Abstract
The cost of attending to a visual event can be the failure to consciously detect other events. This processing limitation is well illustrated by the attentional blink (AB) paradigm, in which searching for and attending to a target presented in a rapid serial visual presentation (RSVP) stream of distractors can impair one’s ability to detect a second target presented soon thereafter. The AB critically depends on ‘top-down’ attentional settings, for it does not occur if participants are asked to ignore the first target. Here we show that ‘bottom-up’ attention can also lead to a profound but ephemeral deficit in conscious perception: Presentation of a novel, unexpected, and task-irrelevant stimulus virtually abolishes conscious detection of a target presented within half a second after the ‘Surprise’ stimulus, but only for its earliest occurrences (generally 1–2 presentations). This powerful but short-lived deficit contrasts with a milder but more enduring form of attentional capture that accompanies singleton presentations in RSVPs. We conclude that the capture of stimulus-driven attention alone can limit explicit perception.
Our attention can be powerfully grabbed by unexpected, salient stimuli (Egeth & Yantis, 1997; Horstmann, 2002; Horstmann & Ansorge, 2006; Horstmann & Becker, 2008; Meyer, Niepel, Rudolph, & Schützwohl, 1991). Such stimulus-driven influence on attention is adaptive, as the ability to detect and respond to novel, unexpected events is crucial to survival (Darwin, 1859/2003). It is therefore unsurprising that animals, including humans, readily demonstrate an orienting response (OR), a reflexive reaction to an unexpected event, whether life-threatening or beneficial (Pavlov, 1927; Sechenov, 1863/1965; Sokolov, Spinks, Näätänen, & Lyytinen, 2002; Gronau, Sequerra, Cohen, & Ben-Shakhar, 2006). In addition to increased arousal and concomitant physiological changes, the orienting response is characterized by facilitated processing of the triggering event to ensure its speedy evaluation and the formulation of an appropriate response to that event (Kahneman, 1973; Sokolov, 1963; Sokolov, Spinks, Näätänen, & Lyytinen, 2002; Spinks & Siddle, 1983; but see Siddle, 1971; Siddle & Mangan, 1971). The widespread mobilization of cognitive processes following the presentation of an unexpected stimulus suggests that the orienting response draws on central attentional resources (Kahneman, 1973). But because such resources are thought to be capacity-limited (Broadbent, 1957; Kahneman, 1973; Chun & Marois, 2002; Marois & Ivanoff, 2005), it is conceivable that the orienting response may not be entirely beneficial. In particular, attending to a novel, unexpected event could leave too few attentional resources available for processing other events, which may therefore go unnoticed.
While the double-sided nature of attention has not been studied with the orienting response, it is well-illustrated by the attentional blink (AB) paradigm. The AB reveals a severe impairment in detecting the second of two targets presented in a rapid serial visual presentation (RSVP) stream of distractors, but only when that target is shown within about half a second of the first (Broadbent & Broadbent, 1987; Chun & Potter, 1995; Raymond, Shapiro, & Arnell, 1992; Weichselgartner & Sperling, 1987). This impairment results from attending to the first target, as participants have little difficulty in identifying the second target when only the latter is to be reported. The AB therefore critically depends on ‘top-down’ attentional settings.
Top-down, or goal-driven, attention is not the only mechanism by which attention is engaged (Egeth & Yantis, 1997). As mentioned above, attention can also be involuntarily summoned in a ‘bottom-up’ manner, a form of attention referred to as stimulus-driven. The extent to which stimulus-driven attention is subject to the same capacity limits as goal-directed attention, though, is unclear. Recent studies suggest that a task-irrelevant stimulus appearing before a target does not produce an AB unless the stimulus is emotionally evocative (Most, Chun, Widders, & Zald, 2005; Smith, Most, Newsome, & Zald, 2006) or shares features with the target, such as having similar form (Ghorashi, Zuvic, Visser, & Di Lollo, 2003; Maki & Mebane, 2006; Visser, Bischof, & Di Lollo, 2004) or having color amongst grayscale distractors (Wee & Chua, 2004; Spalek, Falcon, & Di Lollo, 2006; Maki & Mebane, 2006). The latter finding is consistent with the ‘contingent attentional capture’ hypothesis, which asserts that a task-irrelevant stimulus will capture attention only if it shares features with the goal-directed attentional set that defines the target (Folk, Leber, & Egeth, 2002; but see Horstmann & Becker, 2008; Neo & Chua, 2006; Theeuwes, 2004).
The orienting response suggests that emotionally neutral and task-irrelevant stimuli can still powerfully capture attention so long as those stimuli are novel and unexpected. Consequently, such stimuli would be expected to produce an AB-like deficit for a subsequently presented target. Moreover, this deficit in target detection should be short-lived given the orienting response’s rapid habituation to behaviorally irrelevant stimuli after repeated presentations (Sokolov, 1975), thereby allowing one to ignore recurring inconsequential events. The orienting response’s habituation may also explain why neutral stimuli have not yet been observed to induce a stimulus-driven form of AB: Since AB studies typically involve hundreds of trials (Ghorashi et al., 2003; Maki & Mebane, 2006; Most et al., 2005; Visser et al., 2004; Wee & Chua, 2004; Spalek et al., 2006), any initial target detection impairment may have been washed out by performance on the remaining trials.
In the present study, we show that a novel, task-irrelevant stimulus creates a profound impairment in the subsequent detection of a target, a phenomenon that we have termed Surprise-induced Blindness (SiB). While similar to the AB, SiB has a distinctly brief life span, with the impairment vanishing by the third ‘Surprise’ stimulus presentation. As such, this deficit represents a new, stimulus-driven form of attentional limit to explicit perception.
Experiment 1: Establishing Surprise-induced Blindness
We first tested whether the presentation of an unexpected, task-irrelevant stimulus transiently impairs conscious perception of a subsequent event. The task involved searching for a target in a rapid serial visual presentation (RSVP) stream of distractors drawn from the same visual category as the target (Fig. 1). In four of the 30 trials, a ‘Surprise’ stimulus, drawn from a different stimulus category than the target and distractors, was presented with various stimulus onset asynchronies (SOAs) before the target or in its absence. We reasoned that if the ‘Surprise’ stimulus captured attention, the reallocation of resources toward processing the unexpected event would leave fewer available for detection of the target, causing it to be missed. Owing to the OR’s rapid habituation, we expected that any deficit would endure for only a few trials.
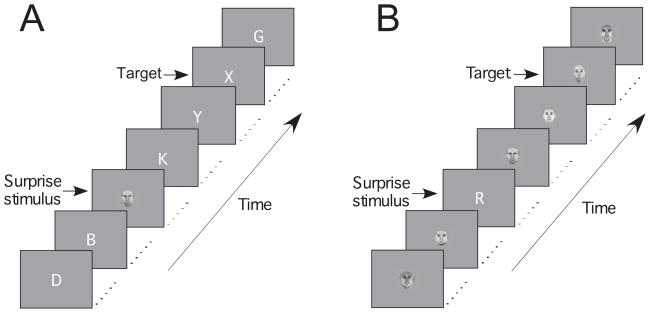
Figure 1.
Trial design of Experiment 1. (A) Half of the participants searched for a target letter in an RSVP of distractor letters. In four of the 30 trials, a Surprise face stimulus was shown before the target. (B) The other half of the participants searched for a target face in an RSVP of distractor faces, with letters serving as Surprise stimuli.
Method
Participants
Forty Vanderbilt University undergraduates (17 males) with normal or corrected-to-normal vision participated for course credit.
Displays
Stimuli were 2.3° × 2.3° light gray faces and white letters presented on a dark gray background. For 20 participants, targets and distractors consisted of faces and Surprise stimuli of letters (both selected from pools of 20). For the 20 other participants, the category assignments were reversed (Fig. 1). These two groups yielded comparable results (Fig. 2A), so their data were combined for most subsequent analyses. Each trial contained an RSVP of 60 items, with each stimulus presented at fixation for 120 ms with a 10 ms inter-stimulus interval. To prevent the perceptual fusion of the serially presented stimuli, the position of each stimulus was randomly jittered by up to 0.3°. The target was shown during 77% of trials, appearing on the 20th, 30th, or 40th frame of the RSVP. Four of the 30 trials of each block (13% of trials) contained a Surprise stimulus. For three of those Surprise stimulus trials, the Surprise stimulus appeared 130 ms, 390 ms, or 780 ms before the target. These SOAs correspond to Lag 1 (wherein the target appears in the frame that immediately follows the Surprise stimulus), Lag 3, and Lag 6, respectively. The fourth Surprise stimulus was presented in a trial that contained no target. Twenty-four of the participants (12 for each category assignment condition) completed nine blocks of trials, while the remaining 16 completed a single block. Except where noted, analyses were limited to the first block of trials, as most effects of interest rapidly habituated.
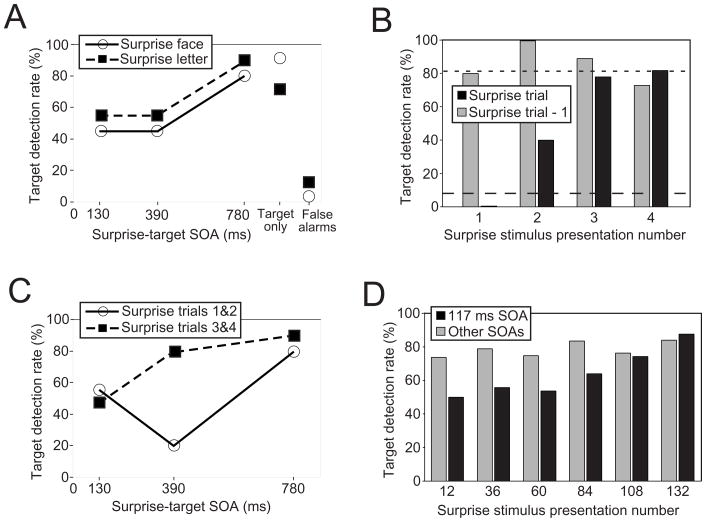
Figure 2.
Results for Experiments 1 and 2. (A) Effect of Surprise stimulus-to-Target SOA on the proportion (%) of participants with correct target detection in the first block of Experiment 1. Results are collapsed across the four Surprise stimulus presentations and shown separately for the Surprise face/Target letter and Surprise letter/Target face experimental groups. (B) Effect of Surprise stimulus presentation number on group target detection performance at the 390 ms Surprise stimulus-to-Target SOA in the first block of Experiment 1. Data are combined across the two experimental groups. Black bars represent performance for Surprise trials; gray bars represent trials immediately preceding Surprise trials. Dotted line indicates Target-only trial performance, while dashed line indicates false alarm rate. (C) Effect of SOA across Surprise stimulus presentation number in the first block of Experiment 1. Data are combined across the two experimental groups. (D) Group target detection performance by block in Experiment 2, plotted as a function of the number of Surprise stimuli that had been observed midway through each block (24 SS per block). “Other SOAs” includes all SS presentations at an SOA other than 117 ms and trials with a target but no SS.
Procedure
Participants initiated each trial by pressing the spacebar. Trials concluded with a response panel (“Target Present or Absent?”) to which participants responded by key press whether the target stimulus, assigned at experiment onset, was absent or present. Before the experiment, participants completed six practice trials at a slower stimulus presentation rate (160 ms per stimulus) followed by six trials at the experimental pace. Auditory feedback was given only during these practice trials. No Surprise stimuli were presented during practice, and participants were not informed about the Surprise stimuli prior to or during the experiment. At the experiment’s conclusion, however, all participants reported the presence of Surprise stimuli, either voluntarily or when asked whether they noticed anything unusual. These reports indicate that participants consciously perceived the Surprise stimuli.
Results and Discussion
Presentation of the Surprise stimuli strongly impaired target detection (Fig. 2A) in the first block of trials, but this effect was dependent on the SOA between the Surprise and target stimuli (Cochran Q test, Q(2) = 12.6, p < 0.01, see Appendix), with poorer group performance for 130 ms and 390 ms SOAs compared to the 780 ms SOA (Fig. 2A; post-hoc sign tests confirmed these differences, p’s < 0.01, see Appendix). Performance for the 780 ms SOA trials was comparable to target-only trials (Fig. 2A), suggesting that target detection was no longer impaired by Lag 6.
The number of Surprise stimuli a participant had observed (presentation number) also influenced target detection performance, a dependency most evident for the 390 ms SOA (Fig. 2B; Omnibus χ2(3) = 17.6, p < 0.001). No participants for whom the first Surprise stimulus appeared 390 ms prior to the target detected the target, and only 40% of participants for whom the second Surprise stimulus appeared with that SOA did so. By contrast, 88% of participants perceived the target when it followed the third or fourth presentation of the Surprise stimulus by 390 ms, and no impairment was detected in subsequent blocks of trials. Pair-wise comparisons revealed that target detection was worse following Surprise Stimulus 1 (SS1) than SS2, SS3, and SS4 (Fisher exact tests, p’s < 0.05), while it was marginally worse following SS2 than SS3 (p = 0.10) and SS4 (p = 0.06). There was no effect of presentation number for the target-only trials immediately preceding the four Surprise trials (Fig. 2B; Omnibus χ2(3) = 3.4, n.s.), ruling out an effect of target detection practice as an account for performance on the Surprise trials. Participants were also not simply learning to associate Surprise stimuli with target presentations, as the target false alarm rate was much lower than the target hit rate for Surprise trials (Sign tests, p’s < 0.001; Fig. 2A).
In contrast to the 390 ms SOA results, target detection with the 130 ms and 780 ms SOAs did not vary across the first four Surprise stimulus presentations (Omnibus χ2(3)’s δ 3.1, n.s.; Fig. 2C). Whereas performance was never affected for the 780 ms SOA, it was evenly impaired across all four Surprise presentations with the 130 ms SOA. This impairment persisted through subsequent blocks of trials, albeit for different lengths of time depending on the identity of the Surprise stimulus. When letters were the Surprise stimuli, the deficit vanished by the third block of trials (mean target performance in letter Surprise trials relative to target-only trials for blocks 3–9: 87% versus 88%; Wilcoxon signed-ranks test, T = 29, n.s.; see Appendix). But with face Surprise stimuli, participants never completely recovered, showing lower performance in every block and a significant overall deficit (mean target performance in face Surprise trials relative to target-only trials for blocks 3–9: 64% versus 91%, T = 5, p < 0.01).
These Lag 1 (130 ms SOA) results reveal a temporal dynamic of target detection performance that is vastly different from the powerful but very short-lived deficit at Lag 3 (390 ms SOA). The differences between the 130 ms and 390 ms SOAs are further explored in the experiments below. Specifically, while Experiments 2 and 3 address the source of the Lag 1 deficit, Experiments 4–7 are aimed at characterizing the Surprise-induced impairment revealed at Lag 3.
Experiment 2: Characterizing the target detection deficit at Lag 1
Experiment 1 revealed an impairment in target detection performance for both the 130 ms and 390 ms SOAs, but while this impairment was highly fleeting for the latter—lasting for only two trials—it persisted for much longer at the shorter SOA. Furthermore, the persistence of the 130 ms SOA deficit depended on stimulus conditions, with a more durable impairment obtained when the Surprise stimulus was a face and the target was a letter than vice versa. By contrast, the deficit at the 390 ms SOA was insensitive to the stimulus conditions. These results suggest that the 130 ms SOA deficit is, at least partly, mechanistically distinct from the 390 ms SOA deficit. The purpose of Experiment 2 was to elucidate the mechanism(s) responsible for the deficit at Lag 1.
We considered three possible accounts for the 130 ms SOA deficit. Given that the Surprise stimulus immediately precedes the target, it may forward mask the target, as forward masking can still be effective up to about 100 ms SOA (Breitmeyer, 1984). Such forward masking could account for the difference in the duration of the Lag 1 deficit in the letter and face Surprise stimulus conditions if a face serves as a more effective forward mask for a letter than vice versa. Alternatively, the lingering 130 ms SOA deficit could result from attention still being captured, albeit in a milder and briefer fashion, by Surprise stimuli well beyond their first two presentations. After all, the Surprise stimuli, even after several blocks of trials, are still rare events, and such rare events have the potential to capture attention (Neo & Chua, 2006; Theeuwes, 2004; Theeuwes & Godjin, 2002; Theeuwes, Atchley, & Kramer, 2000; but see Gibson & Jiang, 1998; Horstmann & Ansorge, 2006). A final account that we considered, which could especially explain the difference in performance between the face and letter Surprise stimuli at the 130 ms SOA, is the special status of faces at capturing attention compared to other objects (Langton, Law, Burton, & Schweinberger, 2008; Devue, Laloyaux, Feyers, Theeuwes, & Brédart, 2009).
To distinguish between these possibilities, we used an experimental design that was similar to Experiment 1 except that the ‘Surprise’ stimuli were scrambled faces that appeared in the majority of trials (75% of trials, compared to 13% in Experiment 1). If the deficit at Lag 1 is due to forward masking rather than to the presence of a rare stimulus, it should be observed even when the stimulus that precedes the target is a frequent event. Similarly, if it is not due to the attention-grabbing power of faces, the deficit should be prolonged even when the Surprise stimulus is not a face.
Method
Fifteen Vanderbilt University undergraduates (5 males) participated for course credit. Stimuli were letters as target/distractors and scrambled faces as Surprise stimuli. The faces were scrambled as a 54-piece tile mosaic using Telegraphic’s scramble filter (http://www.telegraphics.com.au/sw/info/scramble.html) for Adobe Illustrator CS2 (Adobe Systems; San Jose, CA). Each trial contained an RSVP of 50 items, with each item presented at fixation for 100 ms with a 17 ms inter-stimulus interval. There were six blocks of 32 trials comprised of four trial types presented in random order: Target-only (6 trials, accounting for 19% of all trials), Surprise-only (6 trials, 19%), Target+Surprise (18 trials, 56%), and Neither (2 trials, 6%). When present in a trial, the target appeared on either the 20th, 30th, or 40th frame of the RSVP. When present in a trial with a target, the Surprise stimulus appeared 16, 12, 8, 1, −4, or −8 frames before the target (same probability for each SOA). In Surprise-only trials, the Surprise stimulus was presented at one of these SOAs relative to where a target would normally have appeared (frame 20, 30, or 40).
Results and Discussion
The critical SOA (Lag 1, 117 ms SOA) revealed a long-lasting target detection deficit that slowly dissipated across the 144 Surprise stimulus presentations (Fig. 2D; Friedman test for differences across blocks: Q(5) = 13.3, p < 0.05, see Appendix). No other SOAs (or target-only trials) showed such a pattern of results (Friedman tests: all Q(5)’s δ 6.4, n.s.). Performance was lower for the critical 117 ms SOA than for all other SOAs during the first four blocks (two-tailed Wilcoxon signed-rank tests: T’s δ 25 p’s < 0.05 except for block 3 at T = 30, p < 0.10), but not the final two (see Fig. 2D).
As with the 130 ms SOA of Experiment 1, the longevity of the deficit for the 117 ms SOA distinguishes it from the ephemeral SiB. This longevity also argues that the Lag 1 target detection deficit is not a result of the attention-grabbing power of faces, as it was just as strong with scrambled face Surprise stimuli (SS). The Lag 1 deficit, however, did eventually disappear after about 100 SS presentations. This habituation renders the forward masking account unsatisfactory, as forward masking is not known to be so vulnerable to practice effects (Breitmeyer, 1984; Breitmeyer & Ogmen, 2006). Likewise, the ‘attentional capture by a rare event’ account of the Lag 1 deficit is not strongly supported, for the deficit was just as severe and persistent in the present experiment as in Experiment 1 despite the fact that Surprise stimuli were now presented on a majority of the trials. Thus, none of the three accounts examined are entirely consistent with the Lag 1 results of Experiment 2.
Experiment 3: Investigating a singleton account of the Lag 1 deficit
In Experiment 2, increasing the proportion of trials in which a Surprise stimulus is presented by nearly six-fold compared to Experiment 1 did not appreciably affect Lag 1 performance. While these results are not consistent with an ‘attentional capture by a rare event’ account of the Lag 1 deficit, it remains the case that the Surprise stimuli in Experiment 2 were rare occurrences relative to the presentation of other stimuli: Only one of the 50 stimuli shown per trial was a scrambled face; all the others were letters. It is therefore possible that the Surprise stimuli, despite being expected given their frequent trial-to-trial presentations, still captured attention because they ‘popped out’ of the homogenous set of distractor and target stimuli. The goal of Experiment 3 was to examine this possibility. Specifically, we assessed whether the Lag 1 deficit would still be present if face ‘Surprise’ stimuli were now as frequently presented as the standard distractor letter stimuli within each trial. If the enduring Lag 1 deficit was caused by brief attentional capture of the Surprise face/scrambled face singletons in the first two experiments, then this deficit should be absent from Experiment 3.
Method
Twelve members of the Vanderbilt University community (6 females) participated for cash payment. The experimental design was identical to Experiment 2’s except for the following changes. Participants were presented with a 35-item RSVP (117 ms per frame) containing an equal number of distractor faces and letters that appeared in a pseudorandom sequence (3 of each every 6 stimuli). When present (on 80% of trials), the target ‘X’ appeared between items 12 and 32. The crucial manipulation involved the stimulus types that temporally flanked the target, resulting in four target-present conditions (T = target, F = face, L = letter): LTL, LTF, FTL, FTF. Each of the six blocks included six trials of each of these four conditions as well as six no-target trials, all randomly intermixed.
Results and Discussion
Target detection performance in each condition was as follows; LTL: 82.9 ± 2.9%, LTF: 88.2 ± 2.3%, FTL: 79.2 ± 3.3%, FTF: 92.6 ± 3.2%, No Target (false alarms): 6.7 ± 2.2% (errors are standard errors of the mean). There were no effects of block number on target performance in any of these four conditions (Friedman tests: Q(5)’s ≤ 6.8, n.s.). Target detection was worse, however, when the letter target was followed by a letter mask than a face mask (main effect of trailing mask type in two-way ANOVA: F(1, 11) = 12.1, p < 0.01), suggesting that letter masks were, unsurprisingly, more effective backwards masks of the featurally similar targets (Breitmeyer, 1984; Breitmeyer & Ogmen, 2006). Most importantly, target performance was no worse when the target was preceded by a face stimulus than when preceded by a letter stimulus (main effect of preceding mask type: F(1, 11) = 0.027, n.s.). These results indicate that the Lag 1 target deficit is not present when face stimuli occur frequently within each trial. We therefore conclude that the long-lived Lag 1 deficit observed in Experiments 1 and 2 is primarily caused by the Surprise stimuli briefly capturing attention as a result of their status as salient item singletons in the RSVP streams.
In sum, the results of the first three experiments indicate that the presentation of Surprise stimuli impair target detection performance at both short (Lag 1) and middle (Lag 3) lags, but not at long lags (Lag 6, 780 ms SOA). Although the target deficits at short and middle lags are both attention-related, they do not appear to be merely different temporal manifestations of the same capacity-limited process. The Lag 1 deficit is relatively mild but long lasting, with the duration highly dependent on the identity of the Surprise stimulus. By contrast, the Lag 3 impairment is powerful but very fleeting, and is independent of the stimulus’ featural identity. These different characteristics suggest that the Lag 1 deficit may have underlying mechanisms that are partly distinct from the powerful but ephemeral Surprise-induced Blindness revealed at Lag 3. In the remaining experiments of this study, we aim to further understand SiB by examining its characteristics at the critical 390 ms SOA.
Experiment 4: Effect of eye blinks/movements
Abrupt, unexpected stimuli can provoke startle-induced eye blinks (Dawson, Schell, & Böhmelt, 1999). Accordingly, we examined whether the performance deficit observed for the 390 ms SOA in Experiment 1 could be explained by eye blinks/movements caused by the startling effect of Surprise stimulus presentations.
Method
Twenty-three undergraduates (19 females) participated for course credit. The procedure was identical to Experiment 1 (letter targets/distractors and face Surprise stimuli), except that eye blinks/movements were monitored throughout the experiment and six of 52 trials contained a Surprise face stimulus presented with a 390 ms SOA. The Surprise trials occurred randomly after the sixth trial. The eyes were monitored using the ViewPoint Eyetracker chin-rest system (Arrington Research; Scottsdale, AZ) and by video recording. A deviation greater than 2° from fixation was considered an eye movement.
Results and Discussion
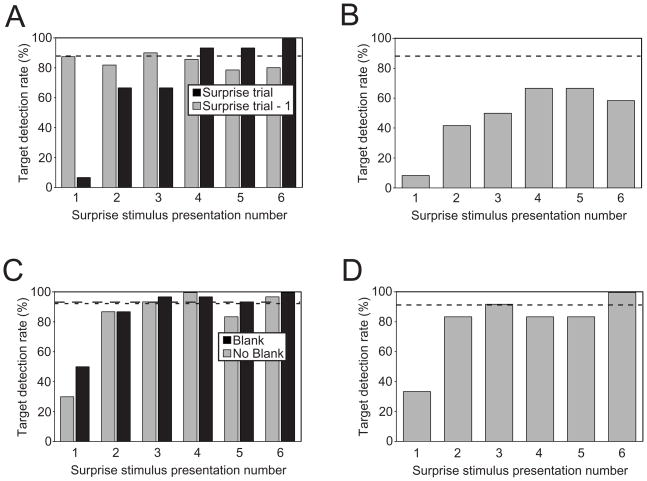
Eye blinks/movements during target presentations were rare, occurring during 11% of all Surprise trials. Moreover, their occurrence was not related to the number of Surprise stimuli (Cochran Q(5) = 0.8, n.s.); that is, eye blinks/movements appeared to occur randomly across the Surprise trials. Of the 23 participants, eight experienced at least one eye blink/movement during the target presentations in a Surprise trial. The majority of participants (15 of 23) experienced no eye blinks during target presentation in Surprise trials, yet their target detection performance still differed across the six Surprise stimulus presentations (Q(5) = 39.8, p < 0.0001). Specifically, only one of the 15 participants detected the target following the first Surprise stimulus (SS1), whereas all participants correctly identified the target following the last Surprise stimulus (Fig. 3A). Target detection was also poorer following SS1 than all others (Sign tests, p’s < 0.05). In contrast, there were no performance differences between the target-only trials immediately preceding the six Surprise trials as a function of presentation number (Fig. 3A). These results replicate our initial finding of a profound but short-lived perceptual deficit following Surprise stimulus presentations and rule out the possibility that this deficit resulted from startle-induced eye blinks/movements.
Figure 3.
(A) Group target detection performance in Experiment 4. Broken line corresponds to the average target hit rate in Target-only trials. (B) Group target detection performance in Experiment 5. (C) Group target detection performance in Experiment 6. Black bars represent participants’ performance for task with no blank interval following the Surprise stimuli; gray bars represent participants’ performance for task with a 130 ms blank interval following each Surprise stimulus. Dashed and dotted lines correspond to the average target hit rate for Target-only trials in the No-Blank and Blank tasks, respectively. (D) Group target performance in Experiment 7.
Experiment 5: Habituation of Surprise-induced Blindness
The rapid recovery from Surprise-induced Blindness could reflect participants’ developing an expectation about the presentation of task-irrelevant stimuli, thereby allowing such stimuli to be filtered out or ignored. Alternatively, since the Surprise stimuli were selected from a homogeneous set (either letters or faces) in Experiments 1 and 4, the recovery could reflect perceptual or semantic habituation to the repeated presentation of the same stimulus type. We distinguished between these possibilities in Experiment 5 by presenting a heterogeneous set of six Surprise stimuli (see Fig. 4).
Figure 4.

The six Surprise stimuli used in Experiment 5.
Method
Experiment 5 was identical to Experiment 4 with the following exceptions: Twelve participants (6 males) were presented with Surprise stimuli consisting of six distinct colorful visual images (Fig. 4) that subtended the same visual angle as the targets.
Results and Discussion
This heterogeneous Surprise stimulus set still showed an effect of presentation number (Fig. 3B; Q(5) = 14.3, p < 0.05). Target detection was lower following the first Surprise stimulus than SS4 and SS5 (Sign test, p’s < 0.05), and marginally so following SS3 (p = 0.06) and SS6 (p = 0.07). Interestingly, although target detection performance stabilized by SS4 (Sign tests, p’s > 0.1), as it had for the 390 ms SOA in previous experiments, it did so at a level of performance that was not only below ceiling, but also below the average performance for trials with no Surprise stimuli (Fig. 3B; Wilcoxon signed-ranks test comparing mean participant performance in target-only trials with performance in last three Surprise stimulus trials, T = 7, p < 0.05). Evidently, the presentation of highly distinct Surprise stimuli prevented the development of an expectation about the identity of any given stimulus, thereby allowing each one to capture some attention for stimulus evaluation (Kahneman, 1973). Nevertheless, this persistent impairment was significantly smaller than the deficit observed with the first few Surprise stimuli. Thus, the characteristic profound initial impairment followed by a quick—albeit incomplete—recovery is still evidenced here.
Experiment 6: Contribution of Trailing Distractor
Surprise-induced Blindness and the Attentional Blink share many important similarities but also have crucial differences. Both phenomena consist of a half-second deficit in conscious target perception following the presentation of an attention-demanding stimulus—another target in the AB and a Surprise stimulus in SiB. Another similarity is superior target detection performance at Lag 1. For the first Surprise stimulus presentation in Experiment 1, Lag 1 target detection (60%) was higher than Lag 3’s (0%), a result that is reminiscent of the AB’s Lag-1 sparing in which the second target is readily perceived when it immediately follows the first target (Raymond et al., 1992; Chun & Potter, 1995; Di Lollo, Kawahara, Ghorashi, & Enns, 2005; Olivers, van der Stigchel, & Hulleman, 2007; Nieuwenstein, Potter, & Theeuwes, 2009). At least one significant distinction between these two deficits of explicit perception does exist, however: The short trial-to-trial lifespan of SiB contrasts markedly with the robustness of the AB, which can last for hundreds of trials (Chun & Potter, 1995; Shapiro, Arnell, & Raymond, 1997).
To further explore the relationship between these two perceptual deficits, we tested whether a manipulation that is known to affect the AB would also affect SiB. The AB is attenuated when the distractor following the first target (T1) is replaced by a blank interval (Breitmeyer, Ehrenstein, Pritchard, Hiscock, & Crisan, 1999; Chun & Potter, 1995; Raymond et al., 1992; Nieuwenstein et al., 2009). This distractor is thought either to interfere with identification of T1, thereby increasing T1’s attentional demands at the expense of T2 (Chun & Potter, 1995; Jolicoeur, Dell’Acqua, & Crebolder, 2001; Nieuwenstein et al., 2009), or to alter top-down settings for the detection of subsequent targets (Di Lollo et al., 2005; Olivers et al., 2007). Given that the Surprise stimulus plays an analogous role to T1 in the classic AB paradigm, here we investigated whether the distractor that follows the Surprise stimulus strongly affects SiB performance by replacing this distractor with a blank interval. If the distractor interferes with Surprise stimulus processing or alters top-down attentional settings, one would expect to find attenuation of the SiB deficit. Alternatively, if the Surprise stimulus itself generates the deficit, removal of the distractor should have little or no effect on target detection.
Method
Experiment 6 was identical to Experiment 4 with the following exceptions: For half of the 60 participants (36 females) the distractor letter immediately following each of the Surprise face stimuli was replaced by a blank of the same duration (130 ms). For the remaining participants, a distractor immediately followed each Surprise stimulus (control condition).
Results and Discussion
Surprise-induced Blindness was still observed in the blank interval condition (Q(5) = 46.3, p < 0.0001), with fewer participants detecting the target following the first Surprise stimulus presentation than targets following subsequent presentations (Sign tests, p’s < 0.01; Fig. 3C). This impairment was not caused by the 130 ms interruption of the RSVP stream; when we substituted a 130 ms blank interval for the Surprise stimulus in a separate group of five participants, all participants detected the target in these ‘Blank’ trials. Thus, it was the Surprise stimulus alone that induced SiB. Most importantly, the magnitude of SiB here was not different from that observed in the group of participants for whom the Surprise stimulus was followed by a distractor (Fisher’s exact test for independent samples for SS1, p = 0.19), although there was a non-significant tendency for a reduced target deficit when SS1 was not followed by a distractor (Fig. 3C). Thus, the removal of the distractor immediately following the Surprise stimulus has, at best, a modest effect on Surprise-induced Blindness. Regardless of how the T1+1 distractor modulates the AB (Chun & Potter, 1995; Jolicoeur, et al., 2001; Di Lollo et al., 2005; Olivers et al., 2007; Nieuwenstein et al., 2009), these results suggest that the trailing distractor is far less important to SiB than it is to the AB. This experiment therefore provides additional evidence that the SiB may have an underlying mechanism partly distinct from the AB.
Experiment 7: Effect of prior expectation
Although the Surprise stimuli captured attention in a ‘bottom-up’ manner, it is conceivable that participants also engaged in goal-directed exploration of these stimuli. Such ‘top-down’ exploration could therefore have contributed to SiB. To test this hypothesis, we informed participants in Experiment 7 that they would occasionally see irrelevant face stimuli that should be ignored. If SiB represents a limitation in goal-directed attention, instructing participants to maintain their attention on the target detection task should alleviate the deficit.
Method
Experiment 7 was identical to Experiment 4 with the following exceptions: Twelve participants (5 males) were specifically instructed that they would see task-irrelevant faces during the experiment and that they were to ignore these stimuli.
Results and Discussion
Despite the instructions to disregard face stimuli, SiB was still observed (Fig. 3D); target detection varied across SS presentation number (Q(5) = 20.1, p < 0.01), and fewer participants detected the target after the first Surprise stimulus than after all other Surprise stimuli (Sign tests, p’s < 0.05). Furthermore, the SiB deficit was similar to those observed in comparable experiments in which participants were not informed about the Surprise stimuli (Fisher tests comparing SS1 and SS2 across Experiments 4, 6 (No Blank condition), and 7: all p’s > 0.10). Indeed, the behavioral performance in this experiment was almost identical to the No Blank condition of Experiment 6 (SS1: 33% versus 30%, SS2: 83% versus 87%). These results demonstrate that instructions to ignore Surprise stimuli are not an effective remedy for SiB, suggesting that stimulus-driven capture, not goal-directed exploration, is responsible for the deficit. By the same token, the results reveal that the presentation of a Surprise stimulus compels reallocation of attentional resources despite running counter to participants’ explicit task-related goals of detecting targets and ignoring Surprise stimuli. Such initial inability to exert top-down control over one’s attention is reminiscent of the reaction time costs that persist even when participants are given sufficient time to switch between known task sets (Rogers & Monsell, 1995; Allport, Styles, & Hsieh, 1994). Rogers & Monsell (1995) argue that a component of task reconfiguration must be triggered exogenously, perhaps akin to SiB suppression being possible only after a Surprise stimulus is observed in the RSVP stream context. This similarity does not imply, however, that the exogenous triggers affect performance via the same mechanism in task switching and SiB paradigms.
General Discussion
The presentation of a novel and unexpected stimulus has long been known to attract attention, compelling a reallocation of resources that is part of the orienting response (Kahneman, 1973; Sokolov, 1963; Sokolov et al., 2002). Although the OR facilitates the rapid and thorough evaluation of these unexpected events, here we reveal that it incurs a significant cost: a profound but temporary impairment in perceiving other visual events. While it remains to be determined whether this ‘Surprise-induced Blindness’ is caused by the Surprise stimulus demanding additional resources or engaging these resources for a longer duration, both of these potential causes are consistent with an attentional capture account (Simons, 2000; Maki & Mebane, 2006; Folk et al., 2002; Horstmann, 2002; Horstmann & Becker, 2008; Theeuwes, 2004; Theeuwes et al., 2000; Theeuwes & Godjin, 2002; Wee & Chua, 2004; Neo & Chua, 2006). Likewise, regardless of whether the target detection impairment results from the Surprise stimulus requiring processing resources to a greater or longer extent than other stimuli, the results are expected to be the same; because attention is unavailable to be deployed for the processing of subsequent events, these events are rendered vulnerable to decay before they reach awareness. As such, Surprise-induced Blindness makes a unique contribution to a growing literature illustrating the importance of attention for conscious perception (Broadbent, 1957; Chun & Marois, 2002; Chun & Potter, 1995; Dehaene, Changeux, Naccache, Sackur, & Sergent, 2006; Enns & Di Lollo, 1997; He, Cavanaugh, & Intrilligator, 1996; Kanwisher, 1987; Mack & Rock, 1998; Most, Simons, Scholl, Jimenez, Clifford, & Chabris, 2001; Raymond et al., 1992; Rensink, O’Regan, & Clark, 1997; Simons, 2000) by demonstrating that deficits of awareness can arise in a stimulus-driven fashion.
Our study has revealed two different temporal manifestations of target detection deficits caused by the presentations of Surprise stimuli. The first, occurring at Lag 1, is relatively mild but long lasting, with a lifespan highly dependent on the featural identity of the Surprise stimulus. This deficit appears to be caused by the presentation of a stimulus that featurally ‘pops out’ from the other items in the RSVP stream. By contrast, the powerful Lag 3 impairment is contingent on the contextual novelty of the Surprise stimulus, but is relatively insensitive to its featural content. This latter deficit, which lasts for only the first few Surprise events, is what we have called Surprise-induced Blindness (SiB), for it occurs when expectations about the occurrence of the surprising events are either absent (Expts 1, 4–6) or imprecise (Expt. 7). While these results suggest that there are important differences between the Lag 1 and Lag 3 deficits, they do not imply that these deficits are mechanistically independent. Rather, it is likely that both impairments result from the effects of attentional capture triggered by the Surprise stimulus, only with attention being captured to a greater and longer extent by a novel and unexpected stimulus (as evidenced at Lag 3) than by an infrequent and featurally distinct stimulus (as evidenced at Lag 1).
Of all attentional phenomena, the AB shares the most features with SiB. In particular, the time courses of these two perceptual deficits are similar, with a profound impairment that peaks around 200–400 ms following the Surprise stimulus (for SiB) or first target (for AB) and recovers by 700–800 ms (Chun & Potter, 1995; Raymond et al., 1992). On the other hand, these two deficits differ markedly in their lifespan across successive trials. In a typical AB experiment, the impairment persists over hundreds of trials (Chun & Potter, 1995), even when participants search for the same T1 (Shapiro & Raymond, 1994). In an SiB experiment, the deficit disappears entirely after the first few presentations of similar Surprise stimuli. In addition, whereas removal of the distractor immediately following the first target dramatically attenuates the AB, SiB is mildly affected by excision of the Surprise stimulus’ trailing distractor. Finally, SiB and the AB appear to impose differential demands on stimulus-driven and goal-directed attention. The attentional blink paradigm stresses goal-directed attention, as an AB is only obtained when the inducing stimulus is goal-relevant or shares features with goal-relevant items (Folk et al., 2002; Ghorashi et al., 2003; Jiang & Chun, 2001; Maki & Mebane, 2006; Visser et al., 2004; Wee & Chua, 2004; Spalek et al., 2006). SiB summons stimulus-driven attention, as the Surprise stimulus is novel, unexpected, and task-irrelevant. In support of a stimulus-driven origin of the SiB, we recently observed that this deficit correlates, both in magnitude and lifespan, with activity in brain regions supporting stimulus-driven attention, but not with activity in those areas largely associated with goal-directed attention (Asplund, Todd, Snyder, & Marois, in press). Neuroimaging studies of the AB have not observed such a dissociation, instead implicating both stimulus-driven and goal-directed attention regions (Marois et al., 2000, 2004; Kranczioch et al., 2005, but see Shapiro et al., 2002). We therefore conclude that although Surprise-induced Blindness and the Attentional Blink are likely to be mechanistically related, SiB is not merely a fleeting form of the AB.
AB-like target detection deficits had previously been observed with task-irrelevant stimuli, but only when such stimuli were emotionally-laden (Most et al., 2005; Smith et al., 2006), shared defining properties with the goal-relevant target (Folk et al., 2002; Ghorashi et al., 2003; Jiang & Chun, 2001; Maki & Mebane, 2006; Visser et al., 2004; Wee & Chua, 2004; Spalek et al., 2006), or appeared in a different location from the target (Horstmann & Becker, 2008; Wee & Chua, 2004). Otherwise, task-irrelevant stimuli, even a salient singleton on its first presentation, caused no such impairment (Gibson & Jiang, 1998; Horstmann, 2002; Horstmann & Ansorge, 2006). At best, singleton or Surprise stimuli have been observed to cause small reaction time costs (Dalton & Lavie, 2006; Gronau et al., 2006), though it remains to be seen whether these RT costs also rapidly habituate.
In contrast to these studies, our results demonstrate that unexpected, task-irrelevant stimuli that share no diagnostic features with the target can nevertheless profoundly impair target detection, implying robust attentional capture (Horstmann, 2002; Horstmann & Becker, 2008; Theeuwes, 2004; Theeuwes et al., 2000; Theeuwes & Godijn, 2002; Wee & Chua, 2004). We surmise that SiB may have been present in previous investigations of attentional capture’s effect on conscious target perception, but given the fleeting nature of this deficit, it may have been undetected because target performance was averaged across the entire experimental session (Ghorashi et al., 2003; Horstmann & Ansorge, 2006; Maki & Mebane, 2006; Most et al., 2005; Visser et al., 2004; Dalton & Lavie, 2006). Moreover, the few studies that did examine the effect of the first few singleton presentations (Horstmann, 2002; Wee & Chua, 2004) may have failed to observe SiB because their stimuli captured attention too briefly (on the order of 150–200 ms) to significantly affect subsequent target performance (Maki & Mebane, 2006; Theeuwes et al., 2000). Along that line, it is interesting to note that the timing of this brief attentional capture is within the range of the Lag 1 (117 and 130 ms SOAs) deficit observed in Experiments 1–3. We therefore conclude that an abridged form of attentional capture can follow the presentation of singletons in RSVPs. This brief capture is rather different from the strong attentional capture triggered by the first two presentations of Surprise stimuli, which is sufficiently powerful to disrupt detection of a target 390 ms after the capturing stimulus.
While the SiB paradigm reveals a powerful form of attentional capture, the effect is also highly fleeting, for capture all but vanishes by the third Surprise stimulus presentation. This characteristic sharply contrasts with the enduring deficits observed in the contingent-capture AB (Folk et al., 2002; Jiang & Chun, 2001) and the affective AB (Most et al., 2005; Smith et al., 2006). How might such rapid adaptation to Surprise stimulus presentation be implemented? Following Sokolov’s interpretation of the OR (Sokolov et al., 2002), we hypothesize that stimulus-driven attention is summoned by a mismatch signal generated whenever a presented stimulus violates one’s expectations. With successive ‘Surprise’ stimulus presentations, participants learn to expect these stimuli, thereby reducing the mismatch signal and capture effects. But regardless of the precise mechanisms that cause SiB’s rapid habituation, our results reveal an important function of such habituation: It allows our cognitive system to ignore behaviorally inconsequential events, permitting limited attentional resources to be freed from such events and therefore available for whatever the world may next throw at us.
Acknowledgments
We thank Isabel Gauthier and Gordon Logan for helpful comments on earlier versions of this manuscript, Ellie Conser for assistance with the experiments, and Anne Hillstrom, Molly Potter, and Brad Wyble for useful criticisms. This work was supported by National Science Foundation grant 0094992 and NIMH grant R01 MH70776 to R.M.
Appendix
Statistical analyses required several parametric and non-parametric tests. To assess the effect of SOA on target detection performance (Experiment 1) and the effect of repeated Surprise stimulus presentations (Experiments 4–7), we used Cochran Q tests for categorical data of dependent samples, employed because each participant provided a single data point for each condition (Sheskin, 2000). We then applied Sign tests—non-parametric exact tests that assess differences between two dependent samples of categorical data (Abdi, 2007)—to determine the significance of the relevant pair-wise comparisons. In Experiment 1, order effects were assessed using Pearson’s chi-square tests for independent samples because each participant saw a single order of presented SOAs. In Experiments 2 and 3, performance across blocks was compared using non-parametric Friedman tests for repeated measures because each participant provided multiple data points, but not enough to ensure a roughly normal distribution of the data. Wilcoxon signed-rank tests were also employed to determine the statistical significance of the comparisons between the critical and control trials (Experiments 1, 2 and 5).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/xhp
References
- Abdi H. Binomial Distribution: Binomial and Sign Tests. In: Salkind NJ, editor. Encyclopedia of Measurement and Statistics. Thousand Oaks (CA): Sage; 2007. [Google Scholar]
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance XV: Conscious and nonconscious information processing. Cambridge (MA): MIT Press; 1994. [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nature Neuroscience. doi: 10.1038/nn.2509. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer B. Visual masking: An integrative approach. Oxford: Oxford University Press; 1984. [Google Scholar]
- Breitmeyer BG, Ehrenstein A, Pritchard K, Hiscock M, Crisan J. The roles of location specificity and masking mechanisms in the attentional blink. Perception & Psychophysics. 1999;61(5):798–809. doi: 10.3758/bf03206898. [DOI] [PubMed] [Google Scholar]
- Breitmeyer B, Ogmen H. Visual masking: Time slices through conscious and unconscious vision. 2. New York: Oxford University Press; 2006. [Google Scholar]
- Broadbent DE. A mechanical model for human attention and immediate memory. Psychological Review. 1957;64:205–215. doi: 10.1037/h0047313. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MH. From detection to identification: Response to multiple targets in rapid serial visual presentation. Perception & Psychophysics. 1987;42(2):105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Chun MM, Marois R. The dark side of visual attention. Current Opinion in Neurobiology. 2002;12(2):184–189. doi: 10.1016/s0959-4388(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(1):109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Dalton P, Lavie N. Temporal attentional capture: Effects of irrelevant singletons on rapid serial visual search. Psychonomic Bulletin & Review. 2006;13(5):881–885. doi: 10.3758/bf03194013. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species by means of natural selection. Peterborough, Ontario: Broadview Press; 1859/2003. [Google Scholar]
- Dawson ME, Schell AM, Böhmelt A. Startle Modification: Implications for neuroscience, cognitive science, and clinical science. Cambridge, England: Cambridge University Press; 1999. [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends in Cognitive Sciences. 2006;10(5):204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Devue C, Laloyaux C, Feyers D, Theeuwes J, Brédart S. Do pictures of faces, and which ones, capture attention in the inattentional-blindness paradigm? Perception. 2009;38(4):552–568. doi: 10.1068/p6049. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J-i, Ghorashi SMS, Enns JT. The attentional blink: Resource depletion or temporary loss of control? Psychological Research. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: Control, representation, and time course. Annual Review of Psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Enns JT, Di Lollo V. Object substitution: A new form of masking in unattended visual locations. Psychological Science. 1997;8(2):135–139. [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics. 2002;64:741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Ghorashi SM, Zuvic SM, Visser TA, Di Lollo V. Focal distraction: spatial shifts of attentional focus are not required for contingent capture. Journal of Experimental Psychology: Human Perception & Performance. 2003;29(1):78–91. [PubMed] [Google Scholar]
- Gibson BS, Jiang Y. Surprise! An unexpected color singleton does not capture attention in visual search. Psychological Science. 1998;9:176–182. [Google Scholar]
- Gronau N, Sequerra E, Cohen A, Ben-Shakhar G. The effect of novel distractors on performance in focused attention tasks: A cognitive-psychophysical approach. Psychonomic Bulletin & Review. 2006;13(4):570–575. doi: 10.3758/bf03193964. [DOI] [PubMed] [Google Scholar]
- He S, Cavanaugh P, Intrilligator J. Attentional resolution and the locus of visual awareness. Nature. 1996;383:334–337. doi: 10.1038/383334a0. [DOI] [PubMed] [Google Scholar]
- Horstmann G. Evidence for attentional capture by a surprising color singleton in visual search. Psychological Science. 2002;13(6):499–505. doi: 10.1111/1467-9280.00488. [DOI] [PubMed] [Google Scholar]
- Horstmann G, Ansorge U. Attentional shifts to rare singletons. Visual Cognition. 2006;14(3):295–325. [Google Scholar]
- Horstmann G, Becker SI. Effects of stimulus-onset asynchrony and display duration on implicit and explicit measures of attentional capture by a surprising singleton. Visual Cognition. 2008;16(2/3):290–306. [Google Scholar]
- Jiang Y, Chun MM. The influence of temporal selection on spatial selection and distractor interference: An attentional blink study. Journal of Experimental Psychology: Human Perception & Performance. 2001;27(3):664–679. doi: 10.1037//0096-1523.27.3.664. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Dell’ Acqua R, Crebolder JM. The attentional blink bottleneck. In: Shapiro K, editor. The limits of attention: temporal constraints in human information processing. New York: OU Press; 2001. pp. 82–99. [Google Scholar]
- Kahneman D. Attention and effort. New York: Prentice Hall; 1973. [Google Scholar]
- Kanwisher N. Repetition blindness: Type recognition without token individuation. Cognition. 1987;27(2):117–143. doi: 10.1016/0010-0277(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Schwarzbach J, Goebel R, Engel AK. Neural correlates of conscious perception in the attentional blink. NeuroImage. 2005;24:704–714. doi: 10.1016/j.neuroimage.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Langton SRH, Law AS, Burton AM, Schweinberger SR. Attention capture by faces. Cognition. 2008;107:330–342. doi: 10.1016/j.cognition.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Mack A, Rock I. Inattentional Blindness. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Maki WS, Mebane MW. Attentional capture triggers an attentional blink. Psychonomic Bulletin & Review. 2006;13:125–131. doi: 10.3758/bf03193823. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of Information processing in the brain. Trends Cogn Sci. 2005;9:296–304. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Marois R, Yi DJ, Chun MM. The neural fate of perceived and missed events in the attentional blink. Neuron. 2004;41(3):465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Meyer WU, Niepel M, Rudolph U, Schützwohl A. An experimental analysis of surprise. Cognition and Emotion. 1991;5:295–311. [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Most SB, Simons DJ, Scholl BJ, Jimenez R, Clifford E, Chabris CF. How not to be seen: The contribution of similarity and selective ignoring to sustained inattentional blindness. Psychological Science. 2001;12(1):9–17. doi: 10.1111/1467-9280.00303. [DOI] [PubMed] [Google Scholar]
- Neo G, Chua FK. Capturing focused attention. Perception & Psychophysics. 2006;68(8):1286–1296. doi: 10.3758/bf03193728. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC, Theeuwes J. Unmasking the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2009;35(1):159–169. doi: 10.1037/0096-1523.35.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL, van der Stigchel S, Hulleman Johan. Spreading the sparing: Against a limited-capacity account of the attentional blink. Psychological Research. 2007;71(2):126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford: Oxford University Press; 1927. [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception & Performance. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rensink RA, O’Regan JK, Clark JJ. To see or not to see: The need for attention to perceive changes in scenes. Psychological Science. 1997;8(5):368–373. [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124(2):207–231. [Google Scholar]
- Sechenov IM. Reflexes of the brain. Cambridge, MA: M. I. T. Press; 1863/1965. [Google Scholar]
- Shapiro KL, Arnell KM, Raymond JE. The attentional blink. Trends in Cognitive Science. 1997;1(8):291–296. doi: 10.1016/S1364-6613(97)01094-2. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Hillstrom AP, Husain M. Control of visuotemporal attetion by inferior parietal and superior temporal cortex. Current Biology. 2002;12:1320–1325. doi: 10.1016/s0960-9822(02)01040-0. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Raymond JE. Temporal allocation of visual attention: Inhibition or interference? In: Dagenbach D, Carr TH, editors. Inhibitory mechanisms in attention, memory and language. Boston, MA: Academic Press; 1994. pp. 151–188. [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. 2. Boca Raton, FL: CRC Press; 2000. [Google Scholar]
- Siddle DAT. The orienting response and distraction. Australian Journal of Psychology. 1971;23:261–265. [Google Scholar]
- Siddle DAT, Mangan GL. Arousability and individual differences in resistance to distraction. Journal of Experimental Research in Personality. 1971;5:295–303. [Google Scholar]
- Simons DJ. Attentional capture and inattentional blindness. Trends in Cognitive Science. 2000;4(4):147–156. doi: 10.1016/s1364-6613(00)01455-8. [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, Zald DH. An “emotional blink” of attention elicited by aversively conditioned stimuli. Emotion. 2006;6:523–527. doi: 10.1037/1528-3542.6.3.523. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. New York: Pergamon Press; 1963. [Google Scholar]
- Sokolov EN. The neuronal mechanisms of the orienting reflex. In: Sokolov EN, Vinogradova OS, editors. Neuronal mechanisms of the orienting reflex. Hillsdale, NJ: Erlbaum; 1975. pp. 217–235. [Google Scholar]
- Sokolov EN, Spinks JA, Näätänen R, Lyytinen H. The Orienting Response in Information Processing. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Spalek TM, Falcon LJ, Di Lollo V. Attentional blink and attentional caputer: Endogenous versus exogenous control over paying attention to two important events in close succession. Perception & Psychophysics. 2006;68(4):674–684. doi: 10.3758/bf03208767. [DOI] [PubMed] [Google Scholar]
- Spinks JA, Siddle D. The functional significance of the orienting response. Chichester, England: John Wiley & Sons, Ltd; 1983. [Google Scholar]
- Theeuwes J. Top-down search strategies cannot override attentional capture. Psychonomic Bulletin & Review. 2004;11(1):65–70. doi: 10.3758/bf03206462. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Atchley P, Kramer AF. On the time course of top-down and bottom-up control of visual attention. In: Monsell S, Driver J, editors. Control of cognitive proceses: Attention and performance XVIII. Cambridge, MA: MIT Press; 2000. pp. 105–124. [Google Scholar]
- Theeuwes J, Godijn R. Irrelevant singletons capture attention: Evidence from inhibition of return. Perception and Psychophysics. 2002;64(5):764–770. doi: 10.3758/bf03194743. [DOI] [PubMed] [Google Scholar]
- Visser TAW, Bischof WF, Di Lollo V. Rapid serial visual distraction: Task-irrelevant items can produce an attentional blink. Perception & Psychophysics. 2004;66:1418–1432. doi: 10.3758/bf03195008. [DOI] [PubMed] [Google Scholar]
- Wee S, Chua FK. Capturing attention when attention “blinks”. Journal of Experimental Psychology: Human Perception & Performance. 2004;30:598–612. doi: 10.1037/0096-1523.30.3.598. [DOI] [PubMed] [Google Scholar]
- Weichselgartner E, Sperling G. Dynamics of automatic and controlled visual attention. Science. 1987;238:778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]