Abstract
Objectives
To examine the association between physical activity (PA) and Alzheimer's disease (AD) course.
Background
PA has been related to lower risk for AD. Whether PA is associated with subsequent AD course has not been investigated.
Methods
In a population-based study of individuals ≥65 in New York who were prospectively followed with standard neurological and neuropsychological evaluations (every ~1.5 years), 357 participants (i) were non-demented at baseline and (ii) were diagnosed with AD during follow-up (incident AD). PA (sum of participation in a variety of physical activities, weighted by the type of activity [light, moderate, severe]) obtained 2.4 (sd. 1.9) years before incidence was the main predictor of mortality in Cox models and of cognitive decline in GEE models that were adjusted for age, gender, ethnicity, education, comorbidities and duration between PA evaluation and dementia onset.
Results
150 incident AD cases (54%) died during the course of 5.2 (sd 4.4) years of follow-up. As compared to incident AD cases who were physically inactive, those with some PA had lower mortality risk, while incident AD participants with much PA had an even lower risk. Additional adjustments for APOE genotype, smoking, comorbidity index and cognitive performance did not change the associations. PA did not affect rates of cognitive or functional decline.
Conclusion
Exercise may affect not only risk for AD but also subsequent disease duration: more PA is associated with prolonged survival in AD.
Keywords: Alzheimer's disease, Epidemiology
INTRODUCTION
Although some studies have failed to detect a significant association (1, 2) between physical activity (PA) and Alzheimer's disease (AD) or dementia risk, most studies (3-8), including a recent one from the present cohort (9) have reported beneficial effects of exercise regarding either rates of cognitive decline or dementia. Nevertheless, whether PA is associated with further AD course and prognosis has not been investigated. At the same time it has been show that PA can slow down or prevent functional decline associated with aging and improve health in the elderly, including reducing overall mortality (4, 10).
We hypothesized that PA may be associated with reduced mortality, and possibly, rates of cognitive decline in AD populations too. We explored this hypothesis using data from a population longitudinal study (Washington Heights-Inwood Columbia Aging Project; WHICAP). More specifically, we sought to examine the association between PA and longevity and cognitive decline in a group of subjects who were non-demented at their baseline assessment, developed AD during follow-up and continued to be prospectively followed.
SUBJECTS AND METHODS
Sample and diagnoses
The sample for the current study has been described in previous studies (11, 12). Briefly, the study included participants of 2 related cohorts recruited in 1992 (WHICAP 1992) and 1999 (WHICAP 1999) which were identified (via ethnicity and age stratification processes) from a probability sample of Medicare beneficiaries residing in an area of 3 contiguous census tracts within a geographically defined area of northern Manhattan (13). The same assessments and study procedures were used in both cohorts. At entry, a physician elicited each subject's medical and neurological history and conducted a standardized physical and neurological examination. All available ancillary information (medical charts, CTs or MRIs) was considered in the evaluation.
Each subject also underwent a structured in-person interview including an assessment of health and function and a neuropsychological battery that contained tests of memory (short and long-term verbal and nonverbal); orientation; abstract reasoning (verbal and non-verbal); language (naming, verbal fluency, comprehension and repetition); and visual-spatial abilities (copying and matching) (14). A global summary score on the Clinical Dementia Rating (CDR) was also assigned.
A consensus diagnosis for the presence or absence of dementia was made at a diagnostic conference of neurologists and neuropsychologists where information of all the above evaluations was presented. Evidence of cognitive deficit (based on the neuropsychological scores as described above), evidence of impairment in social or occupational function (as assessed by the Blessed Dementia Rating Scale, the Schwab and England Activities of Daily Living Scale and the physician's assessment), and evidence of decline in cognitive and social-occupational function as compared to the past were the criteria used for the diagnosis of dementia as required by the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R). The type of dementia was subsequently determined. For the diagnosis of probable or possible AD, the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association were used. PA data were not available to the consensus panel and were not considered in the diagnostic process.
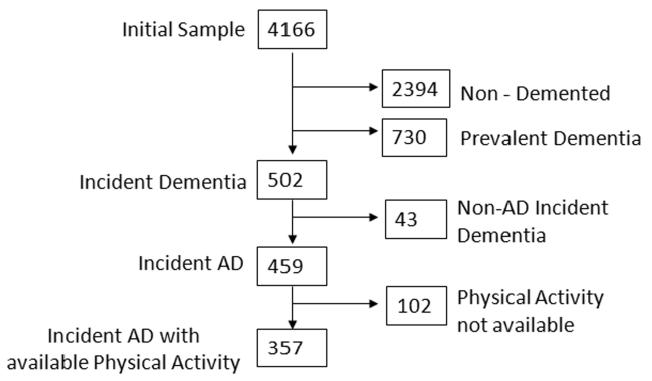
Subjects were followed at intervals of approximately 1.5 years, repeating the baseline examination and consensus diagnosis at each follow-up. Among all potential participants in both WHICAP cohorts, 357 individuals were considered for the present study (figure 1). These were subjects with available PA evaluations who were non-demented at the baseline WHICAP evaluation and developed AD during follow-up (incident AD patients).
Figure 1.
Flow chart describing sample.
Evaluation
Predictors
Exposure: Physical Activity
A modified Godin leisure time exercise questionnaire was used (15). Test-retest (2 weeks to 1 month) reliability correlation coefficients of this instrument have ranged between 0.62 and 0.81 (15-17). Validity of the questionnaire has been demonstrated in relation to multiple measures including body fat (15, 16), VO2 max (15, 16), caltrac actometer (16, 18), treadmill time (16) and other similar activity questionnaires (17, 18).
Two slightly different versions of the questionnaire were used (15). A subset of the participants (n=149) were queried about the most recent two week period in which they engaged in their typical number of activities. The number of times of participation and the number of minutes each time were recorded for 3 different categories of activities: vigorous (aerobic dancing, jogging, playing handball), moderate (bicycling, swimming, hiking, playing tennis) and light (walking, dancing, calisthenics, golfing, bowling, gardening, horseback riding). We constructed a summary PA score for each subject using the following formula: number of minutes x number of times x coefficient (9 for vigorous, 5 for moderate and 3 for light activities [the numbers 9, 5 and 3 corresponding to metabolic equivalents of related activities] (15)). Because of skewed distribution the summary PA score was categorized into a 3-level variable with similar number of subjects / group: No PA, Some PA and Much PA. A different subset of subjects who received an earlier version of the questionnaire (n=208) were queried regarding their PA in a slightly different way: number of hours during the most recent month in which they engaged in their typical number of activities. Using procedures similar to the ones described above, a PA score was again calculated and categorized into a 3-level variable: No PA, Some PA and Much PA. Although categorization of subjects in PA categories was performed within each version of the questionnaire (i.e. separately for the 149 and for the 208 subjects), in supplementary analyses we additionally explored inclusion of a term representing the PA questionnaire version (earlier version as the reference) in the analyses.
PA and Incidence timing
One of the problems of using either clinic-based populations or populations with prevalent dementia is that their current PA may have already been affected by the disease process. In this study of incident AD subjects, PA was queried on average 2.4 (sd. 4.2) years before incidence. We ran survival analysis models using either of the following two timing variables : (i) time from PA assessment to death or last follow-up and (ii) time from AD incidence to death or last follow-up, controlling for time between reported PA and AD incidence. Results were similar, and time from PA assessment is reported in this manuscript. Nevertheless, in order to further control for potential bias, we included as a covariate a term adjusting for the difference in time between PA assessment and AD incidence.
For the vast majority of the AD patients the PA evaluation took place before incidence; however for 81 AD patients PA was ascertained at or after incidence. We performed supplementary analyses excluding these 81 subjects (leaving 276 incident AD subjects for the analyses and 114 deaths during follow-up).
Other covariates
Age at study enrollment (years) and education (years) were used as continuous variables. We also considered period of recruitment (1992 cohort as reference), and gender (men as reference). Ethnic group was based on self-report using the format of the 1990 census. Participants were then assigned to one of four groups: Black (non-Hispanic), Hispanic, White (non-Hispanic) or Other. Ethnicity was used as a dummy variable with White (non-Hispanic) as the reference. In supplementary analyses we considered smoking status (never smoker vs. current or past smoker) and apolipoprotein (APOE) genotype (absence of ε4 allele vs. presence of either 1 or 2 ε4 alleles).
We also considered potential competing causes of mortality by calculating a modified version (19, 20) of the Charlson Index of Comorbidity (21) (referred to as ‘comorbidity index’) which included items for myocardial infarct, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal disease, mild liver disease, diabetes, chronic renal disease, and systemic malignancy. All items received weights of one, with the exception of chronic renal disease and systemic malignancy, which were weighted two.
In the mortality outcome survival analyses we also considered subjects' cognitive performance at the time of PA assessment (cognitive performance at the time of AD incidence produced similar results and is not reported) as a covariate (see below outcomes section for cognitive performance calculation).
Outcomes
Mortality was tracked through follow-up interviews every 18 months and through submission of identifying information for subjects reported to be dead or lost to follow-up to the National Death Index.
Calculation of cognitive performance scores in WHICAP has been previously described (22-25). Briefly, we used factor analysis with principal axis factoring and oblique rotation on 15 neuropsychological test score variables (14) which grouped cognitive performance into four factors: memory, language, processing speed, and visual-spatial ability. Using cognitive test data from all WHICAP participants at their initial visit, Z-scores for each of the cognitive measures that comprised each factor were created and then averaged to create a composite score for each factor. These factor domain scores were then averaged to derive a composite cognitive z-score.
Calculation of functional performance scores was performed as follows. Items from the Disability and Functional Limitations Scale (26-28) were used to elicit self- and observer ratings of basic and instrumental activities of daily living. Basic activities of daily living included using the bath-shower, getting to toilet, dressing, brushing the hair and feeding, while instrumental activities of daily living included dialing a telephone, preparing-cooking meals, shopping, medication administration, light chores (dishes-stove-garbage), and handling of personal business. Using dichotomous forms of these activities a sum of total (basic and instrumental) activities of daily living score was calculated for each subject for each evaluation (range 0-11, with higher scores indicating worse function).
Statistical analyses
Baseline characteristics of subjects by missing PA data, by mortality status and by PA groups were compared using t-test or ANOVA for continuous baseline characteristic of subjects (age, education, comorbidity index, cognition z-score) and χ2 test for categorical baseline characteristics of subjects (gender, ethnicity, APOE).
Mortality
We calculated Cox proportional hazards models with mortality as the dichotomous outcome. The time-to-event variable was time from PA assessment to death; persons who did not die were censored at the time of their last follow-up. In initial models PA was the main predictor. In adjusted models we controlled for the following variables: period of recruitment, age, gender, ethnicity, education and time between PA and AD incidence. All predictors were used as time-constant covariates. We then constructed a series of supplementary models in which we additionally considered smoking, APOE, comorbidity index and cognitive performance (at time of PA evaluation) and PA questionnaire version.
In the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association stroke does not preclude the diagnosis of AD (unless cerebrovascular disease is considered the primary cause of dementia). We conducted additional analyses excluding from our 357 AD patients all subjects with either stroke or other concomitant disease and keeping only subjects with ‘pure’ probable AD.
We finally examined the association between PA and mortality in a separate group of subjects who were non-demented at initial evaluation and remained non-demented throughout the follow-up. We examined for differential associations of PA with mortality in non-demented subjects (as compared to AD patients): we computed a Cox survival model that included terms for (i) dementia, (ii) PA and (iii) dementia × PA interaction.
Rates of cognitive and functional decline
The primary outcome in these analyses was rate of decline in cognition as assessed at each study visit. Cognitive performance at the time of PA assessment and at each follow-up visit was calculated as described in the outcome section above. We used generalized estimating equations (GEE) to test whether PA was associated with differential rates of cognitive change. The repeated cognitive performance scores for each subject were treated as a cluster. The GEE models included the composite cognitive z-score measure as the dependent variable and, as predictors, PA, time (in years from PA assessment) and a PA × time interaction. A significant interaction term would indicate differential rates of change in cognitive function as a function of PA. The models were adjusted for period of recruitment, age, gender, ethnicity, education, smoking, APOE, comorbidity index, time between PA and AD incidence and initial cognitive performance (at time of PA evaluation).
Finally, similar models were calculated with primary outcome rates of functional decline as assessed at the time of PA assessment and at each follow-up visit (calculated as described in the outcome section above).
RESULTS
Missing data analyses
Compared to the 102 subjects with missing PA information, subjects included in the study (n=357) (figure 1) had better cognitive performance (z scores −1.21 for those with missing vs. −0.43 for those included; t=5.72; df=356; p<0.001), better function (5.7 vs. 2.9 activities of daily living score; t=4.24; df=352; p<0.001) and more comorbidities (1.6 vs. 2.1; t=1.70; df =457; p=0.007) and were more commonly Hispanic and less commonly White or Black (20% vs. 10% White, 36% vs. 31% Black,. 42% vs. 58% Hispanic, 2% vs. 1% Other; x2=10.57; df=3; p=0.01), while the groups did not differ in age (79.9 vs. 78.8; t=1.46; df=456; p=0.14), gender (23% male vs. 31% male; x2=0.11; df=1; p=0.11), education (7.3 vs. 7.2 years; t=0.34; df=455; p=0.74), APOE (23% vs. 34% ε4 carriers; x2=0.09; df=1; p=0.09) or smoking status (42% vs. 49% current or past smokers; x2=0.22; df=1; p=0.22).
Clinical-demographic characteristics
Some PA corresponded to approximately a quarter of an hour of vigorous activity / week or half an hour of moderate activity /week or 1 hour of light activity /week (or a combination thereof). Much PA corresponded to approximately 1 hour of vigorous or 2 hours of moderate or 3.5 hours of light activity / week (or a combination thereof). Compared with incident AD subjects who remained alive, those that died were older, had higher education, were more likely to be White or Black and less likely to be Hispanic, had more functional impairment and were less physically active (table 1). The groups did not differ in gender, medical comorbidities, APOE status or cognitive performance. Compared to AD subjects who were physically inactive, those that reported more physical activities had fewer medical comorbidities, better cognitive performance and better function, but did not differ in other clinical demographic characteristics (table 2).
Table 1.
Demographic, clinical and physical activity (PA) characteristics for Alzheimer's Disease patients who did and did not die during follow-up.
| Alive N = 207 |
Dead N = 150 |
All N = 357 |
df | P | ||
|---|---|---|---|---|---|---|
| Age yrs, mean (SD) * | 77.8 (6.4) | 80.2 (6.9) | 78.8 (6.7) | 354 | <0.001 | |
| Gender-Men, N (%) ** | 65 (31 ) | 45 (30) | 110 (31) | 1 | 0.78 | |
| Education yrs, mean (SD) * | 6.5 (4.5) | 8.0 (4.8) | 7.2 (4.7) | 353 | 0.004 | |
| Ethnicity, N (%) ** | White | 18 (9) | 19 (13) | 37 (10) | 3 | 0.03 |
| Black | 54 (26) | 57 (38) | 111 (31) | |||
| Hispanic | 133 (64) | 73 (49) | 206 (58) | |||
| Other | 2 (1) | 1 (1) | 3 (1) | |||
| Physical Activity, N (%) ** | None | 77 (37) | 66 (44) | 143 (40) | 2 | 0.05 |
| Some | 58 (28) | 50 (33) | 108 (30) | |||
| Much | 72 (35) | 34 (23) | 106 (30) | |||
| Presence of ε4 allele (%) ** | 62 (33) | 48 (35) | 110 (34) | 1 | 0.61 | |
| Comorbidity index mean (SD) * | 2.0 (1.4) | 2.1 (1.4) | 2.1 (1.4) | 355 | 0.38 | |
| Cognition z-score, mean (SD) * | −0.47 (0.61) | −0.38 (0.58) | -0.44 (0.60) | 335 | 0.18 | |
| Function ADL score, mean (SD) * | 2.6 (2.8) | 3.3 (3.2) | 2.9 (3.0) | 329 | 0.02 | |
t-test
x2 test
df: degrees of freedom (may vary depending on missing data in some of the analyses).
Table 2.
Demographic and clinical characteristics by Physical Activity (PA) score groups for all Alzheimer's disease subjects.
| No PA N = 143 |
Some PA N = 108 |
Much PA N = 106 |
df | P | ||
|---|---|---|---|---|---|---|
| Age yrs, mean (SD) * | 79.4 (6.8) | 79.0 (6.9) | 77.8 (6.4) | 355 | 0.15 | |
| Gender-Men N (%) ** | 41 (29) | 34 (32) | 35 (33) | 2 | 0.75 | |
| Education yrs, mean (SD) * | 7.1 (5.1) | 7.5 (4.7) | 6.8 (4.1) | 354 | 0.51 | |
| Ethnicity, N (%) ** | White | 14 (10) | 13 (12) | 10 (9) | 6 | 0.34 |
| Black | 39 (27) | 41 (38) | 31 (29) | |||
| Hispanic | 89 (62) | 54 (50) | 63 (59) | |||
| Other | 1 (1) | 0 (0) | 2 (2) | |||
| Presence of ε4 allele (%) ** | 40 (33) | 37 (36) | 33 (33) | 2 | 0.89 | |
| Comorbidity Index, mean (SD) * | 2.3 (1.4) M | 2.1 (1.4) | 1.8 (1.4) N | 356 | 0.02 | |
| Cognition z-score, mean (SD) * | −0.70 (0.60)SM | −0.28 (0.59)N | −0.27 (0.49)N | 336 | <0.001 | |
| Function ADL score, mean (SD) * | 4.6 (3.1) SM | 2.1 (2.6)N | 1.4 (1.9)N | 330 | <0.001 | |
ANOVA; Subgroup comparisons p<0.05 for different PA groups according to post-hoc Scheffe's test. N: No PA, S: Some PA, M: Much PA
x2 test
df: degrees of freedom (may vary depending on missing data in some of the analyses).
PA and mortality
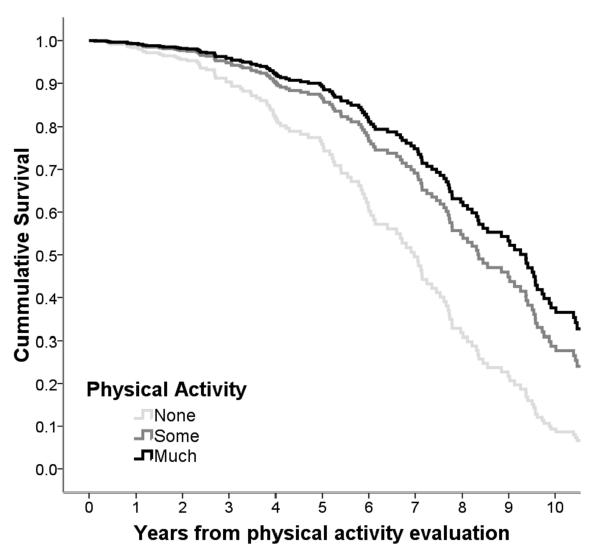
Median survival was 9 years (95% Confidence Interval [CI] 8-10) from PA evaluation (4.8 [4.0-5.5] years since dementia incidence (29)). In unadjusted models (table 3, model 1), compared to physically inactive AD patients (median survival 4.2 [3.2-5.3] years), those reporting some PA had 76% reduced mortality risk (median survival 9.6 [8.7-10.4] years), while those reporting much PA had 80% risk reduction (median survival 12 [10.9-13.1] years). Controlling for demographic variables (table 3, model 2 and figure 2) associations remained strongly significant. Additionally adjusting for APOE genotype, smoking, medical comorbidities and cognitive performance (table 3, model 3) did not change the associations: 43% risk reduction for AD patients reporting some PA and 53% for those reporting much PA.
Table 3.
Hazard Ratios (HRs) for mortality by Physical Activity (PA) score as calculated by Cox survival analyses.
| Model | All | Dead | PA groups |
HR (95%CI) | Wald | df | P value |
|---|---|---|---|---|---|---|---|
| 1 | 357 | 150 | No | 1 (reference) | - | ||
| Some | 0.34 (0.23 – 0.49) | 32.4 | 1 | <0.001 | |||
| Much | 0.20 (0.13 – 0.31) | 53.5 | 1 | <0.001 | |||
| Trend | 0.43 (0.35 -0.54) | 54.2 | 1 | <0.001 | |||
| 2 | 355 | 150 | No | 1 (reference) | - | ||
| Some | 0.53 (0.34 – 0.80) | 8.9 | 1 | 0.003 | |||
| Much | 0.41 (0.25 – 0.67) | 12.6 | 1 | <0.001 | |||
| Trend | 0.63 (0.49 -0.81) | 12.8 | 1 | <0.001 | |||
| 3 | 310 | 131 | No | 1 (reference) | - | ||
| Some | 0.57 (0.36 – 0.89) | 6.0 | 1 | 0.01 | |||
| Much | 0.47 (0.28 – 0.80) | 8.0 | 1 | 0.005 | |||
| Trend | 0.68 (0.52 -0.89) | 8.0 | 1 | 0.005 |
df: degrees of freedom
Model 1 is unadjusted.
Model 2 is adjusted for period of recruitment, age, gender, ethnicity, education and duration between PA evaluation and dementia incidence.
Model 3 is adjusted for all the covariates of model 2, plus APOE genotype, smoking, comorbidity index and cognitive performance at PA evaluation.
Figure 2.
Survival curves based on Cox analyses comparing Alzheimer's disease mortality in subjects belonging to each physical activity group (p for trend <0.001). The figure is derived from a model that is adjusted for cohort, age, gender, ethnicity, education and time duration between PA evaluation and dementia incidence and follow-up is truncated at 10 years.
Although categorization of subjects in PA groups was performed within each version of the questionnaire, in supplementary analyses we additionally adjusted for PA questionnaire version: compared to physically inactive AD subjects, those reporting some PA had a HR 0.51 (0.33-0.77; Wald=10.2; p=0.001) and those reporting much PA a HR of 0.31 (0.20-0.50; Wald=23.6; p<0.001); trend HR 0.56 (0.44-0.71; Wald=23.2; p<0.001). Additionally adjusting for clinical demographic variables did not change the associations: some PA 0.57 (0.36-0.89; Wald=6.1; p=0.01), much PA 0.44 (0.27-0.73; Wald=10; p=0.002), trend 0.66 (0.51-0.86; Wald=9.8; p=0.002).
Excluding the 81 subjects whose PA evaluation took place at or after incidence (leaving 276 incident AD subjects for the analyses and 114 deaths during follow-up) results were as follows. In unadjusted models, compared to physically inactive AD subjects, those reporting some PA had a HR 0.43 (0.28-0.67; Wald=14.5; p<001) and those reporting much PA a HR of 0.25 (0.15-0.40; Wald=31.9; p<0.001); trend HR 0.49 (0.38-0.63; Wald=31; p<0.001). Additionally adjusting for clinical demographic variables did not change the associations: some PA 0.64 (0.40-1.03; Wald=3.5; p=0.06), much PA 0.48 (0.27-0.82; Wald=7.1; p=0.008), trend 0.69 (0.52-0.91; Wald=7; p=0.008).
There were 262 subjects with probable AD (without stroke or other concomitant disease). In models adjusting for clinical demographic variables, compared to physically inactive probable AD patients, those reporting some PA had a HR 0.50 (0.30-0.81; Wald=7.7; p=0.005) and those reporting much PA a HR of 0.36 (0.20-0.65; Wald=11.7; p=0.001); trend HR 0.59 (0.44-0.80; Wald=12; p=0.001).
There were 2185 subjects with available physical activity information who were non-demented at the initial evaluation and remained non-demented during follow-up. In a model adjusted for clinical demographic variables the results were as follows: compared to physically inactive non-demented subjects, those reporting some PA had a HR 0.69 (0.57-0.83; Wald=15; p<0.001) and those reporting much PA a HR of 0.57 (0.46-0.70; Wald=27.2; p<0.001); trend HR 0.75 (0.67-0.84; Wald=27.4; p<0.001). In a model that included terms for (i) dementia, (ii) PA and (iii) their interaction (adjusted for clinical demographic variables) HR for the dementia x PA interaction was 1.05 (0.84 – 1.31; Wald=0.2; p = 0.67). Therefore, formal statistical testing did not provide any evidence for differential association of PA with mortality in non-demented and demented subjects.
PA and rates of cognitive and functional decline
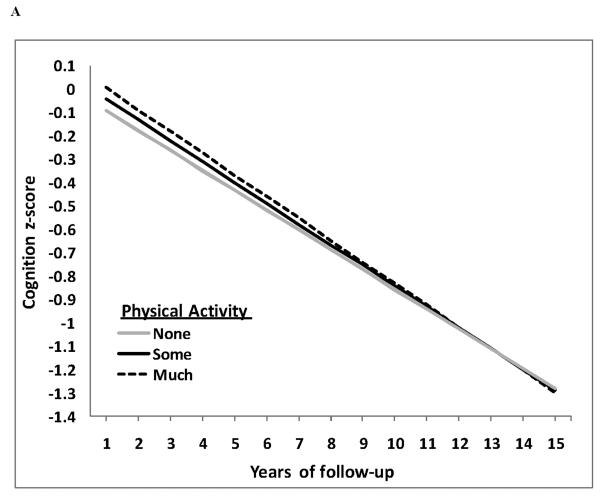
214 incident AD patients had at least 2 cognitive evaluations (permitting calculation of longitudinal changes of cognition) after their PA evaluation (mean 3.7 cognitive assessments; range 2-7). In GEE models adjusted for period of recruitment, age, gender, ethnicity, education, and duration between PA evaluation and dementia onset there was no association between PA and rates of cognitive decline: β = −0.008; z = −0.8; p = 0.42 (figure 3A). Additionally adjusting for APOE genotype, smoking, comorbidity index and cognitive performance at PA assessment did not reveal any association between PA and rates of cognitive decline: β = −0.004; z = −0.4; p = 0.69.
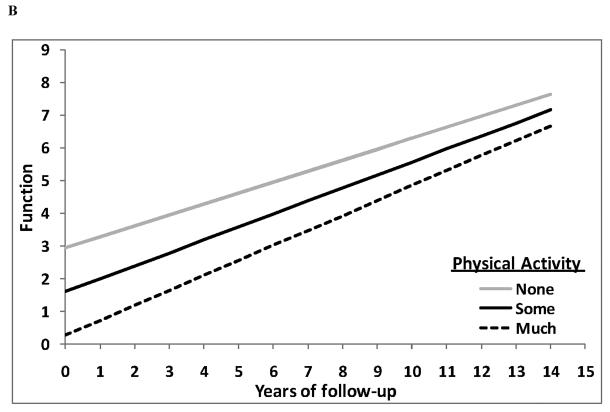
Figure 3.
GEE predicted cognitive z-scores (figure 3A) and GEE predicted function (ADLs) (figure 3B) (y-axis) over the course of follow-up in years (x-axis) separately for the none (grey solid line), some (black solid line) and much (dashed line) PA groups from a fully adjusted model. Lower cognitive z-scores and higher function scores indicate clinical worsening. Neither rates of cognitive (time × PA group interaction β = −0.004; p = 0.69) nor rates of functional (time × PA group interaction β = 0.063; p = 0.18) decline differed among PA groups.
208 incident AD patients had at least 2 complete functional evaluations (permitting calculation of longitudinal changes of function) after their PA evaluation (mean 3.6 functional assessments; range 2-7). In GEE models adjusted for period of recruitment, age, gender, ethnicity, education, and duration between PA evaluation and dementia onset there was no association between PA and rates of functional decline: β = 0.065; z = 1.3; p = 0.18 (figure 3B). Additionally adjusting for APOE genotype, smoking, comorbidity index and cognitive performance at PA assessment did not reveal any association between PA and rates of functional decline: β = 0.063; z = 1.3; p = 0.18.
DISCUSSION
Previous work has suggested that PA may reduce risk of developing AD. According to the current analyses PA does not seem to be associated with rapidity of cognitive change but it seems to relate to prolongation of survival of patients with AD. We noted a gradual reduction in mortality risk with higher reported PA, which suggests a possible dose-response effect. The magnitude of the effect was considerable: as compared to physically inactive subjects, those reporting some PA were 43-47% less likely to die, while those reporting much PA had a 53-59% reduction in mortality. Translating this into median survival times from the PA ascertainment, as compared to physically inactive subjects, those with some PA lived ~5.5 years longer, while those with much PA had a longer survival of ~8 years.
Previous large general population studies indicated that PA can slow down or prevent functional decline associated with aging, improve health in the elderly, and reduce overall mortality in the general population (4, 10). To our knowledge no previous studies have investigated the effect of PA on AD course. The PA-related prolongation of survival in AD patients may be mediated by a direct effect on AD clinical (3, 5-8) or pathological (30) progression. Nevertheless, we found no evidence for modification of rates of cognitive decline. It is possible that while PA before AD onset may have beneficial effects (associated with lower risk for manifesting the disease (3-9)), PA close or after AD onset may be non-differential regarding further course in the face of accumulated AD pathology. The association between PA and survival may relate to reduction of medical comorbidities (31). The fact that the association persisted after adjusting for such comorbidities may indicate measurement error and incomplete adjustment for comorbid conditions. Our analyses in non-demented elderly suggest that the observed association between PA and mortality in AD patients is not dissimilar to the one seen for the general population.
Importantly, the protection from mortality was present despite controlling for multiple potential confounders. Older age has been associated with worse survival in multiple publications (20, 29, 32-34). Many studies have reported shorter survival for demented men, as compared to women (35). Regarding ethnic differences, the literature is mixed with some studies finding higher mortality for Caucasians with AD (29, 36), and some others reporting no race differences (34). Higher education has been associated with faster rates of cognitive decline (25) and increased mortality (37) in AD patients. In our data we detected no association between PA and any of the above factors. Most important, the association between PA and AD mortality persisted despite controlling for all the above characteristics.
The APOE genotype has been related to mortality in the general population (38) although this effect may vary for different ethnicities (39). In AD, the APOE genotype has been shown to relate to clinical (40-42) and physiological (43, 44) heterogeneity, and cognitive decline (45) but its relation to mortality has been debatable (42). Because of the above we considered the APOE genotype in our analyses but detected no differences in PA between ε4 carriers and non-carriers and no modification of the PA–mortality association when adjusting for APOE genotype.
Study limitations include the following. PA was based on reporting and physiological measurement of VO2 maximum (46) or another objective method of physical fitness evaluation has not been performed. Objectively measured PA may relate to cognitive performance better than self-reported one (47). To the extent that PA measurement error is unrelated to AD mortality outcome, this may bias our results towards the null. Two different variants of the PA assessment instrument were used but the ranking of subjects into groups was performed within each version of the instrument and the associations remained when models were additionally adjusted for PA questionnaire. PA was more weighted towards leisure-recreational type of activities while the contribution of physical components of everyday activities was not recorded.
Level of cognitive performance or disease severity may be a confounder since it has been shown to relate to both our predictor (PA may decrease in AD (46)) and our outcome (better cognitive performance at baseline has been associated with longer AD survival (20, 48)). Therefore, another potential explanation for our findings is that differential PA could be an indirect index of AD severity (versus a prognostic marker). Recent reports demonstrating associations between physical frailty (including strength and motoric speed) and AD pathology (49) would support this explanation. A closely related issue is the potential inaccuracies in PA reports by subjects with cognitive problems, such as the AD patients in this study. Finally, incident AD subjects with missing PA information had worse cognitive performance. Arguments against these limitations in our study include the following: (i) PA was ascertained before AD incidence for the vast majority of our subjects, (ii) selecting subjects with PA assessments only before incidence (when cognitively non-demented) did not affect the results, (iii) the associations remain unchanged when we used either time from PA or time from incidence in survival analyses, (iv) we observed no changes in the relation between PA and mortality when adjusting for duration between PA and incidence and (v) there was a significant PA effect even when we adjusted for initial cognitive performance. Despite the above, reverse causality cannot be excluded.
Subjects excluded from the present analyses because of unavailable PA did not differ in most characteristics but had lower cognitive and functional performance, fewer medical comorbidities and were less likely to be Hispanic and more likely to be Black or White. Worse cognition and function suggests that subjects excluded were more likely to be demented. Fewer comorbidities suggests that subjects excluded probably did not have lower levels of PA, while their ethnicity should be non-differential in terms of PA levels. Overall, missing PA data analyses does not suggest a consistent clear pattern of better health and function for subjects included in the analyses. When we adjusted for ethnicity, comorbidities and cognitive performance the associations between PA and mortality were unchanged. Additionally, differential duration of follow-up may have affected our ability to accurately estimate rates of cognitive and functional decline (50). Overall, although it does not seem very likely, we cannot completely exclude that our results could be explained by biases related to missing information.
Elderly adults are often quite physically inactive. Some PA in this cohort of 79 year-old participants corresponded to approximately a quarter of an hour of vigorous activity / week or half an hour of moderate activity /week or 1 hour of light activity /week (or a combination thereof). Much PA corresponded to approximately 1 hour of vigorous or 2 hours of moderate or 3.5 hours of light activity / week (or a combination thereof). Nevertheless, even this relatively small amount of PA was associated with significant reductions in AD mortality. Approximately one third of this population (the ones reporting much PA) were meeting the aerobic PA recommendations of the American College of Sports Medicine and the American Heart Association (51). We had no information collected on additional muscle-strengthening, flexibility and balance exercises, recommended by the American College of Sports Medicine and the American Heart Association (51). Despite the overall small amount of PA (in particular for subjects reporting no and some PA) there was a large relative quantitative difference among the groups: for example subjects reporting much PA report approximately 4 times longer duration of activity compared to subjects reporting much activity. Additionally, to the extent that the reported PA differences represent a long standing lifestyle habit, even small differences may have considerable effects in health. However, this considerably large impact in mortality of relatively small amounts of exercise also raises suspicion for unmeasured factors in socio-economic status or other aspects of lifestyle associated with better health. For the AD patients included in our study, PA was related to medical comorbidities but was not related to indirect indices of socioeconomic status such as education or ethnicity. We addressed this potential bias by adjusting for these variables but, as in all observational epidemiology studies, residual confounding, in particular of the ‘healthy person’ type, cannot be excluded. This issue can be definitely addressed only by the randomization of interventional approaches.
Confidence in our findings is strengthened by the following factors. The study is community-based and the population is multiethnic, increasing the generalizability of the findings compared to clinic-based samples. The assessment instruments for PA have been previously validated and widely used in epidemiological studies. The diagnosis of AD took place in a University hospital with expertise in dementia and was based on comprehensive assessments and standard research criteria. The patients were followed prospectively at relatively short intervals. Measures for multiple potential confounders were carefully recorded and adjusted for in the analyses.
Overall, the effects of PA in AD course have not been explored. Our results provide support that PA is associated with AD duration but they need to be replicated and the mechanisms need to be further investigated.
Acknowledgments
Support: Federal NIA grants AG028506, P01-AG07232.
Footnotes
Disclosures: The authors have nothing to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 3.Weuve J, Kang JH, Manson JE, et al. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 4.Middleton LE, Mitnitski A, Fallah N, et al. Changes in cognition and mortality in relation to exercise in late life: a population based study. PLoS ONE. 2008;3:e3124. doi: 10.1371/journal.pone.0003124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 6.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 7.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Larson EB, Bowen JD, et al. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166:1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 9.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakim AA, Petrovitch H, Burchfiel CM, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;338:94–99. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- 11.Scarmeas N, Stern Y, Mayeux R, et al. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarmeas N, Stern Y, Tang MX, et al. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scarmeas N, Levy G, Tang MX, et al. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49:453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 15.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 16.Jacobs DR, Jr., Ainsworth BE, Hartman TJ, et al. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and science in sports and exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Sallis JF, Buono MJ, Roby JJ, et al. Seven-day recall and other physical activity self-reports in children and adolescents. Med Sci Sports Exerc. 1993;25:99–108. doi: 10.1249/00005768-199301000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Miller DJ, Freedson PS, Kline GM. Comparison of activity levels using the Caltrac accelerometer and five questionnaires. Med Sci Sports Exerc. 1994;26:376–382. [PubMed] [Google Scholar]
- 19.Scarmeas N, Brandt J, Albert M, et al. Delusions and Hallucinations Are Associated With Worse Outcome in Alzheimer Disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Manly JJ, Bell-McGinty S, Tang MX, et al. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 23.Manly JJ, Tang MX, Schupf N, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siedlecki KL, Honig LS, Stern Y. Exploring the structure of a neuropsychological battery across healthy elders and those with questionable dementia and Alzheimer's disease. Neuropsychology. 2008;22:400–411. doi: 10.1037/0894-4105.22.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarmeas N, Albert SM, Manly JJ, et al. Education and rates of cognitive decline in incident Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:308–316. doi: 10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold DP, Andres D, Etezadi J, et al. Structural equation model of intellectual change and continuity and predictors of intelligence in older men [published erratum appears in Psychol Aging 1998 Sep;13(3):434] Psychol Aging. 1995;10:294–303. doi: 10.1037//0882-7974.10.2.294. [DOI] [PubMed] [Google Scholar]
- 27.Gurland B, Golden RR, Teresi JA, et al. The SHORT-CARE: an efficient instrument for the assessment of depression, dementia and disability. J Gerontol. 1984;39:166–169. doi: 10.1093/geronj/39.2.166. [DOI] [PubMed] [Google Scholar]
- 28.Gurland B, Kuriansky J, Sharpe L, et al. The Comprehensive assessment and Referral Evaluation (CARE)--rationale, development and reliability. Int J Aging Hum Dev. 1977;8:9–42. doi: 10.2190/cl3j-0e20-97xx-mv5l. [DOI] [PubMed] [Google Scholar]
- 29.Helzner EP, Scarmeas N, Cosentino S, et al. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71:1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adlard PA, Perreau VM, Pop V, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel T, Brechat PH, Lepretre PM, et al. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63:303–320. doi: 10.1111/j.1742-1241.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 32.Wolfson C, Wolfson DB, Asgharian M, et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med. 2001;344:1111–1116. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 33.Stern Y, Tang MX, Denaro J, et al. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- 34.Heyman A, Peterson B, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part XIV: Demographic and clinical predictors of survival in patients with Alzheimer's disease. Neurology. 1996;46:656–660. doi: 10.1212/wnl.46.3.656. [DOI] [PubMed] [Google Scholar]
- 35.Brookmeyer R, Corrada MM, Curriero FC, et al. Survival Following a Diagnosis of Alzheimer Disease. Arch Neurol. 2002;59:1764–1767. doi: 10.1001/archneur.59.11.1764. [DOI] [PubMed] [Google Scholar]
- 36.Chandra V, Bharucha NE, Schoenberg BS. Patterns of mortality from types of dementia in the United States, 1971 and 1973-1978. Neurology. 1986;36:204–208. doi: 10.1212/wnl.36.2.204. [DOI] [PubMed] [Google Scholar]
- 37.Stern Y, Tang MX, Denaro J, et al. Increased risk of mortality in Alzheimer's disease patients with more advanced educational and occupational attainment. Ann Neurol. 1995;37:590–595. doi: 10.1002/ana.410370508. [DOI] [PubMed] [Google Scholar]
- 38.Schachter F, Faure-Delanef L, Guenot F, et al. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6:29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Tang MX, Schupf N, et al. Mortality and apolipoprotein E in Hispanic, African-American, and Caucasian elders. Am J Med Genet. 2001;103:121–127. doi: 10.1002/ajmg.1528. [DOI] [PubMed] [Google Scholar]
- 40.Scarmeas N, Brandt J, Albert M, et al. Association between the APOE genotype and psychopathologic symptoms in Alzheimer's disease. Neurology. 2002;58:1182–1188. doi: 10.1212/wnl.58.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63:975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern Y, Brandt J, Albert M, et al. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer's disease. Ann Neurol. 1997;41:615–620. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- 43.Scarmeas N, Anderson KE, Hilton J, et al. APOE-dependent PET patterns of brain activation in Alzheimer disease. Neurology. 2004;63:913–915. doi: 10.1212/01.wnl.0000137274.93125.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarmeas N, Habeck C, Anderson KE, et al. Altered PET Functional Brain Responses in Cognitively Intact Elderly Persons at Risk for Alzheimer Disease (Carriers of the {epsilon}4 Allele) Am. J. Geriatr. Psychiatry. 2004;12:596–605. doi: 10.1176/appi.ajgp.12.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burns JM, Cronk BB, Anderson HS, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71:210–216. doi: 10.1212/01.wnl.0000317094.86209.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry. 2008;16:697–701. doi: 10.1097/JGP.0b013e31817945f6. [DOI] [PubMed] [Google Scholar]
- 48.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 49.Buchman AS, Schneider JA, Leurgans S, et al. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71:499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terrera GM, Matthews F, Brayne C. A comparison of parametric models for the investigation of the shape of cognitive change in the older population. BMC Neurol. 2008;8:16. doi: 10.1186/1471-2377-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]