Abstract
The John Henryism active coping (JHAC) hypothesis suggests that striving with life challenges predicts increased risk for cardiovascular disease for those with scarce coping resources. This study examined the moderating role of JHAC in the associations of 1) caregiver status and 2) care recipient functional status with diurnal salivary cortisol patterns among 30 African-American (AA) and 24 White female dementia caregivers and 63 noncaregivers (48 AAs).
Methods
Caregiver participants completed the JHAC-12 Scale, Activities of Daily Living (ADL) scale and Revised Memory and Behavior Problem checklist (RMBPC) and collected five saliva samples daily (at awakening, 9am, 12pm, 5pm, and 9pm) for two successive days.
Results
Univariate ANOVA tests with mean diurnal cortisol slope as the outcome illustrated that among AA caregivers, higher JHAC scores were related to flatter (or more dysregulated) cortisol slopes. The JHAC by ADL and JHAC by RMBPC interactions were each significant for AA caregivers. Among AA caregivers who reported higher ADL and RMBPC scores, higher JHAC scores were associated with flatter cortisol slopes.
Conclusions
These findings extend recent studies by showing that being AA, a caregiver, and high in JHAC may elevate the risk for chronic disease, especially for those with higher patient ADL and behavioral problems. Thus, it is imperative that interventions appreciate the pernicious role of high-effort-coping style, especially for AA caregivers, in order minimize the stressful side effects of patient ADL and memory and behavioral problems for the caregiver.
Keywords: dementia, caregiving, coping, neuroendocrine function, ethnicity
Mace and Rabins (1) refer to the Alzheimer’s and related dementia (ADRD) family caregiver role as the “36-hour workday” because of the uniquely demanding efforts needed to care for the family member. ADRD caregiving can take a sustained toll on health (2–16). Along these lines, dysregulated daily HPA (hypothalamic-pituitary-adrenal cortex axis; e.g., blunted or flattened diurnal cortisol) response is a good indicator of long-term cardiovascular disease risk among highly stressed populations (17–19). Since salivary cortisol varies daily with a normal drop in levels across the day, it is a valuable index of the accumulated health impact of daily stress. However, less is known about the daily HPA profiles of efficacious ADRD caregivers from diverse backgrounds who experience exceptionally difficult challenges of caregiving.
Coping, Caregiver Stress and Health
Problem-focused (e.g., active coping) and emotion-focused (e.g., avoidance) coping styles are critical indices of health among ADRD caregivers (10, 11, 19–21). A general propensity for problem- (vs. emotion) focused approaches of coping is expected to be beneficial to the mental health of ADRD caregivers (23, 24). Also, the influence of coping for ADRD caregivers may be based on unique cultural and sociodemographic factors such as ethnicity (5, 8, 9, 25). For instance, African-American (AA) caregivers tend to report lower levels of stress and depression related to ADRD caregiving than White caregivers with similar levels of caregiver burden (26). Thus, AAs, who are at disparate risk for many psychosocial stressors, cope with the demands of caregiving uniquely (7, 27).
Activities of daily living (ADL) are a classic example of a domain-focused challenge to coping resources, with many ADRD caregivers reporting care recipients who are impaired with independent ADL and assorted memory and behavior problems (22). ADL refer to both the basic (e.g., bathing) and instrumental or higher order self-care abilities (e.g., cooking) that care recipients need assistance with to perform daily activities (28). The extra load of ADL predicts adverse health outcomes for care recipients and caregivers. Impaired ADL reflect greater risk for care recipients in terms of increased hospitalizations, lower Mini-Mental State Examination (MMSE) scores, increased nursing home placement, and higher two-year mortality rates for community-dwelling elders (10, 28). For caregivers, impaired care recipient ADL predict longer duration of caregiving (10), higher depression (10, 29–31), and long-term health decline in the form of extended illness and inpatient hospitalization (13).
Also, over of the course of ADRD, impaired care recipient function in the form of memory and behavior problems (e.g., agitation) has severe consequences for recipients and caregivers (8, 13, 32). Higher levels of impaired memory and behavior problems are correlated with lower MMSE scores (33) and increased caregiver distress in the forms of elevated anxiety, hostility (13), depression (34), lower leisure time satisfaction and less positive affective rewards from caregiving (35), and dysregulated daily cortisol response (4). Moreover, AA (vs. White) caregivers have less negative judgments of disruptive behavior by impaired care recipients (7). Thus, given the powerful role of impaired care recipient function in caregiver health, a relevant question is how high-effort coping impacts the health of diverse overwhelmed caregivers.
High-Effort Coping and Caregiver Stress
Problem-focused coping such as self-efficacy and personal mastery are linked with more adaptive daily HPA responses in most studies (8, 11, 36-38, 39). Schwerdtfeger and colleagues (36) found that high self-efficacy predicts dysregulated (flattened) morning salivary cortisol levels for grade school teachers. A dsyregulated HPA response pattern is prevalent for ADRD caregivers, particularly minority caregivers (4, 6, 9, 15, 16). For instance, McCallum et al. (9) find that AA female caregivers show flatter daily cortisol slopes and more resilience in the caregiving role than White female caregivers.
John Henryism active coping (JHAC), or “the individual's self-perceptionthat he can meet the demands of his environment through hardwork and determination” (40), may have major health implications for diverse ADRD caregivers. James presents JHAC as a cultural adaptation to mainstream American life among members of historically oppressed ethnic groups (e.g., AAs fighting for civil rights in the Jim Crow era; 40). It is based on the premise that one must persevere in demanding times and be resolved and efficacious in achieving goals. The John Henryism hypothesis argues that a discordance between the JHAC coping style and quality of coping resources (e.g., job status, income, support mechanisms), may produce heightened risk for mental illness and chronic disease due to unceasing attentional and emotional activation associated with lowered odds for successful outcomes (17, 41). For instance, high JHAC and high job strain predicted flattened 30-minute awakening cortisol levels among employed AA, but not White, adults in North Carolina (17).
Thus, the JHAC model suggests that the benefits of active (more problem-focused) vs. more emotion-focused forms of coping are reliant upon coping resources. When coping resources are ample then customary active coping approaches are healthy. Yet when coping resources overwhelmed then active coping can intensify existing health risks. Consequently, a secondary aim of the current study was to show that high JHAC levels predict dysregulated diurnal cortisol responses for AA ADRD (but not non-) caregivers. ADRD caregiver status is a challenge to coping resources, and thus may be a risk factor for highly resilient AAs who persevere in the caregiver role.
A primary aim was to show that for AA caregivers with highly impaired care recipients (i.e., high ADL and memory and behavioral problems), higher JHAC would predict even flatter diurnal cortisol responses. Thus, the JHAC by impaired care recipient function hypothesis is a domain specific and thus potentially more reliable version of the traditional John Henryism hypothesis and extends current findings by showing that resilience may have limits for high JHAC AA caregivers with high care recipient problems.
Method
Participants
Participants included AA (N = 30) and White (N = 24) female dementia caregivers who spent at least ten hours per week assisting a family member with memory loss and 63 noncaregivers (48 AAs; 15 Whites). AA noncaregivers averaged 59 years of age and White noncaregivers averaged 71 years of age. The exclusionary criterion of women under the age of 50 was used since HPA response is moderated by age (19). Participants were recruited through the caregiver registry at University Memory and Aging Center of University Hospitals and Case Western Reserve University in addition to flyers posted and presentations given at local senior centers. A more thorough report of our recruitment plan can be found in a separate paper (42).
Procedure
The study received approval from the Institutional Review Board of University Hospitals of Cleveland, OH. Interested persons were contacted by phone and given an overview of the protocol. All participants gave informed consent and data were obtained through in-home interviews. Following the interview, a skilled research assistant outlined and demonstrated the process for self-collecting saliva samples. The interviewer then arranged to come back in three to seven days for the saliva samples. Once the interviewer gathered the saliva samples, participants were compensated $30 by mail for their participation in the study.
Measures
Salivary cortisol measurement
Participants collected saliva at home with “Salivette” devices (Sarstedt Co., Rommelsdorf, Germany) including a cotton swab packed in a plastic holder and sitting within a centrifuge tube. Participants were provided personalized cortisol kits including ten Salivettes with each one labeled by day and time of measurement. Participants stored kits in their refrigerators. They were asked to collect five saliva samples for two successive days at the following times: when awakening, at 9 am, 12 noon, 5 pm, and 9 pm. The samples were largely picked up by the research assistant the day following completion of the protocol and transported to the General Clinic Research Center (GCRC) of University Hospitals. As salivary cortisol levels may vary by medication usage, exercise and sleep patterns, each Salivette kit also contained a form for participants to specify medication, exercise and sleep departures from normal on the days saliva samples were collected.
Laboratory methods
Salivary cortisol samples were tested twice per month in a GCRC wet lab by immunoassay employing microtiter plates and were centrifuged at 3,000 rpm for 15 minutes. The findings are denoted as micrograms per deciliter (μg/dL).
Sociodemographic measures
Demographic and caregiving-related data was collected, with age, ethnicity, menopausal status, education level (on a scale from one to five with the value “2” representing some college), family income (on a scale from one to nine with the value “6” representing income between $25,000 and $30,000), employment status, relationship to the care recipient (e.g., spouse or parent), and duration of caregiving (in months).
Caregiving stressors
Care recipient functional status, as reported by caregivers, was indexed with the Instrumental Activities of Daily Living Scale (IADL; 43) and the Activities of Daily Living Scale (ADL; 44). Six items from the Activities of Daily Living Scale (Cronbach’s alpha in the current sample = .90) assessed the care recipient’s capacity to complete basic tasks of daily functioning independently (i.e., bathing, dressing, toileting, eating, oral/dental care, and transfer). Likewise, seven items from the Instrumental Activities of Daily Living Scale (Cronbach’s alpha = .86) assessed the assistance needed to complete higher level tasks (i.e., shopping, using the telephone, using transportation, preparing meals, doing housework, taking medications, and managing money). Response options included, “1” no help, “2” some help, and “3” a lot of help needed. Overall level of assistance needed for ADL and IADL were summed independently, with higher scores signifying more functional impairment. Overall ADL scores could range from six to 18 points while overall IADL scores could range from seven to 21 points. This sample reported an average of 10.79 (SD = 4.02) ADL problems and 17.29 (SD = 3.74) IADL problems.
The Revised Memory and Behavior Problems Checklist (RMBPC; 45) assesses the number of problems related to memory, depression, and agitation that the patient has experienced over the past week and has bothered the caregiver. The RMBPC has shown good reliability and correlates with care recipient Mini-Mental State Exam (MMSE; 33) scores and increased caregiver depression levels and less positive affective rewards from caregiving (35). The RMBPC includes 24 items with response options ranging from “not at all” (coded as a “0”) to “extremely” (coded as a “4”). Higher RMBPC scores represent more problems for patients. This sample reported an average RMBPC reaction score of 38.13 (SD = 15.01).
Coping Measures
The John Henryism Active Coping scale (JHAC; 40) is a 12-item Likert response scale that measures (a) atypical mental and physical vigor; (b) a focused determination to realize one’s goals; and (c) an unrelenting commitment with hard work. For each item, five response options range from “1” completely true” to “5” completely false”. Since each item has “high-effort” content, responses for each item were reverse-coded and then summed to derive an overall JHAC score that can extend from twelve to sixty points. The JHAC has shown acceptable internal consistency with diverse samples (38). The mean score of 47.72 (SD = 6.54) in the present study is consistent with previous findings from similar populations (17, 39). JHAC is positively correlated with healthy behaviors, religiosity, and life satisfaction.
The Religious Coping (RCOPE) scale is a measure of positive and negative religious coping with demanding experiences that consists of 34 items (46). Response options for each item range from “0” not at all” to “3” a great deal”. The RCOPE includes two dimensions: 1) positive religious coping, which contains items about spiritual relationships and optimistic religious judgments of events; and 2) negative religious coping, which contains items about punitive religious judgments and resentment to God. The positive and negative religious coping subscales have shown excellent internal consistency in recent research (46). The current study used a single “positive” RCOPE score based on the sum of the positive and (reverse-coded) negative subscales. The mean score of 22.78 (SD = 4.88) in the present study is consistent with previous findings.
Mental health measures
Depressive symptomology was evaluated with the Center for Epidemiologic Studies Depression Scale (CES-D; 47). The CES-D is a 20-item measure used to screen for depressive symptoms occurring in the previous week. The response scale ranges from “0” rarely or none of the time to “3” all of the time, with higher scores signifying more depressive symptoms. All items were summed to derive an overall depression score that could extend from zero to sixty points. This sample reported an average depression score of 13.30 (SD = 10.46) and the Cronbach’s alpha was α = .91.
Global stress was measured with the Perceived Stress Scale (PSS, 48). The 10-item version of the PSS is a general index of wide-ranging stress and has acceptable reliability and validity and includes no items germane to caregiving. The response scale ranges from “0” never to “4” very often. Responses for four items were reverse-coded and then all items were summed to derive an overall global stress score that could extend from zero to forty points. This sample reported an average PSS score of 16.24 (SD = 6.99) and the Cronbach’s alpha was α = .91.
Plan for statistical analysis
Log transformed mean diurnal cortisol slope scores were created for each participant by computing the slope of the eight cortisol scores across the two days and then multiplying that value by 1,000.a More positive values which represent flatter daily cortisol slopes, signify more dysregulated HPA functioning (49). A preliminary aim was to assess psychosocial differences in the AA and White caregiver groups by running t-tests and Chi-Square tests using Fisher’s exact or Pearson test (generated with SPSS-PC software). Correlational analyses were run to assess covariation between cortisol response, sociodemographic, caregiving and psychosocial variables.
For hypothesis one, another secondary aim, two Univariate ANOVA models were run: one for AAs and one for Whites. Daily cortisol slope scores were the dependent measure and dichotomized JHAC score and caregiver status were independent variables. Covariates included age, CES-D, and RCOPE.
For hypothesis two, the primary aim, two Univariate ANOVA models were run for AA caregivers. Daily cortisol slope scores were the dependent measure and dichotomized JHAC and extreme-groups measures of ADL and RMBPC (based on tertiles) respectively, were independent variables. High ADL and RMBPC represent less capacity for care recipients to independently complete basic tasks and more care recipient behavioral problems, respectively. Covariates included age and RCOPE.b
Since previous studies have shown significant effects for the JHAC measure when analyzed as a dichotomized score at the median value (40, 41), we assessed JHAC in the ANOVA models as a dichotomized measure. Greenhouse-Geisser tests were applied to correct for violations of sphericity in significant omnibus tests and independent samples t-tests to further investigate the direction of significant interaction effects for daily cortisol slope scores.
Results
Group Demographics
As shown in Table 1, White caregivers were older and more likely than AA caregivers to report post-menopausal status [96% vs. 70%; χ(1, 53) = 5.60; p < .03]. AA caregivers were more likely to care for parents [67% versus 26%; χ(2, 53) = 18.48; p < .0001], scored higher on JHAC and RCOPE (i.e., more positive religious coping) and had flatter daily cortisol slope scores.c Lastly, AA and White caregiver groups did not significantly differ by education level, annual family income, or duration of caregiving.
Table 1.
Demographic and psychosocial data for African American and White American caregivers
| African American Caregivers | White American Caregivers | ||||
|---|---|---|---|---|---|
| Measures | Mean | S.D. | Mean | S.D. | t |
| Age of caregiver | 58.17 | 8.31 | 67.22 | 10.23 | 3.56a |
| Education level | 2.73 | 1.44 | 2.96 | 1.52 | 0.55 |
| Annual family income | 6.83 | 2.14 | 7.65 | 1.30 | 1.71 |
| Duration of caregiving | 60.13 | 54.38 | 70.39 | 31.20 | 0.81 |
| John Henryism active coping | 49.53 | 6.29 | 44.70 | 8.56 | −2.37b |
| ADL | 11.21 | 3.79 | 10.26 | 4.32 | −0.84 |
| IADL | 18.07 | 3.06 | 16.30 | 4.32 | −1.66 |
| RMBPC | 38.17 | 15.17 | 38.09 | 15.16 | −0.02 |
| Religious coping (RCOPE) | 25.37 | 3.46 | 18.22 | 5.29 | −5.62a |
| Depression (CES-D) | 14.70 | 10.69 | 15.61 | 9.08 | 0.33 |
| Global Stress (PSS) | 17.63 | 7.94 | 18.70 | 5.08 | 0.59 |
| Daily cortisol slope | −24.73 | 8.86 | −31.79 | 5.98 | −3.08a |
Note: ADL = Activities of daily living, IADL = Instrumental activities of daily living, RMBPC = Revised memory and behavior problems.
Note: The df = 51 for the t-tests.
Note: For education level, 2 = some college. For annual family income, 6 = between $25,000 and $30,000.
p < 0.001.
p < 0.05.
SD: standard deviation.
John Henryism Active Coping, Caregiver Status, and Cortisol
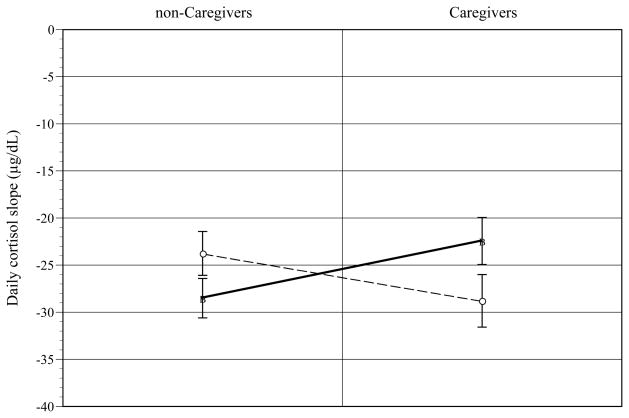
Higher JHAC scores were correlated with lower PSS [r(53) = −0.49; p < .0001] and CES-D (i.e., less depressive symptoms) [r(53) = −0.32; p < .02] scores, higher RCOPE scores [r(53) = 0.46; p < .001], and flatter daily cortisol slopes [r(43) = 0.34; p < .02]. Univariate ANOVA tests showed that the interaction of JHAC and caregiver status was significant for AA caregiversd. As shown in Figure 1, for high JHAC AAs, caregiver status predicted flatter daily cortisol slope than non-caregiver status [t(37) = 2.05; p < .04] (d = .32). For AA caregivers, high JHAC predicted flatter daily cortisol slopes than low JHAC [t(27) = −1.93; p < .06] (d = .35). The JHAC by caregiver status effect or relevant contrasts were not significant for Whites [F(1, 20) = 1.39, p < .25]. For Whites, higher RCOPE scores significantly predicted flatter daily cortisol slope [F(1, 20) = 5.26; p < .03] (d = .32).
Figure 1.
John Henryism (JHAC) by caregiver status effects on daily cortisol slope for African-Americans. [error bars = s. e.]
○Low JHAC ■High JHAC
John Henryism Active Coping, Recipient Functional Status, and Cortisol
Higher ADL scores were correlated with longer duration of caregiving [r(52) = 0.34; p < .01]. RMBPC scores were positively correlated with older age [r(53) = 0.26; p < .05], CES-D [r(53) = 0.50; p < .0001] and PSS [r(53) = 0.34; p < .01] scores. Given that the JHAC by caregiver status effects on daily cortisol slope were only significant for AAs and the marginal sample size for White caregivers, we decided to focus on the role of JHAC and care recipient functional status on daily cortisol slope for AA caregivers. In addition, given the low range of IADL scores we focused on ADL and RMBPC scores.
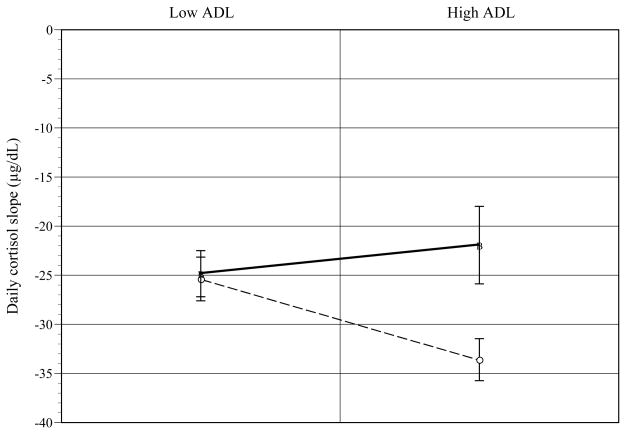
Univariate ANOVA tests showed that the interaction of JHAC and ADL was significant for AA caregivers. As shown in Figure 2, for high ADL AA caregivers, high (vs. low) JHAC predicted flatter daily cortisol slope [t(9) = −2.44; p < .03] (d = .63). At low JHAC, low (vs. high) ADL predicted flatter daily cortisol slope [t(9) = 4.26; p < .002] (d = .82).
Figure 2.
John Henryism (JHAC) by recipient Activities of Daily Living (ADL) effects on daily cortisol slope for African-American caregivers. [error bars = s. e.]
○Low JHAC ■High JHAC
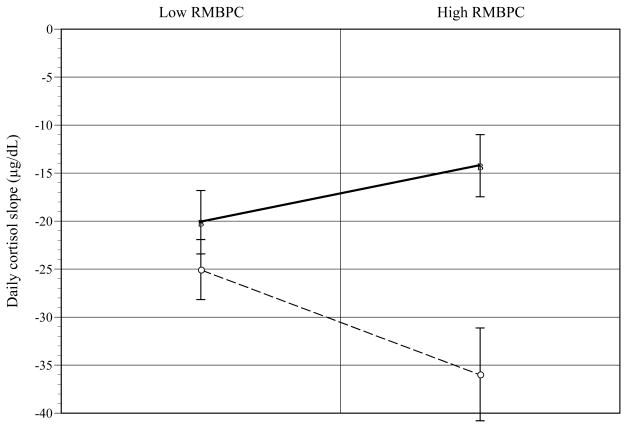
The interaction of JHAC and RMBPC was significant for AA caregivers. As shown in Figure 3, for AA caregivers high on RMBPC, high (vs. low) JHAC predicted flatter daily cortisol slope [t(6) = −5.03; p < .002] (d = .90). At low JHAC, low (vs. high) RMBPC predicted flatter daily cortisol slope [t(8) = 2.52; p < .03] (d = .67). Higher JHAC scores predicted flatter cortisol slopes [F(1, 12) = 14.28, p < .003](d = .74).
Figure 3.
John Henryism (JHAC) by recipient Revised Memory and Behavior Problems Checklist (RMBPC) effects on daily cortisol slope for African-American caregivers. [error bars = s. e.]
○Low JHAC ■High JHAC
Conclusions
The findings that AA caregivers show flatter cortisol slope scores than White caregivers have at least two potential implications. First, AAs experience higher psychosocial stress than Whites. Daily stressors such as perceived discrimination, neighborhood instability, and economic insecurity have excessively negative and complex health effects for AAs compared to Whites (50). The adverse health effects of these stressors appear to be magnified for AA caregivers (6, 25). Second, AA caregivers may have worse pre-existing health profiles than White caregivers. Since AA adults show worse health status at each stage of adulthood than their White counterparts (50–52) the former group may be especially vulnerable to the perils of caregiver stress. For instance, young AA adults show flatter daily cortisol slopes than their White counterparts (49).
The finding that high JHAC AA caregivers who score high (vs. low) on care recipient problems show flatter cortisol slopes suggests 1) that problem-focused coping is contingent on contextual influences and 2) reinforces ethnic disparities in the caregiving experience. The current results suggest that the dichotomous model of coping style (i.e., problem- vs. emotion-focused) is limited as it ignores the powerful moderating role of coping resources.
Notably, AA caregivers are more resilient (9); score lower on caregiver burden and are less likely to institutionalize and seek help for care recipients than White caregivers (5, 27, 53). Yet AA caregivers score similar to White caregivers on depression and distress (9). So the findings indirectly support the McCallum et al. (9) counterbalancing effect where lower amounts of caregiver burden for AAs were offset by a tendency for emotion-focused coping, which was linked with depression. Thus, the current results suggest that high JHAC AA caregivers are likely to score low on caregiver burden and resist institutionalization of care recipient. High JHAC persons are more vigilant and single-minded and thus likely less oriented to utilizing available support resources such as respite or nursing home care.
Many ethnic minority caregivers are socialized to keep their care recipient in the home context regardless of the consequences and thus are likely to make more personal investment and maintain the caregiving role over longer periods of time than White caregivers (5, 8, 25, 27). Thus, by continually striving to maintain social obligations to the care recipient many AA caregivers may experience chronic and excessive cognitive and emotional activation that manifests as chronic HPA hyper-arousal.
A few limitations of the study include moderate sample size for White caregivers, a dearth of comprehensive assessments of daily HPA response, a lack of random sampling, and missing validation of the JHAC measure with ADRD caregivers. The low statistical power for White caregivers may explain the lack of significant results and will be addressed in upcoming studies. We plan to develop a comprehensive study that integrates a wider array of psychosocial and biomedical measures. Given other possible confounding variables that may influence the daily HPA findings we plan to include assessments such as blood chemistry, health behaviors, and specific psychological measures (e.g., neuroticism, self-efficacy, and optimism).
For AA female caregivers with taxing care recipient problems, reducing high effort coping in response to daily challenges may reduce risk for chronically dysregulated HPA responses. Future studies will focus on relevant coping skills interventions that consider support resources for burdened and efficacious AA caregivers.
Acknowledgments
Sources of support: ADRC/NIA grant #AG08012 and NIH (M01 RR000080); No Disclosures to Report
We would like to acknowledge the ADRC/NIA for awarding grant #AG08012 to T. J. McCallum, Ph.D., through the University Memory and Aging Center of University Hospitals and Case Western Reserve University. We also acknowledge partial grant support from the NIH (M01 RR000080) to the General Clinical Research Center of University Hospitals.
The authors show appreciation to the staff of the General Clinical Research Center of University Hospitals for helpful consultation and aid in completing salivary cortisol assays.
Footnotes
Poster presented at the American Psychosomatic Society meeting in Chicago, IL (March 2009).
The values for each time point were also evaluated but will not be included in the current paper for the sake of simplicity in analytical focus.
Hierarchical multiple regression tests were also run. The findings overall were similar to those found for the proposed Univariate ANOVA tests. The only exception was the non-significant interaction of JHAC and RMBPC with age and RCOPE as covariates.
Note that the Bonferroni adjusted criterion for significance for Table 1 given the Type I error rate for multiple t-tests is p < .004.
Note that the criterion for significance for the four Univariate ANOVA tests given the Type I error rate for multiple ANOVA tests is p < .01.
Contributor Information
Marcellus M. Merritt, Email: merrittm@uwm.edu, Department of Psychology & Center on Age and Community University of Wisconsin Milwaukee, Box 413 Milwaukee, WI 53201, Phone: 414-229-6145, Fax: 414-229-5219
T. J. McCallum, Department of Psychology, Case Western Reserve University
Thomas Fritsch, Parkinson’s Research Institute, Milwaukee, WI
References
- 1.Mace NL, Rabins PV, editors. The 36-hour day. 4. Baltimore, MD: The Johns Hopkins University Press; 2006. [Google Scholar]
- 2.Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Surwit RS, Garrett ME, Collins A, Ashley-Koch A, Williams RB. HPA axis function in male caregivers: effect of the monoamine oxidase-A gene promoter (MAOA-uVNTR) Biol Psychol. 2008;79:250–5. doi: 10.1016/j.biopsycho.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark MS, Bond MJ, Hecker JR. Environmental stress, psychological stress and allostatic load. Psychol Health Med. 2007;12:18–30. doi: 10.1080/13548500500429338. [DOI] [PubMed] [Google Scholar]
- 4.de Vugt ME, Nicolson NA, Aalten P, et al. Behavioral problems in dementia patients and salivary cortisol patterns in caregivers. J Neuropsychiatry Clin Neurosci. 2005;17:201–7. doi: 10.1176/jnp.17.2.201. [DOI] [PubMed] [Google Scholar]
- 5.Dilworth-Anderson P, Goodwin PY, Williams SW. Can culture help explain the physical health effects of caregiving over time among African American caregivers? J Gerontol B Psychol Sci Soc Sci. 2004;59:S138–S145. doi: 10.1093/geronb/59.3.s138. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher-Thompson D, Shurgot GR, Rider K, et al. Ethnicity, stress, and cortisol function in Hispanic and non-Hispanic white women: A preliminary study of family dementia caregivers and noncaregivers. Am J Geriatr Psychiatry. 2006;14(4):334–42. doi: 10.1097/01.JGP.0000206485.73618.87. [DOI] [PubMed] [Google Scholar]
- 7.Haley WE, West CA, Wadley VG, et al. Psychological, social, and health impact of caregiving: a comparison of black and white dementia family caregivers and noncaregivers. Psychol Aging. 1995;10:540–552. [PubMed] [Google Scholar]
- 8.Kim JH, Knight BG, Longmire CV. The role of familism in stress and coping processes among African American and White dementia caregivers: effects on mental and physical health. Health Psychol. 2007;26(5):564–76. doi: 10.1037/0278-6133.26.5.564. [DOI] [PubMed] [Google Scholar]
- 9.McCallum TJ, Sorocco KH, Fritsch T. Mental health and diurnal salivary cortisol patterns among African American and European American female dementia family caregivers. Am J Geriatr Psychiatry. 2006;14(8):684–93. doi: 10.1097/01.JGP.0000225109.85406.89. [DOI] [PubMed] [Google Scholar]
- 10.McClendon MJ, Smyth KA, Neundorfer MM. Survival of persons with Alzheimer's disease: caregiver coping matters. Gerontologist. 2004;44(4):508–19. doi: 10.1093/geront/44.4.508. [DOI] [PubMed] [Google Scholar]
- 11.Roepke SK, Mausbach BT, Aschbacher K, et al. Personal mastery is associated with reduced sympathetic arousal in stressed Alzheimer caregivers. Am J Geriatr Psychiatry; 2008;16(4):310–7. doi: 10.1097/JGP.0b013e3181662a80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–19. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 13.Shaw WS, Patterson TL, Semple SJ, et al. Emotional expressiveness, hostility and blood pressure in a longitudinal cohort of Alzheimer caregivers. J Psychosom Res. 2003;54(4):293–302. doi: 10.1016/s0022-3999(02)00412-9. [DOI] [PubMed] [Google Scholar]
- 14.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. 2003;129(6):946–72. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 15.Wahbeh H, Kishiyama SS, Zajdel D, et al. Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimers Dis Assoc Disord. 2008;22(2):181–3. doi: 10.1097/WAD.0b013e31815a9dff. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox S, Bopp M, Wilson DK, et al. Race differences in cardiovascular and cortisol responses to an interpersonal challenge in women who are family caregivers. Ethn Dis. 2005;15(1):17–24. [PubMed] [Google Scholar]
- 17.Bennett GG, Jr, Merritt MM, Sollers JJ, III, et al. Stress, coping, and health outcomes among African-Americans: A review of the John Henryism hypothesis. Psychol Health. 2004;19(3):369–83. [Google Scholar]
- 18.Matthews K, Schwartz J, Cohen S, et al. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–61. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 19.Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Katona C, Livingston G. Validity and reliability of the brief COPE in carers of people with dementia: the LASER-AD Study. J Nerv Ment Dis. 2008;196(11):838–43. doi: 10.1097/NMD.0b013e31818b504c. [DOI] [PubMed] [Google Scholar]
- 21.Gonyea J, O’Connor M, Carruth A, et al. Subjective appraisal of Alzheimer’s disease caregiving: The role of self-efficacy and depressive symptoms in the experience of burden. Am J Alzheimers Dis Other Demen. 2005;20:273–80. doi: 10.1177/153331750502000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mausbach BT, Aschbacher K, Patterson TL, et al. Avoidant coping partially mediates the relationship between patient problem behaviors and depressive symptoms in spousal caregivers. Am J Geriatr Psychiatry. 2006;14:299–306. doi: 10.1097/01.JGP.0000192492.88920.08. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb BH, Wolfe J. Coping with family caregiving to persons with dementia: A critical review. Aging Ment Health. 2002;6:325–42. doi: 10.1080/1360786021000006947. [DOI] [PubMed] [Google Scholar]
- 24.Kneebone II, Martin PR. Coping and caregivers of people with dementia. Br J Health Psychol. 2003;8:1–17. doi: 10.1348/135910703762879174. [DOI] [PubMed] [Google Scholar]
- 25.Knight BG, Silverstein M, McCallum TJ, et al. A sociocultural stress and coping model for mental health outcomes among African American caregivers in Southern California. J Gerontol B Psychol Sci Soc Sci. 2000;3:142–150. doi: 10.1093/geronb/55.3.p142. [DOI] [PubMed] [Google Scholar]
- 26.Janevic MR, Connell C. Racial, ethnic, and cultural differences in the dementia caregiving experience: Recent findings. Gerontologist. 2001;41:334–347. doi: 10.1093/geront/41.3.334. [DOI] [PubMed] [Google Scholar]
- 27.Dilworth-Anderson P, Brummett BH, Goodwin P, et al. Effect of race on cultural justifications for caregiving. J Gerontol B Psychol Sci Soc Sci. 2005;60:S257–S262. doi: 10.1093/geronb/60.5.s257. [DOI] [PubMed] [Google Scholar]
- 28.Carey EC, Walter LC, Lindquist K, et al. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–33. doi: 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joling KJ, van Hout HPJ, Schellevis FG, et al. Incidence of Depression and Anxiety in the Spouses of Patients With Dementia: A Naturalistic Cohort Study of Recorded Morbidity With a 6-Year Follow-Up. Am J Geriatr Psychiatry. 2010;18:146–153. doi: 10.1097/JGP.0b013e3181bf9f0f. [DOI] [PubMed] [Google Scholar]
- 30.Miller B, Guo S. Social support for spouse caregivers of persons with dementia. J Gerontol B Psychol Sci Soc Sci. 2000;55(3):S163–S172. doi: 10.1093/geronb/55.3.s163. [DOI] [PubMed] [Google Scholar]
- 31.Onder G, Finne-Soveri H, Soldato M, et al. Distress of caregivers of older adults receiving home care in European countries: results from the AgeD in HOme Care Study. Am J Geriatr Psychiatry. 2009;17:899–906. doi: 10.1097/JGP.0b013e3181b4beef. [DOI] [PubMed] [Google Scholar]
- 32.Rymer S, Salloway S, Norton L, et al. Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer disease. Alzheimers Dis Assoc Disord. 2002;16(4):248–53. doi: 10.1097/00002093-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Schulz R, Beach SR, Hebert RS, et al. Spousal suffering and partner's depression and cardiovascular disease: the Cardiovascular Health Study. Am J Geriatr Psychiatry. 2009;17(3):246–54. doi: 10.1097/JGP.0b013e318198775b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth DL, Burgio LD, Gitlin LN, et al. Psychometric analysis of the Revised Memory and Behavior Problems Checklist: factor structure of occurrence and reaction ratings. Psychol Aging. 2003;18(4):906–15. doi: 10.1037/0882-7974.18.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Donnell K, Badrick E, Kumari M, et al. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33:601–11. doi: 10.1016/j.psyneuen.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Schwerdtfeger A, Konermann L, Schönhofen K. Self-efficacy as a health-protective resource in teachers? A biopsychological approach. Health Psychol. 2008;27(3):358–68. doi: 10.1037/0278-6133.27.3.358. [DOI] [PubMed] [Google Scholar]
- 38.Vedhara K, Tuinstra J, Miles JN, et al. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology. 2006;31:299–311. doi: 10.1016/j.psyneuen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Gerritsen L, Geerlings MI, Bremmer MA, et al. Personality characteristics and hypothalamic-pituitary-adrenal axis regulation in older persons. Am J Geriatr Psychiatry. 2009;17(12):1077–84. doi: 10.1097/JGP.0b013e3181bd1be6. [DOI] [PubMed] [Google Scholar]
- 40.James SA. John Henryism and the health of African-Americans. In: LaVeist TA, editor. Race, ethnicity, and health: A public health reader. San Francisco, CA: Jossey-Bass; 2002. pp. 350–68. [Google Scholar]
- 41.Merritt MM, Bennett GG, Jr, Williams RB, et al. Low educational attainment, John Henryism and cardiovascular reactivity and recovery to personally-relevant stress. Psychosom Med. 2004;66(1):49–55. doi: 10.1097/01.psy.0000107909.74904.3d. [DOI] [PubMed] [Google Scholar]
- 42.McCallum T, Arlien C. Enhancing the matching model of recruitment with focus groups. Aging Ment Health. 2006;10(3):312–8. doi: 10.1080/13607860500409781. [DOI] [PubMed] [Google Scholar]
- 43.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 44.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 45.Teri L, Truax P, Logsdon RG, et al. Assessment of behavioural problems in dementia: The revised memory and behavior problems checklist. Psychol Aging. 1992;7:622–31. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 46.Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519–43. doi: 10.1002/(sici)1097-4679(200004)56:4<519::aid-jclp6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 48.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 49.Cohen S, Schwartz JE, Epel E, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 50.Williams DR, Neighbors HW, Jackson JS. Racial/ethnic discrimination and health: Findings from community studies. Am J Public Health. 2008;98:S29–S37. doi: 10.2105/ajph.98.supplement_1.s29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cooper C, Tandy AR, Balamurali TB, et al. A systematic review and meta-analysis of ethnic differences in use of dementia treatment, care, and research. Am J Geriatr Psychiatry. 2010;18(3):193–203. doi: 10.1097/JGP.0b013e3181bf9caf. [DOI] [PubMed] [Google Scholar]
- 52.Conner KO, Copeland VC, Grote NK, et al. Mental health treatment seeking among older adults with depression: The impact of stigma and race. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181cc0366. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gray HL, Jimenez DE, Cucciare MA, et al. Ethnic differences in beliefs regarding Alzheimer disease among dementia family caregivers. Am J Geriatr Psychiatry. 2009;17:925–933. doi: 10.1097/JGP.0b013e3181ad4f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]