Abstract
The consequences of biodiversity loss for ecosystem functioning and ecosystem services have aroused considerable interest during the past decade. Recent work has focused mainly on the impact of species diversity within single trophic levels, both experimentally and theoretically. Experiments have usually showed increased plant biomass and productivity with increasing plant diversity. Changes in biodiversity, however, may affect ecosystem processes through trophic interactions among species as well. An important current challenge is to understand how these trophic interactions affect the relationship between biodiversity and ecosystem functioning. Here we present a mechanistic model of an ecosystem with multiple trophic levels in which plants compete for a limiting soil nutrient. In contrast to previous studies that focused on single trophic levels, we show that plant biomass does not always increase with plant diversity and that changes in biodiversity can lead to complex if predictable changes in ecosystem processes. Our analysis demonstrates that food-web structure can profoundly influence ecosystem properties.
The relationship between biodiversity and ecosystem functioning has emerged as a central issue in ecology as human activities are precipitating species extinctions (1–6). Controlled experiments have showed that primary productivity increases with plant species richness but often saturates at high diversity (7, 8). This relationship seems to be fairly general because these experiments were conducted in different localities (7) and throughout several years (8). Theoretical models that consider a single trophic level usually predict the same pattern (9, 10). The positive effects of species diversity on ecosystem processes have been explained by two classes of mechanisms (11): first, a complementarity effect that can arise from niche differentiation or facilitation, and, second, a selection effect that can cause dominance of the most productive species (12).
These mechanisms only imply competitive interactions among species, but trophic cascades are expected to influence biomass at the various trophic levels when several trophic levels are considered (13). An important current challenge is to understand how trophic interactions affect the relationship between biodiversity and ecosystem functioning (1, 3). Understanding the impacts of consumer diversity on ecosystem functioning is particularly important because extinction threats are often higher for animals than for plants (14, 15). Recent experiments suggest that changes in biodiversity may affect ecosystem processes through trophic interactions among species (16–19). Countervailing effects of autotroph diversity and heterotroph diversity were observed recently in some studies; addition of heterotrophs can remove the positive relationship between diversity and production found in autotroph-only treatments (16, 20), and algal biomass was reduced by increasing grazer diversity in a seagrass system (21). One experiment also suggested that food-web structure can affect higher-level ecosystem services (22). However, although experiments and theory on the effects of plant diversity on ecosystem functioning are relatively well developed, little is still known about the effects of consumer diversity and trophic structure on ecosystem processes. In particular, theoretical foundations of the relationship between biodiversity and ecosystem functioning are virtually absent for multitrophic systems, with a very few exceptions (23, 24). We lack theories and models to provide generalizations, predictions, and interpretations for the growing body of experiments simulating biodiversity loss at multiple trophic levels. Here we provide a theoretical model which addresses this need.
The Model
The present model is an extension of a model developed by Loreau (23) for a nutrient-limited ecosystem containing an arbitrary number of plants and specialized herbivores in a heterogeneous environment. Plant nutrient uptake is assumed to decrease the soil concentration of a limiting nutrient in the immediate vicinity of the rooting system, thus creating a local resource-depletion zone around each plant and allowing plant coexistence under some conditions (23). Here we add carnivores and allow both herbivores and carnivores to be generalists. The model is described by the dynamical equations:
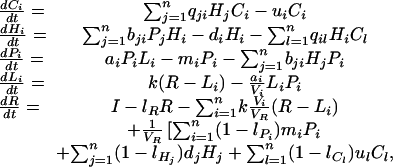 |
where R is the nutrient concentration in the regional soil pool with volume VR, Li is the nutrient concentration in the set of local resource-depletion zones, with total volume, Vi, of plants from species i, and Pi, Hi, and Ci are the biomasses of plant, herbivore, and carnivore species i, respectively. Nutrient is transported between local and regional pools at a rate k per unit time. Plant species i has a growth rate, ai; herbivore species j consumes plant species i at a rate bij, and carnivore species k consumes herbivore species j at a rate qjk. The death rates mi, di, and ui of plants, herbivores, and carnivores, respectively, and the nonrecycled proportions of nutrient lPi, lHi, and lCi from plant, herbivore, and carnivore detritus, respectively, are considered identical in our analysis for the sake of simplicity. The competitive ability of each plant species is measured by its L*, the concentration to which the nutrient in the depletion zone is reduced in monoculture at equilibrium.
When the nutrient transport rate k between local and regional pools is high, the soil nutrient pool becomes effectively homogeneous and our system reduces to the simpler, nonspatial model developed by Grover (23, 25). In this case, plants cannot coexist at equilibrium in the absence of herbivores. Our model allows coexistence of various species of plants, herbivores, and carnivores; in this respect, it differs from Holt and Loreau's model (24), which investigated effects of species sorting on ecosystem processes at the producer and herbivore trophic levels. Here we analyze the impact of food-web structure on the relationship between diversity and ecosystem functioning and consider different scenarios of species extinctions, which has not been done so far for multitrophic systems (23, 24).
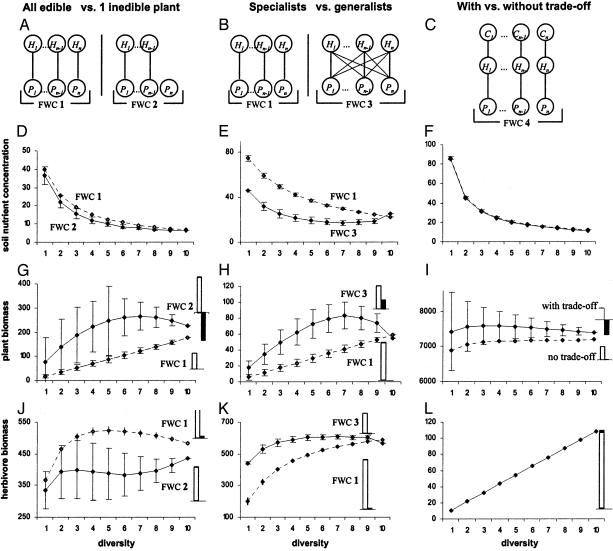
We examine how changes in species richness influence ecosystem properties at equilibrium in systems with different foodweb structures (Fig. 1 A–C), when all plant, herbivore, and carnivore species can coexist (see Appendix for an example with specialist herbivores). This coexistence is possible only if plant species have different principal herbivores. Thus, without loss of generality, we rank herbivores and carnivores such that carnivore species i preferentially consumes herbivore species i, which itself preferentially consumes plant species i. Coexistence further imposes restrictions on parameter value. In particular, when herbivores are generalists, it is possible only if more competitive plants are more consumed by herbivores. For the sake of simplicity in our analysis, unless otherwise stated, we consider the existence of a trade-off between plant competitive ability as determined by plant growth rate ai and plant resistance to herbivory as determined by herbivore consumption rate ci. Through the choice of the matrix values B = (bij) and Q = (qjk), the model can be made to represent the different food-web configurations in Fig. 1.
Fig. 1.
Expected soil-nutrient concentration, total plant biomass, and total herbivore biomass as functions of plant species richness for different food-web configurations (mean ± 1 SD) when the soil-nutrient pool is quasihomogeneous (i.e., the nutrient transport rate k is high). Herbivore species richness varies parallel to plant species richness to keep the same food-web configuration along the diversity gradient. Top grafts show the food-web configurations (FWC) analyzed in the corresponding column. Pi, Hi, and Ci denote plant, herbivore, and carnivore species i, respectively. (Left) Comparison between FWC 1 with no inedible plant (dotted line) and FWC 2 with one inedible plant (solid line). (Center) Comparison between FWC 1 with specialist herbivores (dotted line) and FWC 3 with generalist herbivores (solid line). (Right) Comparison between an ecosystem with a trade-off between plant competitive ability and plant resistance to herbivory (solid line) and an ecosystem without this trade-off (dotted line) for FWC 4. Small histograms on the right show the strengths of the complementarity effect (open) and the selection effect (filled) for the highest-diversity treatment (10 plant species). These effects are measured on the same scale as the y axis, except for G where these effects are shown after a reduction by a factor 4. Parameter values are identical in all parts of the figure, except for k, I, bij, and qij: ai = 0.05i, lH = lN = 0.02, lR = 0.05, m = d = 0.3, and V = VR = 1. (Left) k = 100, I = 4.3, bi = 0.005i.(Center) k = 100, I = 22; for food-web configuration 3 (generalist herbivores), bii = 0.005i, bji = 0.005i + 0.001, if j > i and bji = 0.001 if j > i, in the specialist case, bi is identical with the total consumption rate of each herbivore in the generalist case. (Right) k = 50, I = 87; bi = 0.005i with trade-off, and bi = 0.0275 without trade-off.
Equilibrium values of the various compartments are obtained by solving the dynamical equations above after setting time derivatives to zero. In general, the equilibrium nutrient concentration in the regional soil pool, R*, is a case-specific function of the various species present in the food web and is not presented here. For plants that are consumed by herbivores without predators, the biomasses of plant species i, P*i, and herbivore species i, H*i, are:
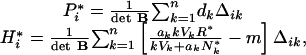 |
where Δik is the cofactor of element bjk in matrix B. When herbivores are specialists, P*i = di/bii, and, consequently, the biomass of each plant species is independent of the presence of other species. This is no longer true, however, when herbivores are generalists. Herbivore biomass depends on soil nutrient availability at equilibrium, R*, and on species composition.
For plants that are either inedible or consumed by specialist herbivores, which themselves are consumed by a carnivore:
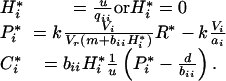 |
Plant biomass then depends on soil nutrient availability.
When herbivores are generalists and the numbers of plant and herbivore species are different, simple analytical expressions cannot be found and numerical analysis was performed.
Results
We analyze two feasible scenarios of biodiversity changes: either (i) plant richness and herbivore richness vary in parallel, or (ii) only herbivore richness changes (in which case plant coexistence with reduced herbivore diversity hinges on spatial heterogeneity of the soil-nutrient pool). Changing plant richness only leads to unfeasible food-web configurations in our model, because herbivore species cannot be more numerous than plant species at equilibrium. To analyze expected ecosystem responses to changes in species richness, we calculate, at each diversity level, the mean and standard deviation of soil-nutrient concentration and of plant, herbivore, and carnivore biomass across random compositional assemblages, as is often done in experiments (7, 8, 26, 27). We present figures with a highest diversity treatment of 10 plant species, but the results are the same whatever the number of species.
Top-Down vs. Bottom-Up Control. Model predictions show striking differences in the expected relationship between species diversity and ecosystem processes for different food-web structures when both plant and herbivore diversities vary (Fig. 1). One important factor that generates these differences is where top-down control occurs in the food web. Compare a food web in which each plant is consumed by a specialist herbivore and an otherwise identical web in which one plant is inedible (Fig. 1, left-hand profiles). In the first case, total plant biomass increases linearly with diversity, whereas total herbivore biomass can show either monotonic or unimodal relationships. In the second case, more complex patterns can occur, including a unimodal relationship for total plant biomass and an initial increase, followed by a decrease at an intermediate level of diversity, and again an increase at high diversity for total herbivore biomass (Fig. 1 G and J). These contrasting patterns are explained by a top-down control imposed by herbivores on edible plants and by a bottom-up dependence of herbivores and inedible plants on soil-nutrient concentration. The biomass of each edible plant is then unaffected by the addition of other plant species, whereas the biomass of herbivore and inedible plant species decreases as soil-nutrient concentration decreases with the addition of other plants (Fig. 1D). Therefore, top-down control of an entire trophic level, as it occurs when all plants are edible, causes mean total biomass to increase linearly with species richness (Fig. 1G), and generates a zero selection effect and a positive complementarity effect. Note that complementarity here does not arise from resource partitioning or facilitation, but from the top-down control by different species of the higher trophic level.
Bottom-up dependence of some species in a trophic level leads to various relationships between total biomass and species diversity, depending on several factors that affect soil-nutrient concentration, in particular, nutrient supply, transport rate, recycling rates, and control of the inedible plant on soil-nutrient concentration. Variation of total biomass as species diversity is increased then depends on the balance between the biomass lost by those species that were already present and the biomass gained by the added species. Dominance by the inedible plant leads to a decreasing mean total plant biomass at high levels of diversity, a high variability across assemblages of different compositions, and a negative selection effect, because the dominant plant is the most affected by an increase in diversity (Fig. 1G). At the herbivore trophic level, the biomass lost by resident herbivores is of the same order of magnitude as the biomass gained by an added species for the parameter values used in Fig. 1, which explains why even small differences in soil-nutrient concentration due to the presence of the inedible plant (Fig. 1D) can generate different relationships between total herbivore biomass and species diversity in the two scenarios considered.
Food-Web Connectivity. Another factor that has an important effect on the relationship between species diversity and ecosystem properties is the degree of herbivore generalization or specialization, a measure of food-web connectivity (Fig. 1, center profiles). When herbivores are generalists, mean total plant biomass does not increase linearly and can even decrease at high diversity (Fig. 1H). Mean total herbivore biomass is usually higher for generalist than for specialist herbivores, but it also decreases more easily at high levels of species diversity (Fig. 1K). Soil-nutrient concentration can even increase when plant richness is high (Fig. 1E). When herbivores are generalists, increased species diversity leads to an increased consumption of each plant species by a wider array of herbivore species, which can result in a decreased total plant biomass. Competition among generalist herbivores is also intense, which results in a smaller resource-use complementarity and a decreased total herbivore biomass at high diversity (Fig. 1K).
Trade-offs. Trade-offs involving trophic interactions in the food web also influence the relationship between species diversity and ecosystem processes. Consider, for instance, a trade-off between plant competitive ability (determined by plant growth rate, ai) and plant resistance to herbivory (determined by herbivore consumption rate, bi) in a food web with specialized herbivores and carnivores (Fig. 1, right profiles). Trophic cascades result in herbivores being controlled by carnivores and plants depending on soil-nutrient concentration, which decreases as more plant species are added (Fig. 1F). Mean total plant biomass can then decrease at high diversity in the presence of the trade-off, whereas it always increases in the absence of the trade-off (Fig. 1I). When the trade-off is present, the biomass of the least competitive plants can be higher in monoculture than that of the most competitive plants, because they are less consumed. Their biomass, however, decreases more with diversity because they suffer more from interspecific resource competition, which leads to a negative selection effect and a high variability of total plant biomass (Fig. 1I). This negative selection effect can cause a decrease in mean total plant biomass at high diversity (Fig. 1I)as more competitive plants strongly reduce the biomass of less competitive ones without themselves gaining much biomass because of high herbivore consumption. When the trade-off is absent, this phenomenon does not occur, and mean total plant biomass still increases at high levels of plant richness. Regardless of the trade-off, mean total herbivore biomass increases linearly with diversity (Fig. 1L), just as total plant biomass did with specialist herbivores when carnivores were absent (Fig. 1 G and H). The presence of carnivores shifts top-down control one trophic level higher and now generates complementarity between herbivores.
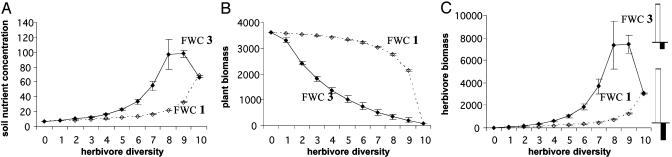
Trophic Position of Species Loss. Changes in diversity at the herbivore trophic level alone have very different effects on ecosystem processes than do simultaneous changes at both the plant and herbivore trophic levels (Fig. 2). In this scenario, the environment is assumed to be sufficiently heterogeneous (i.e., the nutrient transport rate k is small) to allow plant coexistence in the absence of herbivores. Mean total plant biomass always decreases on herbivore addition, but it decreases faster at low diversity when herbivores are generalists because consumption on each plant species increases as herbivores are added (Fig. 2B). Mean total herbivore biomass always increases with diversity when herbivores are specialists, whereas it decreases at high levels of diversity when they are generalists (Fig. 2C). As in the analysis above, a smaller resource-use complementarity between generalist herbivores explains these differences.
Fig. 2.
Expected soil-nutrient concentration, total plant biomass, and total herbivore biomass as functions of herbivore species richness, when plant species richness is held constant (mean ± 1 SD). Comparison between FWC 1 with specialist herbivores (dotted line) and FWC 2 with generalist herbivores (solid line) is shown. Small histograms on the right in c show the strengths of the complementarity effect (in white) and the selection effect (in black), measured on the same scale as the y axis, for the highest diversity treatment (10 herbivore species). Parameter values: ai = 0.05i, lH = lN = 0.02, lR = 0.05, m = d = 0.3, V = VR = 1, k = 20, I = 22; bii = 0.005i and bji = 0.0005i for generalist herbivores, and for specialist herbivores bi is equal to the total consumption rate of each herbivore in generalist case.
Discussion
This work emphasizes several key effects of trophic interactions on the relationship between biodiversity and ecosystem functioning. First, the nature of population controls (top-down vs. bottom-up) in an ecosystem can profoundly affect ecosystem responses to changes in species richness. Thus, heterogeneity within trophic levels and the presence of inedible species are important to consider as they modify top-down control and trophic cascades in food webs (28–30). The impact of trophic interactions may be greater in aquatic systems, because top-down effects of predators are generally stronger in aquatic systems than in terrestrial systems (31). However, trophic cascades lead also to variations in plant species evenness (32), which affects plant biomass and productivity. Second, food-web connectivity, which is determined by the degree of generalization or specialization of consumers, also has a significant influence on the relationship between biodiversity and ecosystem functioning through changes in resource-use complementarity (33) and species abundances due to direct and indirect effects (34). A more complex food web does not lead in this model to synergistic effects of species richness on productivity, as suggested recently (18). Food-web connectivity may enhance the impact of species loss as the effect propagates more through the web, but it can also dampen variations of some species because species are more generalist. Furthermore, compensation by remaining generalist species may also attenuate the effect of species loss (35). Third, trade-offs can play a role as well. Trade-offs between plant competitive ability and resistance to herbivory are known to affect ecosystem responses to nutrient enrichment (36, 37); our work shows that they also affect the relationship between biodiversity and ecosystem functioning. Lastly, the trophic position of species gained or lost in the food web has an important impact because it alters the conditions of species coexistence. Predators, for instance, may enhance prey species diversity through predator-mediated coexistence (38).
Our model supports recent experimental results, showing that increasing consumer diversity may decrease producer biomass and increase secondary production (21, 39). This effect of consumer species richness may be compared with similar effects of plant diversity on soil-resource depletion (40). However, our work also shows that presence of herbivores does not necessarily remove the positive relationship between plant diversity and plant biomass as found in one study (20). Multiple trophic levels in ecosystems are expected to lead to complex relationships between species richness and ecosystem processes, in contrast to the monotonic changes predicted for simplified systems with a single trophic level (7–10). Also, in some of the cases studied, the variability of total biomass around the mean is high, which may give patterns that differ in shape from the expected one in some random assemblages. We have concentrated here on changes in total trophic-level biomass to allow comparison with earlier studies (1–3, 7–10); primary and secondary production show different patterns, but these patterns are often nonmonotonic as well.
This analysis has considered two scenarios of random deletions of plant and herbivore species. However, extinctions in nature are not random (4), may occur across multiple trophic levels (19), and may lead to secondary extinctions (41, 42) or shifts in biodiversity at other trophic levels (43). Various factors can influence coexistence constraints and extinction scenarios; for example, connectance may increase food-web robustness to species extinction (44). Furthermore, changes in diversity at one trophic level can propagate to both higher and lower trophic levels, thus affecting the diversity of all trophic levels in the food web (43). Other constraints in the model can lead to more complex scenarios of extinctions after species removal, which may give different relationships between diversity and biomass. For example, when coexistence is mediated by herbivores as in Grover's model (25), removal of one herbivore leads to extinctions of less competitive plants and their herbivores. In this case, plant biomass can increase with herbivore diversity. Despite these limitations of our work, food-web structure, in particular food-web heterogeneity, connectivity and trade-offs, is very likely to remain essential to understanding the ecological consequences of biodiversity loss in other scenarios. Our model makes several predictions that deserve to be tested experimentally for a better knowledge of the impact of biodiversity on ecosystem functioning.
Acknowledgments
We thank A. Hector, M. Leibold, N. Loeuille, B. Descamps-Julien, and two anonymous reviewers for comments on the manuscript.
Appendix
Here we present an example of model analysis to illustrate the method we used to study the relationship between species diversity and ecosystem properties. The food-web configuration chosen in this example has an identical number of plants and specialist herbivores n, and we consider simultaneous variations in plant and herbivore diversity (Fig. 1 A). The results corresponding to this analysis are shown in Fig. 1 D, G, and J.
To derive the mean soil-nutrient concentration and the mean total biomass of plants and herbivores across random compositional assemblages at equilibrium, we first need to calculate soil-nutrient concentration and total plant and herbivore biomasses in any particular assemblage. In an assemblage of x plant and herbivore species, soil-nutrient concentration, plant biomass and herbivore biomass are found to be at equilibrium:
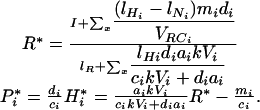 |
All these species can coexist if
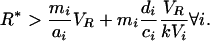 |
If plant and herbivore communities are assembled at random from a pool of n species, each plant or herbivore species has a probability x/n to be in an assemblage of x species. Further, species i has a probability x(x – 1)/[n(n – 1)] to be in the same assemblage as species j. The mean biomass of each plant and herbivore species across all possible assemblages can then be calculated as a function of plant species richness x:
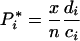 |
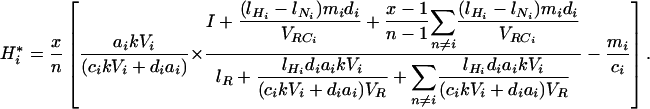 |
It is then possible to obtain the mean soil-nutrient concentration and the mean total plant and herbivore biomasses across random assemblages with plant species richness x. For the sake of simplicity and clarity in the following equations, we assume that plants and herbivores have identical parameters except for plant growth rate, ai, and herbivore consumption rate, ci, and that the ratio ai/ci is constant, i.e., a trade-off exists between plant competitive ability and plant resistance to herbivory.
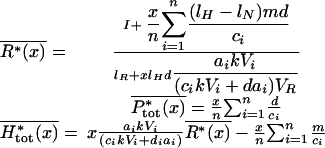 |
The second equation shows that mean total plant biomass increases linearly with plant species richness x. To analyze the variations of mean soil-nutrient concentration and mean total herbivore biomass with diversity, we need to calculate the difference between assemblages with diversities x and x + 1:
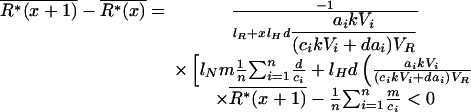 |
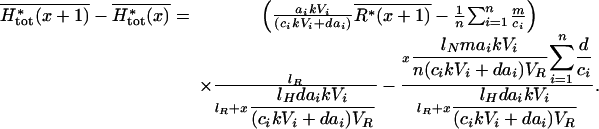 |
Thus, mean soil-nutrient concentration always decreases with diversity, but mean total herbivore biomass can show more complex patterns because it can decrease at high levels of diversity.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., Hector, A., Hooper, D. U., Huston, M. A., Rafaelli, D., Schmid, B., et al. (2001) Science 294, 804–808. [DOI] [PubMed] [Google Scholar]
- 2.Kinzig, A. P., Pacala, S. & Tilman, D. (2002) The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions (Princeton Univ. Press, Princeton).
- 3.Loreau, M., Naeem, S. & Inchausti, P. (2002) Biodiversity and Ecosystem Functioning: Synthesis and Perspectives (Oxford Univ. Press, London).
- 4.Pimm, S. L., Russell, G. J., Gittleman, J. L. & Brooks, T. M. (1995) Science 269, 347–350. [DOI] [PubMed] [Google Scholar]
- 5.Vitousek, P. M., Mooney, H. A., Lubchenco, J. & Melillo, J. M. (1997) Science 277, 494–499. [Google Scholar]
- 6.Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Hanwald, E., Huenneke, L. F., Jackson, R. B., Kinzig, A., et al. (2000) Science 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- 7.Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., et al. (1999) Science 286, 1123–1127. [DOI] [PubMed] [Google Scholar]
- 8.Tilman, D., Reich, P. B., Knops, J., Wedin, D., Mielke, T. & Lehman, C. (2001) Science 294, 843–845. [DOI] [PubMed] [Google Scholar]
- 9.Tilman, D., Lehman, C. L. & Thomson, K. (1997) Proc. Natl. Acad. Sci. USA 94, 1857–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loreau, M. (1998) Proc. Natl. Acad. Sci. USA 95, 5632–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loreau, M. & Hector, A. (2001) Nature 412, 72–76. [DOI] [PubMed] [Google Scholar]
- 12.Huston, M. (1997) Oecologia 110, 449–460. [DOI] [PubMed] [Google Scholar]
- 13.Oksanen, L. & Oksanen, T. (2000) Am. Nat. 155, 703–723. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., et al. (2001) Science 293, 629–638. [DOI] [PubMed] [Google Scholar]
- 15.Duffy, J. E. (2003) Ecol. Lett. 6, 680–687. [Google Scholar]
- 16.Naeem, S., Hahn, D. R. & Schurrman, G. (2000) Nature 403, 762–764. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson, M. & Malmqvist, B. (2000) Oikos 89, 519–523. [Google Scholar]
- 18.Downing, A. L. & Leibold, M. A. (2002) Nature 416, 837–840. [DOI] [PubMed] [Google Scholar]
- 19.Petchey, O. L., McPhearson, P. T., Casey, T. M. & Morin, P. J. (1999) Nature 402, 69–72. [Google Scholar]
- 20.Mulder, C. P. H., Koricheva, J., Huss-Danell, K., Hogberg, P. & Joshi, J. (1999) Ecol. Lett. 2, 237–246. [Google Scholar]
- 21.Duffy, J. E., Richardson. J. P. & Canuel, E. A. (2003) Ecol. Lett. 6, 637–645. [Google Scholar]
- 22.Montoya, J. M., Rodriguez, M. A. & Hawkins, B. A. (2003) Ecol. Lett. 6, 587–593. [Google Scholar]
- 23.Loreau, M. (1996) Math. Biosci. 134, 153–188. [DOI] [PubMed] [Google Scholar]
- 24.Holt, R. D. & Loreau, M. (2002) in The Functional Consequences of Biodiversity: Empirical Progress and Theoretical Extensions, eds. Loreau, M., Naeem, S. & Inchausti, P. (Princeton Univ. Press, Princeton), pp. 246–262.
- 25.Grover, J. P. (1997) Resource Competition (Chapman & Hall, London).
- 26.Hooper, D. U. & Vitousek, P. M. (1997) Science 277, 1302–1305. [Google Scholar]
- 27.Knops, J. M. H., Tilman, D., Haddad, N. M., Naeem, S., Mitchell, C. E., Haarstad, J., Ritchie, M. E., Howe, K. M., Reich, P. B., Siemann, E. & Groth, J. (1999) Ecol. Lett. 2, 286–293. [DOI] [PubMed] [Google Scholar]
- 28.Leibold, M. A. (1989) Am. Nat. 134, 922–949. [Google Scholar]
- 29.Abrams, P. A. (1993) Am. Nat. 141, 351–371. [Google Scholar]
- 30.Steiner, C. F. (2001) Ecology 82, 2495–2506. [Google Scholar]
- 31.Shurin, J. B., Borer, E. T., Seabloom, E. W., Anderson, K., Blanchette, C. A., Broitman, B., Cooper, S. D. & Halpern, B. S. (2002) Ecol. Lett. 5, 785–791. [Google Scholar]
- 32.Schmitz, O. (2003) Ecol. Lett. 6, 156–163. [Google Scholar]
- 33.Norberg, J. (2000) Oecologia 122, 264–272. [DOI] [PubMed] [Google Scholar]
- 34.Wootton, J. T. (1994) Annu. Rev. Ecol. Syst. 25, 443–466. [Google Scholar]
- 35.Duffy, J. E. (2002) Oikos 99, 201–219. [Google Scholar]
- 36.Leibold, M. A., Chase, J. M., Shurin, J. B. & Downing, A. L. (1997) Annu. Rev. Ecol. Syst. 28, 467–494. [Google Scholar]
- 37.Chase, J. M., Leibold, M. A., Downing, A. L. & Shurin, J. B. (2000) Ecology 81, 2485–2497. [Google Scholar]
- 38.Paine, R. T. (1966) Am. Nat. 100, 65–75. [Google Scholar]
- 39.Naeem, S. & Li, S. (1997) Nature 390, 507–509. [Google Scholar]
- 40.Symstad, A. J. & Tilman, J. (2001) Oikos 92, 424–435. [Google Scholar]
- 41.Mittelbach, G. G., Turner, A. M., Hall, D. J. & Rettig, J. E. (1995) Ecology 76, 2347–2360. [Google Scholar]
- 42.Power, M. E., Tilman, D., Estes, J. A., Menge, B. A., Bond, W. J., Mills, L. S., Daily, G., Castilla, J., Lubchenco, J. & Paine, R. T. (1996) Bioscience 46, 609–620. [Google Scholar]
- 43.Dyer, L. A. & Letourneau, D. (2003) Ecol. Lett. 6, 60–68. [Google Scholar]
- 44.Dunne, J. A., Williams, R. J. & Martinez, N. D. (2002) Ecol. Lett. 5, 558–567. [Google Scholar]