Abstract
Ecological character displacement is common in nature but the mechanisms causing divergence are not well understood. The contributions of ecological interactions other than competition have received little attention. We conducted a pond experiment to explore the contribution of both competition and predation to character divergence in threespine stickleback species. We estimated the strength of divergent selection on a morphologically intermediate target population between competition treatments under two alternate predation treatments. Divergent selection on the target population tended to be stronger in the predator-addition treatment than in the predator-reduction treatment, a difference that approached significance (P = 0.09). This trend occurred even though competition was strongest in the predator-reduction treatment. Overall, the strength of divergent selection was best predicted by stickleback mortality (P = 0.025) being strongest where mortality was highest. These results indicate that predation and other agents of mortality can enhance the rate of change in competition per unit of phenotypic divergence and, thereby, divergent selection, even as they lower the overall strength of competition. In this way, predation and other agents of mortality may facilitate, rather than hinder, character displacement.
Ecological character displacement has long been thought to be central to the evolution and maintenance of phenotypic diversity (1–6). Whereas there are now many examples from nature, the underlying mechanisms have rarely been examined (5, 6). Resource competition has been demonstrated between species undergoing character displacement in a few cases (7–14), but resource competition is not the only interaction that can lead to divergence. Populations may also diverge through shared enemies (e.g., predators, parasites, and pathogens; refs. 15–18), by intraguild predation (5), and through behavioral or reproductive interference (5, 6). The role of such interactions during character displacement has received limited attention (refs. 5 and 6, see refs. 16–20).
Here, we address the effect of predators on character displacement between competitors. Our experiment uses threespine stickleback species (Gasterosteus aculeatus complex) from southwestern British Columbia, Canada. Sympatric stickleback species are ecologically and morphologically highly differentiated. In every pair, one species (the limnetic) forages on zooplankton in the open water, whereas the other species (the benthic) forages on invertebrates in the littoral zone (21–24). Allopatric sticklebacks tend to be intermediate between limnetics and benthics in morphology and ecology and exploit both the open water and littoral habitats (24). Prior comparative and experimental work on the group has strongly implicated interspecific resource competition in the origin and maintenance of their phenotypic differences in sympatry (10, 11, 14). However, the role of predation in their character divergence remains untested.
There are good reasons to explore predation's role in this system. In the wild, cutthroat trout (Oncorhynchus clarki), diving birds, and aquatic insects (e.g., dragonfly larvae and backswimmers) are dominant predators of sticklebacks (24–29). The limnetic-benthic species pairs are consistently divergent in armor traits, which is indicative of adaptation to alternate predation regimes or modes of defense (21, 22, 26–28). These species pairs also occur only in a highly nonrandom subset of lakes containing cutthroat trout and no other predatory fish (29).
We tested whether predation alters the effect of competition on divergent selection. How predation should affect divergent selection in this context depends on its effects on the density, behavior, and habitat use of prey. The simplest expectation is that predators, by increasing stickleback mortality, reduce densities and thus decrease interspecific competition (30, 31), thereby weakening divergent selection. This expectation may be overly simplistic, however, because, in addition to their direct effect on prey abundances, predators may also participate in a variety of indirect effects that alter competitive interactions between prey (32–35). Nevertheless, empirical studies support the idea that predation weakens competition, at least over the short term (ref. 36, but see ref. 37), lending justification to this simple expectation. Resulting changes in the form and strength of selection have not been studied before.
Clearly, divergent selection should not occur when interspecific competition is extremely weak (14). However, the strength of divergent selection does not necessarily increase as interspecific competition increases. Theory suggests that the strength of divergent selection is mainly determined by the rate at which interspecific competition is alleviated with increasing phenotypic distance between individuals (see Fig. 6.1 in ref. 5; 38). How predators may affect this rate is not fully understood, although one possibility is through their effects on resource partitioning by prey. If predators elicit different antipredator behaviors in divergent prey phenotypes, leading to increased habitat segregation and reduced resource overlap (39, 40), then divergent selection would strengthen. Conversely, a reduction or elimination of resource partitioning could arise if predators caused divergent prey phenotypes to aggregate in shared refugia and thus forage on similar resources, weakening divergent selection (33, 41).
Given the diversity of ways predation might affect divergent selection between competitors, experimental tests are required to determine the interaction of competition and predation during character displacement. We manipulated insect and trout predation in experimental ponds to investigate impacts on competition and divergent selection between sticklebacks.
Methods
Experimental Design. The effects of competition on divergent selection were tested by using a paired design, similar to that of Schluter (11). Each of nine experimental ponds was divided in two by using an impermeable, plastic barrier. These ponds, measuring 23 × 23 m and 3 m deep in the center, are located on the campus of the University of British Columbia; detailed descriptions of them, and of the procedure for dividing them, can be found elsewhere (10, 11, 14). Introduced into both sides of each pond was the target of the experiment, an ecologically and morphologically intermediate population of sticklebacks with phenotypic variance enhanced by hybridization. Alternate putative competitors were then added to either side of each pond, with benthic sticklebacks being added to one side (+benthic competition treatment) and marine sticklebacks being added to the other (+marine competition treatment). Marine sticklebacks are similar in trophic morphology to limnetics and forage primarily on zooplankton in the open water, but they are more easily distinguished from target populations than are limnetics, because of differences in armor. The initial densities of both the target and the added populations were held constant, with only the phenotype of the added population varying between pond sides.
If competition falls off with increasing phenotypic distance between individuals, then adding marine sticklebacks should reduce most the growth rate of the most limnetic-like individuals in the target population, while adding benthics should depress most the growth rate of the most benthic-like individuals, generating divergent selection on the target population between pond sides. Divergent selection was measured by contrasting the relationships between fitness and trophic phenotype between pond sides. As in past studies (10, 11, 14), we focus here on growth as a component of fitness. Competition has never had a consistent effect on target survival in experiments of similar duration (10, 11, 14). Details concerning selection arising from differences in target survival can be found in Table 2, which is published as supporting information on the PNAS web site.
The effect of predation on divergent selection was tested by repeating the competition experiment under two predation treatments: four of the ponds lacked trout and densities of insect predators were reduced (predator-reduction treatment), whereas five ponds had enhanced insect densities and added trout (predator-addition treatment). The experiment was performed over two summers, with four replicate ponds in 2000 (two predator-addition and two predator-reduction) and five replicate ponds in 2001 (three predator-addition and two predator-reduction). For unknown reasons, all of the trout died in both sides of one predator-addition pond in each year. Although stickleback were retrieved from these ponds, the predation treatments were compromised and they were excluded from all analyses. This finding left a total of three predator-addition and four predator-reduction ponds. When analyzing the results, it was found that stickleback mortality varied widely among ponds and there was overlap between predation treatments, indicating other agents of mortality besides predation or indicating that predator reduction was not completely successful. For this reason, in addition to testing the effect of treatment on divergent selection, we also tested the effect of mortality as a continuous variable.
The ability to detect selection on the target population depends strongly on the frequency of individuals in the tails of the phenotype distribution because these individuals are expected to show the greatest change in fitness between competition treatments (10, 11). Because such individuals are inevitably rare in natural populations, we followed past studies (10, 11) in using hybridization to create a hypervariable target population to increase the sensitivity of our measures of changing selection. The use of hybridization is justified because genetic incompatibilities between local stickleback populations are weak or absent (42, 43) and because any unanticipated effects of hybridization are common to all treatments (11).
Our target population was composed of an equal number of F1 hybrid individuals between a morphologically intermediate allopatric population and benthic populations, and between the same allopatric population and a limnetic population. Allopatric × benthic F1 hybrids were made by using benthic individuals from two different lakes. Benthics from the two lakes differ in armor characteristics (see below) and the use of both enhanced variation in armor phenotypes of more benthic-like individuals in the target population. No selection on armor was detected in the experiment, so details concerning the methods and results of this analysis are presented in Table 2.
Fish Populations. All sticklebacks used in the experiment were juveniles, which were made by artificially crossing wild-caught individuals. Marine sticklebacks were collected from a small tributary of the Salmon River in Fort Langley, British Columbia. This population is morphologically and ecologically similar to other populations of marine sticklebacks in the region. Limnetic sticklebacks were collected from Paxton Lake, whereas benthic sticklebacks were collected from Paxton and Priest lakes. Paxton and Priest lakes are located in separate drainages on Texada Island, British Columbia, and their species pairs have apparently arisen independently (44, 45). While similar in trophic characters, benthics from these lakes differ in their degree of armor reduction with Paxton benthics being the least armored (refs. 22 and 23 and S.M.V. and D.S., unpublished observations). The allopatric population used to make the F1 hybrids was collected from Cranby Lake, which is also on Texada Island. Cranby Lake was chosen because it is similar to Paxton Lake in size, elevation, vegetation, and prey species, and it lies <1 km away in a separate drainage. The morphology of this population is intermediate between limnetics and benthics (24). F1 hybrids were made by crossing females from Cranby Lake with limnetic males and benthic males. The target population consisted of an equal proportion of both types of F1 hybrids (i.e., Cranby × limnetic individuals and Cranby × benthic individuals). The Cranby × benthic crosses were composed of an equal proportion of hybrids made by using benthic males from Paxton and Priest lakes (i.e., 50% Cranby × Paxton benthics and 50% Cranby × Priest benthics). Due to a shortage of some crosses, however, in one pond in 2001 (pond 5) the Cranby × benthic crosses were composed of 13% Cranby × Paxton benthics and 87% Cranby × Priest benthics. To further enhance the frequency of individuals at either morphological extreme we did not include pure Cranby × Cranby individuals in the target population, unlike in past studies (10, 11). All offspring were raised in the laboratory following the methods of past studies (10, 11). The fish were introduced to the experimental ponds ≈1 month after hatching.
Experimental Procedure. The experiment was carried out in the summers of 2000 (ponds 1, 4, and 6) and 2001 (ponds 2, 3, 5, and 7). Sticklebacks were added to the ponds in late June and early July of both summers. Benthics (from Paxton Lake) were added to one randomly chosen side of each pond. Individuals from separate laboratory aquaria were first pooled into larger groups and then allocated haphazardly to the separate ponds. The same procedure was followed to add marine sticklebacks to the opposite side of each pond. Finally, the target populations were added to both sides of every pond. In 2000, we added 1,400 target individuals and 960 benthic or marine sticklebacks to every pond side. In 2001, we used 1,350 target individuals, along with 860 benthics or marine sticklebacks in each pond side. These densities were chosen because previous experiments (10, 11, 14) show that they result in growth rates that are not lower (and are likely higher) than those in nature. Differences between years reflected the availability of the different types of fish.
The predation treatments were created by manipulating the densities of both predatory aquatic insects and cutthroat trout in whole ponds. In the predator-reduction ponds, insect densities were reduced by using minnow traps and dip nets just before introducing the sticklebacks. On average, 237 ± 56 aeshnid and gomphid dragonfly larvae, and 398 ± 181 backswimmers (Notonecta spp.) were removed from each pond (mean ± SD). Although densities of some insects might have recovered fairly rapidly after removal as a result of their mobility, observations suggest that much of the mortality caused by insect predators occurs soon after the introduction of the fish. Nevertheless, mortality was high in one of these ponds (pond 2), leaving open the possibility that insect predators recovered rapidly there.
Insects captured from the predator-reduction ponds were added to the predator-addition ponds. Because qualitative visual surveys suggested that insect densities varied among ponds, captured insects from the predator-reduction ponds were allocated differentially to the predator-addition ponds in an attempt to reduce variation in predator densities among these ponds. Finally, four cutthroat trout were added to both sides of each of the predator-addition ponds ≈1 month after the introduction of the sticklebacks. This delayed introduction allowed the sticklebacks to reach a size at which they would be susceptible to predation by trout while minimizing trout-induced mortality of the predatory aquatic insects. The trout were wild-caught individuals from Placid Lake in the University of British Columbia Research Forest. Although Placid Lake does not contain native sticklebacks, the trout readily prey on sticklebacks when given the opportunity (46). Trout were matched for body size between pond sides.
The experiment ran for 9–10 weeks, at which time sticklebacks were harvested by using minnow traps, and, subsequently, by adding 0.5 kg of 5% rotenone (Syndel International, Vancouver) to each pond side (10, 11, 14). All captured fish were first anaesthetized by using MS-222 (Syndel International) and then preserved in 95% ethanol.
Data Acquisition. Random samples of 175–260 individuals were taken from the total harvest of each pond side and fixed and stained (11). We used a different technique in the two competition treatments to identify individuals belonging to the target population. Armor differences between marine and target individuals (47) allowed target individuals in the +marine treatment to be identified by eye. For the +benthic side of each pond, we used a linear discriminant function derived from the measurement of armor traits of 154 individuals of known cross type. Details of the analysis can be found in Supporting Text, which is published as supporting information on the PNAS web site. All individuals classified as benthics were removed from the samples, leaving only individuals identified as target fish for subsequent analyses. When applied to the known fish, the function misclassified ≈3% of individuals, leaving the possibility that some benthics were mixed in with target individuals in our samples. However, because benthics tend to be larger and grow faster than other phenotypes, the misclassification of a few benthics as target individuals is conservative, tending to reduce our ability to detect divergent selection. Nevertheless, the possibility of classification errors led us to conduct two separate analyses: first, using all individuals classified as targets by the discriminant function, and second, using only the individuals whose posterior probability of being a target individual exceeded 95%. This second analysis, by using a more strict criterion, likely also excluded some of the more benthic-like target individuals as well. Nevertheless, results of this second analysis differed little from the first, and, in no case, was the significance of any statistical test altered. We present the results of the first analysis only.
Pond Conditions. Survival of target individuals was significantly lower in the predator-addition ponds than in the predator-reduction ponds (Table 3, which is published as supporting information on the PNAS web site; nested generalized linear model with pond as a fixed-effect: χ21 = 214.9, P < 0.0001), as was the survival of benthic (χ21 = 28.9, P < 0.0001) and marine (χ21 = 29.3, P < 0.0001) sticklebacks. However, these differences in survival occurred on a background of significant and high variability among ponds within treatments for target (χ25 = 301.4, P < 0.0001), benthic (χ25 = 253.2, P < 0.0001), and marine (χ25 = 70.0, P < 0.0001) sticklebacks. Total survival in one predator-reduction pond (pond 2) was similar to that in the predator-addition ponds, possibly because insect predator density remained high there.
Measuring Selection. We estimated selection on trophic morphology in each target population by examining the growth of target individuals as a function of their gill raker count (number of gill rakers on the first gill arch). More benthic-like individuals within the target population have fewer gill rakers than more limnetic-like individuals. Gill raker count provides an index of underlying differences in trophic morphology of target phenotypes and is not phenotypically plastic in response to diet (48). Final standard length (measured to the nearest 0.02 mm by using Vernier calipers) was used as a measure of growth because fish were introduced to ponds at small and essentially equivalent sizes.
Within each pond, divergent selection on the target population was calculated as the difference between +benthic and +marine pond sides in the slope of the relationship between growth and gill raker count (+benthic slope minus +marine slope). This difference is expected to be positive if the different competitors generate opposing selection on target populations. We evaluated the treatment effect by comparing the within pond measurements of divergent selection between predation treatments by using whole ponds as replicates. The effects on divergent selection of stickleback mortality (one minus absolute survival), competition intensity, and the opportunity for selection were subsequently evaluated as continuous variables, again by using whole ponds as replicates. The intensity of competition was calculated for each pond as the inverse of the mean standard length of sticklebacks retrieved from that pond, because stronger resource competition will result in smaller sticklebacks overall. This measure includes the effects of both intra- and interspecific competition, and we are unable to separate the two, given the design of the experiment. The opportunity for selection was measured for each pond as the squared coefficient of variation (49) in relative standard length of target individuals retrieved from that pond.
Results
Mean Growth. Predation treatment had a strong effect on mean stickleback growth (Table 3). Average growth of target individuals was consistently higher in the predator-addition treatment than in the predator-reduction treatment (t5 = 7.65, one-tailed P (1) < 0.001). Combining treatments, mean growth was inversely related to stickleback survival (r = –0.70, P (1) = 0.04), implying that mortality decreased resource competition. The mean growth of target individuals did not differ significantly between competition treatments (paired t test: t6 = 0.80, P = 0.46), indicating that the overall strength of resource competition did not vary between the +benthic and +marine competition treatments.
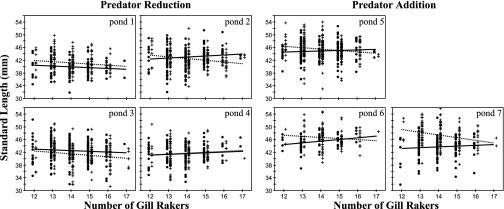
Selection on Trophic Morphology. Differences in the relative growth of target individuals generated selection on trophic morphology (Fig. 1). Divergent selection on the target populations was positive and significant in the predator-addition treatment (Table 1), despite higher mortality and increased mean growth. The growth of the most benthic-like target phenotypes was depressed in the presence of benthic (+benthic competition treatment) as compared with marine (+marine competition treatment) sticklebacks. The opposite results was seen for the most limnetic-like target phenotypes: their growth was depressed in the presence of marine (+marine competition treatment) as compared with benthic (+benthic treatment) sticklebacks. In contrast, divergent selection on the target populations was variable and tended to be weaker in the predator-reduction ponds, a difference between predation treatments that approached statistical significance (t5 = 2.13, P = 0.09). In three of the four predator-reduction ponds, divergent selection was not significant, indicating that selection on the target population did not differ between competition treatments (Table 1). In the fourth pond, however, divergent selection was strong, similar in magnitude to that in the predator-addition treatment (pond 2; Table 1). This is the same pond in which stickleback mortality was also high, which was similar to that observed in the predator-addition ponds.
Fig. 1.
Growth of target individuals as a function of gill raker number when in the presence of benthic (•, solid line) or marine (+, dashed line) sticklebacks. Individuals with higher gill raker counts are more similar to limnetic and marine sticklebacks in their trophic morphology, whereas individuals with lower counts are more benthic-like. For clarity, gill raker counts of individuals in the two competition treatments are displaced slightly.
Table 1. Selection on trophic morphology in target populations.
| Pond | Competition treatment | Sample size | Slope (±SE) | Divergent selection |
|---|---|---|---|---|
| Predator addition | ||||
| 5 | +benthic | 156 | 0.15 (0.19) | 0.56* |
| 5 | +marine | 237 | -0.41 (0.18) | |
| 6 | +benthic | 51 | 0.54 (0.32) | 0.86* |
| 6 | +marine | 106 | -0.32 (0.31) | |
| 7 | +benthic | 149 | 0.22 (0.26) | 1.08* |
| 7 | +marine | 130 | -0.86 (0.26) | |
| Predator reduction | ||||
| 1 | +benthic | 124 | -0.26 (0.22) | 0.09 |
| 1 | +marine | 154 | -0.35 (0.23) | |
| 2 | +benthic | 174 | 0.32 (0.21) | 0.83* |
| 2 | +marine | 161 | -0.50 (0.19) | |
| 3 | +benthic | 204 | -0.21 (0.19) | 0.26 |
| 3 | +marine | 171 | -0.47 (0.23) | |
| 4 | +benthic | 120 | 0.25 (0.24) | -0.01 |
| 4 | +marine | 142 | 0.26 (0.26) |
Slope is the regression of growth (final standard length) of surviving target individuals on their trophic morphology (number of gill rakers; see Fig. 1). Divergent selection is the difference in this slope between competition treatments within ponds (+benthicslope - + marine slope); greater positive values signify stronger divergent selection. *, significance values of P < 0.05 in one-tailed t tests of the difference between slopes within each pond, not treatment effects. See text for tests of treatment effects.
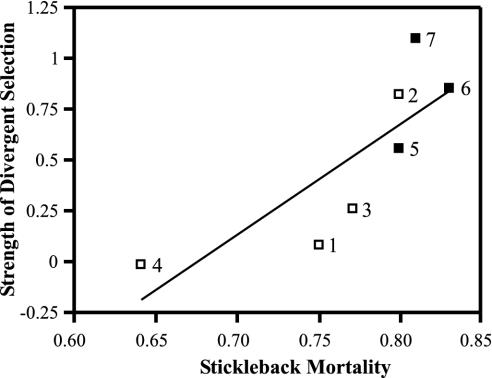
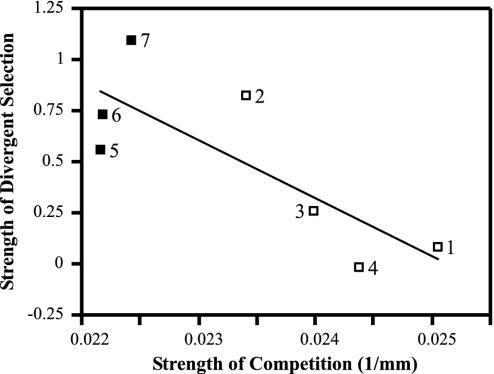
Thus, the strength of divergent selection closely mirrored stickleback mortality, increasing as mortality increased (Fig. 2). This relationship held whether we measured total stickleback mortality (i.e., targets plus benthics plus marines; r = 0.82, df = 5, P = 0.025) or target mortality alone (r = 0.79, df = 5, P = 0.036). Although this relationship appears nonlinear, a quadratic term is not significant (t4 = 1.93, P = 0.13) and a natural-log transformation of the abscissa improves the fit only slightly. The strength of divergent selection was also inversely related to the strength of resource competition (Fig. 3). This relationship also held whether we measured competition strength as the inverse of the mean growth of all sticklebacks (i.e., targets plus benthics plus marines; r = –0.79, df = 5, P = 0.037) or of targets alone (r = –0.79, df = 5, P = 0.036). Finally, the strength of divergent selection was not closely related to the variance in relative standard length of target individuals within each pond (r = 0.17, df = 5, P = 0.15). Therefore, although mortality increased mean target growth, higher growth did not generate a greater opportunity for selection.
Fig. 2.
Strength of divergent selection in relation to stickleback mortality, calculated as one minus the survival of all sticklebacks (number retrieved/number introduced) from a whole pond. Number labels identify individual ponds. □, predator-reduction treatments; ▪, predator-addition treatments.
Fig. 3.
Strength of divergent selection in each pond in relation to the overall strength of resource competition, which was calculated as the inverse of the mean final standard length (mm) of all sticklebacks retrieved from a pond. Greater positive values indicate stronger competition. Pond labels are as in Fig. 2.
Discussion
The study of divergent natural selection resulting from the ecological interaction of populations and species has focused almost exclusively on resource competition, whereas the contribution of other interactions has received little empirical study (4–6). The purpose of the current experiment was to explore the interaction between resource competition and predation in generating divergent natural selection between sympatric populations of sticklebacks, with the additional goal of more fully understanding the ecological interactions that have contributed to the evolution of the present day limnetic and benthic species. We contrasted selection on a morphologically intermediate target population in the presence of alternate competitors to determine how selection on the target population varied under conditions of increased and decreased predation.
Our results were opposite the simplest expectation, deriving from the most common outcome of past field experiments (36), that predation would increase prey mortality and reduce resource competition, thereby weakening divergent selection. Instead, divergent selection tended to be stronger in the predator-addition treatment than in the predator-reduction treatment (Table 1), which was a difference that approached significance. The strongest result was that mortality was a significant predictor of divergent selection (Fig. 2), with divergent selection strengthening as mortality increased. In addition, because mortality lowered stickleback density and weakened resource competition, the strength of divergent selection was inversely related to the strength of competition (Fig. 3). Overall, although mortality was a better predictor of divergent selection than was predation, the two are correlated in our experiment and we are unable to say which of them was most important.
There are two possible explanations for stronger divergent selection under conditions of weaker competition and higher mortality. First, divergent selection may have been generated by predators themselves rather than by competitive interactions between prey. Under certain conditions, sharing predators can cause separate prey populations to compete for enemy-free space, resulting in divergent selection on antipredator traits in the prey populations (16–18). However, this mechanism is unlikely to explain our results because no selection was detected on antipredator traits (number of lateral plates) in target populations (see supporting information). Furthermore, selection arising via shared predators should be manifested as differences in target survival at least as strongly as differences in their growth. However, similar to past pond experiments (10, 11), we detected no consistent selection arising from differences in target survival (see Supporting Text).
While stronger selection under weaker competition seems paradoxical, theoretical models of character divergence reveal that the intensity of divergent selection is determined mainly by the rate at which interspecific competition is reduced per increment of phenotypic divergence (5, 38). Divergent selection is weak when this rate is low, even if both inter- and intraspecific competition are strong. This consideration leads to the second, and most likely explanation for our results: that predation, by increasing resource partitioning among divergent prey phenotypes, caused competition to decline at a greater rate with phenotypic distance even as the strength of competition was lessened. This explanation assumes that competition was still present at the low densities that existed in the predator-addition ponds. Although we have no direct evidence for this assumption, it is difficult to explain the results otherwise.
There are at least two possible ways predators could increase resource partitioning and thereby strengthen divergent selection. First, threat of predation itself could cause changes in prey foraging behavior. Behavioral changes of prey in response to increased predation risk are well documented (35, 50–52), and predators have been implicated in habitat segregation of different prey (e.g., refs. 39 and 40). In our experiment, increased resource partitioning might have arisen if elevated predation risk caused divergent stickleback phenotypes to forage in different habitats in which predation risk was minimized. The second possibility is that changes in resource partitioning could have arisen as a result of predator-induced changes in stickleback density, with density, in turn, altering foraging behavior. For example, low food abundance stemming from greater depletion at high fish density might cause habitat partitioning to weaken between different phenotypes. This may occur if, at high density in both competition treatments, target fish deplete food more rapidly in one of the two main habitats (littoral zone or open water) than the other, forcing all phenotypes into the remaining habitat. Even with uniform food depletion over habitats at high density, there are conditions under which lower food abundance should cause individuals to broaden the range of habitats exploited (53), leading to a reduction in resource partitioning between distinct phenotypes and a weakening of divergent selection between competition treatments. Both of these possibilities concern the important questions of how two prey populations may interact indirectly through effects mediated through other trophic levels (34, 35) and how this leads to selection. Determining the mechanism for the mortality effect in the current study will require experiments that independently manipulate both stickleback density and predation risk. It will also be important to gain a better understanding of the role of predators in altering stickleback foraging behavior and habitat use.
Our findings provide additional support for the hypothesis, originally derived from observational evidence (24), that character displacement has been central to the evolution of limnetic and benthic sticklebacks. Past experiments, all performed in the presence of considerable (unmanipulated) mortality by insect predators, have confirmed key predictions of character displacement theory that competition should generate both density- and frequency-dependent divergent selection (10, 11), and that the strength of competition should decline as divergence proceeds (14). Our current findings, which manipulate predation directly in a test of character displacement, further suggest that character divergence likely involved a richer array of interactions than simply resource competition between sticklebacks. In particular, predation and other agents of mortality may have played a central role. That predation may have been important in character divergence is also suggested by observational (29) and experimental evidence indicating that limnetics and benthics are adapted to alternate predation regimes in their preferred habitats (28, 46). Alternate predation regimes likely strengthen divergent selection between habitats over that occurring from resource differences alone (46).
The generality of our findings remains to be confirmed. They stand in apparent contrast to the outcome of one recent laboratory experiment (54) in which the diversity of sympatric, bacterial niche specialists evolving in spatially structured environments was significantly reduced by a virulent phage. Phage-driven reductions in bacterial density were suggested to have weakened resource competition, and, hence, reduced diversity. Whether predation or parasitism should increase divergent selection arising from competition is expected to depend on how drastically competition is reduced and how predation impacts resource partitioning between distinct phenotypes. An increase in divergent selection with added predation requires an increase in the rate of change of competition per increment of divergence in phenotype. Such an increase cannot occur when competition is reduced to very low levels, nor is it likely without an increase in resource partitioning between phenotypes. Predation's facilitatory effect on divergent selection is therefore unlikely to be universal but it may nevertheless be common. For example, resource competition often persists when predators are present (36).
In conclusion, limnetic and benthic sticklebacks are adapted to alternate suites of predators (28), and mortality generated by these same predators appears to alter selection arising from their competitive interactions in an unexpected way. Stronger divergent selection, despite weaker competition, is nonintuitive and highlights the importance of direct tests of the evolutionary consequences of population interactions.
Supplementary Material
Acknowledgments
We thank J. Boughman, K. Faller, A. Gosline, M. McDonald, S. Latham, J. Mee, K. Rozalska, K. Ryall, and D. Taylor for assistance. Members of the laboratories of D.S., S. Otto, M. Whitlock, and M. Doebeli at the University of British Columbia, and of F. Breden, A. Mooers, and B. Crespi at Simon Fraser University (Burnaby, BC, Canada), and T. Schoener, C. Benkman, and an anonymous reviewer provided helpful comments. This work was supported by the Natural Sciences and Engineering Research Council of Canada (D.S.), I. W. Killam Predoctoral and E. B. Eastburn Postdoctoral fellowships (to H.D.R.), and Natural Sciences and Engineering Research Council of Canada Postgraduate and Postdoctoral scholarships (to S.M.V.).
References
- 1.Lack, D. (1947) Darwin's Finches (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Simpson, G. G. (1953) The Major Features of Evolution (Columbia Univ. Press, New York).
- 3.Grant, P. R. (1986) Ecology and Evolution of Darwin's Finches (Princeton Univ. Press, Princeton).
- 4.Taper, M. L. & Case, T. J. (1992) Oxford Surv. Evol. Biol. 8, 63–109. [Google Scholar]
- 5.Schluter, D. (2000) The Ecology of Adaptive Radiation (Oxford Univ. Press, Oxford).
- 6.Schluter, D. (2000) Am. Nat. 156, Suppl., S4–S16. [Google Scholar]
- 7.Martin, M. M. & Harding, J. (1981) Evolution (Lawrence, Kans.) 35, 975–987. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. H. & Munger, J. C. (1985) Ecology 66, 1545–1563. [Google Scholar]
- 9.Abramsky, Z., Rosenzweig, M. L., Pinshow, B., Brown, J. S., Kotler, B. & Mitchell, W. A. (1990) Ecology 71, 2358–2369. [Google Scholar]
- 10.Schluter, D. (1994) Science 266, 798–800. [DOI] [PubMed] [Google Scholar]
- 11.Schluter, D. (2003) Evolution (Lawrence, Kans.) 57, 1142–1150. [DOI] [PubMed] [Google Scholar]
- 12.Gorbushin, A. M. (1996) Oikos 77, 85–92. [Google Scholar]
- 13.Pfennig, D. W. & Murphy, P. J. (2000) Evolution (Lawrence, Kans.) 54, 1738–1749. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard, J. R. & Schluter, D. (2001) Evol. Ecol. Res. 3, 209–220. [Google Scholar]
- 15.Schoener, T. W. (1986) in Community Ecology: Pattern and Process, eds. Kikkawa, J. & Anderson, D. J. (Blackwell Scientific, Melbourne), pp. 91–126.
- 16.Holt, R. D. (1977) Theor. Popul. Biol. 12, 197–229. [DOI] [PubMed] [Google Scholar]
- 17.Abrams, P. A. (2000) Am. Nat. 156, Suppl., S45–S61. [DOI] [PubMed] [Google Scholar]
- 18.Abrams, P. A. & Chen, X. (2002) Am. Nat. 160, 692–704. [DOI] [PubMed] [Google Scholar]
- 19.Abrams, P. A. (1996) Ecology 77, 1321–1328. [Google Scholar]
- 20.Doebeli, M. & Dieckmann, U. (2000) Am. Nat. 156, Suppl., S77–S101. [DOI] [PubMed] [Google Scholar]
- 21.McPhail, J. D. (1984) Can. J. Zool. 62, 1402–1408. [Google Scholar]
- 22.McPhail, J. D. (1992) Can. J. Zool. 70, 361–369. [Google Scholar]
- 23.McPhail, J. D. (1994) in Evolutionary Biology of the Threespine Stickleback, eds. Bell, M. A. & Foster, S. A. (Oxford Univ. Press, Oxford), pp. 399–437.
- 24.Schluter, D. & McPhail, J. D. (1992) Am. Nat. 140, 85–108. [DOI] [PubMed] [Google Scholar]
- 25.Pressley, P. H. (1981) Evolution (Lawrence, Kans.) 35, 282–295. [DOI] [PubMed] [Google Scholar]
- 26.Reimchen, T. E. (1980) Can. J. Zool. 58, 1232–1244. [Google Scholar]
- 27.Reimchen, T. E. (1994) in Evolutionary Biology of the Threespine Stickleback, eds. Bell, M. A. & Foster, S. A. (Oxford Univ. Press, Oxford), pp. 240–276.
- 28.Vamosi, S. M. (2002) Ann. Zool. Fenn. 39, 237–248. [Google Scholar]
- 29.Vamosi, S. M. (2003) Evol. Ecol. Res. 5, 717–730. [Google Scholar]
- 30.Paine, R. T. (1966) Am. Nat. 100, 65–75. [Google Scholar]
- 31.Connell, J. H. (1975) in Ecology and Evolution of Communities, eds. Cody, M. L. & Diamond, J. M. (Belknap Press, Cambridge, U.K.), pp. 460–490.
- 32.Holt, R. D. (1984) Am. Nat. 124, 377–406. [DOI] [PubMed] [Google Scholar]
- 33.Holt, R. D. & Lawton, J. H. (1994) Annu. Rev. Ecol. Syst. 25, 495–520. [Google Scholar]
- 34.Abrams, P. A., Menge, B. A., Mittelbach, G. G., Spiller, D. A. & Yodzis, P. (1996) in Food Webs: Integration of Patterns and Dynamics, eds. Polis, G. A. & Winemiller, K. O. (Chapman & Hall, New York), pp. 371–395.
- 35.Peacor, S. D. & Werner, E. E. (2001) Proc. Natl. Acad. Sci. USA 98, 3904–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gurevitch, J., Morrison, J. A. & Hedges, L. V. (2000) Am. Nat. 155, 435–453. [DOI] [PubMed] [Google Scholar]
- 37.Chase, J. M., Abrams, P. A., Grover, J. P., Diehl, S., Chesson, P., Holt, R. D., Richards, S. A., Nisbet, R. M. & Case, T. J. (2002) Ecol. Lett. 5, 302–315. [Google Scholar]
- 38.Doebeli, M. (1996) Ecology 77, 510–520. [Google Scholar]
- 39.Kotler, B. P. & Brown, J. S. (1988) Annu. Rev. Ecol. Syst. 19, 281–307. [Google Scholar]
- 40.Lingle, S. (2002) Ecology 83, 2037–2048. [Google Scholar]
- 41.Schmitz, O. J. (1998) Am. Nat. 151, 327–342. [DOI] [PubMed] [Google Scholar]
- 42.Hatfield, T. & Schluter, D. (1999) Evolution (Lawrence, Kans.) 53, 866–873. [DOI] [PubMed] [Google Scholar]
- 43.Rundle, H. D. (2002) Evolution (Lawrence, Kans.) 56, 322–329. [DOI] [PubMed] [Google Scholar]
- 44.Taylor, E. B. & McPhail, J. D. (1999) Biol. J. Linn. Soc. 66, 271–291. [Google Scholar]
- 45.Taylor, E. B. & McPhail, J. D. (2000) Proc. R. Soc. London Ser. B 267, 2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vamosi, S. M. & Schluter, D. (2002) Proc. R. Soc. London Ser. B 269, 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagen, D. W. & Gilbertson, L. G. (1972) Evolution (Lawrence, Kans.) 26, 32–51. [DOI] [PubMed] [Google Scholar]
- 48.Day, T., Pritchard, J. & Schluter, D. (1994) Evolution (Lawrence, Kans.) 48, 1723–1734. [DOI] [PubMed] [Google Scholar]
- 49.Arnold, S. J. & Wade, M. J. (1984) Evolution (Lawrence, Kans.) 38, 709–719. [DOI] [PubMed] [Google Scholar]
- 50.Lima, S. L. & Dill, L. M. (1990) Can. J. Zool. 68, 619–640. [Google Scholar]
- 51.Werner, E. E. & McPeek, M. A. (1994) Ecology 75, 1368–1382. [Google Scholar]
- 52.Mercurio, K. S., Palmer, A. R. & Lowell, R. B. (1985) Ecology 66, 1417–1425. [Google Scholar]
- 53.Schoener, T. W. (1974) Proc. Natl. Acad. Sci. USA 71, 4169–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckling, A. & Rainey, P. B. (2002) Nature 420, 496–499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.