Abstract
Methamphetamine (METH) is a psychostimulant that induces long-term deficits of dopamine terminal markers and apoptotic cell death in the striatum. Our laboratory demonstrated that pharmacological blockade of the neurokinin-1 receptor attenuated the METH-induced damage to the striatal dopamine terminals and the apoptotic cell death of some striatal neurons. Here we employed histological methods to assess the effect of METH on neurokinin-1 receptor trafficking in the striatum as an indirect index of signaling by the neuropeptide substance P (natural ligand for this receptor). Male mice received a single injection of METH (30 mg/kg, i.p.) and were sacrificed 30 minutes later. Immunohistofluorescence confocal microscopy confirmed that the neurokinin-1 receptor is located on cholinergic and somatostatin interneurons of the striatum. METH induced the trafficking of the neurokinin-1 receptor from the membrane into cytoplasmic endosomes primarily in the somatostatin/NPY/NOS interneurons and this phenomenon was attenuated by antagonists of the dopamine D1 (SCH-23390), D2 (raclopride) or neurokinin-1 (WIN-51,708) receptors. These data demonstrate that METH induces the trafficking of the striatal neurokinin-1 receptors principally in the somatostatin/NPY/NOS interneurons and that this phenomenon is dependent on the activity of dopamine D1 and D2 receptors.
Keywords: neurokinin-1 receptor, dopamine receptors, methamphetamine, endocytosis, interneuron, striatum
INTRODUCTION
METH is a psychostimulant that displays high addictive potential and is becoming a major problem with law enforcement because it induces aggressive behavior (Carey et al., 1968; Ellinwood, 1971; Hawks et al., 1969; Szuster, 1990) and it is highly toxic to some neurons in the brain (Yamamoto and Bankson, 2005; Krasnova and Cadet, 2009). Several laboratories have provided unequivocal evidence that exposure to METH induces long-term depletion of dopamine, tyrosine hydroxylase, and dopamine transporters in dopamine terminals of the striatum (Seiden et al., 1975; Woolverton et al., 1989; Eisch et al., 1998), oligomerization and inactivation of striatal dopamine transporters (Baucum et al., 2004), as well as depletion of tryptophan hydroxylase in serotonergic terminals (Schmidt and Gibb, 1985; Hanson et al., 1998). Definitive proof of METH-induced degeneration of neural tissue was obtained with the silver stain (Ricaurte et al. 1982, 1984) and ultrastructural analysis (Ryan et al., 1990). Post-mortem examination of the caudate and putamen of METH users revealed significant reductions of tyrosine hydroxylase, dopamine transporters, and tissue dopamine content (Wilson et al., 1996). METH users abstinent for three years showed reductions in dopamine transporters (McCann et al., 1998). Moreover, detoxified METH users showed reduced glucose utilization and diminished dopamine transporter sites in the striatum (Volkow et al., 2001a,b). METH induces apoptotic cell death in various regions of the rodent brain including the striatum (Deng et al., 2001; Pu et al., 1996; Eisch and Marshall, 1998; Zhu et al., 2005). Similarly, METH appears to cause neuronal loss in human subjects (Thompson et al., 2004). These studies provide compelling evidence supporting the assertion that METH is highly toxic to some neural tissues. However, the mechanism by which METH induces neural damage in the brain remains poorly understood.
The striatum is the major output structure of the basal ganglia. It is comprised of projection neurons (90%) and interneurons. The projection neurons that innervate the substantia nigra reticulata express the neuropeptide substance P (natural ligand of the neurokinin-1 receptor) some of which is released intrastraitally via axon collaterals (Gerfen, 1992; Angulo and McEwen, 1994). The cholinergic and somatostatin interneurons express the neurokinin-1 receptor (Gerfen, 1992). Our laboratory has demonstrated that blockade of the neurokinin-1 receptor with selective non-peptide antagonists that cross the blood-brain barrier protects the dopamine terminals from METH (Yu et al., 2002, 2004) and attenuates the METH-induced apoptotic cell death of some striatal neurons (Zhu et al., 2009). In order to further characterize the mechanism by which the neuropeptide substance P modulates the METH-induced toxicity and cell death through the striatal neurokinin-1 receptor, we have employed a histological assay to assess signaling by substance P (Mantyh et al., 1995b). This assay makes use of the internalization of the neurokinin-1 receptor into endosomes in neurons, an event triggered by binding of substance P to its receptor (Mantyh et al., 1995b). In KNRK cells, internalization of the neurokinin-1 receptor is induced by agonist within minutes of exposure and requires the trafficking of β-arrestins and redistribution of G protein-coupled receptor kinases (McConalogue et al., 1999; Barak et al., 1999). In this study we used a dose of METH (30 mg/kg) known to produce striatal injury and assessed the trafficking of the neurokinin-1 receptor at an early time point post-METH (30 minutes) in order to gain evidence supporting a role for substance P in the METH-induced striatal injury. We demonstrate the internalization of the neurokinin-1 receptors in striatal interneurons soon after a systemic injection of METH. Our results demonstrate that METH induces the internalization of the neurokinin-1 receptors primarily in the somatostatin/NPY/NOS interneurons. Antagonists of the dopamine D1 or D2 receptors attenuate the METH-induced internalization of the neurokinin-1 receptors.
MATERIALS AND METHODS
Animals
Male ICR mice (Taconic, Germantown, NY) between 10 to 13 weeks of age were housed individually on a 12-h light/dark cycle with food and water available ad libitum. The mice were habituated for two weeks prior to commencement of intraperitoneal (i.p.) drug administration. All procedures regarding animal use were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care Committee at Hunter College of the City University of New York.
Drug preparation and treatment
(+)-Methamphetamine hydrochloride (Sigma, St. Louis, MO) was dissolved in 10 mM PBS and injected intraperitoneal (i.p.) at a dose of 30 mg/kg of body weight in a volume of 200 µL. The dopamine D1 receptor antagonist SCH-23390 or the D2 dopamine receptor antagonist raclopride (RBI/Sigma, Natick, MA) were dissolved in vehicle (PBS). The nonpeptide neurokinin-1 receptor antagonist, WIN-51,708 (Sigma/RBI, St. Louis, MI), was dissolved in 45% 2-hydroxylpropyl-β-cyclodextrin (Sigma/RBI, St. Louis, MI) and PBS (1:4). Vehicle, SCH-23390 (0.1 mg/kg), raclopride (1 mg/kg) or WIN-51,708 (5 mg/kg) were given intraperitoneally 30 minutes prior to the injection of METH.
Immunohistochemistry of neurokinin-1 receptor and double label with somatostatin or choline acetyltransferase
The animals were fully anesthetized with Ketamine (100mg/kg) and Acepromazine (3mg/kg) and perfused through the heart with 20 ml of PBS followed by 20 ml of 4% paraformaldehyde and 0.2% glutaraldehyde. The brains were post-fixed overnight in the fixative at 4°C followed by 20% sucrose solution over 24 hours at 4°C for cryo-protection. The brains were frozen at −80 °C until used. Coronal sections 30µm in thickness were cut in a microtome at −20°C and stored in anti-freezing solution (30% glycerin solution in ethylene glycol) at −20 °C until used.
Sections of striatal tissue were washed 3× for 5 minutes each in PBS with 0.3% Triton X-100 followed by a 30-minute incubation at room temperature with 10% Normal Sheep Serum to block nonspecific binding. The sections were incubated overnight at 4°C with polyclonal rabbit anti-neurokinin-1 receptor antibody (1:1000, Chemicon, CA) diluted in the same blocking buffer. After rinsing 3× for 5 minutes each with PBS, the sections were incubated with sheep anti-rabbit secondary antibody conjugated with FITC (1:1000, Chemicon, CA) for 2 hours at room temperature. The sections were washed 3× for 10 minutes each with PBS and mounted onto glass slides.
For double labeling, the primary antibodies used were rabbit anti-neurokinin-1 receptor (1:1000, Chemicon, Temecula, CA) followed by goat anti-somatostatin (1:200, Santa Cruz, CA) or goat anti-ChAT (1:100, Chemicon, CA) diluted in 10% Normal Donkey Serum in blocking buffer with 0.3% Triton X-100. The sections were incubated overnight at 4°C. Secondary antibodies were donkey anti-rabbit conjugated with FITC (1:1000, Novus Biologicals, CO) and donkey anti-goat conjugated with Cy3 (1:100, Chemicon, CA). After 2 hours of incubation at room temperature, the sections were washed 3× for 10 minutes each with PBS and mounted onto glass slides.
Quantification of neurokinin-1 receptor internalization and cell counting
Coronal sections (30µm in thickness) were taken from bregma 0.38±0.1mm (Franklin and Paxinos, 1997). The striatum was subdivided into the following quadrants: dorsal-medial, dorsal-lateral, ventral-medial, and ventral-lateral. An area of 0.26mm2 was assessed for each quadrant as previously described (Zhu et al., 2009). Five sections from each animal (6–9 mice per group) were analyzed using the Leica TCS SP2 Spectral Confocal Microscope (Leica Microsystems). The number of endosomes per neurokinin-1 receptor-positive cell was counted under a magnification of 1000 times. An endosome-like structure was included in the analysis if it was 0.5 µm in diameter, or larger, and was distinctly separated from the somatic plasma membrane. At least 100 neurokinin-1 receptor-positive interneurons were included in the analysis. We relied on one stain for endosomes (neurokinin-1 receptor) because METH may induce trafficking of other kinds of endosomes. Nuclear staining may have resulted in underestimating the number of endosomes.
Statistical analysis
Comparisons between groups were performed from mean +/−SEM values. The difference between groups were analyzed first by ANOVA and then followed by post hoc comparison using Fisher’s protected least significance test. The significance criterion was set at p=0.05.
RESULTS
METH induces trafficking of the neurokinin-1 receptor
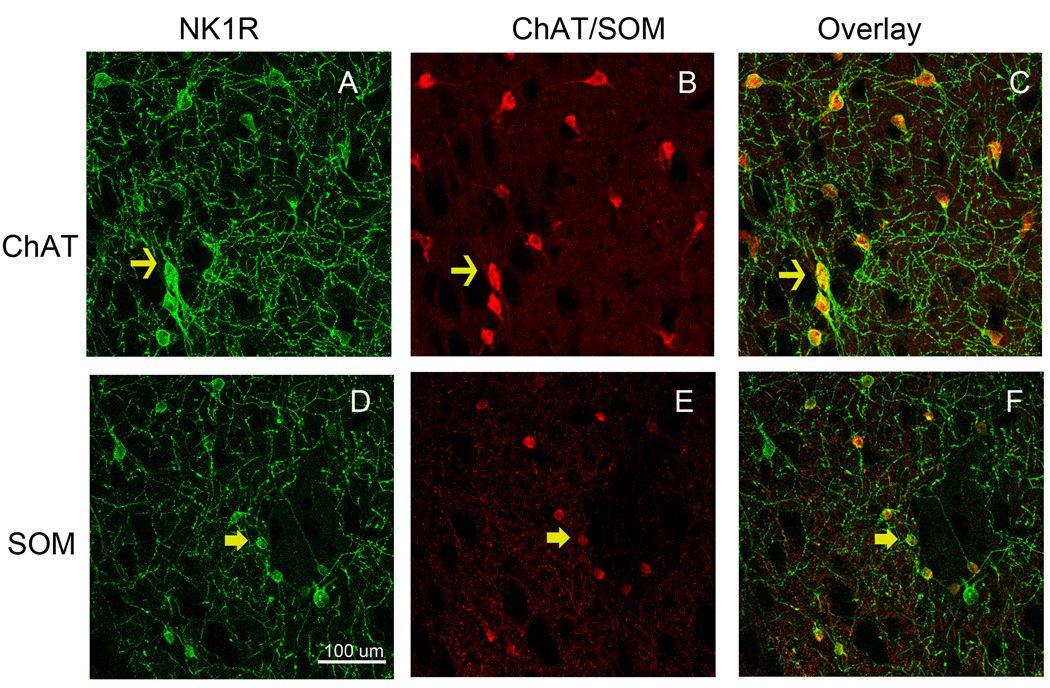
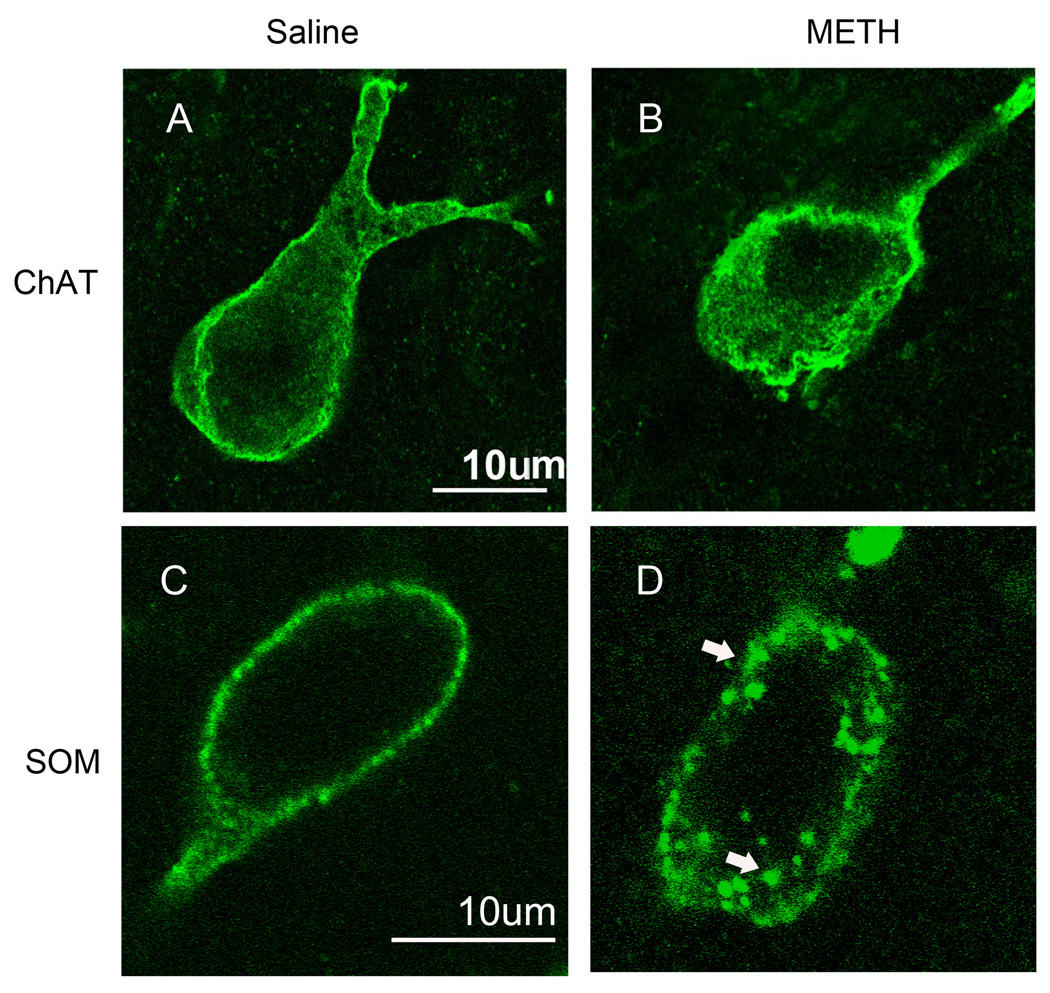
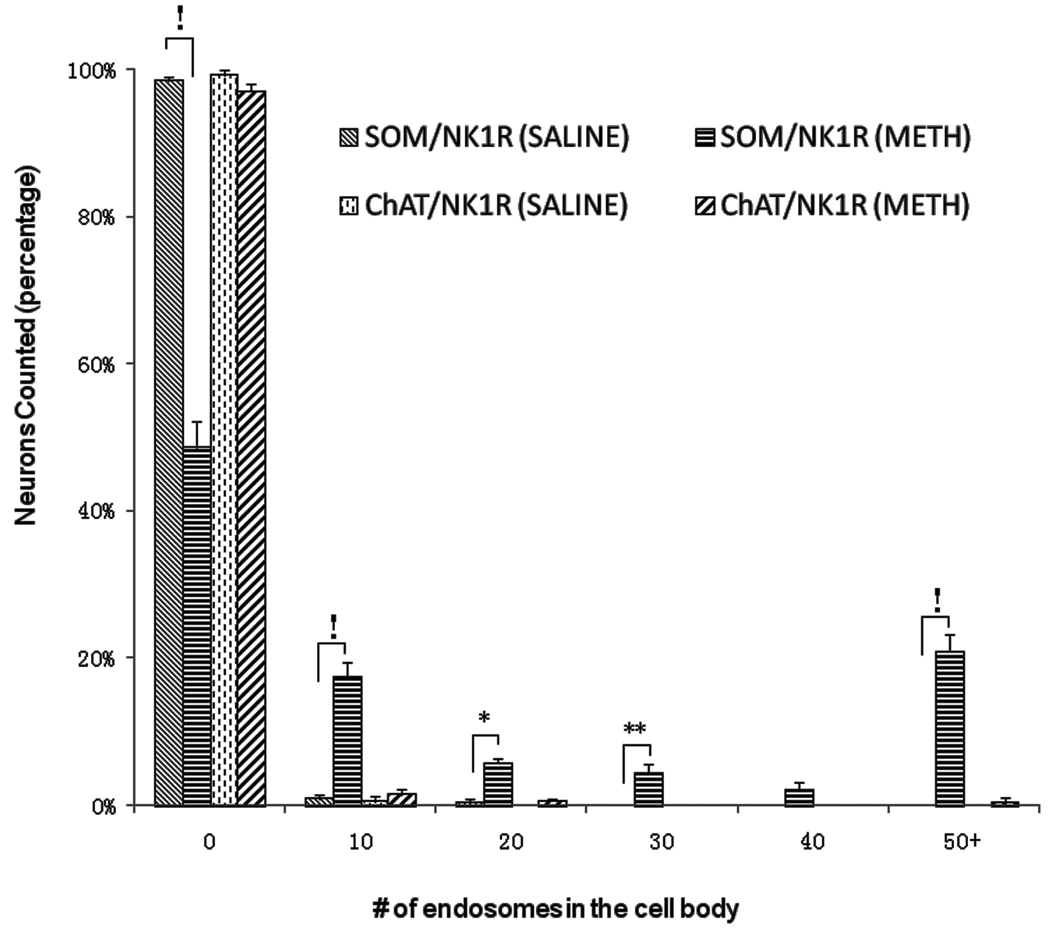
We have utilized immunofluorescence confocal microscopy in order to visualize the effect of METH on internalization of the striatal neurokinin-1 receptors into endosomes. The immunofluorescence for the neurokinin-1 receptor colocalized with immunofluorescence for choline acetyltransferase and somatostatin peptide demonstrating the presence of this receptor primarily on these two subpopulations of striatal interneurons (Figure 1). Note the larger relative size of cholinergic (1B) relative to somatostatin (1E) interneurons. Colocalization was confirmed by performing orthogonal analysis and z-stacks with the confocal microscope. In order to assess the effect of METH on the trafficking of the striatal neurokinin-1 receptors, mice were injected with either saline or METH (30 mg/kg, i.p.) and the animals were sacrificed 30 minutes after the injection of METH. In mice injected with saline, the neurokinin-1 receptor staining is observed primarily in the plasma membrane of the soma and the proximal regions of processes (Figure 2). The cholinergic interneurons show diffuse neurokinin-1 receptor staining throughout the cytoplasm of the soma (Figure 1A), however, the somatostatin interneurons show localization of the neurokinin-1 receptor primarily on the plasmalemmal membrane (Figure 2C). However, 30 minutes after a single injection of METH, the immunofluorescence for the neurokinin-1 receptor was observed in particles that resemble endosomes in the perinuclear region of the cell (Figure 2B & D). Note that the number of METH-induced endosome-like structures is greater and the endosomes are larger in the somatostatin interneurons (Figure 2C & D). Note that a single injection of METH shifted the location of the neurokinin-1 receptors in the somatostatin interneurons from the plasmalemmal membrane to the perinuclear region (compare Figure 2C & D). Visual inspection of the cells with the confocal microscope suggested that METH induced significantly more trafficking of the neurokinin-1 receptor in the somatostatin/NPY/NOS than in the cholinergic interneurons. We counted the number of endosomes per cell in neurons co-labeled with an antibody against somatostatin and the neurokinin-1 receptor. The results are shown in Figure 3 in the form of a histogram expressing the number of cells with varying levels of neurokinin-1 receptor trafficking. Thirty minutes after METH, approximately 20% of the somatostatin/NPY/NOS neurons contain 50 or more endosomes per soma. In contrast, approximately 1% of the cholinergic interneurons display 50 or more endosomes per soma (Figure 3).
Figure 1.
Striatal neurokinin-1 receptors (NK1R, A&D) are expressed by cholinergic (ChAT, B) and somatostatin (SOM, E) interneurons. Striatal sections were processed for immunofluorescence with antibodies against the neurokinin-1 receptor (FITC, green) and choline acetyltransferase (ChAT) or somatostatin (Cy3, red). Overlay of the images shows the expression of the striatal neurokinin-1 receptor within these two subpopulations of interneuron (C&F). Photomicrographs were obtained with the confocal microscope. Space bar: 100 µm. (n = 6)
Figure 2.
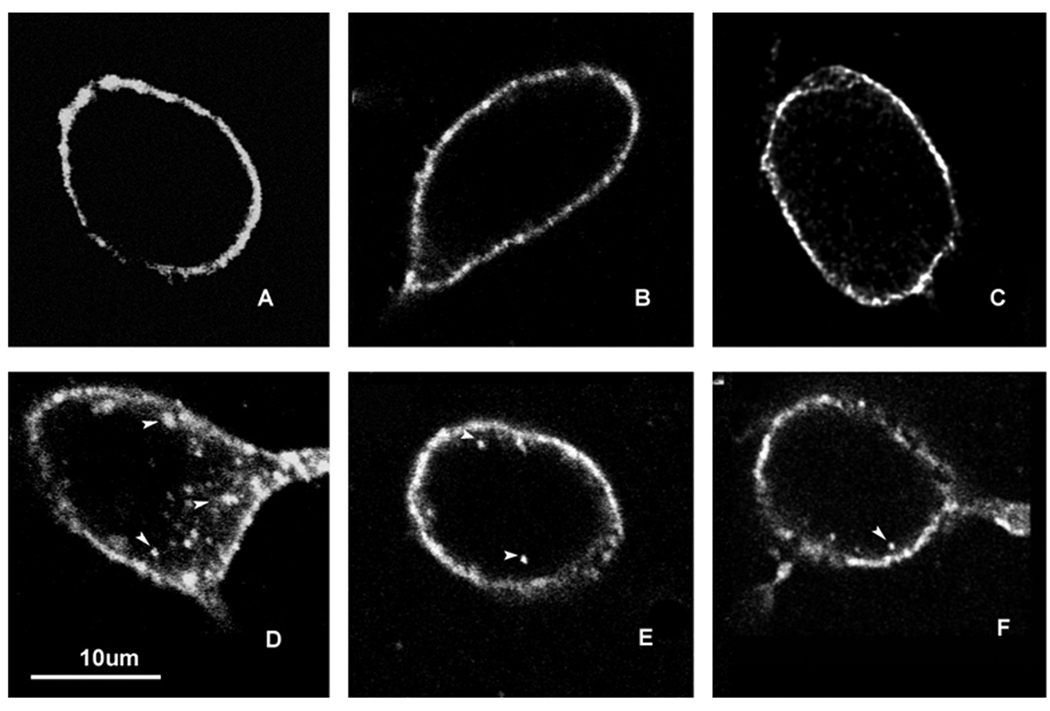
METH induces trafficking of the neurokinin-1 receptor in cholinergic and somatostatin interneurons of the striatum. Mice received a single injection of METH (30 mg/kg, i.p.) and were sacrificed 30 minutes after the injection. Coronal sections of striatal tissue were processed for immunofluorescence with antibodies against the neurokinin-1 receptor and choline acetyltransferase (ChAT, A&B) or somatostatin (SOM, C&D). The staining shown is only for the neurokinin-1 receptor (FITC, green). Photomicrographs were obtained with the confocal microscope. Arrows indicate the position of representative endosomes within a somatostatin interneuron. Scale bars: 10 µm. (n = 8)
Figure 3.
METH induced the endocytosis of the neurokinin-1 receptor primarily in the somatostain/NPY/NOS interneurons. Mice received a single injection of METH (30 mg/kg, i.p.) and were sacrificed 30 minutes after the injection. Coronal sections of striatal tissue were processed simultaneously for immunofluorescence of the neurokinin-1 receptor (NK1R) and somatostatin (SOM) or choline acetyltransferase (ChAT). Note that the somatostatin/NPY/NOS interneurons in the METH group contain more endosomes per soma than the cholinergic interneurons. The number of endosomes per soma was counted manually. !p<0.0001; *p<0.01; **p<0.05. (n = 8)
Antagonists of the substance P, dopamine D1 and D2 receptors attenuate the METH-induced trafficking of the neurokinin-1 receptors
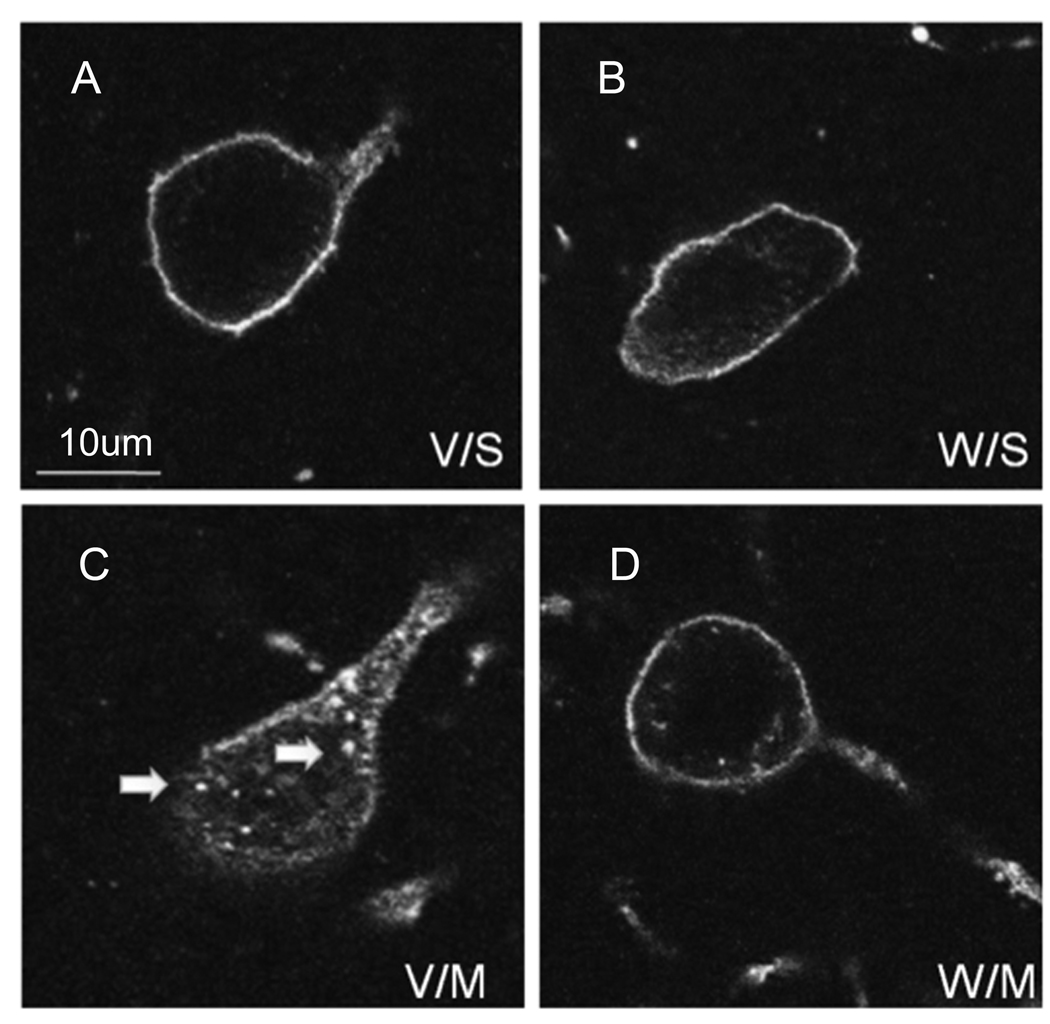
We pre-treated a group of mice with the non-peptide neurokinin-1 receptor antagonist WIN-51,708 (5 mg/kg, i.p.) 30 minutes prior to an injection of saline. The neurokinin-1 receptor antagonist had no effect on the internalization of the substance P receptor when given alone (Figure 4B). However, when given 30 minutes prior to METH it significantly attenuated the METH-induced internalization of the neurokinin-1 receptors into endosomes throughout the striatum (compare Figure 4C & D).
Figure 4.
Pre-treatment with the neurokinin-1 receptor antagonist WIN-51,708 attenuated the METH-induced trafficking of the neurokinin-1 receptors. Mice received vehicle or a single injection of WIN-51,708 (5 mg/kg, i.p.) prior to METH (30 mg/kg, i.p.) and were sacrificed 30 minutes after the METH injection. Coronal sections through the striatum were processed for immunofluorescence for the neurokinin-1 receptor (FITC, green) and somatostatin (Cy3, red). A–D show a representative neuron from each condition. V/S, vehicle/saline (A); W/S, WIN-51,708/saline (B); V/M, vehicle/METH (C); W/M, WIN-51,708/METH (D). Note that blockade of the neurokinin-1 receptor with WIN-51,708 significantly attenuated the METH-induced trafficking of the neurokinin-1 receptors. Photomicrographs were obtained with the confocal microscope. Images are shown in dark field for greater contrast. Scale bar: 10 µm. (n = 6)
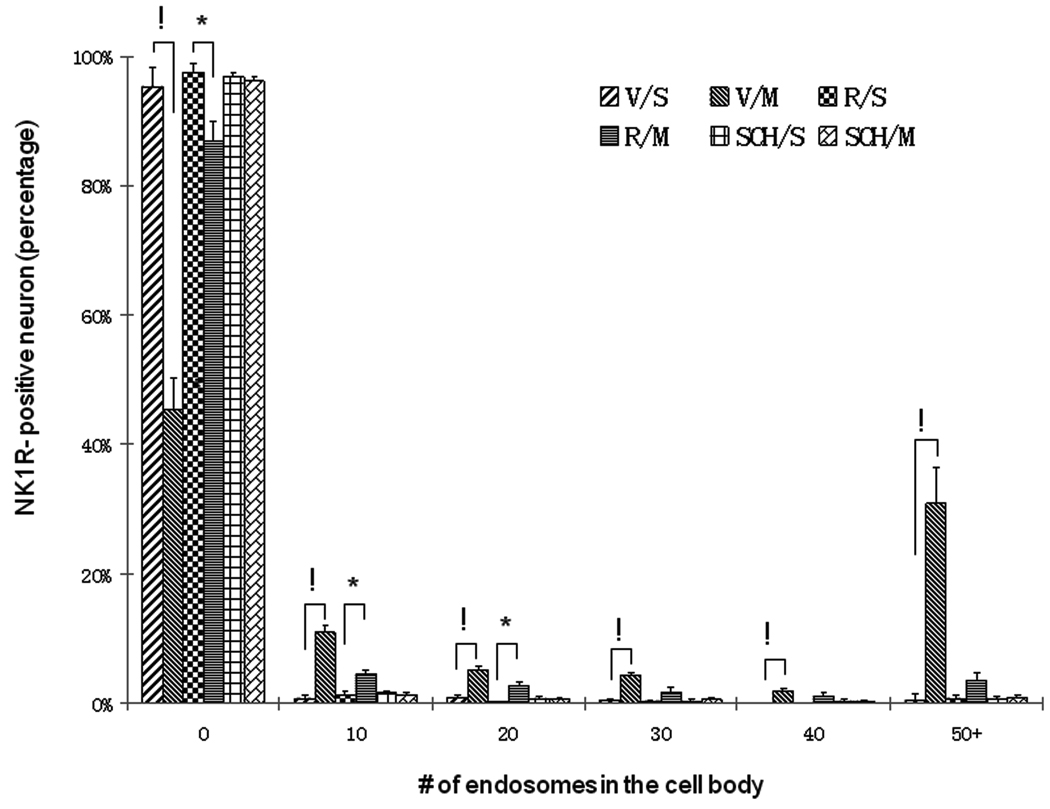
In order to assess the role of the dopamine D1 or D2 receptors on METH-induced trafficking of the neurokinin-1 receptors, a group of mice was injected with the selective D1 receptor antagonist SCH-23390 (0.1 mg/kg) 30 minutes prior to the injection of saline. SCH-23390 when given alone had no observable effect on neurokinin-1 receptor internalization (Figure 5B). However, when the D1 antagonist was injected 30 minutes prior to METH (Figure 5D), it attenuated the METH-induced internalization of the striatal neurokinin-1 receptors into endosomes (Figure 5E). Similarly, pre-treatment with the selective D2 receptor antagonist raclopride (1 mg/kg) had no effect when given alone but attenuated the METH-induced internalization of the neurokinin-1 receptors when given 30 minutes prior to METH (Figure 5F). The dose of the D1 and D2 antagonists employed in this study corresponded to the optimal dose that also prevented METH-induced cell death in the striatum of mice (Xu et al., 2005). A histogram of the number of endosomes per cell in the striatum showed that mice given either SCH-23390 or raclopride in combination with METH displayed less than 4% of the soma with 50 endosomes or more (Figure 6). Thus pre-treatment of mice with SCH-23390 or raclopride 30 minutes prior to METH preserves a distribution of endosomes resembling the control mice.
Figure 5.
Pre-treatment with dopamine D1 or D2 receptor antagonists attenuated the METH-induced internalization of the neurokinin-1 receptors in the striatum. Animals received vehicle (A) or METH (D, 30 mg/kg, i.p.) and were sacrificed 30 minutes after the injection of METH. Note that METH induces neurokinin-1 receptor trafficking (D). In a separate set of animals, SCH-23390 (0.1 mg/kg, i.p.) was administered alone (B) or 30 minutes prior to METH (E). Similarly, raclopride (1 mg/kg, i.p.) was injected alone (C) or 30 minutes before METH (F). Note that blockade of dopamine D1 or D2 receptors (E & F, respectively) significantly attenuated the METH-induced internalization of the neurokinin-1 receptors. Confocal images were photographed in dark field to enhance contrast. Arrowheads indicate the position of endosomes in the cells. Note in D the position of the endosomes surrounding the perinuclear region (dark oval region surrounded by neurokinin-1 receptor staining). (n = 8)
Figure 6.
Histogram showing the effect of dopamine D1 or D2 receptor antagonists on METH-induced trafficking of the neurokinin-1 receptors of the striatum. Treatment groups and conditions were as in Figure 5 above. Note that both SCH-23390 (D1 antagonist) and raclopride (D2 antagonist) were effective in preventing trafficking of the neurokinin-1 receptors in the presence of METH. V, vehicle; R, raclopride; S, saline; SCH, SCH-23390; M, METH; NK1R, neurokinin-1 receptor. !p<0.0001; *p<0.01.
DISCUSSION
The foregoing results demonstrate that METH affects the trafficking of striatal neurokinin-1 receptors as evidenced by the substantial increase in intracellular endosomes in the soma of the somatostatin, and to lesser extent, the cholinergic interneurons of the striatum 30 minutes after METH. This time point was chosen because it has been demonstrated that the peak of METH-inuidced dopamine overflow in the striatum occurs at this time (Jones et al., 1998). The METH-induced redistribution of the neurokinin-1 receptors from the plasma membrane into endosomes can be attenuated by pre-treatment with the neurokinin-1 receptor antagonist WIN-51,708, suggesting that substance P may be interacting with this receptor in the presence of METH. The dose of WIN-51,708 used in this study was found to be the optimal dose for attenuating the METH-induced cell death in the striatum (Zhu et al., 2009). Interestingly, pre-treatment with the dopamine D1 receptor antagonist SCH-23390 or the D2 antagonist raclopride also significantly attenuated the METH-induced trafficking of the neurokinin-1 receptors in the striatum. These observations are consistent with the hypothesis that METH induces the release of substance P in the striatum with subsequent signaling through the neurokinin-1 receptor.
Since its discovery in 1931 as an agent stimulating smooth muscle in the intestine (Euler and Gaddum, 1931), substance P has been shown to influence various physiological functions in mammals. For example, substance P facilitates gastrointestinal motor activity and secretions via the neurokinin-1 receptor (Holzer and Holzer-Petsche, 1997a,b). In the central nervous system, substance P displays antidepressant activity (Kramer et al., 1998). In the periphery, substance P is expressed by small diameter sensory neurons projecting to the dorsal horn where it mediates nociception (Hokfelt et al., 1975; Oku et al., 1987). The release of substance P in the dorsal horn by noxious stimuli results in the internalization of the neurokinin-1 receptor into endosomes in lamina I of dorsal horn neurons of the rat (Mantyh et al., 1995a). Similarly, this receptor is internalized in the striatum after intrastriatal injections of substance P (Mantyh et al., 1995b). In the present study we demonstrate that the neurokinin-1 receptor is internalized in striatal interneurons in the presence of METH. These observations are consistent with our hypothesis that in the presence of METH there is signaling by substance P through the neurokinin-1 receptor. This hypothesis is also supported by the observation that a single injection of METH (15 mg/kg) increased preprotachykinin A messenger RNA levels (encoding the neuropeptide substance P) in the striatum of rats 30 minutes and three hours post-injection (Adams et al., 2001). This result suggests that METH augments the synthesis of substance P to replenish the pool of this peptide putatively exhausted due to increased utilization. The intrastriatal release of substance P in the presence of METH may represent a secondary event preceded by the overflow of pre-synaptic dopamine and activation of the post-synaptic dopamine receptors of the striatum.
The results of this study support the hypothesis that signaling through the striatal neurokinin-1 receptor requires the signaling through the dopamine D1 and D2 receptors since antagonists of the D1 (SCH23390) or the D2 (reclopride) receptor attenuated the METH-induced internalization of the neurokinin-1 receptor into endosomes in striatal interneurons. Moreover, antagonists of the dopamine D1 or D2 receptors protect the dopamine terminals from METH-induced neurotoxicity (Sonsalla et al., 1986). In addition, we have demonstrated that dopamine receptor antagonists also attenuated the METH-induced apoptosis of some striatal neurons (Xu et al., 2005). Therefore, we postulate that the excessive overflow of striatal dopamine induced by METH causes the excessive release of substance P that then signals through the neurokinin-1 receptor potentiating a neurotoxic cascade. Our laboratory is currently investigating the mechanism by which the neurokinin-1 receptors of the striatum potentiate damage of the dopamine terminals and the apoptosis of some striatal neurons in the presence of METH. Preliminary observations point to the biochemical activation of neuronal NOS and the augmented production of nitric oxide.
Approximately 90% of striatal neurons are projection neurons that are devoid of the neurokinin-1 receptor (Gerfen, 1992). The cholinergic and somatostatin/NPY/NOS interneurons comprise 1–2% each of the striatal neurons and are the only striatal neurons expressing the neurokinin-1 receptor (Kawaguchi et al., 1995). Our data demonstrate that in the presence of METH the internalization of the neurokinin-1 receptors occurs primarily in the somatostatin/NPY/NOS interneurons of the striatum. At the very least, the response of the somatostatin interneurons is more robust. This finding is of significance in the light of the observation by our laboratory that this type of interneuron is refractory to the apoptosis induced by METH (Zhu et al., 2006). Our data do not demonstrate why METH induces more trafficking of the neurokinin-1 receptor in the somatostatin/NPY/NOS interneurons. However, one explanation accounting for this observation may involve the dopamine and the neurokinin-1 receptors. For example, the cholinergic interneurons of the striatum express the D2 subtype of dopamine receptor and this receptor has been shown to exert inhibitory effects on this interneuron (Le Moine et al., 1990; Stoof and Kebabian, 1982). Thus, it is plausible that excessive signaling through the D2 receptor on the cholinergic interneurons exerts inhibition on the simultaneous signaling of the neighboring neuronkinin-1 receptors. Alternatively or in conjunction, the neurokinin-1 receptors on the somatostatin/NPY/NOS interneurons may be of higher affinity than the same receptor molecule on the cholinergic interneurons. Also, the conditions needed to induce receptor trafficking in cholinergic interneurons may not have been met in this study. For example, time points longer than 30 minutes may be required. These plausible explanations need to be investigated.
The impact of substance P on the somatostatin interneurons may be one contributory factor to the neural damage induced by METH. This psychostimulant induces the overflow of striatal glutamate (Nash and Yamamoto, 1992) and the activation of a neurodegenerative cascade involving nitric oxide (Fridovich, 1986; Dawson et al. 1998). Pharmacological inhibition of neuronal NOS or deletion of the gene for this enzyme renders the striatum more resistant to METH (Itzhak and Ali, 1996; Imam et al., 2001a,b). Moreover, the METH-induced production of striatal 3-nitrotyrosine, an indirect index of nitric oxide synthesis, was attenuated by an antagonist of the neurokinin-1 receptor (Zhu et al., 2009). A connection between the neurokinin-1 receptor and nitric oxide synthesis has been shown in other systems. For example, in rat skin microvasculature substance P mediates inflammation by augmenting nitric oxide (Ralevic et al., 1995). In cultured synoviocytes substance P acting via the neurokinin-1 receptor increased nitric oxide synthesis (O’Shaughnessy et al., 2006). Thus it is plausible that the neurokinin-1 receptor on the somatostatin/NPY/NOS interneurons of the striatum modulates the synthesis of nitric oxide. This question is under investigation in our laboratory.
In conclusion, our data demonstrate that METH induced the endocytosis of the striatal neurokinin-1 receptor preferentially in the somatostatin/NPY/NOS interneurons. Moreover, the METH-induced endocytosis of the neurokinin-1 receptors required the activity of the dopamine D1 and D2 receptors. These observations are consistent with the hypothesis that the METH-induced overflow of striatal dopamine causes excessive signaling through the neurokinin-1 receptors. Work ongoing in this laboratory is attempting to elucidate the biochemical consequences of the internalization of the neurokinin-1 receptor in the presence of METH in the striatum.
ACKNOWLEDGMENTS
This work was supported by R01 DA020142 from the National Institute on Drug Abuse to JAA. Support for infrastructure came from the Research Centers in Minority Institutions grant awarded to Hunter College by NCRR/NIH.
Abbreviations used
- ChAT
choline acetyltransferase
- FITC
fluorescein isothiocyanate
- ICR
Institute for Cancer Research
- METH
(+)-methamphetamine hydrochloride
- NK1R
neurokinin-1 receptor
- NOS
nitric oxide synthase
- NPY
neuropeptide Y
- PBS
phosphate-buffered saline, pH 7.4
- SCH
SCH-23390
- SOM
somatostatin
- WIN-51,708
17-β-Hydroxy-17-a-ethynyl-5-a-androstano[3,2-β]pyrimido[1,2-a]benzimidazole.
REFERENCES
- Adams DH, Hanson GR, Keefe KA. Differential effects of cocaine and methamphetamine on neurotensin/neuromedin N and preprotachykinin messenger RNA expression in unique regions of the striatum. Neurosci. 2001;102:843–851. doi: 10.1016/s0306-4522(00)00530-3. [DOI] [PubMed] [Google Scholar]
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM. Real-time visualization of the cellular redistribution of G protein-coupled receptor kinase 2 and β-arrestin 2 during homologous desensitization of the substance P receptor. J. Biol Chem. 1999;274:7565–7569. doi: 10.1074/jbc.274.11.7565. [DOI] [PubMed] [Google Scholar]
- Baucum AJ, 2nd, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JT, Mandel J. A San Francisco Bay Area “speed” scene. J Health Soc Behav. 1968;9:164–174. [PubMed] [Google Scholar]
- Dawson TM, Sasaki M, Gonzalez-Zulueta M, Dawson VL. Regulation of neuronal nitric oxide synthase and identification of novel nitric oxide signaling pathways. Prog Brain Res. 1998;118:3–11. doi: 10.1016/s0079-6123(08)63196-9. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH., Jr Assault and homicide associated with amphetamine abuse. Am J Psychiatry. 1971;127:1170–1175. doi: 10.1176/ajp.127.9.1170. [DOI] [PubMed] [Google Scholar]
- Euler UA, Gaddum JH. An unidentified depressor substance in certain tissue extracts. J Physiol (London) 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: The Academic Press; 1997. [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Gibb JW, Metzger RR, Kokoshka JM, Fleckenstein AE. Methamphetamine-induced rapid and reversible reduction in the activities of tryptophan hydroxylase and dopamine transporters: oxidative consequences. Ann N Y Acad Sci. 1998;844:103–107. [PubMed] [Google Scholar]
- Hawks D, Mitcheson M, Ogborne A, Edwards G. Abuse of methylamphetamine. Br Med J. 1969;1:715–721. doi: 10.1136/bmj.2.5659.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Kellerth JO, Nilsson G, Pernow B. Substance P localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997a;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol Ther. 1997b;73:219–263. doi: 10.1016/s0163-7258(96)00196-9. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W, Jr, Ali SF. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem. 2001a;76:745–749. doi: 10.1046/j.1471-4159.2001.00029.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Ageing increases the susceptibility to methamphetamine-induced dopaminergic neurotoxicity in rats: correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001b;78:952–959. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. The neuronal nitric oxide synthase inhibitor, 7-nitroindazole, protects against methamphetamine-induced neurotoxicity in vivo. J Neurochem. 1996;67:1770–1773. doi: 10.1046/j.1471-4159.1996.67041770.x. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons—chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ, Reines SA, Liu G, Snavely D, Wyatt-Knowles E, Swain CJ, Mills SG, MacCoss M, Swain CJ, Harrison T, Hill RG, Hefti F, Scolnick EM, Cascieri MA, Chicchi GG, Sadowski S, Williams AR, Hewson L, Smith D, Carlson EJ, Hargreaves RJ, Rupniak NM. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moine C, Tison F, Bloch B. D2 dopamine receptor gene expression by cholinergic neurons in the rat striatum. Neurosci Lett. 1990;117:248–252. doi: 10.1016/0304-3940(90)90671-u. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Demaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone DA. Receptor endocytosis and dendritic reshaping in spinal neurons after somatosensory stimulation. Science. 1995a;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P-evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci USA. 1995b;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K, Dery O, Lovett M, Wong H, Walsh JH, Grady EF, Bunnett NW. Substance P-induced trafficking of ゲ-arrestins. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy MC, Vetsika EK, Inglis JJ, Carlesson J, Haigh R, Kidd BL, Winyard PG. The effect of substance P on nitric oxide release in a rheumatoid arthritis model. Inflamm Res. 2006;55:236–240. doi: 10.1007/s00011-006-0079-8. [DOI] [PubMed] [Google Scholar]
- Oku R, Satoh M, Takagi H. Release of substance P from the spinal dorsal horn is enhanced in polyarthritic rats. Neurosci Lett. 1987;74:315–319. doi: 10.1016/0304-3940(87)90316-8. [DOI] [PubMed] [Google Scholar]
- Pu C, Broening HW, Vorhees CV. Effect of methamphetamine on glutamate-positive neurons in the adult and developing rat somatosensory cortex. Synapse. 1996;23:328–334. doi: 10.1002/(SICI)1098-2396(199608)23:4<328::AID-SYN11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Khalil Z, Helme RD, Dusting GJ. Role of nitric oxide in the actions of substance P and other mediators of inflammation in rat skin microvasculature. Eur J Pharmacol. 1995;284:231–239. doi: 10.1016/0014-2999(95)00321-b. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Seiden LS, Schuster CR. Further evidence that amphetamines produce long-lasting dopamine neurochemical deficits by destroying dopamine nerve fibers. Brain Res. 1984;303:359–364. doi: 10.1016/0006-8993(84)91221-6. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Linder JC, Martone ME, Groves PM. Histological and ultrastructural evidence that d-amphetamine causes degeneration in neostriatum and frontal cortex of rats. Brain Res. 1990;518:67–77. doi: 10.1016/0006-8993(90)90955-b. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Gibb JW. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur J Pharmacol. 1985;109:73–80. doi: 10.1016/0014-2999(85)90541-2. [DOI] [PubMed] [Google Scholar]
- Seiden LS, MacPhail RC, Oglesby MW. Catecholamines and drug-behavior interactions. Fed Proc. 1975;34:1823–1831. [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J Pharmacol Exp Ther. 1986;238:932–937. [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW. Independent in vitro regulation by the D-2 dopamine receptor of dopamine-stimulated efflux of cyclic AMP and K+stimulated release of acetylcholine from rat neostriatum. Brain Res. 1982;250:263–270. doi: 10.1016/0006-8993(82)90420-6. [DOI] [PubMed] [Google Scholar]
- Szuster RR. Methamphetamine in psychiatric emergencies. Hawaii Med J. 1990;49:389–391. [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychol. 2001a;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding Y-S, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychol. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Xu W, Zhu JPQ, Angulo JA. Induction of striatal pre- and post-synaptic damage by methamphetamine requires the dopamine receptors. Synapse. 2005;58:110–121. doi: 10.1002/syn.20185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Critical Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yu J, Cadet JL, Angulo JA. Neurokinin-1 (NK-1) receptor antagonists abrogate methamphetamine-induced striatal dopaminergic neurotoxicity in the murine brain. J Neurochem. 2002;83:613–622. doi: 10.1046/j.1471-4159.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang J, Cadet JL, Angulo JA. Histological evidence supporting a role for the striatal neurokinin-1 receptor in methamphetamine-induced neurotoxicity in the mouse brain. Brain Res. 2004;1007:124–131. doi: 10.1016/j.brainres.2004.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine-induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049:171–181. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neurosci. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Wang J, Ali SF, Angulo JA. The neurokinin-1 receptor modulates the methamphetamine-induced striatal apoptosis and nitric oxide formation in mice. J Neurochem. 2009;111:656–668. doi: 10.1111/j.1471-4159.2009.06330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]