Abstract
Background
Obesity has been proposed to be a risk factor for the development of childhood asthma.
Objective
To examine weight status from birth to age 5 years in relation to the occurrence of asthma at age 6 and 8 years.
Methods
285 full-term high-risk newborns with at least one asthmatic/atopic parent, enrolled in the Childhood Origin of Asthma (COAST) project, were studied from birth to age 8 years. Overweight was defined by weight-for-length percentiles > 85th prior to age 2 years and body mass index percentile > 85th at ages 2–5 years.
Results
No significant concurrent association was found between overweight and wheezing/asthma occurrence at each year of age. In contrast, longitudinal analyses revealed complex relationships between overweight and asthma. Being overweight at age 1 year was associated with a decreased risk of asthma at age 6 (OR=0.32, p=0.02) and 8 years (OR=0.35, p=0.04) as well as better lung function. However, being overweight beyond infancy was not associated with asthma occurrences. In fact, only children who were overweight at age 5 but not at age 1 year had an increased risk of asthma at age 6 years (OR=5.78, p=0.05).
Conclusion
In children genetically at high risk of developing asthma, overweight at age 1 year was associated with a decreased risk of asthma and better lung function at age 6 and 8 years. However, being overweight beyond infancy did not have any protective effect and even could confer a higher risk for asthma.
Keywords: Asthma, overweight, children, high risk birth cohort
INTRODUCTION
Both asthma and obesity are major public health concerns that often begin in childhood and share some common risk factors. With the increased prevalence of these two conditions in the last 20 years, a natural speculation arose that the development of asthma and obesity might be related.1 Current evidence favors the association that obesity precedes asthma in adults.2 In children, inconsistent findings have been observed3–6; that is, some studies reported an increased risk for asthma in overweight children, whereas others did not. This discrepancy might result from different study designs, especially different ages of the study subjects.
Early childhood is a dynamic period for growth as well as for disease development. For instance, inadequate nutrition during infancy, the period with most rapid growth in life, could lead to consequences that may be lifelong, harming not only the current but also the future growth and development.7 Since both asthma and obesity have roots in fetal and early childhood periods8, 9, it is important to examine the associations between these two conditions beginning from birth. A recent longitudinal study conducted in Netherlands found that asthmatic symptoms at age 8 years were related to late overweight at age 6–7 years but not early overweight at age 1–2 years.10 However, the study did not distinguish the effect of overweight in infancy, i.e., the first year of life, from later years. In addition, the weight and height data were reported by parents, instead of measured by clinicians.
During infancy, human lung parenchyma undergoes a substantial structural remodeling due to alveolar formation and septal restructuring.11, 12 Stimuli or Insults during this critical time period can have profound effects on subsequent development of respiratory systems and lung disease processes, including asthma.13, 14
The objective of this study was to examine the age-related associations between weight status from birth to age 5 years and asthma at age 6 and 8 years. The data used for this study are generated from the Childhood Origin of Asthma (COAST)15 cohort, which comprises of a birth cohort genetically at high risk of developing asthma due to parental history of asthma or respiratory allergies. The frequently collected medical information provided us a unique opportunity for this study.
METHODS
Study Subjects
The design and implementation of the COAST study has been described in detail elsewhere.15 Briefly, a total of 289 full-term newborns with birth weight > 2000 g were enrolled from November 1998 through May 2000. To qualify, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) and/or a history of physician-diagnosed asthma. Of these children, 285 were followed for age 1 year. Among them, 259 children at age 6 years and 238 at age 8 years had asthma diagnosis defined, respectively. Height/length and weight data were collected from medical records. Other information was collected prospectively at each planned clinical visits and sick visits as well. The COAST study was approved by the Human Subjects Committee of the University of Wisconsin.
Wheezing and asthma
Wheezing respiratory illness and asthma were defined as previously described.16 A wheezing illness during the first 5 years was defined as meeting one or more of the following criteria: 1) physician-diagnosed wheezing at an office visit; 2) an illness for which the child was prescribed short or long-acting beta agonists and/or long term controller medications; or 3) an illness given the following specific diagnoses: bronchiolitis, wheezing illness, reactive airway disease, asthma, or asthma exacerbation. Current asthma was defined based on the documented presence of one or more of the following characteristics in the previous year: 1) physician-diagnosed asthma, 2) use of albuterol for coughing or wheezing episodes (prescribed by physician), 3) use of a daily controller medication, 4) step-up plan including use of albuterol or short-term use of inhaled corticosteriods during illness, and 5) use of prednisone for asthma exacerbation. Four separated investigators, blinded to any antecedent histories concerning viral illnesses or patterns of aeroallergen sensitization, independently evaluated each subject for the presence or absence of asthma based on the above criteria.
Pulmonary function test
Pulmonary function tests were conducted yearly beginning at age 5. Three primary indicators of lung function - forced vital capacity (FVC) and forced expiratory volume (FEV) at timed intervals of 0.5 second (FEV0.5), and 1.0 second (FEV1) at ages 6 and 8 years were analyzed. Percent predicted values for FVC (FVC%) and FEV1 (FEV1%) were calculated according to the equations developed by Wang et al.17
Weight status
Height/length and weight data were retrieved from physician records obtained during routine well-child visits at age 2, 4, 6, 9, 12, 18, 24, 30, 36 months and every annual visit after age 3 years. Absolute height/length and weight were converted to gender- and age-specific percentiles by using the 2000 CDC growth references.18 Weight-for length percentile for children at age 2 years or younger and body mass index (BMI) percentile for children at age 3 years or older were calculated. A value greater than 85th percentile was defined as being overweight.19
Birth weight was categorized into 3 groups: (1) low, <15th weight-for-age percentile (only 5 participants had birth weight <2500 g), (2) average, 15th–85th percentile and (3) high, >85th percentile. Rapid weight gain during infancy was defined as an increase in weight-for-age z-scores of greater than 0.67 SD from birth to 6 months, which is equivalent to crossing one percentile channel on the CDC growth chart.
Statistical analyses
All analyses were performed using SAS (version 9.1.3, SAS Institute, Inc., Cary NC, 2001) and R (equivalent to S-Plus, downloadable from http://www.r-project.org). Student t-test or simple regression was used to compare group differences for continuous variables. Chi-square or fisher exact test was used in univariate analyses to compute frequency distributions and test differences in proportions for categorical variables. The associations between asthma and overweight were examined using logistic regression. Other factors that may contribute to the development of wheezing/asthma were also included in logistic regression models; these included breastfeeding, gender, self-reported maternal asthma, as well as various environmental factors during first year of life: dog and cat in household at birth, smoke exposure, daycare attendance, having older children (including siblings and other children) in the household, wheezing with Rhinovirus infection (RV wheezing). Breastfeeding groups were defined based on the duration of exclusively breastfeeding of <2 months, 2–4 months or ≥ 4 months. More detailed descriptions of these factors have been described previously.20–22 Statistical significance was set at an α of 0.05 for all analysis.
RESULTS
Study population
Among 285 COAST children followed up to age 1 year, 259 children (91%) at age 6 years and 238 (84%) at age 8 had sufficient data to be evaluated for asthma using preset criteria. No significant difference was found between these subsets of children and overall 285 children with respect to the descriptive statistics in Table I.
TABLE I.
Descriptive statistics of the study population
| Age 1 y (n = 285) | Age 6 y (n = 259) | Age 8 y (n = 238) | |
|---|---|---|---|
| Gender | |||
| Male (%) | 56% (161) | 57% (147) | 57% (135) |
| Female (%) | 44% (124) | 43% (112) | 43% (103) |
| Maternal allergy | |||
| Yes | 83% (230) | 83% (207) | 83% (195) |
| No | 17% (48) | 17% (44) | 17% (39) |
| Maternal asthma | |||
| Yes | 42% (115) | 43% (106) | 41% (93) |
| No | 58% (160) | 57% (143) | 59% (135) |
| Exclusive breastfeeding | |||
| <2mo | 43% (122) | 42% (110) | 41% (98) |
| 2–4mo | 22% (64) | 22% (57) | 21% (51) |
| ≥4mo | 35% (99) | 36% (92) | 37% (89) |
| Smoking exposure during infancy | |||
| Yes | 25% (71) | 25% (64) | 24% (56) |
| No | 75% (214) | 75% (195) | 76% (182) |
| Daycare attendance during infancy | |||
| Yes | 54% (153) | 55% (142) | 55% (130) |
| No | 46% (132) | 45% (117) | 45% (108) |
| Having older children in the household during infancy | |||
| Yes | 58% (166) | 59% (154) | 60% (143) |
| No | 42% (119) | 41% (105) | 40% (95) |
| Dog in household at birth | |||
| Yes | 35% (101) | 36% (92) | 36% (85) |
| No | 65% (184) | 64% (167) | 64% (153) |
| Cat in household at birth | |||
| Yes | 29% (84) | 29% (76) | 29% (68) |
| No | 71% (201) | 71% (183) | 71% (170) |
| RV wheezing during infancy | |||
| Yes | 16% (47) | 17% (45) | 17% (41) |
| No | 84% (238) | 83% (214) | 83% (197) |
| Birth weight percentile | 53.3 ± 29.1 | 53.6 ± 29.0 | 53.5 ± 28.9 |
| Weight-for-length percentile at 1 y | 49.0 ± 30.6 | 48.8 ± 30.5 | 49.0 ± 30.3 |
| Weight for-length percentile at 2 y | 49.7 ± 32.2 | 50.1 ± 32.3 | 49.8 ± 32.0 |
| BMI percentile at 3 y | 55.8 ± 28.6 | 56.5 ± 28.5 | 56.5 ± 28.2 |
| BMI percentile at 5 y | 59.3 ± 28.2 | 59.4 ± 28.1 | 59.0 ± 27.7 |
p-value < 0.05 from chi-square test.
Prevalence of overweight and wheezing from birth to age 8 years
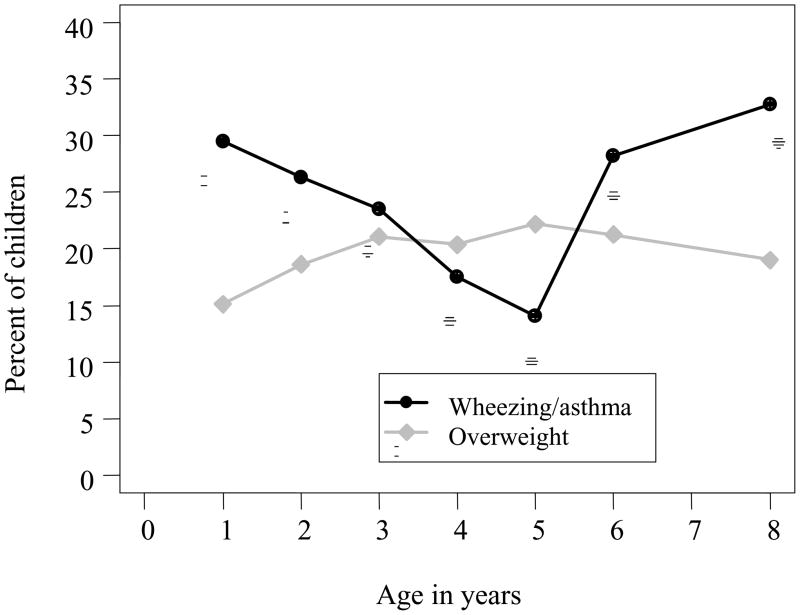
The prevalence of overweight increased from 15% at age 1 year to 22% at age 3 years then stayed about the same between 3–8 years (Figure 1). Proportionately more children with birth weight less than 15th percentile experienced rapid weight gain during the first six month of life (34%) compared to those with average birth weight (20%) and high birth weight (2%), p=0.0005. As a result, a similar percentage of children were found to be overweight at age 1 year among all birth weight groups (11%, 15% and 20% in low, average and high birth weight groups, respectively, p=0.51).
Figure 1.
Prevalence of overweight and wheezing/asthma during the first 8 years of life. Asthma diagnosis was not available for age 5 years or younger. Therefore, the prevalence of wheezing was plotted. At age 6 and 8 years, the prevalence of asthma was plotted.
The prevalence of wheezing illnesses decreased from 29% in the first year of life to 14% at age 5 (Figure 1). Asthma was diagnosed in 28% of children at age 6 and 33% of children at age 8 years. There were no significant concurrent associations between overweight and wheezing/asthma at any given age (all p-values>0.05).
Associations between weight status 0–5 years and asthma at age 6 and 8 years
We conducted a longitudinal analysis to determine whether weight status at any age was associated with the subsequent development of asthma ascertained at age 6 or 8 years (Tables II for univariate analyses and III for multiple regressions). The analyses in Table III show that neither birth weight nor rapid weight gain between 0–6 months was associated with asthma. However, overweight at age 1 year was associated with a lower rate of asthma at age 6 years (p-value=0.02) and asthma at age 8 years (p-value=0.04). Overweight beyond age 1 year (i.e., age 2, or 3 or 5 years) was not related to higher risk of asthma either at age 6 or 8 years (all p-values>0.05).
TABLE II.
Occurrence of asthma at age 6 and 8 y by weight status at different ages
| Asthma 6 y | Asthma 8 y | |||
|---|---|---|---|---|
| % | No. | % | No. | |
| Birth weight | ||||
| < 15th percentile | 30% | 10 (n=33) | 42% | 13 (n=31) |
| 15–85th percentile | 27% | 47 (n=177) | 30% | 49 (n=162) |
| ≥ 85th percentile | 33% | 16 (n=49) | 36% | 16 (n=45) |
| Rapid weight gain 0–6 mo | ||||
| < 0.67 z-score | 27% | 57 (n=209) | 32% | 62 (n=192) |
| ≥ 0.67 z-score | 30% | 14 (n=46) | 32% | 14 (n=44) |
| Weight-for-length at 1 y | ||||
| < 15th percentile | 31% | 16 (n=52) | 38% | 18 (n=48) |
| 15 – 85th percentile | 30% | 51 (n=169) | 33% | 52 (n=456) |
| ≥ 85th percentile | 16% | 6 (n=38) | 24% | 8 (n=34) |
| Weight-for-length at 1 y | ||||
| < 85th percentile | 31%* | 67 (n=214) | 36% | 70 (n=197) |
| ≥ 85th percentile | 16% | 6 (n=38) | 24% | 8 (n=34) |
| Weight-for-length at 2 y | ||||
| < 85th percentile | 28% | 58 (n=201) | 33% | 61 (n=187) |
| ≥ 85th percentile | 27% | 13 (n=48) | 33% | 14 (n=43) |
| BMI at 3 y | ||||
| < 85th percentile | 27% | 53 (n=195) | 33% | 60 (n=181) |
| ≥ 85th percentile | 28% | 15 (n=54) | 27% | 13 (n=48) |
| BMI at 5 y | ||||
| < 85th percentile | 27% | 54 (n=199) | 32% | 60 (n=186) |
| ≥ 85th percentile | 32% | 18 (n=56) | 33% | 16 (n=49) |
| Weight change between 1 and 5 y | ||||
| Never overweight | 30% | 52 (n=173) | 35% | 56 (n=160) |
| Overweight 1 y only | 11% | 2 (n=19) | 21% | 4 (n=19) |
| Overweight 5 y only | 38%^ | 14 (n=37) | 35% | 12 (n=34) |
| Overweight 1 & 5 y | 21% | 4 (n=19) | 27% | 4 (n=15) |
p-value < 0.05 from chi-square test.
p-value < 0.05 compared to overweight 1y only group.
TABLE III.
Adjusted associations between asthma at age 6 and 8 y and weight status at different ages
| Asthma 6 y | Asthma 8 y | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Birth weight * | 0.73 | 0.84 | ||||
| < 15th percentile | 0.78 | 0.24, 2.51 | 1.01 | 0.32, 3.23 | ||
| 15–85th percentile | 0.72 | 0.32, 1.63 | 0.82 | 0.36, 1.90 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| Rapid weight gain 0–6 mo | 0.78 | 0.71 | ||||
| ≥ 0.67 z-score | 1.11 | 0.50, 2.51 | 0.86 | 0.37, 1.96 | ||
| < 0.67 z-score | 1.00 | 1.00 | ||||
| Weight-for-length at 1 y | 0.07 | 0.10 | ||||
| <15th percentile | 3.42 | 0.97, 12.05 | 3.78 | 1.09, 13.13 | ||
| 15–85th percentile | 3.36 | 1.19, 9.54 | 2.71 | 0.97, 7.62 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| Weight-for-length at 1 y | 0.02 | 0.04 | ||||
| < 85th percentile | 3.14 | 1.17, 8.44 | 2.85 | 1.07, 7.57 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| Weight-for-length at 2 y | 0.62 | 0.48 | ||||
| < 85th percentile | 1.23 | 0.55, 2.73 | 1.35 | 0.59, 3.10 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| BMI at 3 y | 0.92 | 0.30 | ||||
| < 85th percentile | 1.04 | 0.50, 2.15 | 1.51 | 0.69, 3.30 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| BMI at 5 y | 0.81 | 0.49 | ||||
| < 85th percentile | 0.91 | 0.45, 1.88 | 1.31 | 0.61, 2.82 | ||
| ≥ 85th percentile | 1.00 | 1.00 | ||||
| Weight change between 1 & 5 y | 0.12 | 0.21 | ||||
| Normal | 4.20 | 0.84, 20.96 | 3.40 | 0.79, 14.64 | ||
| Overweight 1 y only | 1.00 | 1.00 | ||||
| Overweight 5 y only | 5.78 | 1.03, 32.45 | 2.87 | 0.57, 14.42 | ||
| Overweight 1 & 5 y | 1.80 | 0.25, 12.77 | 1.30 | 0.20, 8.39 | ||
Other variables included in the analysis were: gender, maternal asthma and environmental factors during 1st yr: breastfeeding, dog and cat ownership, smoke exposure, daycare attendance and having older children in the household, RV wheezing. Besides these variables, birth weight was included in other models.
We next tested weight change between age 1 and 5 years in relation to the subsequent development of asthma. We discovered that children who were overweight at age 5 years but not at age 1 year had an increased risk of asthma at age 6 years compared to children overweight at age 1 only (p-value=0.05, Table III). Those children overweight at age 5 years only had the highest asthma rate (38% [age 6 years, Table II]), followed by those with normal weight at both age 1 and 5 years (30%), then children overweight at both ages (21%), and the lowest (11%) in those overweight at age 1 year only.
Associations between weight status at age 1 year and lung function at age 6 and 8 years
Weight-for-length at age 1 year was positively correlated with pulmonary function test values at age 6 and 8 years (Table IV). In general, weight-for-length at age 1 was positively correlated with FVC, FEV0.5, and FEV1 at age 6 and 8 years. These correlations remained significant after adjusting for length status, i.e., on percent predicted FVC and percent predicted FEV1 at age 8 (Table IV).
TABLE IV.
Pulmonary function tests by first year weight status
| Correlation1 | Weight-for-length at age 1 y |
||||
|---|---|---|---|---|---|
| <15th %tile | 15–85th %tile | >85th %tile | p-value2 | ||
| At age 6 y | |||||
| FVC% | 0.13 | 103.88 (11.19) | 106.86 (13.90) | 108.44 (13.98) | 0.46 |
| FEV1% | 0.12 | 102.50 (12.02) | 105.55 (14.44) | 105.50 (11.39) | 0.55 |
| FVC | 0.25¶ | 1.42 (0.20)^* | 1.53 (0.24) | 1.60 (0.28) | 0.02 |
| FEV1 | 0.22¶ | 1.25 (0.18)^* | 1.34 (0.22) | 1.37 (0.20) | 0.06 |
| FEV0.5 | 0.21¶ | 0.95 (0.17)* | 1.01 (0.18) | 1.04 (0.16) | 0.11 |
| At age 8 y | |||||
| FVC% | 0.25¶ | 103.74 (12.26)* | 107.20 (12.60) | 112.27 (11.74) | 0.04 |
| FEV1% | 0.24¶ | 96.42 (14.05)* | 101.21 (13.63) | 103.38 (11.19) | 0.10 |
| FVC | 0.30¶ | 1.84 (0.30)* | 1.92 (0.30)* | 2.08 (0.28) | 0.01 |
| FEV1 | 0.29¶ | 1.50 (0.28)* | 1.59 (0.26) | 1.68 (0.20) | 0.04 |
| FEV0.5 | 0.25¶ | 1.12 (0.22)^* | 1.20 (0.22) | 1.24 (0.18) | 0.05 |
Correlation coefficients between weight-for-height percentiles at age 1 y and lung function test scores.
Overall group comparisons from simple regression.
p-value<0.05 for correlation coefficient test.
p<0.05 compared to weight-for-length 15–85th %tile group
p<0.05 compared to weight-for-length >85th %tile group
DISCUSSION
The prospectively followed birth cohort in the COAST project provided us with an excellent opportunity to investigate the associations between weight status in the first five years and having asthma at age 6 and 8 years. The findings demonstrate that in the COAST children, who are genetically at high risk of developing asthma, overweight at age 1 year was associated with decreased risk of asthma and greater lung function at age 6 and 8 years. Beyond age 1, being overweight at 2–5 years of age was no longer protective for asthma development at age 6 or 8 years. In fact, in our study population, late onset of overweight, i.e., overweight at age 5 but not age 1 year, was associated with a higher risk for developing asthma at age 6 and 8 years. These findings provide evidence of the complex relationships between age, overweight status, and wheezing/asthma development.
The effect of overweight status on the subsequent development of asthma during childhood has been previously reported.3–6, 10, 23 Many of the studies focused on weight status at birth, during preschool age, or school age. Very few of them examined overweight status during infancy.8 One study conducted in a general population in the Netherlands8 found that a high BMI between 1–2 year of age was not associated with later asthma symptoms if the weight status became normal. Whether or not being heavier at age 1 year has a protective effect was not analyzed separately in this study.8 However, the study showed that the late onset of overweight at age 6–7 years, instead of “persistent high BMI” between 1–2 years and 6–7 years, was associated with a significantly increased risk of asthma symptoms at age 8, which might indicate protective effects of early overweight status at age 1 and/or 2 years on asthma development.8 Similar findings were observed in our cohort for asthma at age 6 and 8, although the result at age 8 did not reach statistical significance. One possible reason for the insignificant test at age 8 is that some of the children in “Overweight at 1 y only” group became overweight at 6–7 year (data not shown), which might increase their risk of asthma at age 8. As noticed in our study, the percentage of asthmatic children in “Overweight at 1 y only” group at age 8 year was almost doubled compared to age 6 (11% at age 6 vs. 21% at age 8).
It is interesting to speculate why overweight status at age 1 year was associated with a decreased risk of asthma at age 6 and 8 years. One possible explanation is that children on the top 15th percentile of growth chart at age 1 year were in general well nourished, and this promoted maximum postnatal lung development and alveolarization. Accordingly, children with weight-to-height >85% also had increased lung function at age 6 and 8 years. Alveolar development begins during gestation and continues through age 1 and 1.5 years, or even longer.11, 12 Most of the alveoli are added through multiplication during this time.24 Therefore, nutritional influences on alveoarization during infancy could have profound and long lasting effects on lung function, and perhaps the development of lung disease, far beyond infancy. The concept of “programming”, in which growth in early life exerts long-term effects on the structure and function of particular organs and tissues, is well established in epidemiological and animal studies.7, 25 For example, small body size at birth or in infancy has been associated with an increased risk of asthma 26–30 and low lung function.31–34 In addition, low weight gain during infancy was found to be associated with modest reductions in adult lung function.35 On the other hand, abundant nutrition during infancy that would encourage maximum physical growth and development, might reduce the risk of subsequent lung disease.
Current evidence shows that children who were overweight during infancy tend to be overweight in later years, 36, 37 and a similar trend was observed in our study population (data not shown). Establishing a pattern of overweight status could be problematic given that overweight/obesity is a major risk factor for many metabolic diseases.36 However, many of the association studies did not evaluate when children became overweight, and this could lead to biased assumptions about weight status in infancy. It is not clear if health is impaired for children who were overweight in infancy and then subsequently developed normal weight. More studies are needed to thoroughly understand the role of growth during infancy on subsequent health.
The longitudinal design of the COAST study enabled us to examine age related differences in the relationship between weight and asthma risk, and the excellent retention in this cohort added to the validity of the results. Limitations in the present study include the fact that this is a high-risk population for allergic diseases and results from this study need to be confirmed in the general population. Second, the sample size was relatively small and limited subgroup analyses. Third, overweight status was defined by BMI (weight-for-length if a child was younger than 2 years old) instead of the actual percent of body fat. However, in children, using BMI to define overweight has been endorsed by major organizations and it shows a high degree of correlation with percent of body fat.38, 39 Last, parental weight status was not taken into account. This is not likely to introduce bias in the study because evidence shows that parental obesity is not a significant contributor to early childhood obesity, especially in the first two years of life.40, 41
In conclusion, our findings suggest that association between weight and childhood asthma changes with age. While obesity may promote asthma in later life, these findings suggest the possibility that nutrition during infancy should be optimized to promote lung growth and development, and that this could have effects in reducing asthma risk later in childhood. On the other hand, after the first year of life, the attention needs to be focused on balanced nutrition to prevent overweight and obesity beyond infancy, which might be a potential risk factor for asthma. Again, further studies are needed to validate these findings in both high-risk and general populations as well in children premature and/or with very low birth weight.
KEY MESSAGE.
High body weight at age 1 year is associated with a decreased risk of asthma and better lung function at age 6 and 8 years in children with parental history of asthma or atopy.
Acknowledgments
Supported by National Institutes of Health Grants R01 HL61879 and P01 HL70831, U.S. Department of Agriculture Hatch Multi-State Grant WIS01228
ABBREVIATIONS
- BMI
Body mass index
- COAST
Childhood origin of asthma project
- FEV0.5
- Forced expiratory volume (FEV) at timed interval of 0.5 second
- FEV1
Forced expiratory volume (FEV) at timed interval of 1.0 second
- FEV1%
Percent predicted value for FEV1
- FVC
Forced vital capacity
- FVC%
Percent predicted value for FVC
- RV wheezing
wheezing with Rhinovirus infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001 Sep;56(Suppl 2):ii64–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma - A meta-analysis of prospective epidemiologic studies. American Journal of Respiratory and Critical Care Medicine. 2007;175:661–6. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES. The epidemiology of obesity and asthma. Journal of Allergy & Clinical Immunology. 2005 May;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Lucas SR, Platts-Mills TAE. Paediatric asthma and obesity. Paediatric Respiratory Reviews. 2006;7:233–8. doi: 10.1016/j.prrv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Chinn S. Obesity and asthma. Paediatric Respiratory Reviews. 2006;7:223–8. doi: 10.1016/j.prrv.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Peroni DG, Pietrobelli A, Boner AL. Asthma and obesity in childhood: on the road ahead. International Journal of Obesity. 34:599–605. doi: 10.1038/ijo.2009.273. [DOI] [PubMed] [Google Scholar]
- 7.Desai M, Hales CN. Role of fetal and infant growth in programming metabolism in later life. Biological Reviews of the Cambridge Philosophical Society. 1997;72:329–48. doi: 10.1017/s0006323196005026. [DOI] [PubMed] [Google Scholar]
- 8.Gern JE, Lemanske RF, Jr, Busse WW. Early life origins of asthma. Journal of Clinical Investigation. 1999;104:837–43. doi: 10.1172/JCI8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newnham JP, Pennell CE, Lye SJ, Rampono J, Challis JRG. Early Life Origins of Obesity. Obstetrics and Gynecology Clinics of North America. 2009;36:227–44. doi: 10.1016/j.ogc.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Scholtens S, Wijga AH, Seidell JC, Brunekreef B, de Jongste JC, Gehring U, et al. Overweight and changes in weight status during childhood in relation to asthma symptoms at 8 years of age. Journal of Allergy and Clinical Immunology. 2009;123:1312–8. doi: 10.1016/j.jaci.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Zeltner TB, Caduff JH, Gehr P, Pfenninger J, Burri PH. The postnatal development and growth of the human lung. I. Morphometry. Respiration Physiology. 1987;67:247–67. doi: 10.1016/0034-5687(87)90057-0. [DOI] [PubMed] [Google Scholar]
- 12.Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Respiration Physiology. 1987;67:269–82. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 13.Massaro D, Massaro GD. Critical period for alveologenesis and early determinants of adult pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2004;287:L715–7. doi: 10.1152/ajplung.00166.2004. [DOI] [PubMed] [Google Scholar]
- 14.Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Effects of viral respiratory infections on lung development and childhood asthma. Journal of Allergy and Clinical Immunology. 2005;115:668–74. doi: 10.1016/j.jaci.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatric Allergy & Immunology. 2002;13(Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. American Journal of Respiratory and Critical Care Medicine. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiaobin W, Douglas WD, David W, Martha EF, Benjamin GF., Jr Pulmonary function between 6 and 18 years of age. Pediatric Pulmonology. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital & Health Statistics Series. 2002;11:1–190. [PubMed] [Google Scholar]
- 19.Barlow SE the Expert C. Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120:S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 20.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatric Allergy and Immunology. 2002;13:386–93. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 21.Neaville WA, Tisler C, Bhattacharya A, Anklam K, Gilbertson-White S, Hamilton R, et al. Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. Journal of Allergy and Clinical Immunology. 2003;112:740–6. doi: 10.1016/s0091-6749(03)01868-2. [DOI] [PubMed] [Google Scholar]
- 22.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. Journal of Allergy & Clinical Immunology. 2005 Sep;116(3):571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91:334–9. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkus P, tenHaveOpbroek AAW, Quanjer PH. Human lung growth: A review. Pediatric Pulmonology. 1996;21:383–97. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Barker DJP. The developmental origins of adult disease. Journal of the American College of Nutrition. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 26.Yuan W, Basso O, Sorensen HT, Olsen J. Fetal growth and hospitalization with asthma during early childhood: a follow-up study in Denmark. International Journal of Epidemiology. 2002;31:1240–5. doi: 10.1093/ije/31.6.1240. [DOI] [PubMed] [Google Scholar]
- 27.Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. Is low birth weight a risk factor for asthma during adolescence? Archives of Disease in Childhood. 1991;66:584–7. doi: 10.1136/adc.66.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooks AM, Byrd RS, Weitzman M, Auinger P, McBride JT. Impact of low birth weight on early childhood asthma in the United States. Archives of Pediatrics & Adolescent Medicine. 2001;155:401–6. doi: 10.1001/archpedi.155.3.401. [DOI] [PubMed] [Google Scholar]
- 29.Steffensen FH, Sorensen HT, Gillman MW, Rothman KJ, Sabroe S, Fischer P, et al. Low birth weight and preterm delivery as risk factors for asthma and atopic dermatitis in young adult males. Epidemiology. 2000;11:185–8. doi: 10.1097/00001648-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. American Review of Respiratory Disease. 1990 Sep;142(3):555–62. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 31.Canoy D, Pekkanen J, Elliott P, Pouta A, Laitinen J, Hartikainen AL, et al. Early growth and adult respiratory function in men and women followed from the fetal period to adulthood. Thorax. 2007;62:396–402. doi: 10.1136/thx.2006.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tennant PWG, Gibson GJ, Pearce MS. Lifecourse predictors of adult respiratory function: results from the Newcastle Thousand Families Study. Thorax. 2008;63:823–30. doi: 10.1136/thx.2008.096388. [DOI] [PubMed] [Google Scholar]
- 33.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–8. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orfei L, Strachan DP, Rudnicka AR, Wadsworth MEJ. Early influences on adult lung function in two national British cohorts. Archives of Disease in Childhood. 2008;93:570–4. doi: 10.1136/adc.2006.112201. [DOI] [PubMed] [Google Scholar]
- 35.Hancox RJ, Poulton R, Greene JM, McLachlan CR, Pearce MS, Sears MR. Associations between birth weight, early childhood weight gain and adult lung function. Thorax. 2009;64:228–32. doi: 10.1136/thx.2008.103978. [DOI] [PubMed] [Google Scholar]
- 36.Ong KK. Size at birth, postnatal growth and risk of obesity. Hormone Research. 2006;65:65–9. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 37.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. British Medical Journal. 2005;331:929–31. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000 May 6;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. American Journal of Clinical Nutrition. 2002 Jun;75(6):978–85. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 40.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. American Journal of Clinical Nutrition. 1999 Mar;69(3):524–30. doi: 10.1093/ajcn/69.3.524. [DOI] [PubMed] [Google Scholar]
- 41.Stunkard AJ, Berkowitz RI, Schoeller D, Maislin G, Stallings VA. Predictors of body size in the first 2 y of life: a high-risk study of human obesity. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004 Apr;28(4):503–13. doi: 10.1038/sj.ijo.0802517. [DOI] [PubMed] [Google Scholar]