Abstract
Age-related tongue weakness may contribute to swallowing deficits in the elderly. One contributing factor may be an alteration in muscle fiber type properties with aging. However, it is not clear how muscle fiber types within the aged tongue may vary from those found in young adults, or how fiber types may vary across the anteroposterior axis of the extrinsic tongue muscles. We examined myosin heavy chain (MHC) composition of anterior, medial, and posterior sections of the genioglossus muscle (GG) in 10 old male Fischer 344/Brown Norway rats and compared findings to previously reported data from young adult male rats. Significant differences (p< .01) between young adult and old rats were found in the distribution of MHC isoforms along the anteroposterior axis of the muscle. In the anterior, medial, and posterior regions, there was a significantly smaller proportion of type IIb MHC in the old rat GG muscles, while the proportion of type IIx MHC was significantly greater. In the medial region, the proportion of type I MHC was found to be significantly greater in the old rats. Thus, we found a shift to more slowly contracting muscle fibers in the aged rat tongue.
Keywords: Tongue, Dysphagia, Myosin heavy chain, Aging, Deglutition, Deglutition Disorders
Introduction
The tongue plays a vital role in swallowing by serving as a major contributor to three of the four phases of the swallow [1]. Due to the complex nature of deglutition, it is possible that even seemingly small changes to the tongue musculature may result in a disruption of swallowing function. Therefore, it is important to understand the manner in which characteristics of the tongue musculature may change as a function of aging or disease as a precursor to the study of potential interventions.
Loss of muscle mass and strength and increased fatigability are phenomena associated with aging in skeletal muscle systems and have been linked to functional changes such as reduced maximal and specific force in the limb musculature [2–5]. Similar changes may occur in the cranial musculature involved in swallowing, but have not been sufficiently studied. Cranial muscle changes with aging are suspected because several studies have reported age-related changes in swallow actions [6–15]. In particular, these studies have found that older individuals swallow more slowly, with longer durations in the oral and pharyngeal stages of the swallow, during which the tongue plays a major role. As such, temporal parameters of muscle contraction in the tongue may be altered as a function of aging, and these alterations may be a factor in the longer swallowing durations observed in elderly people.
One possible etiology for age-related tongue weakness may be a shift from rapidly-contracting muscle fibers to more slowly contracting muscle fiber types. A number of studies have shown that there is a change in MHC isoform distribution and muscle fiber type in skeletal muscles with age [16–19]. Specifically, an increase in more slowly-contracting muscles fibers and slow MHC isoforms has been reported, along with a decrease in more rapidly-contracting fibers and fast MHC isoforms [18, 19]. These studies have focused on MHC isoform distribution within the muscle because MHC type is closely associated with muscle contraction speed [20, 21, 22]. However, specific changes to MHC isoform distribution and other structural and physiological properties have not been as widely studied in cranial muscles [23]. Further study of MHC isoform distribution in the tongue musculature, and changes due to aging, is needed because cranial muscles may not respond to aging in the same manner as other skeletal muscles [24, 25, 23].
It was shown in the young adult rat that MHC isoform distribution varied across the anteroposterior axis of the genioglossus muscle (GG), the main muscle of tongue protrusion. Specifically, more rapidly contracting muscle fibers (associated with type IIb MHC isoforms) were found in the posterior portion of the GG [26]. It is not known if MHC isoform distribution in the old rat tongue varies along the anteroposterior axis in a manner similar to that found previously in young adult animals [26]. If differences are found in anterior, medial, and posterior GG muscle fibers in old versus young adult rats, then inferences may be made and hypotheses developed regarding biological properties underlying the complex motor control patterns observed during deglutition in the elderly.
To determine the manner in which aging affects the distribution of MHC characteristics along the anteroposterior axis of the GG muscle, myosin heavy chain composition was examined in aged rats. Our hypothesis was that statistically significant differences would be found in MHC isoform distribution along the anteroposterior axis of the GG muscle in the aged rat. It was further hypothesized that these differences would not be reflective of a greater proportion of more rapidly contracting muscle fibers in the posterior tongue, as had been found in young adult rat GG muscles [26]. Therefore, the purpose of this study was to investigate MHC isoform distribution along the anteroposterior axis of the GG in the aged rat tongue to gain insight into changes in muscle biochemistry that might underlie muscle weakness and fatigue in the aged tongue.
Materials and Methods
Protocols used in a prior study by Volz et al. (2007) were followed to ensure that data from the present study from aged rats could be directly compared with data from the young adult rats collected and reported by Volz et al.
Experimental Animals and Surgical Procedures
Ten aged male (32 months old) Fischer 344/Brown Norway rats, genetically identical to the rats used by Volz et al. (2007), were anesthetized with an intraperitoneal injection of ketamine hydrochloride (70 mg/kg) and xylazine hydrochloride (7 mg/kg) and then euthanized with a 0.2-mL intracardiac injection of phenytoin sodium. Under a Zeiss surgical microscope, the GG muscles were identified, harvested, and dissected. Once the GG muscle was harvested, it was cut into three equal sections; the anterior, medial, and posterior sections (Figure 1). A total of 60 sections were collected, 30 sections from the right side of the animals and 30 sections from the left. The samples were snap-frozen in liquid methylbutane chilled in liquid nitrogen immediately following the dissection. The muscles sections were stored at −80 degrees Celsius until they were ready to be prepared.
Figure 1.
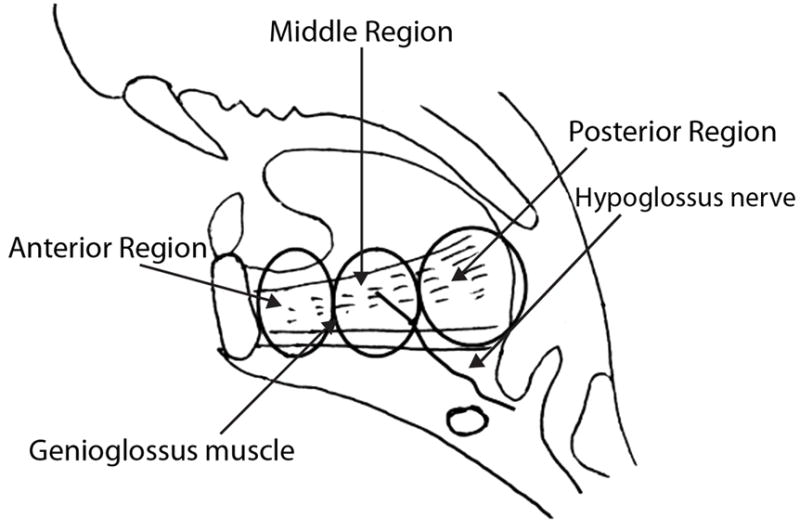
Sagittal schematic of the genioglossus (GG) muscle in the adult rat. The muscle is sectioned to illustrate anterior, medial, and posterior GG regions (courtesy of Dr. Hiromi Nagai).
Muscle Section Preparation
The sections of the anterior, medial, and posterior GG muscle blocks were cut into 60 μm serial sections using a cryostat. Muscle sections were mounted on microscope slides. Freehand dissection under a stereomicroscope was performed with 27-gauge stainless-steel needles to remove excess adipose, connective and nerve tissue. Once the extra material was removed, the small bundle of muscle fibers was isolated and broken down into smaller pieces with the needle. Each specimen was placed in 20 mL of sample buffer (15% glycerol, 1% sodium dodecyl sulfate, 62.5 mmol/L Tris, 15 mmol/L dithiothreitol, 0.75 mmol/L leupeptin, 0.0625% bromophenol blue), centrifuged at 14,000 rpm for 1 min, homogenized in an ultrasonic bath for 25 minutes, centrifuged again for 1 min, diluted five times in sample buffer and stored at −80 degrees Celsius until SDS-PAGE gels were run.
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis
Muscle samples were centrifuged and then diluted 10, 20, and 40 times in sample buffer to achieve a working range of protein concentrations. In addition, soleus muscle, a predominately slow twitch muscle in the rat, was diluted in sample buffer and loaded in the gel to serve as a control for the type I MHC isoform. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 0.75-mm-thick 6% acrylamide/30% glycerol separating gel (18 cm × 16 cm) and a 4% acrylamide/30% glycerol stacking gel, using the Hoefer SE 600 system (Amersham Biosciences, Piscataway, NJ). The running conditions were set at 275 V, 40 mA, and 15 W for 24 hours at 10 degrees Celsius. The gels were stained using SilverQuest Silver Staining Kit (Invitrogen, Carlsbad, CA) and developed to visualize protein bands. As previously demonstrated, individual MHC isoforms were identified according to increasing electrophoretic mobility as follows: IIa < IIx < IIb < I [27].
Western Blotting
To further identify the location of the individual MHC isoforms within the SDS-PAGE gel, an immunoblotting procedure was performed. Once the electrophoresis was finished, the proteins were transblotted onto a Polyvinylidine Fluoride (PVDF) membrane at 100mA overnight at 4° C, according to a previously established method [28]. Following the transfer, the blot was incubated in blocking buffer consisting of TBS and 5% nonfat milk for 2 hours at room temperature. Then the blot was incubated with each of the primary antibodies separately overnight at 4° C. Four monoclonal antibodies specific to each MHC isoform were used to identify each protein band in the SDS-PAGE gel. SC-71 was used to identify the type IIa isoform, 6H1 was used to identify the type IIx isoform, BF-F3 was used to identify the type IIb isoform, and BA-D5 was used to identify the type I isoform [29, 30]. All antibodies used (supernatant form) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology. The secondary antibodies used were peroxidase labeled anti-mouse IgG for SC-71 and BA-D5 (Bio-Rad) and peroxidase labeled anti-mouse IgM for 6H1 and BF-F3 (Invitrogen). Enhanced chemoluminescence (ECL) was used to detect antibody binding (Thermo-Scientific). Following detection of each individual MHC isoform with each monospecific monoclonal antibody, membranes were stripped in a solution containing 7% beta-mercaptoethanol, 2.5% SDS and 63 mmol/L Tris (pH 6.7) for 1 hour at 65° C [28]. After stripping, the membrane was incubated in the next monoclonal antibody followed by the secondary antibody, until all four antibodies were detected in a single membrane. Due to the small percentage of the type I isoform in the GG muscle (<2%), the soleus muscle, a primarily slow twitch muscle in the rat, was used to further verify the location of the type I isoform within the gel. All other isoforms were located in the GG muscle alone (Figure 2). The results of these immunoblotting experiments confirm that individual MHC isoforms can be identified within a SDS-PAGE gel according to increasing electrophoretic mobility as follows: IIa < IIx < IIb < I, as has been shown in previous research [27].
Figure 2.
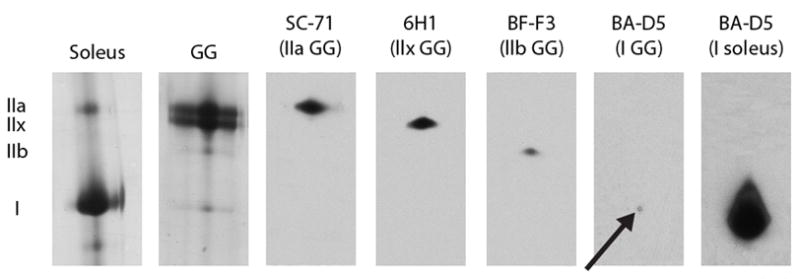
Identification of myosin heavy chain (MHC) isoforms with Western blotting technique in the genioglossus (GG) and soleus muscle of the rat. The soleus, a muscle composed of predominantly type I MHC, is provided as a control to demonstrate accuracy of identification of type I MHC. In the first lane from the left in the panel, MHC isoforms (IIa and I) from a protein stained gel from the soleus muscle. In the second lane in the panel, MHC isoforms (IIa, IIx, IIb, I) from a protein stained gel from the GG muscle. In the next four lanes, Western blots of the four MHC isoforms in the GG muscle stained with monoclonal antibodies SC-71 for type IIa, 6H1 for type IIx, BF-F3 for type IIb, and BA-D5 for type I. Arrow indicates the small percentage of type I found in the GG muscle. In the final column of the panel, Western blot of MHC isoform type I in the soleus muscle stained with BA-D5.
Data Analysis
Densitometry was performed on individual silver-stained gels using a flatbed scanner and computer-assisted image analysis (UN-SCAN-IT gel) to determine the percentages of MHC isoforms in each channel of the gel. Individual variability in gel development was accounted for by subtracting the average number of pixels of the gel background from the average number of pixels of each band, in order to control for varying background densities. The image analysis program used by Volz et al. (2007) was Scion Image 1.62, which was used to perform densitometry analyses and also measured the density of protein bands on the gel.
To assess the reliability of the data analysis method and to control for individual variation in the selection and definition of each of the MHC bands in a given gel channel, a second analysis was performed on 50% of the gels. The second analysis was conducted by the second author who was familiar with the UN-SCAN-IT gel program and was trained in this specific data analysis technique. The second set of measurements was made without reference to or knowledge of the initial set of measurements. Reliability was measured using an interclass correlation coefficient test.
The proportions of MHC isoforms within the anterior, medial, and posterior GG were compared using a Wilcoxon matched-pairs signed-ranks test. Results from old animals in this study were compared with previously reported results in the young adult rat from Volz et al. (2007), using a Wilcoxon rank sum test. A critical α-level of 0.01 was used to determine statistical significance.
Results
Reliability
Intraclass correlation coefficients for repeated densitometry measurements were 0.96 for type IIa, 0.89 for type IIb, 0.88 for type IIx, and 0.79 for type I. Accordingly, reliability of measurement was good to excellent.
Myosin Heavy Chain Characteristics in Old GG Muscles
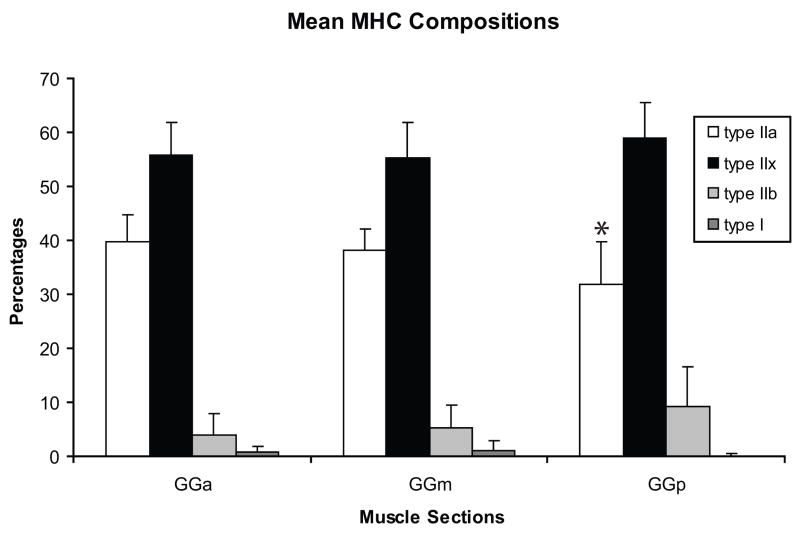
As shown in Figures 3 and 4, type IIx MHC was predominant in the anterior, medial, and posterior regions of the old GG muscle followed by IIa, then IIb, then type I. Only one significant difference in MHC composition was found along the anteroposterior axis of the aged rat GG muscle. Specifically, the anterior GG contained a significantly greater proportion of type IIa MHC (p = 0.004) than the posterior region, but did not differ significantly from the medial region (p=.43). There were no statistically significant differences across regions in the proportion of type IIx, type IIb, or type I MHC isoforms.
Figure 3.
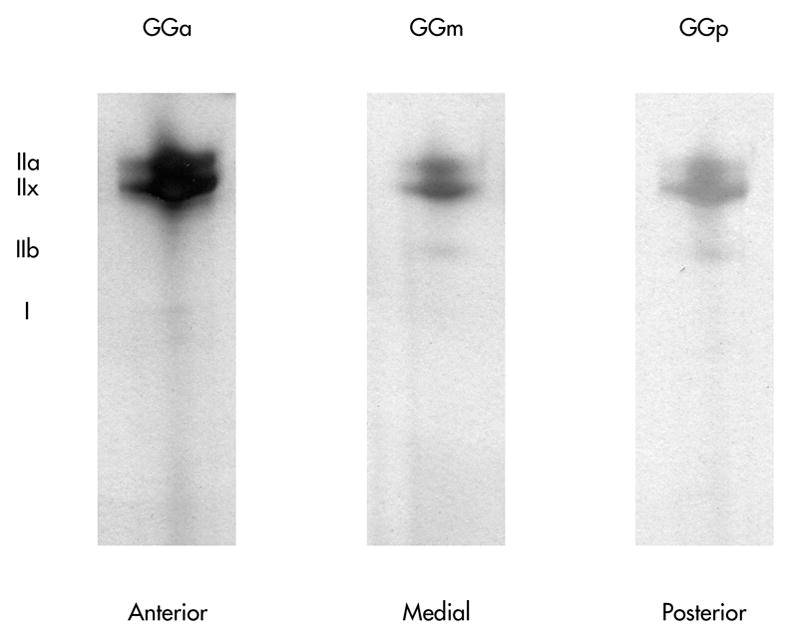
Representative silver-stained SDS-PAGE gels from one animal. Varying myosin heavy chain isoform distribution in genioglossis (GG) muscle regions is shown. The anterior GG contained a significantly greater proportion of type IIa than the posterior (p<.01), but not the medial. No other significant differences were found.
Figure 4.
The percent of type IIa, type IIx, type IIb and type I myosin heavy chain (MHC) isoforms in the genioglossus (GG) muscles from old rats. Type IIx MHC predominated in the anterior, medial, and posterior regions of the GG muscle, followed by IIa, IIb, and type I. The proportions of MHC isoforms within the anterior, medial, and posterior GG were compared using a Wilcoxon matched-pairs signed-ranks test. A significantly greater proportion of type IIa MHC was found in the anterior GG versus the posterior GG (p <.01). Error bars represent standard deviation. GGa= anterior, GGm= medial, GGp=posterior.
Comparison of Old and Young GG Myosin Heavy Chain Composition
Significant differences were found in the distribution of MHC isoforms along the anteroposterior axis of the GG muscles obtained from old and young adult rats. In the anterior, medial, and posterior regions, there was a significantly smaller proportion of type IIb MHC in the old rat GG muscles (p = 0.0002; p = 0.0002; p = 0.0003, respectively), while the proportion of type IIx MHC was significantly greater (p = 0.0004; p = 0.0013; p = 0.0008, respectively). In the medial region alone, the proportion of type I MHC was found to be significantly greater in the old rats (p = 0.006). No significant differences were found in type IIa (Figure 5).
Figure 5.
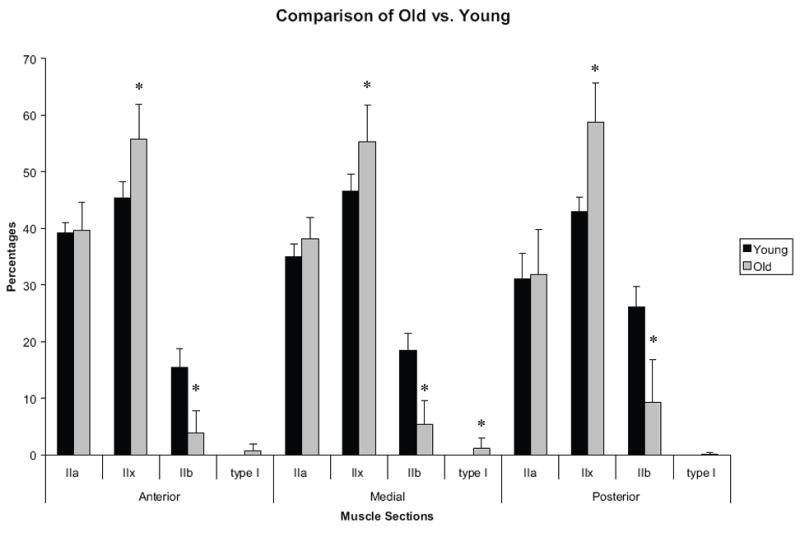
Percentage of myosin heavy chain (MHC) in young adult rats (from Volz et al., 2007) and old rats from the present study. Type IIx was found to be significantly greater in old rats, while type IIb was found to be significantly greater in young rats, across all regions (p<.01). Error bars represent standard deviations.
Discussion
The hypothesis of this study was that statistically significant differences would be found in MHC isoform distribution along the anteroposterior axis of the GG muscle in the aged rat, but that these differences would not reflect a greater proportion of more rapidly contracting muscle fibers in the posterior tongue, as has been found in young adult rat GG muscles [26]. The results of this study allow us to accept this hypothesis.
In old rats, we found that the proportion of type IIa MHC was significantly greater in the anterior portion of the GG versus the posterior portion, thus demonstrating a change in the biochemical properties of the muscle when different regions were compared. The type IIa MHC is associated with the most slowly contracting of the Type II myosins, therefore, these findings are associated with a greater proportion of more slowly contracting, fatigue-resistant muscle fiber types in the anterior tongue.
It is notable that there was not a significant difference in the proportion of type IIb MHC across regions of the GG muscle in old rats. The type IIb MHC isoform is associated with the most rapidly contracting muscle fibers. Thus, as hypothesized, aged rat GG muscles did not demonstrate a greater proportion of more rapidly contracting muscle fibers in the posterior tongue, as evidenced in prior research of the GG muscle in young adult rats [26].
In the aged rats in this investigation, a shift toward more slowly contracting muscle fiber types, across all regions of the GG, was observed when compared with data from young adult rats studied previously [26]. That is, there was a significant increase in the mean proportion of type IIx MHC, and a decrease in the type IIb MHC in the old rats compared with the young adult rats. Volz and colleagues [26] suggested that regional differences in the distribution of MHC isoforms may be related to functional differences. It was suggested that the more slowly contracting, but oxidative and fatigue-resistant MHC isoforms found in the anterior portion of the GG were potentially beneficial for the sustained muscle contractions needed to carry out early accommodation of the bolus, while the more rapidly contracting MHC isoforms found in the posterior tongue were necessary for rapid propulsion of the bolus in the later phases of the swallow. These hypotheses were supported by the fact that the posterior portion, and not the anterior portion of the GG, has been found to be active during the later phases of the swallow [31]. If a shift toward more slowly contracting isoforms in the posterior portion of the GG muscle is also found in humans, and in other muscles involved in the oropharyngeal swallow, some of the physiological changes observed in the aged swallow, such as an increased pharyngeal transit time, may be partially explained.
In the current study, we reported the presence of the type I MHC isoform within the anterior, medial, and posterior sections of the GG muscle. This finding was unexpected because previous work in the rat lingual muscles, in particular the genioglossus, found almost entirely fast-twitch or Type II muscle fibers [32]. However, this prior study was conducted with young rats. Based on the findings of the current study, it appears that the type I isoform is present in small quantities in the aged rat tongue. More investigation is required to better understand the physiological implications of the type I isoform in the aged rat tongue muscles in the current study and to determine how this finding relates to aging lingual muscles in humans.
Differential representation of muscle phenotypes along the anteroposterior axis of the tongue may reflect adaptation to different motor control demands. That is, the anterior versus posterior tongue may have different roles in goal-directed movements, such as bolus manipulation and propulsion during the swallow. For example, in an electromyographic study in humans, the posterior portion of the GG was active in tongue-to-palate pressure generation, while the anterior portion of the GG was not [31]. The difference in activation in separate regions of the GG muscle may be explained by the complex neuromuscular organization of the GG muscle. The GG muscle may contain at least two separate neuromuscular compartments, horizontal and oblique, each with different muscle fiber insertion points and innervation patterns. In the horizontal compartment the nerve fibers extend throughout the whole length of the muscle, while in the oblique compartment the nerve fibers form a grid-like pattern [33]. The distinct and separate neuromuscular compartments in the GG muscle may account for the differences in MHC isoform distribution found in different regions of the GG muscle because innervation patterns may be associated with the maintenance and establishment of specific muscle fiber types [34]. As such, small changes to innervation patterns along the anteroposterior axis of the GG muscle may contribute to the varied distribution of MHC isoforms within the GG muscle.
Clinical Implications
In young adult rats, it was hypothesized that the varied distribution of MHC isoforms along the anteroposterior axis of the GG may assist with performance of the complex tongue movements necessary for deglutition [26]. In particular, the predominance of more rapidly-contracting muscle fibers (associated with type IIb MHC isoforms) in the posterior portion of the GG may be necessary for rapid propulsion of the bolus, whereas more fatigue resistant oxidative fibers (associated with type IIa MHC isoforms) may be necessary in the anterior portion of the GG to perform repetitive movements during bolus manipulation in the initial preparatory portion of the oral swallow [26]. The shift to more slowly contracting, but oxidative and therefore fatigue-resistant, MHC isoforms seen in the posterior portion of the GG in aged rats may have interesting physiological implications in regards to the aging swallow. It has been shown that older individuals swallow more slowly thereby increasing the length of their mealtimes, and thus the act of eating a meal may be considered an endurance event [35, 36]. Therefore, if a shift to more fatigue-resistant fibers in the posterior tongue is present in the muscles of elderly individuals, there may be increased fatigue resistance during mealtimes. However, the loss of rapidly contracting fibers in the posterior tongue may reduce an elderly individual’s ability to rapidly propel the bolus into the pharynx. As such, the occurrence of the types of biochemical changes found in our study with aging in GG muscle may suggest that speed is sacrificed in the interest of preserving endurance. While a reduction in speed may not have a significant effect on the swallow of healthy elderly individuals, it may provide functional complications for individuals with neurologic or respiratory pathology, because they may not have the functional reserve and normal coordination necessary to compensate for the additional deficits associated with secondary disease processes [6, 8, 37, 38, 39].
We have speculated that the changes in MHC composition reported here with aging and across the anteroposterior axis of the GG muscle of the rat may be used to explain, in part, facets of altered muscle actions associated with swallowing in elderly people. However, it is equally plausible that biochemical changes found in the GG muscle may be the product of, and not the cause of, age-related alterations in swallowing behaviors. Although there have been no studies that address the issue of causation directly, previous studies involving immobilization of the hindlimb musculature to model age-related disuse or reduction in use suggest that predominately fast contracting limb muscles, like the extensor digitorum longus (EDL) muscle, respond differently to disuse than to aging alone [40]. In particular, hindlimb immobilization was not found to cause changes in the properties of the EDL muscle, while age related changes to the EDL were reported in the absence of disuse [40]. In addition it has been shown that there is a reduction in limb strength and muscle mass in elderly individuals that cannot be correlated with a reduction in physical activity alone [41]. These results suggest that while behavioral factors, such as disuse, may contribute to changes in muscle strength and function, it also appears that separate and unique biochemical changes occur as a result of aging in the absence of disuse. Future studies that combine measurement of message, protein and function or physiology may be able to employee designs to address this issue more directly.
Limitations and Future Directions of the Study
The comparisons of young adult versus old animals made during this study should be interpreted with caution due to a few study limitations. First, due to the length of time between data collected from the young adult and old animals, animal batch differences may have occurred. Thus, although animals in the two studies were genetically identical there maybe slight differences in animal care and other factors between the two cohorts. As such, it is difficult to be absolutely certain that age is the only factor affecting MHC isoform composition within these two groups. Secondly, due to the fact that the gels from the studies were run at separate times, it is possible that different gel running conditions may have affected the density measures used to analyze the two gel groups. However, the normalization procedure of comparing the proportion of each MHC isoform within a given gel channel using the background of each channel as a control measure, does help to eliminate possible confounding gel running conditions. Based on the fact that the animals were genetically identical and as a result of the normalization procedure, these limitations are minor and have only a remote chance of influencing the findings of the study. The benefit of using existing data for the young adult GG muscles, and thus not euthanizing an additional group of control animals unnecessarily, outweighed these potentially minor limitations.
The results of the current study have provided new information on normal aging changes that occur in swallowing musculature in an animal model. If these muscular changes occur in humans, they could partially explain one possible mechanism of age related decline in muscle mass, strength, and fatigue in the tongue musculature, which has been linked to swallowing difficulties in the elderly population [42]. Specifically, our results indicated a change in the myosin heavy chain distribution of the GG muscle in aged rats, which is a strong determinant of muscle fiber type, particularly in the posterior portion of the muscle. These results may be physiologically important during the swallow because the posterior portion of the GG is active during generation of the tongue-to-palate pressure needed to carry out a normal pharyngeal swallow [31]. Thus a shift to more slowly contracting muscle fibers in the posterior portion of the GG may contribute to tongue-related swallowing difficulties in the elderly. Continued research, however, is needed to further define the manner in which changes in muscle fiber composition are associated with the functional complications seen in the elderly swallow.
Acknowledgments
This study was supported by National Institute on Deafness and Other Communication Disorders Grants R01DC005935 and R01DC008149. We are grateful for the assistance of Glen Leverson, Victoria Rajamanickam, Aaron Johnson, John Russell, Michelle Ciucci, and Jamie Shier in the completion of this work.
References
- 1.Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev. 2008;14(2):77–86. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 2.Doherty TJ, Brown WF. Age-related changes in the twitch contractile properties of human thenar motor units. J Appl Physiol. 1997;82(1):93–101. doi: 10.1152/jappl.1997.82.1.93. [DOI] [PubMed] [Google Scholar]
- 3.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 4.Campbell MJ, McComas AJ, Petito F. Physiological changes in ageing muscles. J Neurol, Neurosurg, Psychiatry. 1973;36(2):174–182. doi: 10.1136/jnnp.36.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: Underlying mechanisms. Medicine and Science in Sports and Exercise. 1994;26(4):432–439. [PubMed] [Google Scholar]
- 6.Kendall KA, Leonard RJ, McKenzie S. Common medical conditions in the elderly: Impact on pharyngeal bolus transit. Dysphagia. 2004;19(2):71–77. doi: 10.1007/s00455-003-0502-z. [DOI] [PubMed] [Google Scholar]
- 7.Logemann JA. Effects of aging on the swallowing mechanism. Otolaryngol Clin North Am. 1990;23(6):1045–1056. [PubMed] [Google Scholar]
- 8.Sonies BC. Oropharyngeal dysphagia in the elderly. Clin Geriatr Med. 1992;8(3):569–577. [PubMed] [Google Scholar]
- 9.Robbins J. Normal swallowing and aging. Semin Neurol. 1996;16(4):309–317. doi: 10.1055/s-2008-1040989. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: Radiologic findings in 56 cases. Am J Roentgenol. 1991;156(6):1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 11.Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, et al. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: Scintigraphic study. J Physiol. 1994;266(6 Pt 1):G972–7. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson T, de Picciotto J. Swallowing problems in the normal ageing population. S Afr J Commun Disord. 1999;46:55–64. [PubMed] [Google Scholar]
- 13.Schindler JS, Kelly JH. Swallowing disorders in the elderly. Laryngoscope. 2002;112(4):589–602. doi: 10.1097/00005537-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Jaradeh S. Neurophysiology of swallowing in the aged. Dysphagia. 1994;9(4):218–220. doi: 10.1007/BF00301913. [DOI] [PubMed] [Google Scholar]
- 15.Gleeson DC. Oropharyngeal swallowing and aging: A review. J Commun Disord. 1999;32(6):373–95. doi: 10.1016/s0021-9924(99)00017-9. quiz 395–6. [DOI] [PubMed] [Google Scholar]
- 16.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, et al. Function, morphology and protein expression of ageing skeletal muscle: A cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140(1):41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 17.Harridge SD, White MJ, Carrington CA, Goodman M, Cummins P. Electrically evoked torque-velocity characteristics and isomyosin composition of the triceps surae in young and elderly men. Acta Physiol Scand. 1995;154(4):469–477. doi: 10.1111/j.1748-1716.1995.tb09932.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272 (2 Pt 1):C638–49. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- 19.Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellof F. Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. J Histochem Cytochem. 2005;53(4):445–454. doi: 10.1369/jhc.4A6257.2005. [DOI] [PubMed] [Google Scholar]
- 20.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77(2):493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 21.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottinelli R, Betto R, Schiaffino S, Reggiani C. Maximum shortening velocity and coexistence of myosin heavy chain isoforms in single skinned fast fibres of rat skeletal muscle. J Muscle Res Cell Motil. 1994;15(4):413–419. doi: 10.1007/BF00122115. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Bless DM, Connor NP, Ford CN, Kyungah L, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. Ann Otol, Rhinol Laryngol. 2002;111(11):962. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- 24.Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13:48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- 25.Kjellgren D, Thornell LE, Andersen J, Pedrosa-Domellof F. Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44(4):1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- 26.Volz LM, Mann LB, Russell JA, Jackson MA, Leverson GE, Connor NP. Biochemistry of anterior, medial, and posterior genioglossus muscle in the rat. Dysphagia. 2007;22(3):210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]
- 27.Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75(5):2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- 28.Li ZB, Lehar M, Nakagawa H, Hoh JF, Flint PW. Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg. 2004;130(2):217–22. doi: 10.1016/j.otohns.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Lucas CA, Kang LH, Hoh JF. Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun. 2000;272(1):303–8. doi: 10.1006/bbrc.2000.2768. [DOI] [PubMed] [Google Scholar]
- 30.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 31.Palmer PM, Jaffe DM, McCulloch TM, Finnegan EM, Van Daele DJ, Luschei ES. Quantitative contributions of the muscles of the tongue, floor-of-mouth, jaw, and velum to tongue-to-palate pressure generation. J Speech Lang Hear Res. 2008;51(4):828–835. doi: 10.1044/1092-4388(2008/060). [DOI] [PubMed] [Google Scholar]
- 32.Sato I, Suzuki M, Sato M, Sato T, Inokuchi S. A histochemical study of lingual muscle fibers in rat. Okajimas Folia Anat Jpn. 1990;66(6):405–415. doi: 10.2535/ofaj1936.66.6_405. [DOI] [PubMed] [Google Scholar]
- 33.Mu L, Sanders I. Neuromuscular organization of the canine tongue. Anat Rec. 1999;256:412–424. doi: 10.1002/(SICI)1097-0185(19991201)256:4<412::AID-AR8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 35.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000;55(11):M634–40. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 36.Kays S, Robbins J. Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Semin Speech Lang. 2006;27(4):245–259. doi: 10.1055/s-2006-955115. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson H, Ekberg O, Olsson R, Hindfelt B. Quantitative aspects of swallowing in an elderly nondysphagic population. Dysphagia. 1996;11(3):180–184. doi: 10.1007/BF00366381. [DOI] [PubMed] [Google Scholar]
- 38.Burkhead LM, Sapienza CM, Rosenbek JC. Strength-training exercise in dysphagia rehabilitation: Principles, procedures, and directions for future research. Dysphagia. 2007;22(3):251–265. doi: 10.1007/s00455-006-9074-z. [DOI] [PubMed] [Google Scholar]
- 39.Galvao DA, Taaffe DR. Resistance exercise dosage in older adults: Single- versus multiset effects on physical performance and body composition. J Am Geriatr Soc. 2005;53(12):2090–2097. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- 40.Brown M, Hasser EM. Differential effects of reduced muscle use (hindlimb unweighting) on skeletal muscle with aging. Aging (Milano) 1996;8(2):99–105. doi: 10.1007/BF03339562. [DOI] [PubMed] [Google Scholar]
- 41.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: Influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–17. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 42.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103(3):823–9. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]