Abstract
Parkinson’s disease (PD) is a neurodegenerative disorder caused by the loss of dopaminergic neurons. Adult human endometrial derived stem cells (HEDSC), a readily obtainable type of mesenchymal stem-like cell, were used to generate dopaminergic cells and for transplantation. Cells expressing CD90, platelet derived growth factor (PDGF)-Rβ and CD146 but not CD45 or CD31 were differentiated in vitro into dopaminergic neurons that exhibited axon projections, pyramidal cell bodies and dendritic projections that recapitulate synapse formation; these cells also expressed the neural marker nestin and tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis. Whole cell patch clamp recording identified G-protein coupled inwardly rectifying potassium current 2 channels characteristic of central neurons. A 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine induced animal model of PD was used to demonstrate the ability of labelled HEDSC to engraft, migrate to the site of lesion, differentiate in vivo and significantly increase striatal dopamine and dopamine metabolite concentrations. HEDSC are a highly inducible source of allogenic stem cells that rescue dopamine concentrations in an immunocompetent PD mouse model.
Keywords: stem cells, adult stem cells, MSC, endometrium, Parkinson’s disease, transplantation
Introduction
Stem cell research has vastly expanded in recent years, with the promise of revolutionizing medical therapy. An active target of this research has been Parkinson’s disease (PD), a chronic, progressive, degenerative disease of the central nervous system that debilitates both motor function and speech due to the insufficient production of dopamine by pigmented cells in the substantia nigra. 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine (MPTP) is a selective neurotoxin of dopaminergic cells that induces PD in both animals and human beings.
Initial excitement about the therapeutic potential of embryonic stem cells for PD was damped by ethical concerns and technical difficulties, including tumour formation. Similarly, the initial enthusiasm for foetal tissue transplantation for PD was tempered in double-blind clinical trials with poor long-term results [1, 2]. The role of mesenchymal stem cells (MSC) in neuro-transplantation has shown recent promise due to the ability of this subtype of stem cells to migrate to sites of damaged neural tissue following both intravenous and intracranial transplantation of bone marrow derived MSC [3–5] or amnionic fluid derived stem cells [6] in preclinical studies. MSC have the additional advantage of being readily manipulated for use as delivery vehicles for gene therapy in PD models [7, 8].
Adult human endometrial derived stem-like cells (HEDSC) are a type of MSC that have only recently been characterized [9–16]. The endometrium displays tenacious regeneration ability due to the demands of menstruation and pregnancy, which make this tissue a promising source of dynamic stem cells suitable for use in regenerative medicine therapies. In vitro transdifferentiation of HEDSC into cartilage, bone, fat and muscle has recently been demonstrated [10, 17, 18]; however, neither transdifferentiation in vivo, transplantation, nor differentiation into a neurogenic cell type has previously been demonstrated. Here we demonstrate the ability of HEDSC to differentiate into dopamine-producing neurons. We also demonstrate their ability to be used for transplantation, where HEDSC engraft, migrate to the site of lesion, and are spontaneously differentiated in vivo. Furthermore, we show a therapeutic benefit, where transplantation rescues dopamine concentrations in an immunocompetent PD mouse model. To our knowledge this study is the first to demonstrate dopamine improvement using MSC in the absence of gene therapy, which could indicate a particular penchant for HEDSC use in PD transplantation.
Results
Flow cytometry
Flow cytometry was performed to characterize the HEDSC used in this study. After two passages in culture, HEDSC displayed the following surface markers: CD31+ 1.4%, αSMA+ 5.5%, CD90+ 99.6%, CD45+ 0.3%, which is consistent with a non-haematogenously derived endometrial stromal cell. HEDSC were strongly positive for both PDGF-Rβ+ 99.7% and CD146+ 99.7%, which have been shown to isolate HEDSC from fresh endometrial samples.
Neurogenic in vitro differentiation of HEDSC
In vitro transdifferentiated HEDSC exhibited neurogenic morphology including long axon projections, pyramidal cell bodies and dendritic projections that appear to recapitulate synapse formation in culture (n= 3) (Fig. 1). Neurogenic cell identity was demonstrated using immunostaining. Cytoplasmic expression of the neural stem cell marker nestin was observed in in vitro differentiated cells using a human nestin antibody. Dense staining in the soma and axon hillock region is evidence in some neurons, which appear to overlay the nucleus in other cells. In addition, almost all HEDSC that remained adherent after neurogenic differentiation in vitro expressed the rate-limiting enzyme involved in dopamine production tyrosine hydroxylase (TH). The presence of TH production suggests a functional phenotype, specifically dopamine synthesis. Control cells not differentiated with neurogenic media failed to demonstrate any of these indicators of neuronal identity (Fig. 1).
Fig 1.
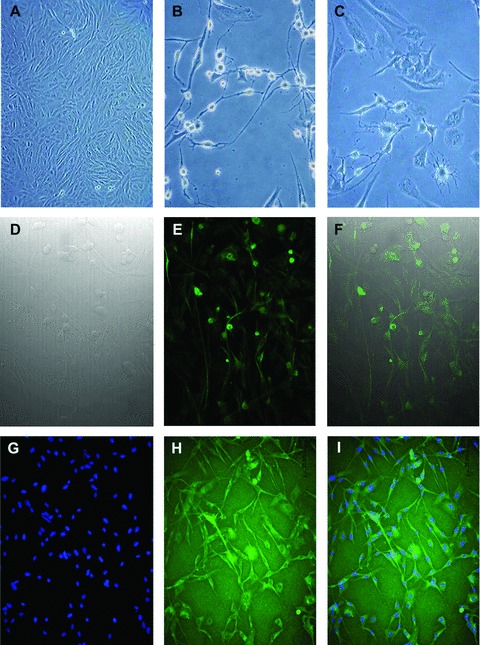
In vitro neurogenic differentiation of HEDSC. HEDSC cultured in control media demonstrate typical stromal cell morphology (A), whereas cells cultured in neurogenic media demonstrated both pyramidal and dendritic cell morphology as is pictured using light microscopy (B, C). Differentiated cells visualized using: differential interference contrast (D), IF for neural stem cell marker nestin expression (E), and a merge of both (F). Differentiated cell cultures, also express TH (H), DAPI nuclei staining (G), and merge of both (I).
Electrophysiological properties of in vitro differentiated cells
In addition to morphological and immunostaining characteristics, in vitro differentiated cells expressed electrophysiological properties of neurons. A whole cell patch clamp recording method was used to measure the current characteristics of individual cells to look for evidence of barium sensitive potassium channels, which are characteristic of central neurons, including dopaminergic cells. The experiments were performed on 10 separate experiments derived from samples differentiated from three separate patients. In the differentiated cells, a series of voltage steps from −60 mV to −120 mV induces inward currents, which were dramatically decreased in the presence of barium (200 μM), a non-specific blocker of the inwardly rectifying potassium current (Kir). The Kir current, resembling the G-protein coupled inwardly rectifying potassium current (GIRK), was only present in differentiated cells, therefore no Ba2+-sensitive inward currents were present in undifferentiated cells (Fig. 2).
Fig 2.
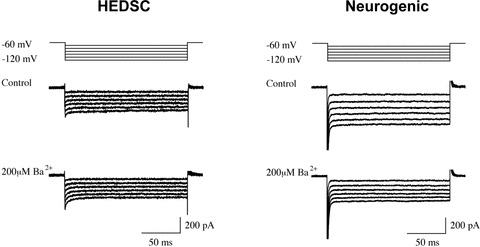
Electrophysiology using whole cell patch clamp testing. HEDSC-derived neurogenic cells display GIRK2 current characteristic of central neurons that diminishes with barium administration (right), whereas control cells do not (left).
Transplantation of HEDSC in Parkinson’s disease mouse model
HEDSC were successfully transplanted into both immunodeficient and immunocompetent MPTP lesioned mice, where engraftment was demonstrated up to 5 weeks following transplantation using multiple techniques. First, human genomic DNA was detected within transplanted mouse brains using PCR (Fig. 3A). Next, engrafted cells were visualized within the mouse brain using four different techniques. A human mitochondrial antibody, which does not cross react with the mouse antigen, was used to detect human cells in mice brains. Human cells were found around the transplantation site in the striatum; however, they were also found to have migrated to the substantia nigra (Fig. 3B). In contrast, when transplantations were performed with differentiated HEDSC, localization to the substantia nigra was not observed.
Fig 3.
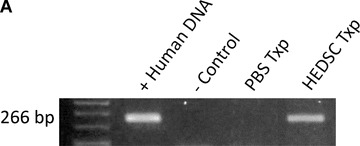
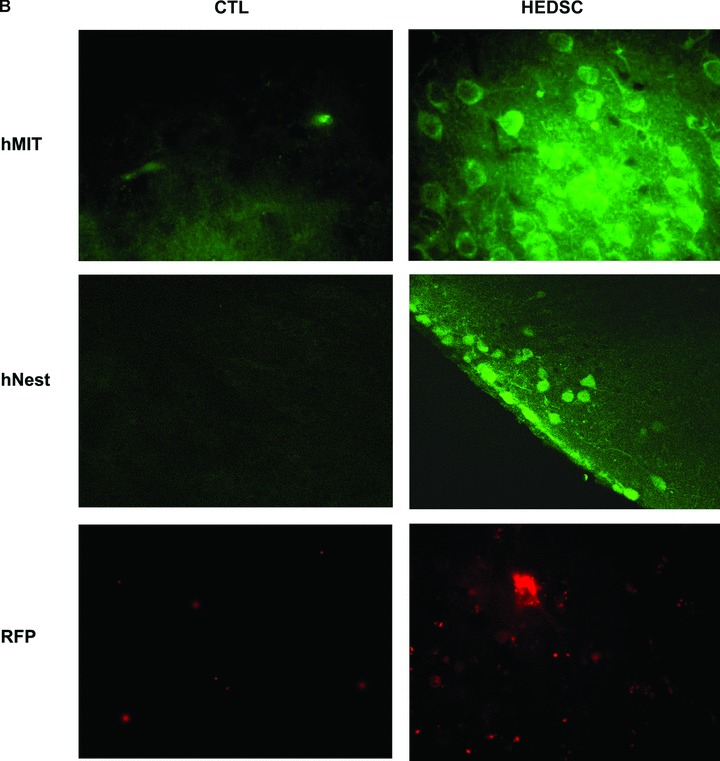
HEDSC Engraft, differentiate in vivo, and migrate to the lesioned site in mice brains. (A) PCR detecting human DNA in mouse brain transplanted with HEDSC (n= 14), but not with sham transplants (n= 8) using PBS. (B) Low power view of murine brain section from sham treated [control (CTL)] and HEDSC-treated animals. An area that includes the substantia nigra (SN) is outlined. IHC using a human nestin antibody identifies cells localized to the SN in the transplanted animals. Human cells are visualized in mouse brains in the right column, and controls are shown on the left. In the top panel, all human cells are detected using a human mitochondrial antibody (hMit), which are seen here at the site of transplantation in the striatum. Spontaneous in vivo differentiation of transplanted HEDSC was observed, where they expressed nestin (hNestin). Transplanted cells adapted a neurogenic phenotype morphologically, as is visualized using red fluorescent surface labelling. Human cells were observed remote from the initial transplantation site (striatum), where they migrated to the lesioned brain area (substantia nigra) which is the area pictured in the bottom right panel (red fluorescent surface labelling).
The transplanted human cells were shown to exhibited neural stem cell markers by staining with the human nestin antibody (Fig. 3B). Mice that were transplanted with HEDSC were also found to express human TH by RT-PCR, whereas sham transplanted animals did not (data not shown). As cells were observed to successfully engraft in both immunodeficient as well as immunocompetent mice, wild-type mice were used for subsequent experiments.
Engraftment and migration was confirmed using two different types of fluorescently labelled HEDSC for transplantation: PKH26 was used for surface labelling and whereas green fluorescent protein (GFP) transfection was used for cytoplasmic labelling. First, PKH26 red labelled HEDSC were identified at the site of transplantation in the striatum; however, they also demonstrated the ability to migrate to the site of lesion, localizing in the substantia nigra. Furthermore, these migrating cells were able to differentiate from an endometrial phenotype into a neurogenic phenotype in vivo (Fig. 3B). GFP transfected HEDSC were also able to be visualized within the mouse brains, but this method was limited by the low transfection efficiency of approximately 10% of HEDSC in culture prior to use for transplantation.
Intracranial transplantation with HEDSC resulted in a significant improvement of striatal dopamine (DA) and dihydroxyphenylacetic acid (DOPAC) concentrations in this MPTP mouse model of PD as measured by high-performance liquid chromatography (HPLC). Mean DA concentrations (ng/ml) were significantly higher in MPTP lesioned mice after HEDSC transplant (n= 8, 113.1 ± 5.5 S.E.M.) compared to MPTP lesioned mice treated with sham phosphate buffered saline (PBS) transplant (n= 14, 78.6 + 7.0 S.E.M.), whereas mean concentrations of unlesioned mice were 134.6 ± 3.2 (n= 5), P < 0.0001. Mean DOPAC concentrations (ng/ml) were also significantly higher in HEDSC transplanted (n= 8, 5.5 ± 0.3 S.E.M.) versus sham mice (n= 14, 4.0 ± 0.4 S.E.M.), whereas unlesioned mice exhibited baseline concentrations of 5.3 ± 0.2 S.E.M. (n= 5), P= 0.008 (Fig. 4).
Fig 4.
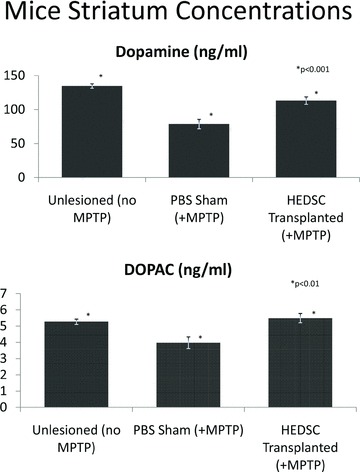
HEDSC Transplantation increases dopamine concentrations in mouse striatum. Dopamine concentrations (mean ± S.E.M.) were measured in mice brains. In the first graph, animals not treated (unlesioned) with MPTP (n= 5) are shown in the left column with baseline levels. In the middle column, MPTP lesioned mice showed an expected decrease in dopamine concentrations when given sham operations with PBS (n= 8). In the right column, MPTP lesioned mice demonstrated rescued dopamine concentrations when treated with HEDSC transplantation (n= 14), P < 0.0001. A similar therapeutic effect of HEDSC is seen in the bottom graph by measuring the dopamine metabolite DOPAC concentrations in mice striatum, P= 0.008.
Discussion
Here we demonstrate the ability of HEDSC to differentiate into dopamine-producing neurons, where in vitro cultures demonstrate characteristic neuron morphology, express markers of neural cell phenotype and enzymatic function, and display electrophysiological properties specific to dopamine-producing neurons. Furthermore, we demonstrate the ability of HEDSC to be used for transplantation for the first time, even in immunocompetent animals. This was shown by detecting human DNA in mouse brains after HEDSC transplantation, visualizing human HEDSC in mouse brains using antibodies specific to human cells, identifying HEDSC labelled with red fluorescent dye and identifying GFP fluorescing human HEDSC. These cells survive in the location they are transplanted, but also spontaneously migrate to areas of damage and spontaneously differentiate in vivo. HEDSC exert a therapeutic benefit by rescuing dopamine concentrations in this PD animal model.
HEDSC represent an important source of stem cells that can be obtained from a routine office procedure. Further, they can be used as an autologous or allogenic stem cell source, thereby obviating concerns regarding rejection in human beings. The lack of rejection of human stem cells in this murine model could be due to several possibilities. First it is possible this is a result of immune-privilege provided by the blood–brain barrier. Lack of rejection after neuro-transplantation has been demonstrated in several studies [3, 4, 19–27]. However, evidence suggests that MSC have immuno-privileged properties themselves compared to other types of stem cells [26, 28–30], lending additional support to the potential of stem cells from the endometrium. In fact, spontaneous microchimerism of maternal tissue from foetal cells is well established in the setting of pregnancy [31, 32] and following blood transfusions. In addition, MSC can home to and engraft mouse bone marrow [33]. It is also possibly that MSC, or HEDSC in particular, display a particular penchant for neural regeneration such as PD treatment due to an underlying disposition of these cells towards neural cells [34]. An additional advantage of MSC is their low potential for teratoma formation. It is also possible that PD disease is particularly amenable to exogenous stem cells transplantation due to the lack of gliosis, which impedes MSC influx in pathological disease pathologies like stroke.
To date, stem cells derived from the endometrium that demonstrate the ability to transdifferentiate have been isolated in two ways: (1) by performing flow cytometry to select for cells that are both PDGF-Rβ+ and CD146+[9, 18] and (2) by passaging routine stromal cell endometrial cultures [10, 11, 17]. It is interesting that merely passaging cells in a routine manner selects cells that are strongly positive for the same markers shown to prospectively isolate HEDSC, and serves to mutually validate both reports. However, the differences in cells collected in these two manners remains to be more fully characterized.
As our understanding of stem cell biology grows, we are re-evaluating their role in tissue repair. It now appears that stem or progenitor cells are present in most tissues [35]. An expanding body of evidence suggests that stem cells play a role in processes that were previously unrecognized; for instance, it was once thought that neurons in the brain did not undergo regeneration after completing development, when in fact neurons are involved in adult tissue remodelling [36]. It is possible that stem cells may actually be involved in a dynamic state of disease and repair relevant to many chronic disease processes. In fact, it is possible that chronic disease may actually be an indicator of the lifetime burden of regeneration and stem cell depletion. This theory can be illustrated by the cases of early onset PD in high regenerative burden states such as boxing, post-concussion syndrome or drug abuse.
Stem cells are defined by the ability to clonally proliferate and self-replicate, but do exhibit a terminal, albeit extremely long proliferation capacity in vivo. Variations in observed cell culture may reflect a similar naturally occurring variation in vivo. The life time burden of regeneration may help to explain the high levels of variation observed in culture between patients; e.g. tissue donors with low burdens produce cell cultures with robust replication and differentiation potential, where cultures derived from donors with high burdens of regeneration produce cultures with decreased activity. This could also help explain the remarkable plasticity of newborn brains, but relatively limited neural plasticity in adults. Perhaps endogenous MSC do not treat diseases like PD well in vivo because the PD phenotype occurs after a long burden of autotransplantation with endogenous MSC, which then are depleted. The therapeutic potential of MSC, in particular HEDSC, will likely vary depending on the lifetime regeneration burden of the donor source. Based on the therapeutic potential we demonstrate here, HEDSC may become an important source of allogenic stem cells to be used for regenerative medicine.
Materials and methods
Sample collection
Human endometrial tissue was collected by curettage from nine reproductive aged women undergoing surgery for benign gynaecological conditions. Standard endometrial stromal cell cultures were generated in a routine fashion, which produced an unfractionated stromal cell population. Briefly, endometrial tissue was minced and then digested in Hank's balanced salt solution (HBSS) (Gibco, Invitrogen, Carlsbad, CA, USA) containing 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (25 mM), collagenase B (1 mg/ml, Roche Diagnostics, Indianapolis, IN, USA) and DNase I (0.1 mg/ml, Sigma-Aldrich, St. Louis, MO, USA) for 30–45 min. at 37°C with agitation. Resultant dispersed cell solutions were then passed through a 70 μM sieve (BD Biosciences, Beford, MA, USA) to remove glandular epithelial components. Filtered cell solutions were then centrifuged, supernatant decanted and resuspended in Dulbecco’s modified Eagle’s medium: Ham’s F12 (DMEM, Gibco, Invitrogen) with phenol red containing 1% antibiotics-antimycotics (ABAM, Gibco, Invitrogen) and 10% foetal bovine serum (FBS, Gibco, Invitrogen). Resuspended cells were then plated in plastic flasks, maintained at 37°C in a humidified chamber (5% CO2). Thereafter, cells were passaged using standard trypsinization methods.
Flow cytometry
Human endometrial derived stromal cultures were characterized using flow cytometry after passage two. Cells were trypsinized and washed with staining buffer, which comprised PBS with 3% FBS and 0.05% sodium azide. Cells were passed through a 70 μM sieve to minimize cell clumping for analysis, centrifuged and supernatant decanted. Cell pellets were incubated on ice with the following antibodies for 1 hr: CD90 directly conjugated with antigen-presenting cell (APC; BD Pharmingen, San Jose, CA, USA), CD146 directly conjugated with phycoerythrin (PE) (BD Pharmingen), CD45 directly conjugated with fluorescein isothiocyanate (FITC) (BD Pharmingen), CD31 directly conjugated with FITC (BD Pharmingen) and platelet derived growth factor receptor β directly conjugated with APC (PDGF-Rβ, R&D Systems, Minneapolis, MN, USA) for 1 hr. For intracellular staining, cells were treated using BD Cytofix/Cytoperm Fixation/Permeabilization Kit before incubation with α smooth muscle actin antibody directly conjugated with Cy3 (αSMA, Sigma, St. Louis, MO) for 1 hr. Cells were then washed and resuspended with sorting buffer, which comprised PBS with 0.1% bovine serum albumen. Directly conjugated isotype controls were used set electronic gates to <3% positive cells. Cells were then analysed on the BD FACSVantage SE Cell Sorter using FACSDiVa (BD Biosciences, San Jose, CA, USA).
Neurogenic in vitro differentiation
After the second passage, cells were treated with a two-step dopaminergic differentiation protocol adapted from Blondheim et al. [34]. HEDSC were first treated with differentiation medium I for 24–48 hrs, which consisted of DMEM with 10% FBS, 1% ABAM, 2 mM L-Glutamine (Invitrogen), recombinant human fibroblast growth factor (rhFGF) (10 ng/ml, rhFGF basic, R&D Systems), recombinant human epidermal growth factor (rhEGF) (10 ng/ml, rhEGF, R&D Systems) and N2 supplement-B (StemCell Technologies, Vancouver, CA, USA). Cells were then changed to differentiation medium II for up to 96 hrs, which consisted of DMEM with 2 mM L-glutamine, 1% ABAM, N2 supplement-B, butylated hydroxyanisole (200 μM, Sigma), dibutyryl cyclic AMP (1 mM, Sigma), 3-isobutyl-1-methyl-xanthine (0.5 mM, Sigma) and all-trans-retinoic acid (1 μM, Sigma). Undifferentiated endometrial stromal cell cultured in DMEM w/10% FBS were grown until limited by confluence, then harvested or fixed and used as controls.
In vitro immunostaining
For in vitro immunofluorescent experiments, cells were cultured in chamber slides and then fixed with methanol. To examine for evidence of nestin production (a neural stem cell marker), cells from in vitro experiments were washed with PBS, permeabilized by incubating in PBS containing 0.2% Triton X-100 (PBS-TX) for 10 min., blocked with 10% normal goat serum for 1 hr, and then stained overnight with human nestin antibody (Abcam, Cambridge, MA, USA) diluted 1:250 with 2.5% normal goat serum in PBS. The following day cells were incubated with FITC-labelled anti-rabbit IgG prepared in goats (Vector Laboratories, Burlingame, CA, USA) for 1 hr. Slides were mounted with VECTASHIELD Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories).
To examine for evidence of TH production (the rate-limiting enzyme in dopamine synthesis), fixed in vitro cell cultures were washed with PBS and blocked using donkey antimouse serum for 1 hr. Cells were then stained using a mouse monoclonal TH antibody (DiaSorin, Stillwater, MN, USA) diluted at 1:5000 in PBS-TX overnight. After a series of washes, sections were incubated with the fluorescent secondary antibody donkey antimouse IgG 488 Alexafluore (1:200; Molecular Probes, Carlsbad, CA, USA) for 1 hr to visualize TH-immunoreactive cells. Slides were mounted with VECTASHIELD Mounting Medium with DAPI.
In vitro electrophysiology
Whole cell patch clamp recording was then performed on in vitro differentiated and undifferentiated endometrial cells from three different patients to examine for evidence of GIRK2 channels, which are characteristic of central neurons including dopaminergic cells. Briefly, cells were maintained in a recording chamber with artificial cerebrospinal fluid (bubbled with 5% CO2 and 95% O2) containing (in mM): NaCl 124, KCl 3, CaCl2 2, MgCl2 2, NaH2PO4 1.23, NaHCO3 26, glucose 10, pH 7.4 with NaOH. Whole-cell voltage clamp (at −60 mV) was performed to observe inwardly rectifier potassium currents with a Multiclamp 700A amplifier (Axon Instruments; Molecular Devices Corporation, Sunnyvale, CA, USA). The patch pipettes with a tip resistance of 4–6 MΩ were made of borosilicate glass (World Precision Instruments, Sarasota, FL, USA) with a Sutter pipette puller (P-97) and filled with a pipette solution containing (mM): K-gluconate 135, MgCl2 2, HEPES 10, ethylene glycol tetraacetic acid (EGTA) 1.1, Mg-ATP 2, Na2-phosphocreatine 10, and Na2-GTP 0.3, pH 7.3 with KOH. After a giga-Ω (GΩ) seal and whole-cell access were achieved, the series resistance (between 20 and 40 MΩ) was partially compensated by the amplifier. A series of voltage steps from −60 mV to –120 mV was applied to recorded cells under voltage clamp in the presence of high concentration of K+ (60 mM) to monitor inwardly rectifier potassium current. The existence of inwardly rectifier potassium currents was verified by applying Ba2+ (200 μM) containing bath solution to the recorded neurons. Both input resistance and series resistance were monitored throughout the experiments. Only those recordings with stable series resistance and input resistance were accepted. All data were sampled at 3–10 kHz and filtered at 1–3 kHz with an Apple Macintosh computer using Axograph 4.9 (Axon Instruments). Electrophysiological data were analysed with Axograph 4.9 and plotted with Igor Pro software (WaveMetrics, Lake Oswego, OR, USA).
HEDSC fluorescent labelling
Fluorescent labelling of HEDSC was performed in two ways. Trypsinized cells after passage two were labelled with PKH26 (Sigma) according to the manufacturer’s instructions prior to use for transplantation. Alternately, cells were transfected with a GFP plasmid using Lipofectamine for 24 hrs. Cell media was then changed and cultures continued for an additional 48 hrs prior to trypsinization and transplantation. Cell labelling with PKH26 and GFP transfection was confirmed by visualization immediately prior to transplantation.
HEDSC transplantation into mice
For HEDSC transplantations, an established PD mouse model was generated by injecting 8-week-old male mice with 30 mg/kg intraperitoneal MPTP on two consecutive days in both immunocompetent (C57-Black 6) and immunodeficient (non-obese diabetic severe combined immunodeficiency knockout on a Black 6 background) mice. All mice were maintained under standard laboratory conditions with water and food available ad libitum; lights were maintained on a 12 hr light/dark cycle. Transplantations were performed with undifferentiated as well as differentiated HEDSCs. Five days after MPTP treatment, anesthetized mice underwent transplantation using a stereotaxic frame. Using an aseptic technique, a burr hole (0.5 mm) was made on both sides of the skull. Each mouse received a total of four injections: 2 mm lateral to Bregma at both 0.5 mm rostral and caudal of Bregma. Mice were transplanted with either 105 undifferentiated HEDSC in PBS, 105 differentiated HEDSC in PBS, or PBS control over 5 min. at a depth of 4, 3.5 and 3 mm. The needle remained in the striatum for an additional 5 min. interval before slowly being retracted to avoid HEDSC reflux.
Mice were killed 5 weeks later and one striatum was used for DNA analysis and the other striatum was used to measure dopamine concentrations.
Detection of human DNA in mouse brains
DNA was harvested using the QIAamp DNA MiniKit (Qiagen, Valencia, CA, USA). Human DNA was amplified using the genomic primers: forward 5′-CGTTGGAACAGAGGTTGGAG-3′ and reverse 5′-TCCTGAAAGCTGAGGGAAG-3′ at 65°C annealing temperature using high-fidelity Taq polymerase (Invitrogen). Human genomic DNA was used as a positive control. To detect expression of human TH in mice that received transplantation, mRNA was extracted from mice brains using the RNAeasy kit (Qiagen). cDNA was generated using iScript cDNA synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Primers used for TH were specific for human and for mRNA by crossing introns: forward 5′-TGG ACC ACC CGG GCT-3′ (15 bp) and reverse 5′5′-GTC GCC GTG CCT GTA CTG-3′ (18 bp). Human DNA was amplified using high-fidelity Taq polymerase (Invitrogen). PCR products were resolved on a 1.5% agarose gel with ethidium bromide and visualized under ultraviolet light.
Visualization of human cells in mice brains
Mice were killed at intervals up to 5 weeks following HEDSC transplantation. Some mice were overdosed with dimethyl ether and transcardially perfused with fixative (4% paraformaldehyde, 0.1% glutaraldehyde and 15% picric acid in PBS) for immunofluorescent experiments. To examine for evidence of human cell engraftment, brains were removed from mice and were post-fixed overnight in fixative without glutaraldehyde. Serial 50 μm coronal sections were cut through mouse brains using a vibratome and were collected in microtitre plates.
To detect all human cells present in the mouse brains, a generic antibody against human cells that does not cross react with mouse tissue was used: human 60 kD mitochondrial antibody (Millipore, Bedford, MA, USA). Brain sections were then counterstained with the fluorescent secondary antibody donkey antimouse IgG 488 Alexafluore (1:200; Molecular Probes) for 1 hr. Engraftment in mouse brains was further demonstrated by identifying immunofluorescent PKH26 labelled HEDSC (n= 6) and GFP transfected HEDSC (n= 3) in situ. To detect transplanted human cells that expressed neural stem cell markers, sections of mouse brain were also stained with a human nestin antibody using the same protocol described above for in vitro immunostaining.
Dopamine concentrations in transplanted mice
The functional ability of HEDSC to rescue dopamine production in a PD mouse model was evaluated by measuring DA and DOPAC from the striatum of HEDSC transplanted (n= 14) and control (n= 8) immunocompetent C57-Black 6 mice. Five unlesioned mice were injected with intraperitoneal saline to be used as an MPTP model control. Control mice were treated with MPTP, but underwent a sham intracranial transplant with PBS. Test mice were treated with MPTP and underwent a therapeutic transplant with HEDSC. Five weeks after transplantation, mice were killed. Striata from each mouse were rapidly dissected on a chilled glass plate and frozen at −80°C. Samples from each mouse were processed and concentrations of dopamine and metabolites were measured using HPLC as previously described [37]. Results were expressed as nanograms per milligram of protein (mean ± S.E.M.). N-values represent number of mice samples that were examined.
Statistical analysis
Mean dopamine and DOPAC concentrations between groups were analysed using One way anova and result with a P-value <0.05 were considered significant using SPSS statistical software (Chicago, IL, USA).
Ethics statement
Endometrial tissue was collected under an approved Human Investigations Committee protocol at Yale University. Informed written consent was obtained prior to surgery and to collect research samples. Mice were used under an approved Institutional Animal Care and Use Committee protocol at Yale University.
Acknowledgments
This study was supported by the Reproductive Scientist Development Program (Berlex Foundation and NIH K12HD00849) and Specialized Cooperative Centers Program in Reproduction and Infertility Research (NIH U54 HD052668).
References
- 1.Freed C, Greene P, Breeze R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–9. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 2.Olanow C, Goetz C, Kordower J, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–14. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 3.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Chen J, Wang L, et al. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neurosci Lett. 2001;316:67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 5.Hellmann M, Panet H, Barhum Y, et al. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395:124–8. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 6.Cipriani S, Bonini D, Marchina E, et al. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int. 2007;31:845–50. doi: 10.1016/j.cellbi.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Zhao C, Liu Y, et al. Therapeutic benefit of TH-engineered mesenchymal stem cells for Parkinson’s disease. Brain Res Brain Res Protoc. 2005;15:46–51. doi: 10.1016/j.brainresprot.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Ye M, Wang X, Zhang Y, et al. Transplantation of bone marrow stromal cells containing the neurturin gene in rat model of Parkinson’s disease. Brain Res. 2007;1142:206–16. doi: 10.1016/j.brainres.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 9.Schwab K, Gargett C. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrov R, Timeva T, Kyurkchiev D, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–8. doi: 10.1530/REP-07-0428. [DOI] [PubMed] [Google Scholar]
- 11.Sasson I, Taylor H. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci. 2008;1127:106–15. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargett C. Review article: stem cells in human reproduction. Reprod Sci. 2007;14:405–24. doi: 10.1177/1933719107306231. [DOI] [PubMed] [Google Scholar]
- 13.Gargett C, Chan R, Schwab K. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288:22–9. doi: 10.1016/j.mce.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Schwab K, Hutchinson P, Gargett C. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23:934–43. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 15.Gargett B, Chan R. Endometrial stem/progenitor cells and proliferative disorders of the endometrium. Minerva Ginecol. 2006;58:511–26. [PubMed] [Google Scholar]
- 16.Gargett C. Identification and characterisation of human endometrial stem/progenitor cells. Aust NZ J Obstet Gynaecol. 2006;46:250–3. doi: 10.1111/j.1479-828X.2006.00582.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolff E, Wolff A, Du H, et al. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14:524–33. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 18.Gargett C, Schwab K, Zillwood R, et al. Isolation and Culture of Epithelial Progenitors and Mesenchymal Stem Cells from Human Endometrium. Biol Reprod. 2009;80:1136–45. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–9. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Chen J, Chen X, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Mahmood A, Wang L, et al. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–63. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 22.Lu D, Sanberg P, Mahmood A, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–81. [PubMed] [Google Scholar]
- 23.Mahmood A, Lu D, Wang L, et al. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–203. [PubMed] [Google Scholar]
- 24.Mahmood A, Lu D, Lu M, et al. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 25.Mahmood A, Lu D, Qu C, et al. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–31. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu C, Mahmood A, Lu D, et al. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. 2008;1208:234–9. doi: 10.1016/j.brainres.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansilla E, Marin G, Sturla F, et al. Human mesenchymal stem cells are tolerized by mice and improve skin and spinal cord injuries. Transplant Proc. 2005;37:292–4. doi: 10.1016/j.transproceed.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein D, Shearer G. Suppression of human cytotoxic T lymphocyte responses by adherent peripheral blood leukocytes. Ann NY Acad Sci. 1988;532:207–13. doi: 10.1111/j.1749-6632.1988.tb36339.x. [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Zhang L, Ren G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–8. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 30.Zinkernagel R, Doherty P. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- 31.Adams Waldorf K, Nelson J. Autoimmune disease during pregnancy and the microchimerism legacy of pregnancy. Immunol Invest. 2008;37:631–44. doi: 10.1080/08820130802205886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lapaire O, Hösli I, Zanetti-Daellenbach R, et al. Impact of fetal-maternal microchimerism on women’s health–a review. J Matern Fetal Neonatal Med. 2007;20:1–5. doi: 10.1080/14767050601144834. [DOI] [PubMed] [Google Scholar]
- 33.Mouiseddine M, François S, Semont A, et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80:S49–55. doi: 10.1259/bjr/25927054. [DOI] [PubMed] [Google Scholar]
- 34.Blondheim N, Levy Y, Ben-Zur T, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141–64. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- 35.Kørbling M, Estrov Z. Adult stem cells for tissue repair – a new therapeutic concept. N Engl J Med. 2003;349:570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 36.Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 37.Elsworth J, Brittan M, Taylor J, et al. Restoration of dopamine transporter density in the striatum of fetal ventral mesencephalon-grafted, but not sham-grafted, MPTP-treated Parkinsonian monkeys. Cell Transplant. 1996;5:315–25. doi: 10.1177/096368979600500220. [DOI] [PubMed] [Google Scholar]


