Summary
The aggregation by antigen of the IgE bound to its high affinity receptor on mast cells initiates a complex series of biochemical events that result in the release of inflammatory mediators. The essential role of the protein tyrosine kinase Syk has been appreciated for some time; newer results have defined the mechanism of its activation. The use of siRNA had defined the relative contribution of Syk, Fyn and Gab2 to signaling and has made possible screening to identify previously unrecognized molecules that are involved in these pathways.
Keywords: mast cells, FcεRI signal transduction, Syk, Fyn, Gab2, phosphatases
Introduction
The activation of mast cells or basophils initiates a series of biochemical events that result in the release of biologically active mediators that cause allergic reactions. A major mechanism for the stimulation of these cells is the interaction of antigen with the IgE bound to high affinity IgE receptor (FcεRI) on the cell surface. A series of biochemical events then result in the release of preformed mediators from granules and the generation of newly synthesized mediators.
This review first summarizes the current model for the FcεRI-induced signaling based on studies from our and other groups [1–4]; it then reviews studies on the important role of the tyrosine kinase Syk in these reactions, and then recent studies from our laboratory on these processes.
Model of Mast Cell Activation
FcεRI is a member of the immunoreceptor family that includes the B cell receptor, T cell receptor and immunoglobulin receptors. These receptors have subunits which contain a sequence named the immunoreceptor tyrosine-based activation motif (ITAM) that has two tyrosine residues separate by 6 to 8 amino acid residues. FcεRI is a tetrameric formed by the complex αβγ2 chains. The α-chain binds the Fc portion of IgE at ratio of 1:1 while the β- and γ-chains contain ITAMs in their cytoplasmic domains. Because FcεRI has no intrinsic enzymatic activity, the activation of non-receptor protein tyrosine kinases are essential for cell activation. Aggregation of FcεRI results in phosphorylation of the two tyrosines residues within the ITAMs by Lyn, or another member of the Src family of tyrosine kinases that associate with the receptor.
Phosphorylated ITAM then serves as a docking site for Syk. This binding of Syk through its SH2 domains to the phosphorylated ITAM results in a conformational change of Syk which increases its enzymatic activity.
Once activated, Syk is tyrosine phosphorylated predominantly due to autophosphorylation [5–7] and leads to downstream propagation of signals. Syk then tyrosine phosphorylates the adaptors LAT1 and LAT2 (NTAL) which are membrane proteins, activates the PI3K pathway and the protein tyrosine kinase BTK. Phosphorylated LAT functions as a scaffold for multimolecular signaling complexes that include cytosolic adaptors, such as Gads, Grb2, SLP-76, and SHC; guanosine triphosphate exchangers, including Sos and Vav1; and the enzymes phospholipase Cγ1(PLC) and PLCγ2. Phospholipase Cγ activated through these pathways together with contributions by the protein tyrosine kinase Btk, and PI3K hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) in the plasma membrane to produce the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) which, respectively, result in the activation of protein kinase C (PKC) and the rise in intracellular calcium [Ca2+]i [1]. Binding of IP3 to IP3 receptors (IP3R) in the ER (endoplasmic reticulum) membrane, causes the release of Ca2+ from the ER stores, resulting in a decrease in the free Ca2+ concentration in the ER. This results in only a small and transient increase in [Ca2+]i that is not sufficient for most cellular responses. The depletion of ER Ca2+ stores results in Ca2+ influx across the plasma membrane by a mechanism that has been called store-operated calcium entry (SOCE). Although the electrophysiology of these channels, termed CRAC (calcium release activated calcium), were well know, their molecular identity was only recently determined [8,9]. Two molecules, STIM1 and Orai1 were identified as being responsible for this store-operated calcium entry in immune cells; ORAI1 is the pore subunit of the CRAC channel while STIM1 is ER Ca2+ sensor that sense ER Ca2+ concentration. The depletion of calcium in the ER induces STIM1 formation of multimers in the ER membrane which move to sites of ER plasma membrane apposition and interact directly with ORAI channels. A number of intracellular signals include the activation of several members of the protein kinase C family, phospholipase D and sphingosine kinases are thought to play a role in subsequent events FcεRI aggregation results in downstream signals [10]. These early events activate the small GTPases such as Rac, Ras and Rho resulting in the stimulation of the ERK, JNK and p38 MAP kinase pathways. The MAP kinases are important signaling mediators between the cytosol and the nucleus that regulate a variety of transcription factors. Further the increase in [Ca2+]i with the participation of SNARE complex of proteins allow granular fusion and release of their contents.
The increase in intracellular calcium concentration also activates the Ca2+/calmodulin-dependent serine phosphatase calcineurin. Calcineurin dephosphorylates the nuclear factor for T cell activation (NFAT) which exposes NFAT nuclear-localization signals. Once in the nucleus, NFAT regulates the transcription of several cytokine genes. Transcriptions of other pro-inflammatory mediators are regulated by NFκB proteins which are transcription factors retained in the cytoplasm by binding to the inhibitor IκB. FcεRI stimulation leads to phosphorylating, and degradation of IκB which allows the release and nuclear translocation of the NFκB proteins. Protein kinase Cβ (PKCβ), CARMA1, Bcl10, and MALT1 are other proteins involved in this pathway. Activation of the NFAT and NFκB transcription factors and the MAP kinases then results in the synthesis of several cytokines. Another substrate of PKC and the MAP-kinases are cytoplasmic phospholipase A2 which release arachidonic acid that is metabolized by the cycloxygenase or the lipoxygenase pathways to form the inflammatory mediators, prostaglandins and leukotrienes.
Receptor aggregation also activates and recruits negative regulators which limit the intensity and during of the positive signals. Among these are is c-Cbl which by ubiquinating signaling molecules like Syk, decrease their levels in the cell, PTEN and SHIP both of which hydrolyze PIP3 products of PI3K and SHP-1 that can dephosphorylate signaling molecules. Interestingly, the tyrosine phosphorylation of these are upstream of Syk [11]. Other negative signals are generated by recruitment of negative signaling molecules such as SHIP or SHP to the membrane by ITIM-bearing receptors (e.g. FcγRIIB). The tyrosine in the ITIM is phosphorylated during the activation steps by Lyn then recruits of inhibitory effectors such as SHIP, SHP-1 or SHP-2. In some but not all cases, the negative signals are generated by the co-aggregation of the two receptors (such as FcεRI and the negative receptor).
Syk regulating FcεRI function
Syk and Zap-70 are members of a non-receptor tyrosine kinase family one or both of which are expressed in the cytoplasm of hematopoietic cells Syk [12]. Structurally Syk/Zap-70 consists of two Src-homology 2 domains (SH2) and a kinase domain followed by a short COOH-terminal extension (tail). The interdomain A is located between the NH2-terminal SH2 (SH2-N) and COOH-terminal SH2 (SH2-C), while interdomain B or linker region is between the SH2-C and the kinase domains [12,13].
Syk−/− mice have high rates of perinatal lethality, and exhibit cardiovascular, immune, and hematopoietic defects showing that this kinase is required for development and function of various tissues (ref). Although several tyrosine kinases have been implicated in FcεRI-mediated signaling, only the absence of Syk results in complete loss of degranulation and release of cytokines [14]. Syk negative cells are defective in most of the signaling events that occur after FceRI stimulation; except for the tyrosine phosphorylation of submits of the receptor and the negative regulatory proteins SHIP1, SHP1 and SHP2. Syk−/− mast cells also are unable to activate NFAT or NFκB [15,16]; but transfection of these cells with Syk reconstitutes their activation. In addition, treatment of bone marrow mast cells (BMMC) with siRNA targeted to Syk efficiently decreases receptor-induced cell activation [17].
Phosphorylated ITAM serves as the docking site for the two SH2 domains of Syk with an anti-parallel binding orientation: the SH2-N of Syk binds to the COOH-terminal phospho-tyrosine of the ITAM while the SH2-C binds to the NH2-terminal phospho-tyrosine [18,19]. Experiments using peptides corresponding to the β- and γ-ITAM of FcεRI showed that Syk binds preferentially to the biphosphorylated γ-ITAM [18]. Although this association requires both SH2 domains of Syk, the SH2-C binds stronger to the phosphorylated γ-ITAM than the SH2-N [20].
The binding of Syk to the phosphorylated ITAM results in conformational changes of Syk molecule by exposing its COOH-terminal region [21]. Structural studies of ZAP-70 suggest an autoinhibitory state for Syk family proteins where, during the inactive state, the COOH-terminal region interacts with the inter-SH2 domain by hydrogen-bonding network. This stabilizes the autoinhibitory structure of the enzyme [22]. The binding of Syk to the phosphorylated ITAM disrupts the COOH-terminal/SH2-interdomain interactions which “opens” the molecule resulting in conformational changes which leads to Syk activation [16].
Tyrosine phosphorylation of Syk after receptor aggregation is due to mostly autophosphorylation with some contribution by other tyrosine kinases [5]. There are ten tyrosines that are autophosphorylated in Syk in vitro and several of these are also found to be phosphorylated in vivo after cell activation [23,24]. Such phosphorylations can regulate Syk function and also generate potential docking sites for other molecules. One of these phosphotyrosines, Tyr-130, located in the inter-SH2 domain regulates Syk binding to phosphorylated ITAM; phosphorylation of Try-130 inhibits Syk binding while Y130F mutation enhances this association [25]. Phosphorylation of other tyrosines in Syk may also play a role in regulating ITAM binding including tyrosines in the linker region [16,26].
The linker region plays an important role in regulating Syk function. An alternatively spliced form of Syk, termed SykB, lacks a 23 amino acid sequence in the linker domain, although both isoforms are expressed in mast cells, Syk is more efficient that SykB in immunoreceptor signaling [27]. There are three conserved tyrosines in the linker region of both Syk and ZAP70 (Tyr-317, Tyr-342 and Tyr-346). Substitution of Tyr-317 with Phe enhances Syk-mediated signaling in mast cells and increases its kinase activity [28]. Phosphorylation of Tyr-317 also creates a binding site for Cbl family proteins which are negative regulators of tyrosine kinases; c-Cbl mediates ubiquitination of both Syk and FcεRI after antigen activation [29]. Furthermore, studies with c-Cbl and Cbl-b deficient cells indicate that Cbl-b functions as a negative regulator of FcεRI-induced degranulation while c-Cbl is more involved in MAP kinase regulation [30].
Analysis of the other two tyrosines (Tyr-342 and Tyr-346) indicate that phosphorylation of Tyr-342 is required for Syk-mediated phosphorylation of SLP-76, LAT and PLC-γ2, calcium mobilization and mast cell degranulation [26]. This can be as a result of decreased kinase activity and reduced binding of Syk to phosphorylated γ-ITAM when the Tyr-342 was mutated to Phe. Interesting, Tyr-346 is minimally phosphorylated after receptor stimulation and its substitution with Phe has minimal effects on receptor-mediated mast cell degranulation.
In the kinase domain of Syk the two adjacent tyrosines Tyr-591/Tyr-520, in the activation loop of the enzyme, are phosphorylated after Syk activation both in vitro and in cells. Substitution of these with Phe reduces signaling and degranulation in mast cells, although it does not cause significant changes on Syk kinase activity [31]. Interesting, the binding of anti-ganglioside antibody of the surface of mast cells increased Syk tyrosine phosphorylation but not histamine release or phosphorylation of the activation loop tyrosines [5]. This demonstrates that phosphorylation of Tyr-519 and Tyr-520 is required for Syk function in vivo.
The COOH-terminal region of Syk has three conserved tyrosines (Tyr-623, Tyr-624 and Tyr-625) the last two of which are also conserved in human ZAP-70. Tyr-624 and Tyr-625 are phosphorylated in both Syk and ZAP-70 by autophosphorylation or following receptor stimulation [23,32]. Mutation of these three Tyr to Phe disrupts the auto-inhibitory state of Syk which allows it to be more tyrosine phosphorylated by another tyrosine kinase in cells which activates Syk and results in autophosphorylation. Without such phosphorylation by another tyrosine kinase, these mutations result in Syk that has very low enzymatic activity and capacity for autophosphorylation. In immunoreceptor signaling, Syk alone is sufficient to propagate downstream signaling, whereas ZAP-70 requires the additional activity of another tyrosine kinase, Lck. Therefore mutation of these tyrosines results in Syk which functionally is more similar to ZAP-70 in its requirement for another tyrosine kinase for activation. In mast cells this mutated Syk is less efficient in FcεRI signaling because of decreased binding to phosphorylated ITAM independent of phosphorylation of Tyr-130. Together with the decreased kinase activity, this leads to reduced mast cell degranulation, and decreased phosphorylation of MAP kinases, and lower activation of NFAT and NFκB [16].
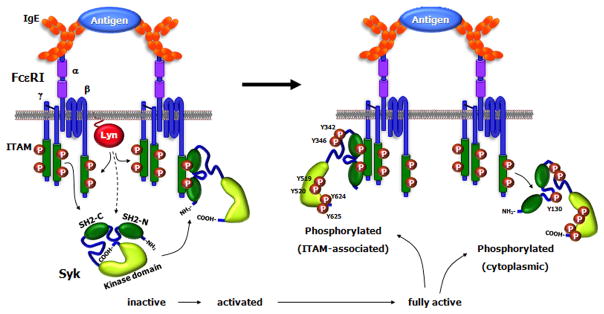
The present model for activation of Syk is shown in Fig 2. Structural studies of ZAP-70 suggest that this family of kinases has an autoinhibitory state where there are interactions of Tyr in the tail with both the kinase and the inter-SH2 domains. The globular representation of Syk shows the molecule in its autoinhibitory state with the COOH-terminal tail interacting with the kinas and inter-SH2 domains thereby contributing to keeping the molecule in a closed conformation. The binding of Syk to the phosphorylated ITAM results in a conformational change that exposes the COOH-terminal tail region. This leads to the phosphorylation of the two tyrosines (Tyr-624 and Tyr-625), which then keeps the molecule in an open conformation allowing for further phosphorylation of other tyrosine residues on Syk both by other tyrosine kinases but mostly by autophosphorylation. Therefore, the phosphorylation of Tyr-624 and Tyr-625 are important for the activation and regulation of the activity of Syk.
Fig 2.
Syk Activation. The globular representation of Syk shows the molecule in its autoinhibitory state with the COOH-terminal tail interacting with the inter-SH2 domains thereby contributing to keeping the molecule in a closed conformation. The binding of Syk to the phosphorylated ITAM results in a conformational change that exposes the COOH-terminal region. This leads to the phosphorylation of two tyrosines in the tail, which then keeps the molecule in an open conformation allowing for further phosphorylation of other tyrosine residues mostly by autophosphorylation, although there could be contributions by other tyrosine kinases. Phosphorylation of Tyr-130 in the inter-SH2 domain results in Syk dissociating from the ITAM.
The Limited Contribution of Fyn and Gab2 to FcεRI Signaling
Studies with Fyn and Gab2 deficient cells suggest a role for these proteins in signaling from FcεRI. Although Lyn is thought to be the enzyme that phosphorylates the tyrosine residues in ITAMs of the β and γ subunits[33][33], BMMC from Lyn−/− mice have normal or enhanced FcεRI-induced degranulation, suggesting that other tyrosine kinases such as Fyn could substitute for Lyn [34]. Fyn is activated following IgE-FcεRI stimulation and its tyrosine phosphorylation is increased in Lyn−/− cells. In BMMC from Fyn deficient mice, there is decreased FcεRI-induced PI3K activation with reduced activation of JNK and p38 MAPKs, and of the transcription factor NFκB; these changes parallel decreases in degranulation, release of leukotrienes and generation of cytokines [35]. In Fyn−/− BMMC there is decreased tyrosine phosphorylation of Gab2 suggesting an interaction between Fyn and this adaptor protein [36–38]. Gab2 is tyrosine phosphorylated after FcεRI stimulation downstream of Syk and has potential binding sites for signaling molecules such as Grb2, SHP2, p85 subunit of PI3K and Shc. BMMC from Gab2−/− mice have decreased FcεRI-induced PI3K activation, reduced tyrosine phosphorylation of phospholipase Cγ1, Akt and JNK resulting in reduced degranulation and cytokine generation [17]. Gab2 may also regulate granule translocation during degranulation through a calcium-independent mechanism that also involves Fyn and the small GTPase RhoA.
Experiments using transient transfections with small interference RNA (siRNA) targeting Syk, Fyn or Gab2 indicate that decreased expressions of these proteins have distinct consequences on FcεRI-mediated mast cell signaling [15]. The siRNA treatment efficiently decreases the expression of these proteins by more than 80%. For Syk there is a good correlation between the reduction in protein expression and the decrease in the FcεRI-mediated immediate, late cellular responses and signaling events; degranulation and TNF-α release, activation of NFκB and NFAT, as well as Akt phosphorylation are all significantly reduced. In contrast, comparable decrease in Fyn expression has little or no effects on degranulation, cytokine release and the same signaling events. The decreased expression of Fyn did not change the FcεRI-induced phosphorylation of Gab2 at two sites (Ser159 and Tyr452) that are important for signaling by this protein. The reduced expression of Gab2 but not Fyn decreases the receptor-induced PI3K activation, confirming the role of Gab2 in regulating PI3K. The decreased PI3K activation results in reduced phosphorylation of the MAPK p38 and JNK, as well as reduced NFAT activation with a decrease in TNF-α release but minimal changes in degranulation. The results were very similar in BMMC and two different mouse mast cell lines, one of which is growth factor dependent strongly suggesting that these changes were due to the applied siRNA treatment. These findings indicate that Fyn does not appear to play a major role in IgE-mediated mast cell responses whereas Gab2 has a role in the generation of cytokines.
These experiments with siRNA decrease in expression of Fyn and Gab2 are different than results with BMMC derived from mice with knockout of the same proteins. The amount of a protein required for signaling in these pathways may be in such excess that even >80% decrease in expression as was achieved in the present experiments will not be a limiting factor in signal transduction. However, whatever the reason these observations have important implications for pharmacological modulation of the activity of these molecules: inhibitors of Syk would be effective in reducing both early and late responses, while those that act on Gab2 would be effective on late responses. In contrast, inhibition of Fyn would be ineffective in regulating mast cell responses.
Single Cell Analysis of Mast Cell Responses
The FcεRI-induced late mast cell responses was analyzed at a single cell basis using an RBL-2H3 cell line transfected with a reporter plasmid containing three tandem NFAT binding sites fused to enhanced green fluorescent protein. Surprisingly, with this sensitive detection system, there is activation of IgE sensitized cells at concentrations of antigen which are as much as 10-fold lower than was detected by degranulation. There are also differences in signaling pathways leading to degranulation compared to NFAT-mediated gene activation. Both signaling to NFAT activation and degranulation required Syk and calcineurin. However inhibitors of the PI3K pathway block degranulation but do not decrease NFAT activation [39,40]. Studies of the FcεRI-induced increase in intracellular calcium and the decrease in response with inhibitors indicated that NFAT was activated at lower intracellular signals compared to degranulation. Therefore, FcεRI activation can result in nuclear signals in the absence of the release of mediators. These findings can explain some biological functions of mast cells that do not correlate or require release of inflammatory mediators which could occur due to contact with low concentrations of antigen. The generation of nuclear signals would then regulate mast cell survival, growth and differentiation.
FcεRI-induced Morphological and Cytoskeletal Changes Depend on Syk
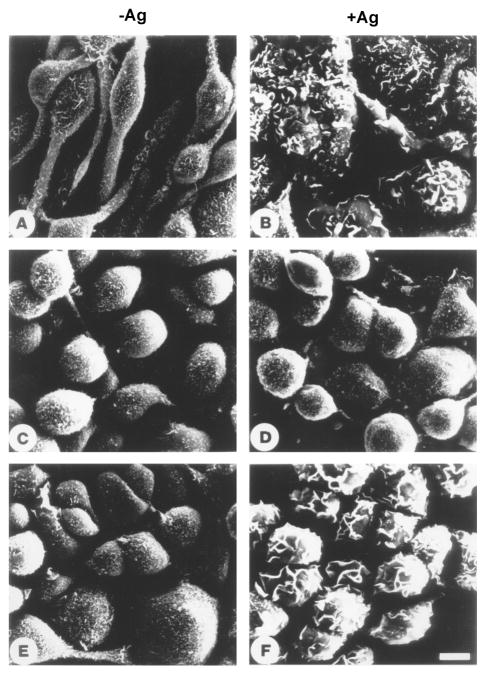
Aggregation of FcεRI in RBL-2H3 cells results in transformation of the cell surface topography from finely microvillous to highly folded or plicated, increases cell spreading and fluid pinocytosis with redistribution of actin [40,41]. These morphological changes parallel mediator release. The receptor-induced morphological changes and redistribution of actin are also observed in the absence of extracellular calcium suggesting that they are very early events, independent of the major influx of calcium [42] although they are inhibited by the addition of a chelating agent such as EGTA or EDTA [43]. Even in the absence of extracellular calcium there is the transient increase in intracellular calcium that is due to the release of calcium from the ER. However, experiments with Syk kinase inhibitors as well as Syk negative cells indicate that the FcεRI-induced morphological/cytoskeletal changes are downstream of Syk. In Syk negative cells FcεRI-aggregation does not induce increased tyrosine phosphorylation of the cytoskeletal protein paxillin and the focal adhesion kinases FAK and Pyk2 [44,45]. Similarly tyrosine phosphorylation of Vav which regulates GTP/GDP exchange activity for members of the Rho/Rac family of GTP-binding proteins, Ras-GTPase activating protein and MAP kinases are absent in Syk negative cells. Transfection of Syk reconstitutes these phosphorylations. Syk is also essential for the surface topographical changes, cell spreading and redistribution of F-actin (Fig 3). Therefore, these biochemical and morphological changes induced by FcεRI aggregation are all downstream of Syk.
Fig. 3.
The FcεRI-induced morphological changes in mast cells require Syk. RBL-2H3 (Syk+), TB1A2 (Syk−), and the Syk transfected 3A5 cell line were cultured with antigen specific IgE, washed and then either incubated with medium alone (A,C,E) or activated with antigen (B,D,F). After 20 minutes the cells were prepared for scanning electron microscopy. The nonstimulated RBL-2H3 cells are spindle-shaped with their surface covered with small short microvilli (A); FcεRI activation induced cell spreading and transformed the cell surface to a lamellar topography with deep folds and ruffles (B). The Syk-negative variant are less spindle-shaped and more round and transfection of Syk into these cells did not change their appearance (C and E). FcεRI aggregation did not induce any morphological changes in the Syk-negative cells (D) while these receptor-induced morphological changes are reconstituted by transfection of Syk (F).
siRNA screens to identify molecules that regulate FcεRI-stimulated signaling
Protein tyrosine phosphorylation is one of the earliest detectable events after FcεRI aggregation and plays an essential role in this signal transduction pathway [46]. There is also phosphorylation on other residues such as Ser/Thr and also of lipids all of which are critical for signal transduction in cells. The extent of these receptor induced-phosphorylations is regulated by the balance between protein kinases and phosphatases. As described in previous sections, several kinases are responsible for these cellular responses. However, there is only limited information about the role of protein phosphatases in the IgE-receptor induced pathways. So far studies of phosphatases have been restricted to several well characterized phosphatases, such as SHP1, SHP2, SHIP1, SHIP2, and PTEN. The role of such molecules has been established by using inhibitors or cell lines that are deficient in one of these molecules. Such cells are usually derived from either in vitro selection of established cell lines or from genetically modified mice that lack those molecules. Genome sequencing has predicted the presence of many phosphatases about which there is little or no other information. The development of siRNA technology has made it possible to perform genetic screens in mammalian cell lines to determine the function of such phosphatases, although such screening has not been commonly applied to complex pathways such as degranulation induced by antigen-receptor signaling. This siRNA approach is also challenging as differentiated immune cells are notoriously difficult to transfect. After testing different mast cells lines and delivery systems a protocol suitable for a large-scale screen was developed that yielded more than 80% transfection efficiency in the mouse mast cell line MMC-1 without affecting cell survival or function. In control experiments Syk siRNA dramatically decreased targeted protein expression with a comparable reduction in the FcεRI-initiated mast cell degranulation. Compared to stably transfected cell lines, this transient transfection system is suitable for large scale screening in immortalized or primary cell lines and it also eliminates the possibility of clonal variation. This siRNA approach therefore, appears to be highly practical for large scale screening to identify molecules in the signaling pathway when combined with a specific assay, such as measurement of the β-hexosaminidase release [46]
This siRNA screening approach was used to identify phosphatase genes that are involved in FceRI-stimulated degranulation by screening a siRNA library, which targets 198 known or predicted phosphatases. Out of 198 targets, 10 enhanced and 7 inhibited FcεRI induced degranulation in the MMC-1 mouse mast cell line. The majority of the hits were poorly characterized molecules that on the basis of their sequence had been classified as protein tyrosine, serine or dual specific phosphatases. However, there were also some well characterized proteins, for example the siRNA that targeted both the regulatory (Ppp3r1) and the catalytic (Ppp3cc) subunit of calcineurin and PTEN (Pten) inhibited degranulation. For 7 of the strongest hits, four different siRNAs per target were tested, and at least 2 out of the 4 single siRNA per target had similar effects as the pool suggesting that these were true hits. Bone marrow derived mast cells from normal mice further validated these results for six definite positive results; except for Pten, all the other 6 positive hits including Inpp5b, Ppp3r1, Mtmr4, Ptpn4, Ptpn9, and Ptpn14 had similar effects on FcεRI-induced mast cell degranulation in both MMC-1 and BMMC cells.
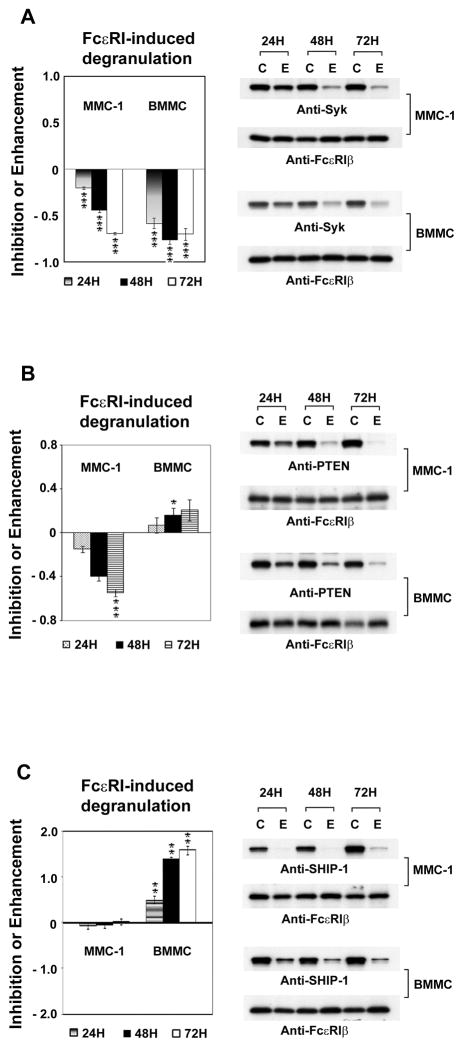
Among the positives, Pten knockdown had the opposite effect in MMC-1 and BMMC; it strongly inhibited FcεRI-induced β-hexosaminidase release in MMC-1 cells, while it slightly enhanced it in BMMC (Fig 3). In both cell types immunoblotting confirmed the specific knockdown of PTEN by siRNA. PTEN is a negative regulator of the PI3K pathway by dephosphorylating phosphatidylinositol phosphate (PI) at the 3′ position. The disparity in degranulation responses due to decreased Pten expression between MMC-1 and BMMC is probably due to a difference in the status of PI3K activation in the two cell types; the MMC-1 cells have a high basal PI3K activity. Another negative regulator of the PI3K pathway is SHIP-1 that hydrolyzes PI-3,4,5-triphosphate at the 5′ position. We therefore, compared the response on the MMC-1 and BMMC to the decreased expression of SHIP-1 (Fig 3C). Although SHIP-1 is well known to be a negative regulator of degranulation, decreased expression enhanced release only in the BMMC. These data indicate that the balance between PI(4,5)P2 and PI(3,4,5)P3 is different in the MMC-1 and BMMC and show the importance of confirming siRNA screening results with primary cells.
The siRNA screen also identified the calcium-dependent serine/threonine protein phosphatase calcineurin as a regulator of degranulation in mast cells; knockdown of either its regulatory subunit, (Ppp3r1) or catalytic subunit (PPP3CC) inhibited degranulation in MMC-1 cells. The decreased expression of calcineurin B (Ppp3r1) reduced the phosphorylation of PKD/PKCμ and PKCδ, which is required for FcεRI-induced mast cell degranulation. Calcineurin also regulates nuclear signals and gene transcription by dephosphorylating the NFAT transcription factor allowing its translocation into the nucleus. The knockdown of the regulatory subunit Ppp3r1 decreased NFAT activation (−44%), but was less effective on NFkB activation (−27%). In turn, knockdown of the catalytic subunit PPP3CC did not change NFAT activation but increased NFκB. These effects on the activity of transcription factors were mirrored by changes in the release of cytokines. Thus, Ppp3r1 knockdown decreased secretion of TNFα, IL-13 and CCL-4, while PPP3CC knockdown decreased TNFα, but increased IL-13 and MIP-1β (Barbu et al. unpublished results). These results indicate that in mast cells calcineurin controls both the FcεRI-induced late cellular events by regulating the activity of transcription factors and regulates degranulation by a mechanism that involves PKC [47].
The synthesis and secretion of cytokines after immune receptor activation depends on NFAT and NFκB transcription factors. Mouse mast cell lines that have NFAT or NFκB reporter systems were also screened with this siRNA phosphatase library to determine molecules that regulate late events after receptor stimulation (Zhang, Barbu, Groves, and Siraganian unpublished observations). There was no correlation in the effect of these siRNA on the NFAT, NFκB or degranulation responses, suggesting that distinct phosphatases regulate the FcεRI-mediated early and late responses. Among the 198 phosphatases, 16 consistently enhanced or inhibited the signals generated after FcεRI-initiated NFAT or NFκB activation. The positive hits were validated by testing in BMMC for their effects on FcεRI-induced degranulation and the release of the cytokines. There were 12 hits that enhanced the NFAT or NFκB response; all of these enhanced the release of at least one cytokine. Therefore, these genes are positive regulators of the FcεRI-induced late cellular responses. The largest increase was after the knockdown of Inpp5d (SHIP-1), a known negative regulator of the FcεRI pathway. Among four hits that inhibited the activation of NFAT or NFκB, three inhibited the receptor induced cytokine generation and two of these also the degranulation response. This strongly suggests that these two molecules control FcεRI-induced immediate and late cellular events. These screens using degranulation, NFAT or NFκB end-points have identified molecules not previously associated with the FcεRI pathway. Therefore, these methods allow high throughput whole-genome RNAi screening to identify many new molecules involved in the FcεRI-mediated cellular responses.
Caveats on interpreting signaling studies in cells
The early studies to identify cell signaling molecules used protein biochemical analysis of cells. The use of inhibitors also suggested the role of certain enzyme classes although it was complicated by the non-specificity of most such compounds. The analysis of changes in molecules (e.g. phosphorylation of proteins) suggested their involvement in the pathway without indicating their exact role. The overexpression of the protein whether it was the wild-type, constitutively active or dominant negative was also used to analyze signal transduction, however, this was complicated by the presence of the endogenously wild-type proteins. The analysis of variants of cell lines as well as cells from gene-knock mice then added a new dimension for the study of the role of individual molecules in signal transduction. The knock-out mice also allowed the analysis of the role of the gene in the whole animal in vivo immune responses and allergic reactions.
The use of bone marrow derived mast cells from gene targeted mice can often provide useful information; however, the phenotype resulting from the deletion of a gene may not be due simply to the loss of the function of the corresponding protein. There can be compensatory effects by increased or decreased expression of other molecules which become pronounced with prolonged loss of the molecule. This would be more of a problem in long-term experiments such as in BMMC, compared to the short-term knockdown with siRNA. For example, in Lyn negative BMMC there is decreased expression of TRPC4 protein that may explain the delayed calcium response of these cells [48]. Another complication is that deletion of a gene can result in a developmental block and embryonic lethality (for example knock-out of the tyrosine kinase FAK). Although in this case mast cells can be cultured from early embryos, there are other genes that are required for cell maturation and those cases require alternative strategies [49,50]. A secondary problem is when a protein has several functions or domains then the phenotypic consequences of gene knockout may manifest as a combination of several different perturbations. For example, a protein tyrosine kinase can function both as a positive and negative regulator of signaling. The use of knock-ins with mutations or deletions of parts of molecules can help in these signaling studies. The absence of a protein could also result in compensatory increase in other family members or changes in other proteins with which it interacts. A problem is that there frequently could be modifying genes, for example the phenotype of gene deletion can vary depending on the genetic background of the mice. In these cases, gene targeting with siRNA in a transient system might provide more accurate insight into the function of the protein in the signaling pathway.
Concluding remarks
The availability of genomic sequences and the development of new techniques have revolutionized the study of signal transduction. Gene targeting has allowed the introduction of mutations into the mouse genome; including the knock-out of whole genes and the knock-in of specific mutations in proteins. Whole genome sequences have also made possible the high-throughput use of siRNA to test for the role of any expressed molecules in multiple signaling pathways. Transient transfection with siRNA can also be a powerful tool to unravel the relative function of different molecules in signaling pathways. Data from these different techniques should result in a better understanding of the signal transduction pathways in mast cells that are crucial for the release of different inflammatory mediators. Such knowledge should lead to the development of more effective pharmacological agents that modulate these pathways.
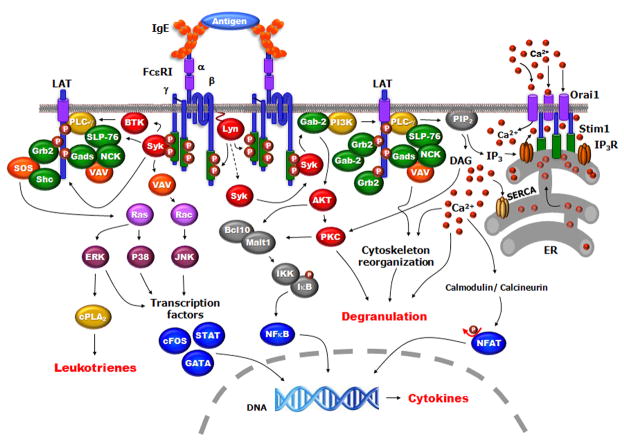
Fig 1.
Model Depicting major molecules and events in mast cell activation. Antigen binding to IgE cross-links two FcεRI leading to Lyn phosphorylating the tyrosines in the ITAMs of the β and γ-subunits of the receptor. Syk kinase is recruited and activated by binding to the phosphorylated ITAM of the γ subunit; the activated Syk autophosphorylates and activates downstream signaling which includes phosphorylation of Btk, PI3K, the adaptors LAT1, LAT2, SLP-76 and PLCγ. Multimolecular complexes form on LAT which includes Gads, SLP76, PLCγ and Vav. The activated PLCγ hydrolyzes PI(4,5)P2 to form DAG and IP3. The IP3 activates the IP3-receptor on the ER releasing calcium, the calcium sensor STIM1 then interacts with the ORA1 membrane protein opening the CRAC channels allowing the increase in intracellular calcium. DAG activates PKC and also interacts with calmodulin to activate calcineurin leading to NFAT translocation into the nucleus. The activation of GTPases lead to MAP kinase activation. These pathways lead to degranulation, release of leukotrienes and cytokine synthesis.
Fig. 4.
The comparisons of targeting Syk, PTEN and SHIP-1 in MMC-1 and BMMC. The mouse mast cells line (MMC-1), or Bone Marrow Derived Mast Cells (BMMC) were transfected with siRNA for Syk (A), PTEN (B) or SHIP-1 (C). Control cells were transfected with scrambled siRNA “C”. The cells were sensitized and then stimulated with antigen after 24, 48 and 72 hours. The antigen-induced β-hexosaminidase release is expressed as the fraction of that in controls. The lysates of the cell pellets were analyzed by immunoblotting with the indicated antibodies using the anti-FcεRIβ as a loading control. “C” for scrambled siRNA controls, “E” experimental siRNA.
Acknowledgments
We thank Jacqueline Groves for helpful discussions and review of the manuscript; we also thank William Swaim for help with morphological experiments. This work was supported by the Intramural Research Program of the National Institutes of Health, NIDCR.
Abbreviations
- BMMC
bone marrow derived mast cells
- ER
endoplasmic reticulum
- FcεRI
high affinity IgE receptor
- Grb2
growth factor receptor-bound protein 2
- Gads
Grb2-related adaptor protein
- ITAM
immunoreceptor tyrosine-based activation motif
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- LAT
linker for activation of T cells
- MMC-1
mouse mast cell line
- NFAT
nuclear factor for T cell activation
- NFkB
nuclear factor κ B
- PLC
phospholipase C
- PI3K
phosphatidylinositol-3 kinase
- RBL-2H3
rat basophilic leukemia 2H3 mast cell line
- SH2
Src homology 2
- SLP-76
SH2 domain-containing leukocyte protein of 76 kDa
- siRNA
small interference RNA
- Syk
spleen tyrosine kinase
Footnotes
Note all residue designations are for rat Syk, NCBI Reference Sequence NM_012758.1.
References
- 1.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Errico D, Lessmann E, Rivera J. Adapters in the organization of mast cell signaling. Immunol Rev. 2009;232:195–217. doi: 10.1111/j.1600-065X.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639–46. doi: 10.1016/j.jaci.2009.08.035. quiz 647–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–46. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J Biol Chem. 2000;275:35442–7. doi: 10.1074/jbc.M004549200. [DOI] [PubMed] [Google Scholar]
- 6.Benhamou M, Ryba NJ, Kihara H, Nishikata H, Siraganian RP. Protein-tyrosine kinase p72syk in high affinity IgE receptor signaling. Identification as a component of pp72 and association with the receptor gamma chain after receptor aggregation. J Biol Chem. 1993;268:23318–24. [PubMed] [Google Scholar]
- 7.Minoguchi K, Benhamou M, Swaim WD, Kawakami Y, Kawakami T, Siraganian RP. Activation of protein tyrosine kinase p72syk by FcεRI aggregation in rat basophilic leukemia cells. p72syk is a minor component but the major protein tyrosine kinase of pp72. J Biol Chem. 1994;269:16902–8. [PubMed] [Google Scholar]
- 8.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vig M, Kinet JP. Calcium signaling in immune cells. Nat Immunol. 2009;10:21–7. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitomi T, Zhang J, Nicoletti LM, Grodzki AC, Jamur MC, Oliver C, Siraganian RP. Phospholipase D1 regulates high-affinity IgE receptor-induced mast cell degranulation. Blood. 2004;104:4122–8. doi: 10.1182/blood-2004-06-2091. [DOI] [PubMed] [Google Scholar]
- 11.Kimura T, Zhang J, Sagawa K, Sakaguchi K, Appella E, Siraganian RP. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J Immunol. 1997;159:4426–34. [PubMed] [Google Scholar]
- 12.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siraganian RP, Zhang J, Suzuki K, Sada K. Protein tyrosine kinase Syk in mast cell signaling. Mol Immunol. 2002;38:1229–33. doi: 10.1016/s0161-5890(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–9. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grodzki AC, Moon KD, Berenstein EH, Siraganian RP. FcεRI-induced activation by low antigen concentrations results in nuclear signals in the absence of degranulation. Mol Immunol. 2009;46:2539–47. doi: 10.1016/j.molimm.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Castro RO, Zhang J, Jamur MC, Oliver C, Siraganian RP. Tyrosines in the carboxy-terminus regulate Syk kinase activity and function. J Biol Chem. 2010 doi: 10.1074/jbc.M110.134262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbu EA, Zhang J, Siraganian RP. The limited contribution of Fyn and Gab2 to the high affinity IgE receptor signaling in mast cells. J Biol Chem. 2010;285:15761–8. doi: 10.1074/jbc.M110.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura T, Kihara H, Bhattacharyya S, Sakamoto H, Appella E, Siraganian RP. Downstream signaling molecules bind to different phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) peptides of the high affinity IgE receptor. J Biol Chem. 1996;271:27962–8. doi: 10.1074/jbc.271.44.27962. [DOI] [PubMed] [Google Scholar]
- 19.Chen T, et al. Interaction of phosphorylated FcεRIγ immunoglobulin receptor tyrosine activation motif-based peptides with dual and single SH2 domains of p72syk. Assessment of binding parameters and real time binding kinetics. J Biol Chem. 1996;271:25308–15. doi: 10.1074/jbc.271.41.25308. [DOI] [PubMed] [Google Scholar]
- 20.Kihara H, Siraganian RP. Src homology 2 domains of Syk and Lyn bind to tyrosine-phosphorylated subunits of the high affinity IgE receptor. J Biol Chem. 1994;269:22427–32. [PubMed] [Google Scholar]
- 21.Kimura T, Sakamoto H, Appella E, Siraganian RP. Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol Cell Biol. 1996;16:1471–8. doi: 10.1128/mcb.16.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–46. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, Geahlen RL. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta. 1997;1355:177–90. doi: 10.1016/s0167-4889(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, et al. Quantitative time-resolved phosphoproteomic analysis of mast cell signaling. J Immunol. 2007;179:5864–76. doi: 10.4049/jimmunol.179.9.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Oh H, Burton RA, Burgner JW, Geahlen RL, Post CB. Tyr130 phosphorylation triggers Syk release from antigen receptor by long-distance conformational uncoupling. Proc Natl Acad Sci U S A. 2008;105:11760–5. doi: 10.1073/pnas.0708583105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Berenstein E, Siraganian RP. Phosphorylation of Tyr342 in the linker region of Syk is critical for FcεRI signaling in mast cells. Mol Cell Biol. 2002;22:8144–54. doi: 10.1128/MCB.22.23.8144-8154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latour S, Zhang J, Siraganian RP, Veillette A. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signalling. EMBO J. 1998;17:2584–95. doi: 10.1093/emboj/17.9.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sada K, Zhang J, Siraganian RP. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J Immunol. 2000;164:338–44. doi: 10.4049/jimmunol.164.1.338. [DOI] [PubMed] [Google Scholar]
- 29.Paolini R, et al. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of FcεRI and Syk in RBL cells. J Biol Chem. 2002;277:36940–7. doi: 10.1074/jbc.M204948200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Chiang YJ, Hodes RJ, Siraganian RP. Inactivation of c-Cbl or Cbl-b differentially affects signaling from the high affinity IgE receptor. J Immunol. 2004;173:1811–8. doi: 10.4049/jimmunol.173.3.1811. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Kimura T, Siraganian RP. Mutations in the activation loop tyrosines of protein tyrosine kinase Syk abrogate intracellular signaling but not kinase activity. J Immunol. 1998;161:4366–74. [PubMed] [Google Scholar]
- 32.Kulathu Y, Hobeika E, Turchinovich G, Reth M. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. Embo J. 2008;27:1333–44. doi: 10.1038/emboj.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizumi H, Yamamoto T. Impaired tyrosine phosphorylation and Ca2+ mobilization, but not degranulation, in lyn-deficient bone marrow-derived mast cells. J Immunol. 1997;158:2350–5. [PubMed] [Google Scholar]
- 34.Gomez G, Gonzalez-Espinosa C, Odom S, Baez G, Cid ME, Ryan JJ, Rivera J. Impaired FcεRI-dependent gene expression and defective eicosanoid and cytokine production as a consequence of Fyn deficiency in mast cells. J Immunol. 2005;175:7602–10. doi: 10.4049/jimmunol.175.11.7602. [DOI] [PubMed] [Google Scholar]
- 35.Parravicini V, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–8. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 36.Xie ZH, Ambudkar I, Siraganian RP. The adapter molecule Gab2 regulates Fc epsilon RI-mediated signal transduction in mast cells. J Immunol. 2002;168:4682–91. doi: 10.4049/jimmunol.168.9.4682. [DOI] [PubMed] [Google Scholar]
- 37.Gu H, et al. Essential role for Gab2 in the allergic response. Nature. 2001;412:186–90. doi: 10.1038/35084076. [DOI] [PubMed] [Google Scholar]
- 38.Yu M, Lowell CA, Neel BG, Gu H. Scaffolding adapter Grb2-associated binder 2 requires Syk to transmit signals from FcεRI. J Immunol. 2006;176:2421–9. doi: 10.4049/jimmunol.176.4.2421. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 1985;101:2145–55. doi: 10.1083/jcb.101.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Apgar JR. Regulation of the antigen-induced F-actin response in rat basophilic leukemia cells by protein kinase C. J Cell Biol. 1991;112:1157–63. doi: 10.1083/jcb.112.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishida K, et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–26. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sahara N, Siraganian RP, Oliver C. Morphological changes induced by the calcium ionophore A23187 in rat basophilic leukemia (2H3) cells. J Histochem Cytochem. 1990;38:975–83. doi: 10.1177/38.7.1693935. [DOI] [PubMed] [Google Scholar]
- 43.Okazaki H, Zhang J, Hamawy MM, Siraganian RP. Activation of protein-tyrosine kinase Pyk2 is downstream of Syk in FcεRI signaling. J Biol Chem. 1997;272:32443–7. doi: 10.1074/jbc.272.51.32443. [DOI] [PubMed] [Google Scholar]
- 44.Benhamou M, Gutkind JS, Robbins KC, Siraganian RP. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990;87:5327–30. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benhamou M, Siraganian RP. Protein-tyrosine phosphorylation: an essential component of FcεRI signaling. Immunol Today. 1992;13:195–7. doi: 10.1016/0167-5699(92)90152-w. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Mendoza M, Guiraldelli MF, Barbu EA, Siraganian RP. Small interfering RNA screen for phosphatases involved in IgE-mediated mast cell degranulation. J Immunol. 2010;184:7178–85. doi: 10.4049/jimmunol.0904169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–50. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- 48.Vial D, Oliver C, Jamur MC, Pastor MV, da Silva Trindade E, Berenstein E, Zhang J, Siraganian RP. Alterations in granule matrix and cell surface of focal adhesion kinase-deficient mast cells. J Immunol. 2003;171:6178–86. doi: 10.4049/jimmunol.171.11.6178. [DOI] [PubMed] [Google Scholar]
- 49.Kambayashi T, Larosa DF, Silverman MA, Koretzky GA. Cooperation of adapter molecules in proximal signaling cascades during allergic inflammation. Immunol Rev. 2009;232:99–114. doi: 10.1111/j.1600-065X.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita Y, et al. Genetic variation influences FcεRI-induced mast cell activation and allergic responses. J Immunol. 2007;179:740–3. doi: 10.4049/jimmunol.179.2.740. [DOI] [PubMed] [Google Scholar]