not statistically significant. Mice deficient in RNS, however, were most affected and exhibited the highest average number of lesions per 10 low-power fields (32.0 ± 35.2) followed by the CGD mice treated with AG (i.e. deficient in ROS and RNS) with an average of 18.2 ± 14.6 lesions, CGD (ROS deficient) mice with an average of 7.6 ± 3.36 lesions, and WT mice with an average of 2.40 ± 2.6 lesions. Lesions with microsporidia infection also were observed in lung, small intestine, kidney, colon, pancreas, brain, and peritoneum.
Figure 3.
Effect of RNS and ROS deficiency on E. cuniculi infection in mice. Groups of WT mice and mice deficient for RNS (WT treated with AG), ROS (CGD mice with gp91phox−/−), or both RNS and ROS (CGD treated with AG) were inoculated ip with 1 × 107 E. cuniculi. Each group comprised 5 animals. After 1, 2, and 4 weeks, 3 ml of saline were introduced into the peritoneum of anesthetized mice and 0.5 ml peritoneal fluid was aspirated. Cytospins of PEC were fixed, stained with Calcofluor White (and Evan’s blue counterstain), and the percent of infected macrophages was counted. ANOVA was used to compare results of each of the deficient groups of mice against infectivity results of control WT mice. Note change in y axis range over time.
3.4 Effects of L-tryptophan and ferric citrate on microsporidia infection in macrophages in vitro
The results of the murine studies indicated that RNS and ROS contribute to macrophage-mediated responses against E. cuniculi but that macrophages employ additional mechanisms of control that likely contribute to survival of the mice. For example, macrophages are known to sequester nutrients and co-factors to inhibit intracellular microbial growth [18]. To investigate this, L-tryptophan, an essential amino acid degraded by activated macrophages, was added at concentrations up to 10 mM to cultures of LPS + IFNγ-activated macrophages, but this failed to rescue E. cuniculi replication (data not shown).
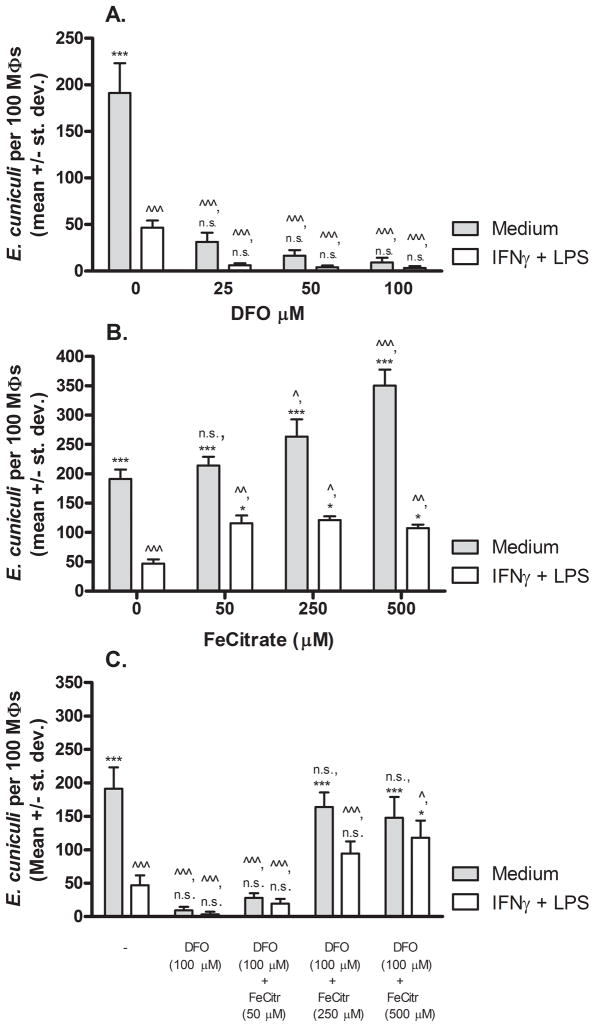
To address the role of iron sequestration as another possible mechanism for macrophage inhibition of microsporidia, a series of experiments was performed to remove iron and/or replace iron in resting or activated macrophages (Fig. 4). Addition of desferoxamine (DFO), a chelator of iron, inhibited replication of E. cuniculi in medium-treated and activated macrophages at all concentrations tested when compared to respective controls (i.e. wells not treated with DFO) as shown in Fig. 4, panel A. Conversely, addition of ferric citrate to activated macrophages (i.e. treated with LPS and IFNγ) partially rescued microsporidia growth. For example, addition of 50 μM ferric citrate to activated macrophages resulted in an average of 116 ± 26 organisms per 100 macrophages compared to a mean of 48 ± 15 organisms per 100 activated macrophages that had not been treated with ferric citrate (P < 0.001). Addition of higher concentrations of ferric citrate to activated macrophages retained similar levels of rescued infectivity, but did not approach the level of infectivity observed in non-activated resting macrophages. Interestingly, iron supplementation via ferric citrate to non-activated macrophages increased E. cuniculi replication over that in the non-supplemented resting macrophages. For example, medium-treated macrophages incubated with 500 μM ferric citrate exhibited statistically significantly enhanced replication of E. cuniculi with a mean of 350 ± 53 organisms per 100 macrophages compared to macrophages not given ferric citrate that exhibited an average of 191 ± 32 organisms per 100 non-activated macrophages (P < 0.001). To further corroborate this effect, macrophages treated with 100 μM DFO were also treated with ferric citrate in attempt to reverse the DFO chelation effect. A concentration of 500 μM ferric citrate was required to statistically significantly reverse activated macrophage destruction of E. cuniculi. Ferric citrate added at 250 μM or 500 μM was able to rescue E. cuniculi replication in DFO-treated resting macrophages to levels observed in non-treated medium control macrophages.
Figure 4.
Effect of iron deprivation or supplementation on E. cuniculi replication in murine peritoneal macrophages in vitro. PEC macrophages from WT mice treated with medium (filled bars) or activated with IFNγ plus LPS (open bars) were inoculated with E. cuniculi and various concentrations of the iron chelator, desferoxamine (DFO, Panel A), ferric citrate iron supplement (Panel B), or combination of both (Panel C). Three days later (72 hrs), the cultures were fixed, stained with Calcofluor White, and the numbers of E. cuniculi per 100 macrophages were counted. ANOVA was used to compare infectivity in treated groups of macrophages in comparison to medium-treated (^) or activated (*) macrophages as described in Fig. 1.
4. Discussion
The recognition of microsporidia species associated with emerging and opportunistic infections grew tremendously during the AIDS pandemic, but the incidence and prevalence of microsporidia infections has declined in HIV-infected individuals due to the use of highly active antiretroviral therapies (HAART)[1]. In addition to reducing HIV levels and retaining T cell levels, HAART also may induce oxidative stress to affect secondary opportunistic pathogens [19]. Microsporidiosis, however is increasingly identified in other populations such as organ transplant recipients, chemotherapy patients, children, travelers, and the elderly, and continues to be identified in HIV-infected individuals unable to access antiretroviral treatment [1; 20]. The best characterized and studied species affecting humans is E. cuniculi. It was the first mammalian species grown in culture and it naturally infects a wide spectrum of mammals including rodents, nonhuman primates, and humans [5]. The majority of immune-competent animals infected with E. cuniculi rarely develop clinical signs, but infections persist unless hosts are treated with appropriate drugs [4; 5; 6]. It is currently unclear if humans also carry persistent microsporidia infections. Positive serology for Encephalitozoon-specific antibodies in otherwise healthy individuals, and the detection of another microsporidian, Enterocytozoon bieneusi, in immune-competent children and adults, however, suggest that persistent microsporidioses in humans do occur [21; 22; 23; 24; 25]. Immune-deficient hosts, on the other hand, develop clinical signs that may include diarrhea and weight loss, and these hosts often succumb to infection [15; 26; 27].
Resistance to microsporidiosis, and E. cuniculi infection specifically, requires intact T-cell-mediated immune responses [26]. CD4+ and CD8+ T cells contribute via production of IFNγ to activate macrophages and cytotoxic T cell lysis (CTL) activity to destroy infected host cells [27; 28]. It is believed that the majority of organisms in the CTL-targeted cells are then phagocytized but that a few organisms escape to infect new cells. Whereas microsporidia infect and replicate within resting macrophages, activated macrophages are currently the only cells known to be capable of killing microsporidia and contribute to immune responses via induction of inflammation and secretion of chemokines [1; 29; 30]. IFNγ plus LPS can activate macrophages to kill E. cuniculi in vitro and resistance in vivo has been demonstrated to depend on IFNγ, as well [8; 9; 10; 11; 12; 31].
RNS were implicated in IFNγ + LPS-activated macrophage destruction of E. cuniculi in vitro in earlier studies [14; 15], but a subsequent report indicated that iNOS-deficient mice were able to withstand and survive infection with a high dose of these parasites [8]. This raised questions about the contribution of RNS in macrophage responses to controlling infection. To address this issue, the studies presented here were designed to address the mechanisms of innate immunity mediated by macrophages against E. cuniculi infection in vitro and in vivo. The in vitro studies continued to support a role for both RNS, as described in earlier reports [14; 15] and ROS in macrophage-mediated resistance to E. cuniculi. The in vivo results also were consistent with those of Khan and Moretto [8] in that mice deficient for RNS and/or ROS, survived infection. The report by Khan and Moretto [8] did not assess parasite burdens, and the results in this study demonstrated that the mice deficient for RNS and/or ROS did exhibit higher parasite burdens in PEC macrophages that required longer periods of time to resolve in comparison to WT mice. This suggests that RNS and ROS contributed to resistance but that other factors, including iron sequestration, probably also contributed to macrophage-mediated control of these microsporidia. The mice deficient in RNS, (with or without concurrent ROS-deficiency) exhibited higher microsporidia burdens than the ROS-deficient mice, although differences among these three groups of deficient mice were not statistically significant. These results suggested, however, that RNS may be more important for controlling infection than ROS.
RNS are well known for mediating destruction of intracellular parasites but are also known to modulate host cell apoptosis (anti-inflammatory) and necrosis (pro-inflammatory) that in turn, may benefit either the pathogen or the host under varying situations [32; 33; 34; 35]. For example, relatively lower levels of RNS contributed to pneumococcal killing and macrophage necrosis whereas higher concentrations of RNS induced macrophage apoptosis and resolution of inflammation [36]. Similarly, RNS contributed to destruction of intracellular Toxoplasma gondii organisms and increased apoptosis of macrophages that then helped reduce proliferation of lymphocytes and induced effector T cell apoptosis, thereby resolving inflammation [37]. The observation that mice treated with AG and deficient in RNI exhibited higher parasite burdens than the WT mice, but that this infection eventually subsided to allow survival of the mice supports a dual effect of RNS in microsporidiosis. On the one hand, RNS appear to contribute to macrophage-mediated killing for control of E. cuniculi. On the other hand, RNS may be important for resolving inflammation to reduce toxicity and tissue destruction. In the study reported here, unresolved inflammation in the AG-treated, RNS-deficient mice may have contributed to continued recruitment of macrophages and effector lymphocytes that controlled the microsporidia infection, but the price of continued or unresolved inflammation may have been the failure of the mice to thrive as evidenced by significantly lower body weights of these mice compared to weight gains exhibited in the WT mice and CGD ROS-deficient mice.
RNS function as effectors and regulators of apoptosis, and microsporidia also appear to affect apoptosis of the host cell. Scanlon et al. [38] and DelAguila et al. [39] demonstrated that epithelial cells infected with microsporidia exhibited significantly lower susceptibility to apoptosis in conjunction with increased anti-apoptotic bcl-2 and decreased bak pro-apoptotic signals as well as absence of p53 translocation to the nucleus, respectively. Preliminary studies in our lab further corroborate these findings whereby E. cuniculi-infected human THP-1 macrophage cells were able to inhibit apoptosis whereas THP-1 cells inoculated with dead E. cuniculi were more susceptible to apoptosis stimuli (Sokolova et al., unpublished). Bcl-2 has been reported to protect macrophages from RNI-induced apoptosis signals [40] and RNS can nitrosylate caspase 9 to prevent cytochrome c release and thereby inhibit apoptosis, as well [41].
The results of this study support roles for RNS and ROS in macrophage-mediated control of microsporidia growth based on the early higher parasite burdens and longer times required to control infection in the RNS- and/or ROS-deficient mice. The survival of these mice, however, suggests that additional mechanisms, such as iron sequestration, also contribute to innate resistance. Alternatively, the results also may indicate that RNS and/or ROS may be exploited by the microsporidia to support growth sufficiently long enough to replicate, mature, and then escape to infect newly-recruited macrophages. In the absence of RNS, for example, continued inflammatory responses may help control infection but contribute to host toxicity, as observed in the RNS-deficient mice. In summary, RNS and ROS, along with iron sequestration, contribute to macrophage-mediated innate immunity against E. cuniculi infection. Continued studies will likely indicate that replicating microsporidia conversely, modulate these responses to enable establishment of persistent infection.
Acknowledgments
This work was supported by funding from the National Institutes of Health, Bethesda, MD, U.S.A. (RR00164, AI039968, and AI071778) and the Tulane Research Enhancement Bridge Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Didier ES, Weiss LM. Microsporidiosis: current status. Curr Opin Infect Dis. 2006;19:485–492. doi: 10.1097/01.qco.0000244055.46382.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. doi: 10.1016/j.actatropica.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Mertens RB, Didier ES, Fishbein MC, Bertucci DC, Rogers LB, Orenstein JM. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol. 1997;10:68–77. [PubMed] [Google Scholar]
- 4.Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microb Infect. 2000;2:709–720. doi: 10.1016/s1286-4579(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 5.Didier ES, Snowden KF, Shadduck JA. Biology of microsporidian species infecting mammals. Adv Parasitol. 1998;40:283–320. doi: 10.1016/s0065-308x(08)60125-6. [DOI] [PubMed] [Google Scholar]
- 6.Snowden KF, Didier ES, Orenstein JM, Shadduck JA. Animal models of human microsporidial infections. Lab Anim Sci. 1998;48:589–592. [PubMed] [Google Scholar]
- 7.Weidner E, Sibley LD. Phagocytized intracellular microsporidian blocks phagosome acidification and phagosome-lysosome fusion. J Protozool. 1985;32:311–317. doi: 10.1111/j.1550-7408.1985.tb03056.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan IA, Moretto M. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect Immun. 1999;67:1887–1893. doi: 10.1128/iai.67.4.1887-1893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salat J, Jelinek J, Chmelar J, Kopecky J. Efficacy of gamma interferon and specific antibody for treatment of microsporidiosis caused by Encephalitozoon cuniculi in SCID mice. Antimicrob Agents Chemother. 2008;52:2169–2174. doi: 10.1128/AAC.01506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Didier ES, Shadduck JA. IFN-gamma and LPS induce murine macrophages to kill Encephalitozoon cuniculi in vitro. J Eukaryot Microbiol. 1994;41:34S. [PubMed] [Google Scholar]
- 11.Choudhry N, Korbel DS, Zaalouk TK, Blanshard C, Bajaj-Elliott M, McDonald V. Interferon-gamma-mediated activation of enterocytes in immunological control of Encephalitozoon intestinalis infection. Parasite Immunol. 2009;31:2–9. doi: 10.1111/j.1365-3024.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 12.El Fakhry Y, Achbarou A, Desportes I, Mazier D. Resistance to Encephalitozoon intestinalis is associated with interferon-gamma and interleukin-2 cytokines in infected mice. Parasite Immunol. 2001;23:297–303. doi: 10.1046/j.1365-3024.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- 13.Jelinek J, Salat J, Sak B, Kopecky J. Effects of interferon gamma and specific polyclonal antibody on the infection of murine peritoneal macrophages and murine macrophage cell line PMJ2-R with Encephalitozoon cuniculi. Folia Parasitol (Praha) 2007;54:172–176. [PubMed] [Google Scholar]
- 14.Didier ES. Reactive nitrogen intermediates implicated in the inhibition of Encephalitozoon cuniculi (phylum Microspora) replication in murine peritoneal macrophages. Parasite Immunol. 1995;17:405–412. doi: 10.1111/j.1365-3024.1995.tb00908.x. [DOI] [PubMed] [Google Scholar]
- 15.Didier ES, Varner PW, Didier PJ, Aldras AM, Millichamp NJ, Murphey-Corb M, Bohm R, Shadduck JA. Experimental microsporidiosis in immunocompetent and immunodeficient mice and monkeys. Folia Parasitol (Praha) 1994;41:1–11. [PubMed] [Google Scholar]
- 16.Hoffman RA, Simmons RL. Nitric Oxide Modulation of the Allograft Response in Vivo. Methods. 1996;10:43–50. doi: 10.1006/meth.1996.0077. [DOI] [PubMed] [Google Scholar]
- 17.Juan-Salles C, Garner MM, Didier ES, Serrato S, Acevedo LD, Ramos-Vara JA, Nordhausen RW, Bowers LC, Paras A. Disseminated encephalitozoonosis in captive, juvenile, cotton-top (Saguinus oedipus) and neonatal emperor (Saguinus imperator) tamarins in North America. Vet Pathol. 2006;43:438–446. doi: 10.1354/vp.43-4-438. [DOI] [PubMed] [Google Scholar]
- 18.Appelberg R. Macrophage nutriprive antimicrobial mechanisms. J Leuk Biol. 2006;79:1117–1128. doi: 10.1189/jlb.0206079. [DOI] [PubMed] [Google Scholar]
- 19.Mondal D, Pradhan L, Ali M, Agrawal KC. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovasc Toxicol. 2004;4:287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- 20.Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet Parasitol. 2004;126:145–166. doi: 10.1016/j.vetpar.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Nkinin SW, Asonganyi T, Didier ES, Kaneshiro ES. Microsporidian infection is prevalent in healthy people in Cameroon. J Clin Microbiol. 2007;45:2841–2846. doi: 10.1128/JCM.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Gool T, Vetter JC, Weinmayr B, Van Dam A, Derouin F, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175:1020–1024. doi: 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- 23.Leelayoova S, Subrungruang I, Suputtamongkol Y, Worapong J, Petmitr PC, Mungthin M. Identification of genotypes of Enterocytozoon bieneusi from stool samples from human immunodeficiency virus-infected patients in Thailand. J Clin Microbiol. 2006;44:3001–3004. doi: 10.1128/JCM.00945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mungthin M, Subrungruang I, Naaglor T, Aimpun P, Areekul W, Leelayoova S. Spore shedding pattern of Enterocytozoon bieneusi in asymptomatic children. J Med Microbiol. 2005;54:473–476. doi: 10.1099/jmm.0.45832-0. [DOI] [PubMed] [Google Scholar]
- 25.Pagornrat W, Leelayoova S, Rangsin R, Tan-Ariya P, Naaglor T, Mungthin M. Carriage rate of Enterocytozoon bieneusi in an orphanage in Bangkok, Thailand. J Clin Microbiol. 2009;47:3739–3741. doi: 10.1128/JCM.01606-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Didier ES. Immunology of microsporidiosis. Contrib Microbiol. 2000;6:193–208. doi: 10.1159/000060361. [DOI] [PubMed] [Google Scholar]
- 27.Khan IA, Moretto M, Weiss LM. Immune response to Encephalitozoon cuniculi infection. Microb Infect. 2001;3:401–405. doi: 10.1016/s1286-4579(01)01397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan IA, Schwartzman JD, Kasper LH, Moretto M. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J Immunol. 1999;162:6086–6091. [PubMed] [Google Scholar]
- 29.Fischer J, West J, Agochukwu N, Suire C, Hale-Donze H. Induction of host chemotactic response by Encephalitozoon spp. Infect Immun. 2007;75:1619–1625. doi: 10.1128/IAI.01535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathews A, Hotard A, Hale-Donze H. Innate immune responses to Encephalitozoon species infections. Microb Infect. 2009;11:905–911. doi: 10.1016/j.micinf.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Almeida-Leite CM, Galvao LM, Afonso LC, Cunha FQ, Arantes RM. Interferon-gamma induced nitric oxide mediates in vitro neuronal damage by Trypanosoma cruzi-infected macrophages. Neurobiol Dis. 2007;25:170–178. doi: 10.1016/j.nbd.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Melino G, Catani MV, Corazzari M, Guerrieri P, Bernassola F. Nitric oxide can inhibit apoptosis or switch it into necrosis. Cell Mol Life Sci. 2000;57 :612–622. doi: 10.1007/PL00000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James SL. Role of nitric oxide in parasitic infections. Microbiol Rev. 1995;59:533–547. doi: 10.1128/mr.59.4.533-547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 35.Luder CG, Gross U, Lopes MF. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends Parasitol. 2001;17:480–486. doi: 10.1016/s1471-4922(01)02016-5. [DOI] [PubMed] [Google Scholar]
- 36.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- 38.Scanlon M, Leitch GJ, Shaw AP, Moura H, Visvesvara GS. Susceptibility to apoptosis is reduced in the Microsporidia-infected host cell. J Eukaryot Microbiol. 1999;46:34S–35S. [PubMed] [Google Scholar]
- 39.del Aguila C, Izquierdo F, Granja AG, Hurtado C, Fenoy S, Fresno M, Revilla Y. Encephalitozoon microsporidia modulates p53-mediated apoptosis in infected cells. Int J Parasitol. 2006;36:869–876. doi: 10.1016/j.ijpara.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Messmer UK, Reed UK, Brune B. Bcl-2 protects macrophages from nitric oxide-induced apoptosis. J Biol Chem. 1996;271:20192–20197. doi: 10.1074/jbc.271.33.20192. [DOI] [PubMed] [Google Scholar]
- 41.Torok NJ, Higuchi H, Bronk S, Gores GJ. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res. 2002;62:1648–1653. [PubMed] [Google Scholar]