Abstract
Sarcopenia is the age-related loss of skeletal muscle mass and function and is characterized by a reduction in muscle mass and fiber cross-sectional area, alterations in muscle fiber type and mitochondrial functional changes. In rhesus monkeys, calorie restriction (CR) without malnutrition improves survival and delays the onset of age-associated diseases and disorders including sarcopenia. We present a longitudinal study on the impact of CR on early stage sarcopenia in the upper leg of monkeys from ~16 years to ~22 years of age. Using dual-energy X-ray absorptiometry we show that CR delayed the development of maximum muscle mass and, unlike Control animals, muscle mass of the upper leg was preserved in CR animals during early phase sarcopenia. Histochemical analyses of vastus lateralis muscle biopsies revealed that CR opposed age-related changes in the proportion of Type II muscle fibers and fiber cross-sectional area. In contrast the number of muscle fibers with mitochondrial electron transport system enzyme abnormalities (ETSab) was not significantly affected by CR. Laser capture microdissection of ETSab fibers and subsequent PCR analysis of the mitochondrial DNA revealed large deletion mutations in fibers with abnormal mitochondrial enzyme activities. CR did not prevent stochastic mitochondrial deletion mutations in muscle fibers but CR may have contributed to the maintenance of affected fibers.
Keywords: sarcopenia, calorie restriction, rhesus monkey
1. Introduction
Calorie restriction (CR) is the only dietary regimen that slows aging and extends lifespan in diverse species. The beneficial effects of CR extend to primates with improved health span and survival outcome for rhesus monkeys [1]. In humans, CR improves indicators of cardiovascular health [2] and, although controlled short-term CR studies have been conducted [3], analysis of its long-term effects on aging muscle at the cellular level has not been conducted. The rhesus monkey closely models human aging in many respects with the advantage of a rate of aging ~ 3 times that of humans [4]. We have undertaken a longitudinal study of aging and the impact of CR in male rhesus monkeys (Macaca mulatta). One goal of this work is to determine the effects of CR on sarcopenia, the process of skeletal muscle aging. We have previously reported that CR preserves total muscle mass in rhesus monkeys [5]. Herein we present the impact of CR on the early stages of sarcopenia through longitudinal examination of intact upper leg musculature using dual-energy X-ray absorptiometry (DXA) and at the cellular level using vastus lateralis (VL) biopsies.
In humans, sarcopenia has a significant impact on daily living for ~45% of adults over 60 years of age [6] and is associated with both muscle fiber atrophy and fiber loss. Muscle fiber types are grouped based on dominant myosin isoform expression and metabolism, factors that contribute to the contractile properties of individual fibers. The impact of age is not equivalent among fiber types; unlike the slow-twitch myosin Type I fibers, the fast-twitch glycolytic myosin Type II fibers are vulnerable to age-associated atrophy and loss [7]. These negative phenotypes of aging skeletal muscle are conserved in the rhesus monkey [8].
A proposed mechanism of muscle fiber loss involves age-dependent changes in mitochondrial DNA [9, 10]. The mitochondrion is unique among cellular organelles in that it contains its own genome, a 16kb double stranded circular DNA molecule encoding 22 tRNA and 13 polypeptides of the electron transport system (ETS). The activity of Complex IV of the ETS resides with the multimeric enzyme cytochrome c oxidase (COX) containing both nuclear and mitochondrial encoded subunits. MtDNA is susceptible to age-dependent mutations, including large deletion mutations that remove one or all of the COX subunits encoded by the mitochondrial genome [11]. Characteristic phenotypes of muscle cells that have a high concentration of mutant mitochondria are the absence of COX activity and an over-abundance of succinate dehydrogenase (SDH) activity. In rodents, ETS abnormal (ETSab) fibers often become atrophic within the region of the abnormality and some affected fibers break [12], suggesting a possible mechanism for permanent fiber loss.
Lean mass loss in humans is estimated at 1–2% per year after the age of 50 [13, 14]. Rhesus monkeys begin to undergo muscle mass loss at ~16 years of age [4]. As previously reported, age-dependent changes in muscle fibers of the vastus lateralis (VL) in monkeys between the ages of 16- and 22-years include decreases in the number of Type II muscle fibers, decreases in Type II muscle fiber cross-sectional area (CSA) and an increase in the number of ETSab muscle fibers [8]. In the present study, we determine the impact of CR on the cellular phenotypes of early stage sarcopenia from 11 monkeys assessed at 3 year intervals; 6, 9 and 12 years from initiation of the CR diet and compare these results with those observed in Control monkeys.
2. Materials and Methods
2.1. Animals and Diet
The CR monkeys are part of an ongoing longitudinal study at the Wisconsin National Primate Research Center (WNPRC) [1, 15, 16]. The median life expectancy of rhesus monkeys in captivity is ~26 years with some of the monkeys in this colony living into their late 30's [17]. The animals at year 6 of the study had an average age of 15.8 y (15 to 21y) and at the end, 21.8y (21 to 27y) representing their late middle years and into early old age. We have previously reported on the age-related changes in muscle from Control animals in this cohort [8] and, herein, report findings in the CR animals.
Briefly, 30 males, between 8- and 14-years of age, were monitored for baseline food intake and then randomized to either a Control (C, n = 15) or Calorie Restricted (CR, n = 15) diet. Food allotments for CR animals (Teklad diet 93131, enriched by 30% in vitamins and minerals) were reduced 10% per month for 3 months to reach a 30% CR. Control animals were fed ~20g more than their average daily intake to assure ad libitum access to food (purified lactalbumin based diet containing 10% fat and 15% protein [Teklad #85387, Madison, WI]). VL biopsies were collected at time points 6-, 9-, and 12-years after introduction of the CR diet. Over the course of this study, 4 CR monkeys died and we report data from 11 of the remaining CR monkeys. All animal procedures were performed at the WNPRC under approved protocols from the Institutional Animal Care and Use Committee of the Graduate School of the University of Wisconsin, Madison.
2.2 Body Composition
Body weight of each animal was assessed throughout the study. Appendicular lean mass and fat mass were assessed biannually using whole body DXA (Model DXP-L, GE/Lunar Corp., Madison, WI) scans as previously described [5]. Estimated skeletal muscle mass (ESM) of the upper leg was determined by summing the lean mass from the thigh region of both limbs. Muscle mass loss for each individual animal was determined by dividing the upper leg lean mass at each time point by the maximum upper leg lean mass measured for that animal in the 12 years of the study. Fat mass, determined by DXA measurements, was used to calculate percent body fat (%BF = [fat mass / body weight] × 100).
2.3. Biopsy Collection
Six, nine and twelve years post-initiation of the study, VL biopsies were performed immediately following DXA, alternating legs with successive biopsies. Biopsy tissue was bisected with one half of the sample flash frozen in liquid nitrogen and the other embedded in Optimal Cutting Temperature Medium (OCT, Sakura Inc., Torrance, CA) and frozen in liquid nitrogen. Samples were stored at −80°C until use. Frozen muscle biopsies were sectioned using a cryostat. For each biopsy, 200 consecutive 10μm-thick sections were cut and stored at −80°C.
2.4. Histochemistry
Slide sections were stained with hematoxylin and eosin [18] for muscle morphology and muscle fiber counts. Muscle fiber types were identified using standard immunohistochemical detection of the Type II isoform of myosin heavy chain (monoclonal antibody MY32, Sigma St. Louis, MO) followed by the 3,3'-Diaminobenzidine tetrachloride (DAB) reaction for visualization [18]. General muscle fiber atrophy was assessed using slides from the immunohistochemical analysis. Five 10× images per section were taken. The cross-sectional area (CSA) of Type I and Type II muscle fibers were measured using ImagePro. The CSA of at least 200 muscle fibers were measured for each type from each biopsy.
Histochemical staining for mitochondrial enzyme activities, COX and SDH were performed on air-dried sections according to Seligman et al. [19] and Dubowitz [20], respectively. Twenty-nine slide triplicates (the 2nd, 3rd and 4th, the 9th, 10th and 11th etc.) from the 200 slide sections from each biopsy were stained for COX, SDH and both COX and SDH enzyme activity staining. Unique ETSab fibers were identified for each muscle sample and the percentage of ETSab fibers determined from the total number of cell present. To measure atrophy specifically associated with ETSab regions, fibers were followed along their length and the cross-sectional area was measured at 70μm intervals. Cross-sectional area ratios were determined for 54 ETSab fibers (and 55 normal fibers) where the minimum CSA of the ETS abnormal region (or the minimum CSA of the normal fiber) was divided by the mean CSA of the normal region to give a minimum CSA ratio. Abnormal fibers with CSA ratios ± 2 standard deviations from the distribution of normal fiber CSA ratios were defined as atrophic or hypertrophic. The length of the ETS abnormality within a fiber was also measured.
2.5 Laser capture microdissection and Mt DNA Amplification
Laser capture microdissection (LCM) was used to isolate 10μm thick sections of ETSab and normal muscle fibers to determine the mitochondrial genotype. Frozen sections adjacent to those used for identification of ETSab phenotypes were stained for SDH activity, dehydrated in ethanol and cleared in xylenes. Muscle fibers of interest were microdissected using a PixCell II laser capture microscope (Arcturus Bioscience, Inc., Mountainview, CA, USA) as previously described [11]. Total DNA was extracted, primary PCR reactions were performed using mtDNA primers to amplify ~14,400 bp of the ~16,000 bp rhesus mitochondrial genome (1176F : 725R), followed by a nested amplification (3499F : 16245R). Specifics of techniques and sequencing of PCR products are as described before [8, 11].
2.6. Statistical Analysis
A linear mixed model approach was used to estimate longitudinal trends in the data while accounting for the dependency in the data due to multiple observations per subject. SAS PROC MIXED was used to estimate the correlation among the repeatedly measured outcomes [21]. The effects of CR on overall outcome levels and differences in longitudinal trends were tested by including diet main effect and diet-by-year interaction terms in the model. Age was used as a covariate to control for the slight differences in age among the animals within a given assessment year.
3. Results
Longitudinal results from each CR animal are reported at 6-, 9- and 12-year of the study. To put these values in context, we compare mean values of CR animals to mean values from Control animals generated within the same time-frame. The values for the Controls were reported previously [8].
3.1 Body Composition
To determine the long-term impact of CR on age-associated changes in body composition and muscle mass, percent body fat and upper leg muscle mass were determined for the 11 CR monkeys using DXA analysis. The scale weight of each animal was measured on the morning of DXA assessments. Not surprisingly, CR monkeys weighed less than Control animals over the 6-year period of this study; however, in contrast to the Control animals, CR monkeys had a significant increase in weight with age (r = 0.58; p < 0.01, Table 1). Similar to Control animals, the percentage of body fat also increased with age in CR animals (r = 0.64; p < 0.01, Table 1).
Table 1.
Longitudinal whole body, muscle and muscle fiber data for 11 rhesus monkeys collected after 6-, 9- and 12-years of calorie restriction.
| Animal | Diet | Age @ Biopsy | Weight | % Fat | Upper Leg MM % (MM / Max) | % Type II | Type I CSA | Type II CSA | % ETS Abn |
|---|---|---|---|---|---|---|---|---|---|
| 1 | R | 21 | 8.0 | 4.8 | 97.9 | ** | ** | ** | 0.29 |
| 24 | 8.5 | 10.4 | 95.8 | 97.2 | 4181 | 8654 | 0.44 | ||
| 27 | 10.3 | 23.3 | 95.4 | 95.1 | 6159 | 13000 | 0.00 | ||
| 2 | R | 16 | 9.4 | 5.5 | 99.5 | 93.5 | 3693 | 7799 | 0.097 |
| 19 | 9.4 | 9.62 | 93.3 | 98.1 | 5860 | 11378 | 0.33 | ||
| 22 | 9.9 | 15.2 | 82.8 | 83.6 | 10483 | 29592 | 0.00 | ||
| 3 | R | 16 | 7.4 | 3.9 | 90.3 | 91.8 | 5943 | 7425 | 0.04 |
| 19 | 7.8 | 6.5 | 95.9 | 93.6 | 4963 | 7774 | 4.54 | ||
| 22 | 10.9 | 28.0 | 97.0 | 93.7 | 6656 | 8465 | 0.16 | ||
| 4 | R | 16 | 12.3 | 15.5 | 99.2 | ** | ** | ** | ** |
| 19 | 11.3 | 14.1 | 90.3 | 99.9 | 3159 | 11110 | 1.216 | ||
| 22 | 10.2 | 11.7 | 82.5 | 85.7 | 6020 | 10217 | 4.20 | ||
| 5 | R | 15 | 9.2 | 6.01 | 95.0 | 94.3 | 3738 | 9128 | 0.00 |
| 18 | 9.9 | 8.9 | 96.8 | ** | ** | ** | ** | ||
| 21 | 11.6 | 23.6 | 95.2 | 97.6 | 3944 | 8858 | 0.34 | ||
| 6 | R | 15 | 9.4 | 12.9 | 96.9 | 99.0 | 2936 | 8569 | 0.45 |
| 18 | 9.4 | 18.7 | 87.7 | 99.7 | 3570 | 8909 | 0.00 | ||
| 21 | 11.1 | 31.4 | 84.5 | 99.9 | 5901 | 9319 | 0.12 | ||
| 7 | R | 15 | 7.3 | 3.9 | 70.0 | 94.8 | 4170 | 6349 | 0.05 |
| 18 | 10.1 | 8.0 | 92.4 | 95.9 | 5378 | 13293 | 0.13 | ||
| 21 | 12.4 | 20.9 | 94.7 | 88.9 | 6361 | 13206 | 0.80 | ||
| 8 | R | 15 | 8.0 | 4.4 | 81.4 | ** | ** | ** | 0.47 |
| 18 | 8.2 | 6.5 | 79.1 | 85.4 | 5167 | 9486 | 0.49 | ||
| 21 | 11.3 | 17.9 | 98.2 | 86.6 | 7873 | 16871 | 0.30 | ||
| 9 | R | 15 | 7.4 | 3.8 | 87.2 | 96.5 | 6817 | 9760 | 0.00 |
| 18 | 8.3 | 7.7 | 93.4 | 95.6 | 4928 | 8551 | 0.11 | ||
| 21 | 10.7 | 20.4 | 99.6 | 92.8 | 3228 | 12681 | 0.60 | ||
| 10 | R | 15 | 8.0 | 4.1 | 88.4 | 95.5 | 5742 | 15701 | 0.13 |
| 18 | 8.9 | 11.6 | 84.3 | ** | ** | ** | ** | ||
| 21 | 10.3 | 21.7 | 87.3 | 96.5 | 4796 | 8212 | 0.31 | ||
| 11 | R | 15 | 11.1 | 3.9 | 95.4 | 99.5 | 2560 | 7678 | 0.15 |
| 18 | 12.3 | 7.9 | 100.0 | 99.7 | ** | 8445 | 0.13 | ||
| 21 | 14.2 | 19.5 | 96.2 | 99.0 | 7702 | 10533 | 0.26 | ||
| r values | 0.58 | 0.64 | −0.15 | −0.16 | 0.30 | 0.02 | 0.22 | ||
| p values | < 0.01 | < 0.01 | 0.48 | 0.46 | 0.15 | 0.93 | 0.30 |
Data points not available
Although recorded food intakes did not change, a steady weight gain was appreciated in the DR animals beginning in the spring/summer of 2000. All equipment and procedures were fully evaluated; however, no definitive cause was discovered. The contemporaneous nature of the weight gain led us to believe that this was not a clear aging effect.
3.2 Upper Leg Muscle Mass
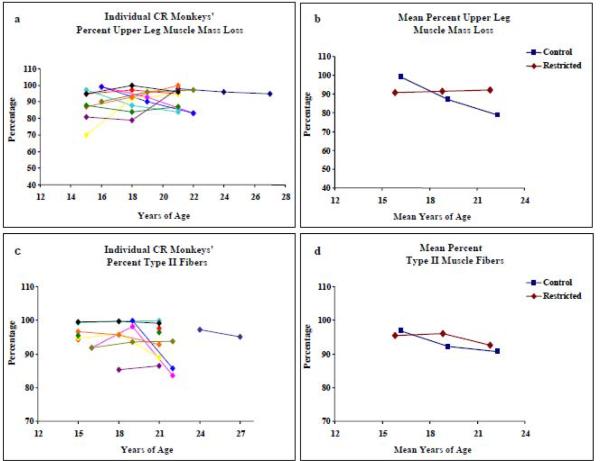
After 6 years on CR when animals were ~16 years of age, the average muscle mass of the upper leg was 90.8 ± 9.0% of their maximum upper leg muscle mass, at 9y it was 96.6 ± 6.1% and at 12y, 92.1 ± 5.4%. The correlation between muscle mass and increasing age, examining all CR animals over the length of the study, adjusted for repeated measures was not statistically significant (r = −0.15; p = 0.48, Table 1, Figure 1a). Over the course of the 12 year study, the average upper leg muscle mass loss of the CR monkeys was less than a half percent (0.4%) compared to 20% loss for Controls (p<0.01) (Figure 1b, Table 2). While the Control monkeys presented peak upper leg muscle mass at 16.6 ± 1.6 years of age, the average age for peak upper leg muscle mass in the CR monkeys was marginally higher at 18.2 ± 2.2 years (p=0.06).
Figure 1.
Left panels describe longitudinal muscle mass loss and percentage of Type II fibers in VL biopsies from 11 CR rhesus monkeys. Right panels compare mean values for the CR monkeys to the mean values from Control monkeys (McKiernan et al., 2009). Standard deviations for mean Control and Restricted measures are listed in Table 2.
Table 2.
Estimated correlations for measured variables with age and for differences in trends for variables between Control and CR monkeys.
| Diet | 6y | 9y | 12y | Est Corrd | p value | Fe | p value | |
|---|---|---|---|---|---|---|---|---|
| Age | C | 16.2 ± 0.5 | 19.2 ± 0.5 | 22.3 ± 0.5 | ||||
| R | 15.8 ± 0.5 | 18.8 ± 0.5 | 21.8± 0.5 | |||||
| %Mma | C | 99.0 ± | 87.1 | 78.8 | r = −0.70 | <0.01 | 16.41 | < 0.01 |
| R | 90.8 ± | 91.6 | 92.1 | r = −0.15 | 0.48 | |||
| % Type II | C | 96.7 ± 1.35 | 92.3 ± 1.5 | 90.7 ± 1.9 | r = 0.44 | 0.03 | 3.88 | 0.03 |
| R | 95.4 ± 1.03 | 96.1 ± 1.6 | 92.7 ± 2.0 | r = −0.16 | 0.46 | |||
| CSAb Type I | C | 5044 ± 598 | 4694 ± 448 | 6271 ± 762 | r = −0.06 | 0.67 | 0.32 | 0.73 |
| R | 4450 ± 543 | 4650 ± 329 | 6322 ± 631 | r = 0.23 | 0.11 | |||
| CSA Type II | C | 12073 ± 299 | 10152 ± 370 | 8579 ± 359 | r = −0.62 | 0.00 | 11.15 | < 0.01 |
| R | 9051 ± 1021 | 9733 ± 602 | 10983 ± 732 | r = 0.23 | 0.16 | |||
| ETSAbc | C | 0.14 ± 0.05 | 0.31 ± 0.06 | 1.05 ± 0.19 | r = 0.44 | 0.03 | 1.07 | 0.35 |
| R | 0.17 ± 0.06 | 0.82 ± 0.51 | 0.64 ± 0.36 | r = 0.22 | 0.30 |
% Muscle Mass (upper leg muscle mass / maximum upper leg muscle mass) × 100
Cross-sectional area (μm2)
Electron transport system enzyme abnormalities
Correlation with age
Difference between trends in Control and Restricted
3.3 Fiber Type Proportion and Fiber Cross-sectional Area
On the cellular level, aging is associated with a shift in fiber type distribution in skeletal muscle where the relative proportion of Type II muscle fibers decreases and the proportion of Type I fibers increases. In biopsies from CR animals the proportion of Type II muscle fibers did not change over the 6-year period of analysis (Table 1: r = −0.16; p = 0.46, Figure 1c). In contrast, a significant decrease in Type II fiber percentage was observed in Control monkeys (Figure 1d) demonstrating a significant difference in muscle fiber type composition in aging control and restricted monkeys (F=3.88, p = 0.03; Table 2).
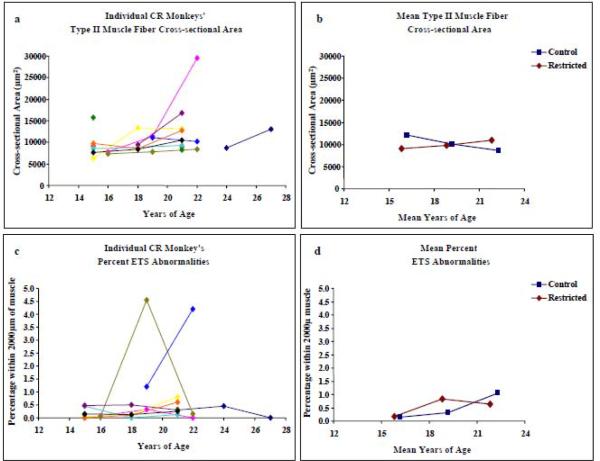
The average CSA of Type II muscle fibers in CR monkeys was 9051 ± 2887μm2 at year 6; the same monkeys at year 9 had an average muscle fiber CSA of 9733 ± 602μm2. At year 12 of the study, muscle fiber CSA was 10983 ± 732μm2 (Figure 2a). Among all CR animals in the study, the correlation between fiber CSA and age, adjusted for repeated measures, was not significant (r = 0.23, p = 0.16, Table 2). In contrast, the CSA of Type II muscle fibers in Control monkeys decreased >25% over the six years of the study, such that a difference was observed between Control and CR monkeys (F=11.15, p < 0.01, Figure 2b, Table 2). In CR monkeys, the CSA of Type I muscle fiber was not reduced with age; on the contrary, there was a tendency for Type I fiber CSA to increase (8/11 monkeys displayed this trend), although the difference in Type I fiber CSA between CR and Control was not statistically significant (p=0.73).
Figure 2.
Left panels describe longitudinal Type II fiber cross-sectional area (CSA) and the percentage of ETS abnormalities (ETSab) in 2mm of VL biopsy tissues from 11 CR rhesus monkeys. Right panels compare mean values for the CR monkeys to the mean values from Control monkeys (McKiernan et al., 2009). Standard deviations for mean Control and Restricted measures are listed in Table 2.
3.4 Muscle Fibers with Abnormal Mitochondrial Enzyme Activities
To determine the effect of CR on mitochondrial encoded Complex IV (COX) activity, the percentage of ETSab fibers per 2000μm of sectioned tissue was assessed. On average, there were 2173 ± 1070 muscle fibers per biopsy. Muscle fibers that had abnormal COX (negative) and SDH (hyper-reactive) enzyme activities were counted and followed along the length ETSab fibers The age-dependent accumulation of ETSab fibers observed in each of the 11 rhesus monkeys, over time, is presented in Figure 2c and Table 1. There was no significant correlation between the percentage of ETSab fibers and age in VL muscle from CR animals (r = 0.22; p = 0.30). In Controls, there was a significant increase in the percentage of ETSab over time; however; there was no significant difference between Control and CR monkeys with respect to the trends in the age-dependent accumulation of ETSab (Figure 2d, Table 2).
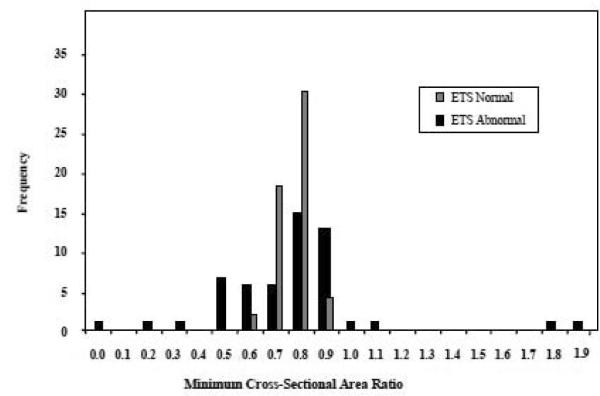
In rodents, muscle fibers bearing ETSab often display localized fiber atrophy. The CSA ratio (minimum CSA/average CSA) of ETSab fibers from CR monkeys (n=54) reveals that 64% of fibers had minimum CSA ratios within the normal range (0.6 – 0.9), 19% were hypertrophic and 17% were atrophic (Figure 3). The CSA ratio for ETSab fibers from Control monkeys (n=55) was 58% within the normal range, 16% hypertrophic and 26% atrophic (McKiernan et al., 2009). No difference in atrophy (minimum CSA ratio) trends was observed between normal and ETSab muscle fibers in CR animals (p= 0.46); however, similar to Control fed animals, the variance in the CSA ratio was greater for ETSab fibers than for normal fibers. The mean length of an ETS abnormality in CR monkey muscle fibers was 643 ± 385μm and no different from the mean length of ETS abnormalities in Control fibers (547 ± 292μm, p = 0.21).
Figure 3.
Fifty four ETS abnormal and 55 ETS normal muscle fibers were followed along their length and CSA of the fiber measured every 70μm. The minimum CSA recorded in the ETS abnormal region was divided by the mean CSA for the normal region (for normal fibers the minimum CSA of that fiber was divided by the mean CSA of the rest of the fiber), to provide the minimum CSA ratio. The frequency of fibers with a specific CSA ratio was plotted. The variance in minimum CSA ratio was greater in ETS abnormal fibers compared to ETS normal fibers.
3.5 Mitochondrial DNA Deletion Mutations
In Control animals, mtDNA deletion mutations ranging in size from 4.6 to 10.6 Kb were detected in all ETSab fibers that were tested [8]. To determine whether deletions within the mtDNA genome were also responsible for the ETSab observed in the CR animals, we used laser capture microdissection, DNA extraction and PCR amplification of mtDNA from ETSab regions of muscle fibers. In this approach 97% of the mitochondrial genome is amplified in the primary reaction, and in a secondary reaction, a 12Kb region spanning the major arc (containing the three mitochondrial encoded genes of Complex IV) is amplified as previously described [8]. We selected biopsies from two CR animals for this analysis, 39 ETSab and 18 normal fibers were laser captured. Mitochondrial DNA from 20 ETSab and 10 normal fibers were successfully amplified. In fibers with normal mitochondrial ETS enzyme activities, the primary amplification resulted in the predicted full length PCR product (>15kb). Twenty phenotypically abnormal fibers had PCR products that were less than full length. All deletion mutations were within the range of 6–8Kb. Deletion mutations removed a large portion of the major arc and involved at least one the three mitochondrial encoded COX subunits. No common deletions were observed among those sequenced. Representative sequence analysis of the mitochondrial DNA from three ETSab fibers is presented in Table 3.
Table 3.
mtDNA breakpoint analysis of ETSab fibers
| Fiber # | Breakpoints | Direct Repeats | Deletion Size | Deleted Genes |
|---|---|---|---|---|
| Fiber 7 | 15487 - 7878 | 5 | 7609 bp | CytB thru COX II |
| Fiber 12 | 15140 – 7415 | 0 | 7725 bp | CytB thru tRNA(S) |
| Fiber 22 | 15202 – 8837 | 9 | 6365 bp | CytB thru ATP(6) |
4. Discussion
It is widely accepted that sarcopenia is characterized by a combination of fiber loss and fiber atrophy. We have investigated the impact of long-term CR on cellular aspects of skeletal muscle aging in rhesus monkeys, a species in which CR significantly retards whole body muscle mass loss [5]. We have previously shown that muscle mass loss begins soon after muscles reach their maximum mass in Control monkeys. During early-stage sarcopenia this loss can be attributed to Type II muscle fiber atrophy. This change in CSA is concomitant with a decrease in the percentage of Type II muscle fibers and an increase the number of muscle fibers expressing mitochondrial deletion mutations [8].
The upper leg muscle aging profile from CR rhesus monkeys differed from that of the Controls. The most important effect of CR, as examined in this study, was the retention of muscle mass over the six years of the study. The maintenance of muscle mass in CR animals (a loss of 0.4%) contrasts with the loss of muscle mass over the same time period for the Controls (a loss of 20%).
Skeletal muscle is highly adaptive and fiber type composition can be altered in response to changes in energetic demand [22, 23]. VL is one of the largest muscles in the upper leg and is predominantly oxidative [7]. We have previously shown that the relative proportion of Type II fibers decreases between 15 and 26 years of age in rhesus monkey VL [8]. Type I fibers are typically more resistant to fiber atrophy than Type II muscle fibers. It is unclear if the change in fiber type proportion in Control monkeys is due to preferential loss of Type II fibers or due to fiber type conversion of Type II to the more resilient Type I. In this study, we show that the relative distribution of fiber type was unaltered in CR monkeys over the 6 year period of analysis.
Previously, we reported [8] that the CSA of Type II fibers from Control monkeys decreased by ~2000μm2 every three years between 6-, and 12-years of study. Over the same period of time, the CSA of Type II fibers from CR monkeys did not change. Furthermore, the change in Type II fiber CSA over the course of the study was significantly different between Control and CR. CR monkeys are smaller than Control animals with lower body mass and lower estimated muscle mass [5]. The average CSA of Type II muscle fiber from CR monkeys was smaller than that from Control monkeys at 6- and 9-years of the study (p=0.004 and p=0.003, respectively). At the 12-year time point of the study, however, Type II fiber CSA was greater (p= 0.01) in VL from the CR monkeys (10954 ± 2684 μm2) compared to the Controls (8400 ± 1276 μm2). These findings demonstrate a delay in the onset of sarcopenia at the cellular level with CR, and link cellular atrophy to age associated whole muscle atrophy.
We have proposed that age-dependent accumulation of ETSab within a muscle fiber may contribute to fiber atrophy and subsequent fiber loss [12]. Unlike the VL from Control monkeys, where increased numbers of ETSab fibers were detected over the course of the study, no significant increase in ETSab was detected in the VL from CR monkeys with age. Two CR monkeys had unusually high numbers of ETSab muscle fibers (#3 at 19y and #4 at 22y with 4.5% and 4.2%, respectively), greater than any observed in Control animals. The high percentage and fluctuation in the percentage of ETS abnormalities in two CR animals is difficult to interpret and may be due to sampling. Biopsies were taken from alternating limbs. Also, we have examined cross-sections of whole VL muscles from animals in the study that have died. Within the VL ETS abnormalities are not randomly distributed, a significantly higher proportion of ETS abnormalities appear in a specific quadrant of the muscle. Biopsies may have been taken from this ETS abnormality rich quadrant of the muscle and another biopsy from a less susceptible area of the VL. Neither of these animals had any condition known to negatively impact muscle.
Laser capture of ETSab fibers and subsequent PCR analyses from CR monkeys revealed large mtDNA deletion mutations. These data are consistent with PCR analysis of ETSab fibers from rats [12, 24, 25] and rhesus monkeys [11]. The etiology of the ETSab fibers (deletion mutations) appears to be similar in both Control and CR monkeys.
The progression of an ETS abnormality (length and fiber atrophy) was not different between Control and CR monkeys. Unlike rodent studies, we did not detect a correlation between fiber atrophy and ETSab for CR animals or for Controls [12, 26]. Permanent fiber loss, as observed in the rat model, is significant at 33 months of age (median lifespan), and highly correlates with an increase in ETS abnormalities [27]. The monkeys in this study had not yet advanced to median lifespan age (~26y). Also, monkey muscle fibers are large compared to those of most mammals. The progression to cell death (via atrophy) due to a single mitochondrial DNA deletion mutation may simply take a longer period of time. The fact that CR fed monkeys' Type II and Type I fibers maintain their cross-sectional area in the early stages of sarcopenia may provide a certain degree of tolerance for ETSab.
The etiology of fiber atrophy is complex and a number of processes have been implicated as being causative including muscle disuse [28], oxidative stress [29], systemic inflammation [30], increased protein degradation [31], impaired regenerative potential [32], loss of neuronal contact [33, 34] and selective apoptosis within the multinucleated fiber [35, 36]. In Control monkeys we have shown that the distribution of fiber types shifts with age and at the cellular level, fiber atrophy correlates with aging. CR prevents the shift in fiber type distribution and delays cellular atrophy in monkeys. It will be of interest to explore the potential role of mTOR and SIRT1, factors implicated in mitochondrial energy metabolism in CR studies [37]. A better understanding of how CR delays the events that trigger the rearrangement of fiber type distribution and cellular atrophy will reveal novel targets for treatment and prevention of sarcopenia.
Acknowledgements
We acknowledge the efforts of the veterinary staff of the Wisconsin National Primate Research Center. This work was supported by NIH grants P01 AG-11915; P51 RR000167 and the Ellison Medical Foundation Senior Scholar Award (Judd Aiken). This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01.
Abbreviations
- CR
calorie restriction
- DXA
dual-energy X-ray absorptiometry
- ETSab
electron transport system enzyme abnormalities
- VL
vastus lateralis
- mtDNA
mitochondrial DNA
- COX
cytochrome c oxidase
- SDH
succinate dehydrogenase
- CSA
cross-sectional area
- ESM
estimated skeletal muscle mass
- LCM
laser capture microdissection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Colman R, Anderson RM, Johnson SC, Dastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Holloszy JO, Fontana L. Caloric restriction in humans. Exp. Gerontol. 2007;42(8):709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Phys. and Behav. 2008;94:643–648. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in rhesus monkeys: Age of onset. Exp. Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [5].Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J. Gerontol. A. Biol. Sci. 2008;63(6):556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 2004;52(1):80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- [7].Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? J. Neuro. Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- [8].McKiernan SH, Colman R, Lopez M, Beasley TM, Weindruch R, Aiken JM. Longitudinal analysis of early stage sarcopenia in aging rhesus monkeys. Exp. Geron. 2009;44:170–176. doi: 10.1016/j.exger.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res. Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- [10].McKenzie D, Bua E, McKiernan S, Cao Z, Wanagat J, Aiken JM. Mitochondrial DNA deletion mutations: A causal role in sarcopenia. Eur. J. Biochem. 2002;269:2010–2015. doi: 10.1046/j.1432-1033.2002.02867.x. [DOI] [PubMed] [Google Scholar]
- [11].Gokey NG, Cao Z, Pak J, Lee D, McKiernan SH, McKenzie D, Weindruch R, Aiken JM. Molecular analyses of mtDNA deletion mutations in microdissected skeletal muscle fibers from aged Rhesus monkeys. Aging Cell. 2004;3:319–326. doi: 10.1111/j.1474-9728.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- [12].Herbst A, Pak J, McKenzie D, Bua E, Bassiouni M, Aiken J. Accumulation of mitochondrial DNA deletion mutations in aged muscle fibers: Evidence for a causal role in muscle fiber loss. J. Gerontol. 2007;62A:235–245. doi: 10.1093/gerona/62.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Singh MAF. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity and health. J. Gerontol. 2001;56A:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- [14].Thomas DR. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- [15].Kemnitz JW, Weindruch R, Roecher EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J. Gerontol. Biol. Sci. 1993;48:B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- [16].Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp. Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- [17].Gresl. TA, Colman RJ, Roecker EB, Havighurst TC, Huang Z, Allison DB, Bergman RN, Kemnitz JW. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am. J. Physiol. Endocrinol. Metab. 2001;281:E757–765. doi: 10.1152/ajpendo.2001.281.4.E757. [DOI] [PubMed] [Google Scholar]
- [18].Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. Battelle Press; Columbus, Ohio: 1980. [Google Scholar]
- [19].Seligman AM, Karnovsky MJ, Wasserkrug HL, Hanke,r JS. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB) J. Cell. Biol. 1968;38:1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dubowitz V. Muscle biopsy: a practical approach. Bailliere Tindall; London: 1985. [Google Scholar]
- [21].Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biometrical J. 2006;48:286–301. doi: 10.1002/bimj.200510192. [DOI] [PubMed] [Google Scholar]
- [22].Pette D. The adaptive potential of skeletal muscle fibers. Can. J. Appl. Physiol. 2002;27(4):423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- [23].Zierath JR, Hawley JA. Skeletal muscle fiber type: Influence on contractile and metabolic properties. PLoS Biology. 2004;2(10):1523–1527. doi: 10.1371/journal.pbio.0020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cao A, Wanagat J, McKiernan SH, Aiken JM. Mitochondrial DNA deletion mutations are concomitant with ragged red regions of individual, aged muscle fibers: analysis by laser-capture microdissection. Nucleic Acids Res. 2001;29(21):4502–4508. doi: 10.1093/nar/29.21.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15(2):322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- [26].Bua E, McKiernan SH, Aiken JM. Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle. FASEB J. 2004;18(3):582–584. doi: 10.1096/fj.03-0668fje. [DOI] [PubMed] [Google Scholar]
- [27].Lushaj EB, Johnson JJ, McKenzie D, Aiken J. Sarcopenia accelerates at advanced aged in Fisher 344×Brown Norway rats. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:921–927. doi: 10.1093/gerona/63.9.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Degens H. Age-related skeletal muscle dysfunction: causes and mechanisms. J Musculoskelet Neuronal Interact. 2007;7(3):246–252. [PubMed] [Google Scholar]
- [29].Rossi P, Marzani B, Giardina S, Negro M, Marzatico F. Human skeletal muscle aging and the oxidative system: cellular events. Curr Aging Sci. 2008;1(3):182–191. doi: 10.2174/1874609810801030182. [DOI] [PubMed] [Google Scholar]
- [30].Roth SM, Metter EJ, Ling S, Ferrucci L. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- [31].Nair KS. Age-related changes in muscle. Mayo Clinic Proc. 2000;75(suppl):S14–S18. [PubMed] [Google Scholar]
- [32].Degens H, Always AE. Control of muscle size during disuse, disease, and aging. Int. J. Sports Med. 2006;27(2):94–99. doi: 10.1055/s-2005-837571. [DOI] [PubMed] [Google Scholar]
- [33].Larsson L. Motor units: remodeling in aged animials. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- [34].Hollmann W, Struder HK, Tagarakis CV, King G. Physical activity and the elderly. Eru J Cardiovasc Prev Rehabil. 2008;14(6):730–739. doi: 10.1097/HJR.0b013e32828622f9. [DOI] [PubMed] [Google Scholar]
- [35].Tews DS. Muscle-fiber apoptosis in neuromuscular diseases. Muscle and Nerve. 2005;32:443–458. doi: 10.1002/mus.20348. [DOI] [PubMed] [Google Scholar]
- [36].Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anderson RM, Weindruch R. Metabolic reprogramming, caloric restriction and aging. Trends Endocrinol Metab. 2010;21(3):134–141. doi: 10.1016/j.tem.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]