Abstract
Several sets of male mice were given dietary melatonin over a series of experiments performed during a nine year period. Overall, melatonin-supplemented mice aged ≥26 months at sacrifice had significantly fewer tumors with lower severity than similarly aged control animals. The studies were originally designed to explore the potential of this agent for reducing the rate of onset of some genetic indices of brain aging. When these animals were sacrificed they were routinely examined for overt evidence of tumors and when these were found, a note was made of their occurrence, and of their size. Tumors are commonly found during senescence of several strains of mice. Since tumorigenesis was not the original intent of the study, these observations were recorded but not pursued in greater detail. In this report, these data have now been collated and summarized. This analysis has the disadvantage that tumor origin and morphology were not recorded. However, the study also has the advantage of being conducted over an extended period of time with many groups of animals. In consequence, many extraneous factors, which could be potential confounders, such as seasonal or dietary variations, are unlikely to have interfered with the analysis. The use of more than one mouse strain strengthens the possibility that the findings may have general relevance. Both aged and young animals were included in the original experiments but the tumor incidence in animals younger than 25 months was very low.
Keywords: Aging, Melatonin, Cancer, Tumors
1. Introduction
In addition to the well-known relation between darkness and melatonin production, there are many previous reports suggesting that melatonin may be oncostatic or cancer-preventive [1]. Cancer incidence greatly increases in older animals at the same time that the circadian pulsatile production of melatonin in the pineal gland declines; there is increasing mechanistic evidence relating these two phenomena [2]. Human epidemiological studies are necessarily focused on specific cancer types, notably breast cancer [3,4]. Studies involving blindness suggest a lower incidence of cancer in those lacking sight [5]. This correlation has been further refined and the degree of protection shown proportional to the extent of visual impairment [6.7]; this observation has been attributed to the absence of light-induced depression of circulating melatonin. Melatonin levels are often inferred rather than assayed in such studies, which may also involve other endocrine factors influenced by the absence of normal circadian cycles. Other human studies have been carried out on populations such as night workers exposed to abnormal circadian rhythms [8], and the IARC unit of the World Health Organization has classified circadian rhythm-disrupting shift-work as potentially carcinogenic [9]. Suggestive results reporting protective effects of melatonin against the spread of pre-existing cancer in humans [10-12] are reinforced by several animal studies in which melatonin treatment is reported to give a degree of protection against cancer implants and carcinogens [13,14]. Further confirmation of this concept comes from evidence that hormone-dependent cancers are less common in those who live north of the Arctic Circle and thus experience a light deficit during extended periods of winter darkness. This is unlikely to be compensated for by extended light during summer months due to protective measures employed to permit sleep [15]. Several reports concerning the effect of administered melatonin on the onset and progression of tumors in experimental animals reinforce human epidemiological and clinical studies. Using carcinogens and tumorigenic cell lines, melatonin treatment has been reported to give a degree of protection against both the onset and rate of progression of tumors [13, 14, 16].
2. Methods
The total number of animals used in these studies was 43 melatonin-treated mice and 52 controls over 20 months old for the cancer analysis; and 74 and 77, respectively, older than 16 months for the death-rate analysis. All animals were males. The corresponding values for mice aged under 7 months of age were 57 and 58 respectively. Each individual study comprised sets of between 7 and 10 mice. The period of treatment with dietary melatonin was always between 9 and 14 weeks immediately prior to sacrifice, while the melatonin content of the experimental diet was 40ppm. The age at sacrifice of adult mice was 4.5 to 7 months while that of senescent mice was 26-27 months. Some groups included only in the death-rate analysis were sacrificed earlier, at 16.7 and 21.7 months. The strains of mouse used were B6C3F1 (C57BL/6J F × C3H M) and CB6F1 (BALB/cJ F × C57BL/6J M). All mice were from Harlan Labs (Indianapolis, IN). Mice were housed two to four per cage and maintained on a 12 hour light/dark cycle in a temperature controlled (22±1 °C) room. Food and water were provided ad libitum. Young and old control animals were fed a pelleted minimal basal diet (AIN-93M, Dyets #100900, Dyets Inc., Bethlehem, PA) consisting of 10% sucrose and 14% casein (w/w) as well as a minimal salt and vitamin mix. This basal diet was supplemented with 40 ppm (w/w) melatonin (Sigma-Aldrich, St. Louis, MO) in separate groups of mice. The dosage level was confirmed by independent testing (Irvine Nutri-Chemical Laboratories, Irvine, CA). Tissue and serum levels of melatonin, but not of other hormones, were measured in one group of mice [17]. Separately caged sentinel animals were maintained in the same room to monitor for the occurrence of infectious disease; no disease was detected during the treatment periods. All experiments were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine, and conformed to the National Institute of Health guide for the care and use of laboratory animals.
Tumor severity was scored to evaluate the size and multiplicity of tumors occurring in each animal. A score of 1 was assigned to a single tumor recorded as small, a score of 2 to a single (unremarkable) tumor, and a score of 3 was assigned if an animal had developed multiple tumors or if a single tumor was recorded as large. A severity index for each treatment was computed by summing the severity scores and dividing by the number of animals in the treatment group.
3. Results
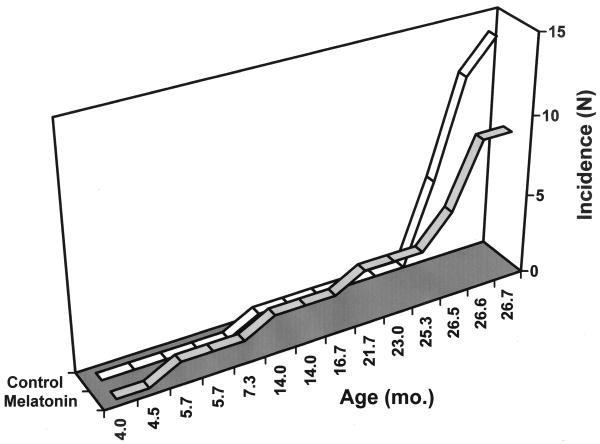
As expected, a large increase of observable tumors occurred with age. However, an effect of the melatonin dosing was apparent in aged mice where the tumor incidence was markedly reduced (Fig. 1). Furthermore, the severity of any visible tumors was lessened in melatonin-treated animals (Fig. 2). Melatonin's protective effect was apparent only in older animals (Fig. 3); in younger mice ≤ 25 months of age, melatonin treatment did not significantly affect cancer incidence.
Fig. 1.
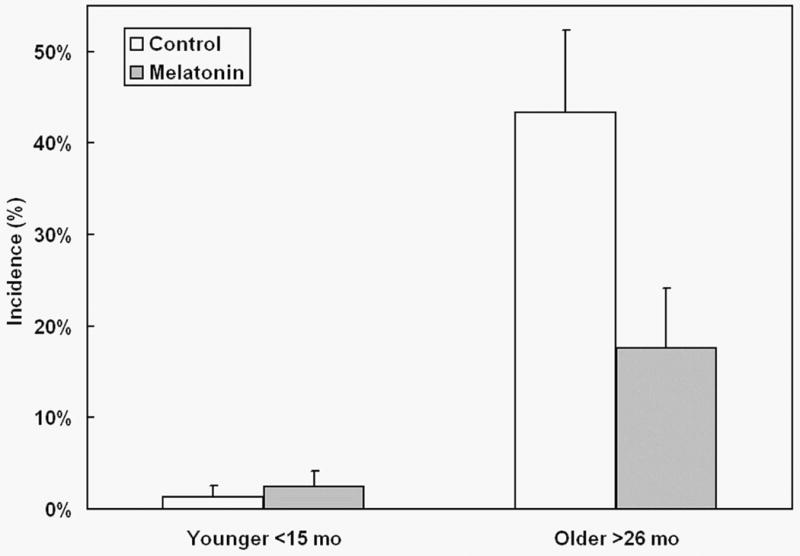
Effect of melatonin supplementation on tumor incidence in mice of two different age ranges. Mice aged less than 15 months (Younger) or over 26 months (Older) at sacrifice were fed chemically purified diets either without (open bars) or with 40 ppm melatonin (shaded bars). Y-axis displays cancer incidence as percentage of animals in each group with observable cancers at necropsy.
Fig. 2.
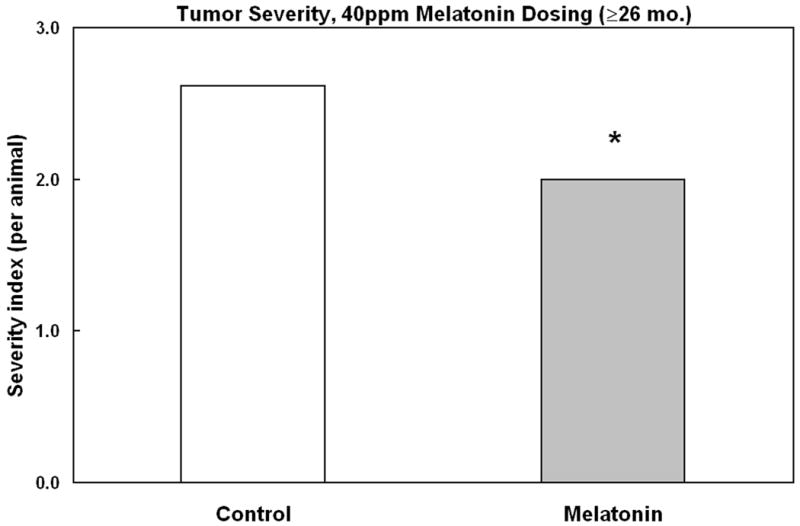
Effect of melatonin supplementation on tumor severity in mice aged over 26 months at sacrifice. Mice were fed chemically purified diets either without (open bar) or with 40 ppm melatonin (shaded bar). Y-axis displays cancer disease severity index (see text).
Fig. 3.
Cumulative effect of melatonin supplementation on tumor incidence in mice of increasing age. Mice were fed chemically purified diets either without (open segments) or with 40 ppm melatonin (shaded segments). Y-axis displays cancer incidence as number of animals in each group with observable cancers at necropsy.
While tumor size may be considered rather subjective, all observations over the period of the work were performed by a single person. This would tend to minimize variation. The mice were all originally used to study the effect of melatonin upon cortical immune function in a series of experiments ranging over six years. Non-neurological pathological changes were only noted in order to allow systematic recording of overall health. Thus, the objectivity of these examinations is enhanced by their original documentation being motivated merely by thoroughness rather than for hypothesis development.
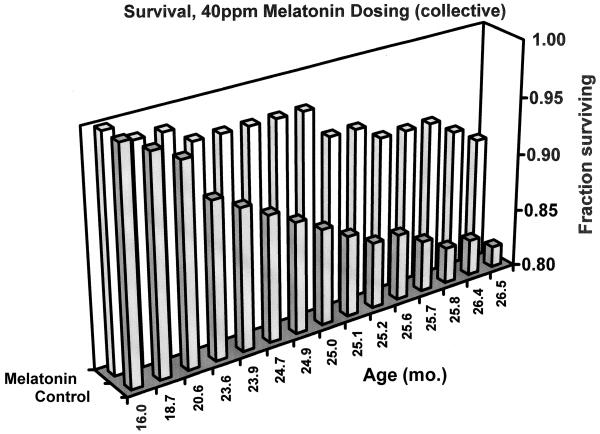
The reduced incidence of visible tumors in melatonin-treated mice raised the issue whether normal mortality taking place during the long experimental retention periods could be affected by this treatment. After 16 months of age the overall spontaneous death rate was markedly reduced in mice receiving melatonin (Fig 4). Over 91% of melatonin treated mice survived to 26.5 months age, while only 82% of untreated mice attained this age. Thus treatment with melatonin led to the overall death rate in the most aged mice being halved from 18% to 9%.
Fig. 4.
Survival fraction with age, of mice receiving dietary melatonin after 16 months of age. Mice were fed chemically purified diets either without (open segments, N=77) or with 40 ppm melatonin (shaded segments, N=74). Y-axis displays proportion of surviving mice in either group, calculated from records of spontaneous deaths occurring prior to planned sacrifice dates.
4. Discussion
The results presented here confirm that melatonin can reduce the risk of onset and delay the progression of tumors. Our extensive prior work on the effects of melatonin on the brain, suggests some of the mechanisms underlying its oncostatic effects. The levels of melatonin encountered within tissues are sufficiently low that a direct anti-oxidant effect is unlikely to play a significant role [17]. However, melatonin is able to reverse some key genetic changes associated with senescence. Thus melatonin can specifically prevent the age-related increased expression of genes relating to inflammation [18,19]. This increase is not provoked by extrinsic inflammatory stimuli but reflects a change with age, in basal gene expression. In consequence, the immune response to exogenous stimuli is also altered with age [20]. Thus the protective effect of melatonin may be by way of reversal of age-related changes in gene expression (Table 1) [21,22]. These findings are likely to be relevant to human populations since age-related changes of gene expression occurring in mice have close counterparts in humans [23]. Since age is a very important factor in determining cancer risk, restoration of a more youthful profile of mRNA production may reduce the likelihood of an aged organism from developing cancer.
Table 1.
Comparison between cancer-associated, age-related and melatonin-induced changes in gene expression. Age and melatonin data from Sharman et al., 2007 [22]. GEO = Gene Expression Omnibus website http://www.ncbi.nlm.nih.gov/geo/
| Gene | Age-induced change | Melatonin-induced change | Cancer-associated change (trend) | Reference to cancer-associated change |
|---|---|---|---|---|
| LCN2 |
|
|
[31] | |
| EST AV057155 |
|
|
GEO link, from [39] | |
| Igk-V1 |
|
|
GEO link, from [39] | |
| Igk-V1 |
|
|
GEO link, from [37] | |
| Igh-6 |
|
|
GEO link, from [37] | |
| Lrg1 |
|
|
[40] | |
| Igj |
|
|
[41] | |
| Gvin1 |
|
|
GEO link, from [37] | |
| TiPARP |
|
|
GEO link, from [37] | |
|
|
[35, 36] | |||
| Agxt2l1 |
|
|
GEO link, from [42] |
Our results indicating a reduction in spontaneous tumor incidence by melatonin are supported by studies of chemically-induced or implanted cancers. Melatonin (5 mg/kg bw, i.p.) prevented N-nitrosodiethylamine/CCl4-induced hepatocarcinoma in rats [24]. Growth of hepatoma tumors implanted in mice is inhibited by 200 μg/day of melatonin administered either s. c. or in the diet over a 32 day period [25]. In a model of pancreatic cancer induced in Syrian hamsters by N-nitrosobis (2-oxopropyl)amine, melatonin (20 μg/ml supplied in tap water for 12 weeks) reduced the number of tumors and presence of differentiated adenomacarcinoma tissue [26].
There is evidence that melatonin treatment reduces tumor incidence in female mice as well as males. In particular, reductions in mammary cancers by melatonin treatment is consonant with the generally inhibitory seasonality input it provides to the female reproductive system of many mammalian species, and with its antiestrogenic and aromatase-inhibiting actions [27]. Melatonin has been shown to reduce tumor incidence and/or latency in a number of mammary cancer-prone mouse models, including virus-associated [28], and the SHR strain [29].
A potential gene of especial relevance for carcinogenesis is that for the glycoprotein lipocalin 2 (lcn2, ngal). Substantially increased levels of lipocalin 2 are associated with a wide number of human cancers, including esophageal [30], rectal, pancreatic, ovarian [31], thyroid and breast cancers [32]. High levels of the lcn2 gene are also expressed in human hepatocellular carcinoma tissue [33]. Lcn2 gene expression is substantially increased in old mouse brains [21] and in liver (unpublished observation). In brain, dietary melatonin supplementation reduces this expression level to that found in young animals [21]. In a breast cancer cell line, increased lcn2 expression is associated with a more aggressive and invasive morphology, and repression of lcn2 expression with siRNA reduced cell migration and engendered a more clustered morphology [34]. There is clearly a mechanistic involvement of lcn2 in at least one type of cancer and high levels of the hepatic lcn2 gene are associated with hepatocellular carcinomas. Therefore any reduction of gene expression of this gene in liver paralleling that found in brain following administration of melatonin, may account for the reduced numbers of liver cancers observed in the melatonin-treated animals. It should be kept in mind, however, that even though elevated lcn2 levels are associated with all the organ cancers listed above—particularly at early stages of progression—heightened levels are not always associated with more advanced disease, and in some cases may subdue disease aggressiveness [34]. Although our studies were carried out with male animals, the link between elevated Lcn2 and breast cancer in females is particularly strong [32]. This link suggests that inclusion of female animals in future studies should be both important and fruitful. Some genes become over-represented as a consequence of the aneuploidy typical of many cancers. The expression of one such gene, TiPARP, is increased about 10-fold in human head and neck cancer [35,36], and is also increased in a cancer cell line [37]. TiPARP (Parp7) is a member of the poly-ADP-ribose polymerase (PARP) protein family, other members of which are intimately involved with DNA damage sensing and repair, cell death, and immunity – all functions that become dysregulated in cancers. In contrast to the overexpression of TiPARP in human head and neck cancer, its transcription is decreased by age in non-cancerous tissue, but restored by melatonin (Table 1).
Very few tumors were observed in animals sacrificed prior to 15 months of age (1 in 78 control animals and 2 in 82 melatonin-fed). At such low levels of tumor incidence it was not possible to measure the effect of melatonin feeding. Assuming a binomial distribution about the observed probabilities of incidence, the difference between these tumor incidence levels was not significant.
Since melatonin administration decreased the size of detectable tumors, it is possible that, in addition to reducing their overall incidence, melatonin was able to slow down the rate of growth of pre-existing tumors. It is also noteworthy that the overall mortality rate of mice irrespective of the cause of death was reduced in the melatonin-treated group.
Melatonin increased the proportion of mice not dying before reaching the fully aged phase. Since malignancies, especially hepatocellular tumors and lymphoma, are the major cause of death in mature male B6C3F1 mice [38], this finding may be related to the reduced number of tumors found in treated mice. However, the results do not directly address the issue of whether melatonin affects maximum life span.
These results serve to emphasize the importance of continuing to investigate the beneficial qualities of melatonin especially in relation to the interface between cancer and aging. Melatonin is very non-toxic, inexpensive and readily available and this further adds to its attractiveness as a potential carcinostatic agent.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (AG 16794) and in part by a UC Davis Center for Human and Nutrition Research Pilot Award (CHNR08-318) to E.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Srinivasan V, Spence DW, Pandi-Perumal SR, Trakht I, Cardinali DP. Therapeutic actions of melatonin in cancer: possible mechanisms. Integr Cancer Ther. 2008;7:189–203. doi: 10.1177/1534735408322846. [DOI] [PubMed] [Google Scholar]
- 2.Jung-Hynes B, Reiter RJ, Ahmad N. Sirtuins, melatonin and circadian rhythms: building a bridge between aging and cancer. J Pineal Res. 2010;48:9–19. doi: 10.1111/j.1600-079X.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Schernhammer ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281:1–7. doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feychting M, Osterlund B, Ahlbom A. Reduced cancer incidence among the blind. Epidemiology. 1998;9:490–494. [PubMed] [Google Scholar]
- 6.Verkasalo PK, Pukkala E, Stevens RG, Ojamo M, Rudanko SL. Inverse association between breast cancer incidence and degree of visual impairment in Finland. Br J Cancer. 1999;80:1459–1460. doi: 10.1038/sj.bjc.6690544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pukkala E, Ojamo M, Rudanko SL, Stevens RG, Verkasalo PK. Does incidence of breast cancer and prostate cancer decrease with increasing degree of visual impairment. Cancer Causes Control. 2006;17:573–576. doi: 10.1007/s10552-005-9005-6. [DOI] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer. 2007;90:941–943. doi: 10.1038/sj.bjc.6601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantermann T, Roenneberg T. Is light-at-night a health risk factor or a health risk predictor? Chronobiol Int. 2009;2:1069–1074. doi: 10.3109/07420520903223984. [DOI] [PubMed] [Google Scholar]
- 10.Lissoni P, Chilelli M, Villa S, Cerizza L, Tancini G. Five years survival in metastatic non-small cell lung cancer patients treated with chemotherapy alone or chemotherapy and melatonin: a randomized trial. J Pineal Res. 2003;3:12–15. doi: 10.1034/j.1600-079x.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 11.Lissoni P. Biochemotherapy with standard chemotherapies plus the pineal hormone melatonin in the treatment of advanced solid neoplasms. Pathol Biol (Paris) 2007;55:201–204. doi: 10.1016/j.patbio.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360–366. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 13.Otálora BB, Madrid JA, Alvarez N, Vicente V, Rol MA. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res. 2008;4:307–315. doi: 10.1111/j.1600-079X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Yasui Y, Tanaka M, Tanaka T, Oyama T, Rahman KM. Melatonin suppresses AOM/DSS-induced large bowel oncogenesis in rats. Chem Biol Interact. 2009;177:128–136. doi: 10.1016/j.cbi.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Erren TC, Piekarski C. Does winter darkness in the Arctic protect against cancer? The melatonin hypothesis revisited. Med Hypotheses. 1999;53:1–5. doi: 10.1054/mehy.1999.0810. [DOI] [PubMed] [Google Scholar]
- 16.Vesnushkin GM, Plotnikova NA, Semenchenko AI, Anisimov VN. Dose-dependent inhibitory effect of melatonin on carcinogenesis induced by benzo[a]pyrene in mice. J Exp Clin Cancer Res. 2006;25:507–513. [PubMed] [Google Scholar]
- 17.Lahiri DK, Ge YW, Sharman EH, Bondy SC. Age-related changes in serum melatonin in mice: higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J Pineal Res. 2004;36:217–223. doi: 10.1111/j.1600-079X.2004.00120.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharman KG, Sharman EH, Yang E, Bondy SC. Dietary melatonin selectively reverses age-related changes in cortical cytokine mRNA levels, and their responses to an inflammatory stimulus. Neurobiol Aging. 2002;23:633–638. doi: 10.1016/s0197-4580(01)00329-3. [DOI] [PubMed] [Google Scholar]
- 19.Sharman E, Sharman KG, Lahiri DK, Ge YW, Bondy SC. Age-related changes in murine CNS mRNA gene expression are modulated by dietary melatonin. J Pineal Res. 2004;36:165–170. doi: 10.1046/j.1600-079x.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 20.Sharman KG, Sharman E, Campbell A, Bondy SC. Reduced basal levels and enhanced LPS response of IL-6 mRNA in aged mice. J Gerontology. 2001;56:B520–B523. doi: 10.1093/gerona/56.12.b520. [DOI] [PubMed] [Google Scholar]
- 21.Perreau VM, Cotman CW, Sharman KG, Bondy SC, Sharman EH. Melatonin treatment in old mice enables a more youthful response to LPS in the brain. J Neuroimmunology. 2007;182:22–31. doi: 10.1016/j.jneuroim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharman EH, Bondy SC, Sharman KG, Lahiri D, Cotman CW, Perreau VM. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem Int. 2007;50:336–344. doi: 10.1016/j.neuint.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharman EH, Sharman KG, Bondy SC. Parallel changes in gene expression in aged human and mouse cortex. Neurosci Lett. 2005;390:4–8. doi: 10.1016/j.neulet.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian P, Mirunalini S, Dakshayani KB, Pandi-Perumal SR, Trakht I, Cardinali DP. Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res. 2007;43:305–312. doi: 10.1111/j.1600-079X.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 25.Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999;59:4693–4701. [PubMed] [Google Scholar]
- 26.Ruiz-Rabelo JF, Vázquez R, Perea MD, Cruz A, González R, Romero A, Muñoz-Villanueva MC, Túnez I, Montilla P, Muntané J, Padillo FJ. Beneficial properties of melatonin in an experimental model of pancreatic cancer. J Pineal Res. 2007;43:270–275. doi: 10.1111/j.1600-079X.2007.00472.x. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez-Barcelo EJ, Cos S, Mediavilla D, Martinez-Campa C, Gonzalez A, Alonso-Gonzalez C. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38:217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian A, Kothari L. Melatonin, a suppressor of spontaneous murine mammary tumors. J Pineal Res. 1991;10:136–140. doi: 10.1111/j.1600-079x.1991.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 29.Anisimov VN, Alimova IN, Baturin DA, Popovich IG, Zabezhinski MA, Rosenfeld SV, Manton KG, Semenchenko AV, Yashin AI. Dose-dependent effect of melatonin on life span and spontaneous tumor incidence in female SHR mice. Exp Gerontol. 2003;38:449–461. doi: 10.1016/s0531-5565(02)00240-1. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XF, Zhang Y, Zhang XH, Zhou SM, Yang GG, Wang OC, Guo GL, Yang GY, Hu XQ. Clinical significance of Neutrophil gelatinase-associated lipocalin (NGAL) expression in primary rectal cancer. BMC Cancer. 2009;9:134. doi: 10.1186/1471-2407-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang Z, Zhang X, Niu Y, Shen Z, Shen J, Wu X, Li E. Upregulation of neutrophil gelatinase-associated lipocalin in oesophageal squamous cell carcinoma: significant correlation with cell differentiation and tumour invasion. J Clin Pathol. 2007;60:555–561. doi: 10.1136/jcp.2006.039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–3918. doi: 10.1073/pnas.0810617106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil MA, Chua MS, Pan KH, Lin R, Lih CJ, Cheung ST, Ho C, Li R, Fan ST, Cohen SN, Chen X, So S. An integrated data analysis approach to characterize genes highly expressed in hepatocellular carcinoma. Oncogene. 2005;24:3737–3747. doi: 10.1038/sj.onc.1208479. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Moses MA. Lipocalin 2: a multifaceted modulator of human cancer. Cell Cycle. 2009;8:2347–2352. doi: 10.4161/cc.8.15.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redon R, Hussenet T, Bour G, Caulee K, Jost B, Muller D, Abecassis J, du Manoir S. Amplicon mapping and transcriptional analysis pinpoint cyclin L as a candidate oncogene in head and neck cancer. Cancer Res. 2002;62:6211–6217. [PubMed] [Google Scholar]
- 36.Katoh M, Katoh M. Identification and characterization of human TIPARP gene within the CCNL amplicon at human chromosome 3q25.31. Int J Oncol. 2003;2:541–547. [PubMed] [Google Scholar]
- 37.Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, Kim DJ, Jaffee E, Pardoll D, Wu TC. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res. 2007;67:1832–1841. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haseman JK, Hailey JR, Morris RW. Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: a National Toxicology Program update. Toxicol Pathol. 1998;26:428–441. doi: 10.1177/019262339802600318. [DOI] [PubMed] [Google Scholar]
- 39.Katzenellenbogen M, Pappo O, Barash H, Klopstock N, Mizrahi L, Olam D, Jacob-Hirsch J, Amariglio N, Rechavi G, Mitchell LA, Kohen R, Domany E, Galun E, Goldenberg D. Multiple adaptive mechanisms to chronic liver disease revealed at early stages of liver carcinogenesis in the Mdr2-knockout mice. Cancer Res. 2006;66:4001–4010. doi: 10.1158/0008-5472.CAN-05-2937. [DOI] [PubMed] [Google Scholar]
- 40.Kakisaka T, Kondo T, Okano T, Fujii K, Honda K, Endo M, Tsuchida A, Aoki T, Itoi T, Moriyasu F, Yamada T, Kato H, Nishimura T, Todo S, Hirohashi S. Plasma proteomics of pancreatic cancer patients by multi-dimensional liquid chromatography and two-dimensional difference gel electrophoresis (2D-DIGE): up-regulation of leucine-rich alpha-2-glycoprotein in pancreatic cancer. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:257–267. doi: 10.1016/j.jchromb.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann K, Firth MJ, Beesley AH, Freitas JR, Ford J, Senanayake S, de Klerk NH, Baker DL, Kees UR. Prediction of relapse in paediatric pre-B acute lymphoblastic leukaemia using a three-gene risk index. Br J Haematol. 2008;140:656–664. doi: 10.1111/j.1365-2141.2008.06981.x. [DOI] [PubMed] [Google Scholar]
- 42.Nindl I, Dang C, Forschner T, Kuban RJ, Meyer T, Sterry W, Stockfleth E. Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Mol Cancer. 2006;5:30. doi: 10.1186/1476-4598-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]