Abstract
Objectives
To develop a method for drug dosing and pharmacokinetic (PK) sampling in children with cancer from a single indwelling central venous catheter that minimized drug contamination.
Methods
A bench top system was designed to simulate dosing and clearing actinomycin-D (AMD) and vincristine (VCR) from central venous catheters. The authors evaluated the effects of flush volume, composition, and pH, timed drug instillation, and number of blood-draw return cycles on residual drug concentrations. A proof-of-principle study was conducted in three pediatric cancer patients with paired PK samples obtained via both central and peripheral catheters.
Results
Nearly complete removal of drug from catheter was obtained after five blood-draw return cycles consisting of 5 mL of whole blood. Residual concentration of AMD was 0.18 ± 0.02 ng/mL or 0.16% of the initial infusion concentration. VCR exhibited lower propensity for catheter adsorption than AMD with residual concentrations undetectable after three blood-draw return cycles. In patients in which the clearance procedure was utilized, higher drug concentrations were generally observed from centrally cleared samples at most time points, but differences relative to peripherally-obtained samples were not statistically significant for either AMD or VCR. Two out of three patients had higher exposure for AMD based on PK samples obtained from central catheter, whereas exposure for VCR was similar for both sampling catheters in all patients.
Conclusions
A reliable procedure to efficiently reduce AMD and VCR contamination during PK sampling has been established and is currently being used in a PK study being conducted by the Children’s Oncology Group.
Keywords: Actinomycin-D, vincristine, catheter, pediatric, pharmacokinetics
INTRODUCTION
For children with cancer, pharmacokinetic (PK) studies have a significant role in studying and deriving optimal dosing strategies for both established as well as new agents. Most children with cancer are treated with intravenous chemotherapeutics administered through an indwelling central venous catheter. For PK studies, in order to avoid specimen contamination by residual drug concentration, sampling is typically performed through a separate intravenous catheter. Residual concentration refers to the amount of drug quantified in the final blood sample following the clearing procedure that represents in part the “residual” attached to the catheter lumen. Although the placement of a peripheral intravenous catheter represents a minimal risk or a minor increase over minimal risk, the discomfort and pain associated with the additional catheter requirement often presents a barrier for participation in pediatric PK studies.
Several studies suggest that dosing and sampling from a single indwelling catheter can result in falsely elevated plasma drug concentrations due to adsorption of drug molecules onto the catheter lumen.1-6 The discrepancy between drug concentrations sampled through a central venous catheter and a peripheral intravenous catheter can be significant, but simple flushing of the central catheter does not eliminate drug adsorption. A limited number of approaches have been undertaken by other investigators in efforts to circumvent this problem. Evaluation of flushing solutions of various volumes through catheters dosed with high concentration of digoxin, aminophylline, and phenytoin showed almost complete clearance after a 5 mL flush followed by a 5 mL blood discard.7 Analysis of tobramycin demonstrated similar drug concentrations from analyzed samples obtained simultaneously by venipuncture and by indwelling Hickman® catheters following a 30 minute flush,8 and studies of 2-deoxy-2-[18F] fluoro-glucose (FDG) in patients undergoing positron emissions tomography have shown minimal to no increase in radioactivity in samples obtained from the FDG administration catheter following a 10 mL saline flush, t-port replacement, and 1 mL blood discard.9
Although these studies suggest that for certain drugs clearing procedures may minimize contamination, there are currently no uniformly accepted approaches that reliably address this challenge. Our laboratory has conducted a pilot study to quantify the PK of actinomycin-D (AMD) and vincristine (VCR) in children with cancer. Despite its longstanding and widespread use in treating rhabdomyosarcoma and Wilms tumor,10 there is very little PK information on AMD from which safe and appropriate age-based pediatric dosing can be derived. Likewise, VCR is an integral component in pediatric cancer treatment regimens, and is used in the treatment of lymphoma, acute leukemia, Wilms tumor, Ewing sarcoma, neuroblastoma, hepatoblastoma, and rhabdomyosarcoma.11 Little information is known regarding its binding affinity for biomaterials and catheters.
The ability to use a single catheter to obtain PK samples and accurately report drug concentration values may increase participation of children onto important PK studies. In this report, our goals were to (1) assess the extent of drug contamination from simultaneous dosing and sampling through a single catheter in an in vitro system; (2) establish a clearing procedure that will minimize catheter drug adsorption; (3) evaluate the clinical utility of the optimized clearing procedure in a proof of principle study in children.
MATERIALS AND METHODS
Reagents
AMD was supplied in a lyophilized powder as Cosmegen (500 μg/vial plus 20 mg mannitol; Merck and Company; West Point, PA and Ovation Pharmaceuticals; Deerfield, IL) and was reconstituted in 1.1 mL sterile water to a final concentration of 0.5 mg/mL. VCR was supplied as 1 mg/mL in sterile water (Mayne Pharma Incorporated; Paramus, NJ). 0.9% normal saline was supplied by Becton Dickinson. Hydrochloric acid (HCl) and sodium hydroxide (NaOH) 1 N were obtained from Fisher Scientific (Fair Lawn, NJ) and were diluted into HPLC-grade water (Fisher Scientific) to a final concentration of 0.1 and 0.01 N. Sodium bicarbonate (NaHCO3) 7.5% was obtained from American Reagent (Shirley, NY) and diluted into HPLC-grade water to a concentration of 4.25%. Whole blood, plasma, and albumin were obtained from the general inventory of the blood bank at the Children’s Hospital of Philadelphia.
In Vitro Catheter Apparatus
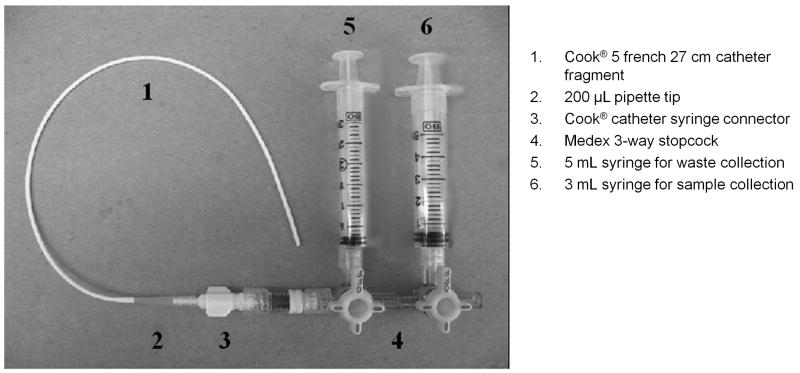
An in vitro catheter apparatus was created to replicate the administration of AMD and VCR in patients. A series of modifications to the apparatus was implemented throughout the experimental phase. In early experiments, a 27 cm fragment of a 5 french (Fr) central venous catheter (Cook Incorporated; Bloomington, IN) fit with a syringe connector (Cook Incorporated) was joined to a 3-way stopcock (Medex; Dublin, OH) via a needle-free valve (Alaris; San Diego, CA) (Figure 1). Sampling was accomplished through the 3-way stopcock using a 3 mL syringe, and blood to be returned was obtained through a 5 mL syringe (Becton Dickinson; Franklin Lakes, NJ). Later experimentation was performed with a series of full-length commercial indwelling central venous catheters supplied by Cook Incorporated and by CR Bard Incorporated (Murray Hill, NJ). Sampling was again accomplished through a 3-way stopcock via a needle-free valve using a 3 mL and 5 mL syringe for sample and blood to be returned, respectively. Final experimentation was accomplished using only a series of full-length indwelling central venous catheters (Cook Inc.; CR Bard, Inc.) and a needle-free valve. The 3-way stopcock was omitted and sample and blood to be returned were obtained directly via a 3 mL and 5 mL syringe, respectively, connected to the needle-free valve. In early in vitro experiments, only Hickman®- or Broviac®-style catheters were emulated; in later experiments using full-length catheters, Port-A-Cath® reservoir-style catheters were also employed. The dead space volume for these catheters used in the experiments ranged from 100 μL (catheter fragments) to 1.8 mL (9 french Broviac® catheters). On average the dead space for full-length indwelling catheters was 1.5 mL.
Figure 1.
Design of the in vitro sampling apparatus. The internal diameter of the catheter was 5 french, or 1.67 mm. This corresponded to a volume of approximately 200 μL. Modifications to the types of catheters and stopcock were implemented in later experiments.
Dosing and Sampling Method
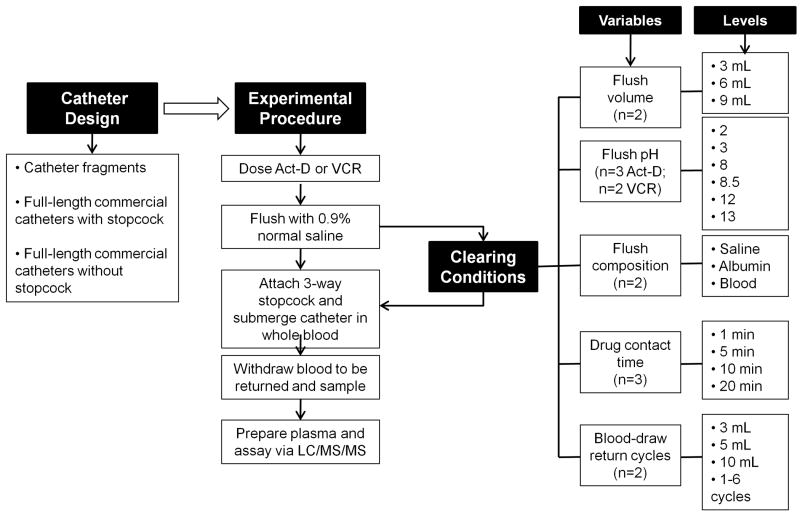
In early experiments using catheter fragments, fragments were primed with 3 mL 0.9% normal saline prior to drug infusion. 100 μL of 0.5 mg/mL AMD or 1 mg/mL VCR, followed by a 3 mL 0.9% normal saline flush, was infused directly through the catheter using a micropipette, followed by different clearing conditions (Figure 2): (1) Flush volume: 3 mL, 6 mL, or 9 mL of 0.9% normal saline; (2) Flush pH: 200 μL of 0.1 N HCl (pH 2), 0.01 N HCl (pH 3), 0.1 N NaOH (pH 13), 0.01 N NaOH (pH 12), 4.25% NaHCO3 (pH 8), or 7.5% NaHCO3 (pH 8.5); (3) Flush composition: 200 μL of 0.9% normal saline, human albumin, or whole blood; (4) Drug contact time: drug was allowed to remain in contact with the luminal surface of the catheter for 1, 5, 10, or 20 minutes; (5) Blood-draw return cycles: 3, 5 or 10 mL of whole blood were pulled from and then returned to a fresh whole blood reservoir in 1 to 6 cycles. A 3-way stopcock was then attached to the catheter via a needle-free valve for sampling. The catheter tip was submerged in 45 mL of whole blood. 2.5 mL blood-draw return volumes were pulled from fresh whole blood, followed by a 2 mL or 2.5 mL (blood-draw return cycle optimization only) sample volume. Blood-draw return and samples were aliquotted into sodium heparin blood collection tubes (Becton Dickinson), and centrifuged at 400 × g for 10 minutes. Plasma was separated into 500 μL samples and frozen at -80 °C until assayed.
Figure 2.
Schematic of experimental design and procedures for the in vitro catheter studies. Dosing and sampling of AMD or VCR were carried out using several configurations of the catheter apparatus. Various clearing conditions were tested to minimize residual drug adsorption to the catheter. Each experiment was repeated 2 to 3 times as indicated by n.
In both later and final experiments using full-length indwelling catheters, catheters were primed with a volume of whole blood equal to that of the catheter volume, followed by a 3 mL 0.9% normal saline flush. In the case of double-lumen catheters, each lumen was primed and flushed. One mL (0.5 mg) AMD, followed by 0.5 mL (0.5 mg) VCR, each followed by a 3 mL 0.9% normal saline flush, were infused directly through the catheter via a needle-free valve. A 3-way stopcock was then attached to the valve. The catheter tip was submerged in whole blood, and blood-draw return volumes and a sample volume (2.5 mL) were obtained, aliquotted, centrifuged, and stored as above. In double lumen catheters, samples and blood-draw return were obtained from both the dosing (infusion) lumen, and the unused lumen; after obtaining blood-draw return and sample from the dosing lumen, a new 3-way stopcock was attached to the unused lumen, and a single 5 mL blood-draw return followed by a 2.5 mL sample were obtained. In final experiments, five blood-draw return volumes of 5 mL each, followed by a sample volume (2.5 mL) were obtained from the blood reservoir; in double lumen catheters, blood-draw return and sample were obtained from both the dosed and non-dosed lumen following the clearing procedure in each lumen. Samples were aliquotted, centrifuged, and stored as above. In vitro experiments were repeated two to three times on different days.
Analytical Method
AMD and VCR were quantified in human plasma using a validated analytical method based on LC/MS/MS detection.12 The lower limit of quantitation (LLOQ) of AMD and VCR were 0.05 ng/mL. The lower limit of detection (LOD) was 0.025 ng/mL. The intraday precision (as defined by the coefficient of variation, CV) based on the standard deviation of replicates of quality control samples ranged from 4.9 to 7.5% and 6.5 to 11.3% for AMD and VCR, respectively. The interday precision ranged from 7.2 to 10.0% and 11.3 to 13.0% for AMD and VCR, respectively.
Proof-of-Principle Study
Based upon the results of the in vitro catheter clearance data, a procedure was created to clear drug from the luminal surface of the central venous line in patients dosed with AMD and VCR. The study of AMD and VCR protocol was approved by the institutional review board of The Children’s Hospital of Philadelphia. Children between the ages of 6 months to 21 years with weights greater than 5 kg, who were to receive AMD and VCR on a dose and schedule prescribed by their treating physicians, were invited for participation. Four (5, 30 minute, 4, 20-24 hour post-dose) to nine (5, 10, 30, 60, 90, 150 minute, 4-6, 8-12, 20-24 hour post-dose) sets of plasma PK samples were obtained from each patient during the treatment cycle that followed enrollment on the study; one set from an indwelling central venous catheter (e.g. Broviac®, Port-a-Cath®) from which drug administration took place, and a second set from a newly placed peripheral venous catheter (PIV). Patients had either Port-A-Cath or Hickman- or Broviac-style catheters, appropriate for weight and age. Prior to drawing the first and second samples from the indwelling catheter, a 3-way stopcock was fitted to the catheter hub, and 5 mL of blood was removed and then returned to the patient. This was repeated four times, followed by the withdrawal of a 5 mL blood-draw return, then a 2.5 mL sample. Thus, a total of five waste cycles were returned to the patient (Table 1). Samples were placed on ice, centrifuged, and plasma separated within 30 minutes of collection. Plasma was frozen at -80 °C until analysis. Bland-Altman analysis, intraclass correlation analysis, and the Student’s paired t-test were used to compare drug concentrations from the two sets of blood samples. Nonlinear compartmental analysis was used to calculate area under the curve for total drug exposure (WinNonLin version 4.1, Pharsight Corporation; Mountain View, CA).
Table 1.
Catheter clearing procedure
| Catheter Clearing |
|
| Catheter clearing procedure for double lumen catheters | |
| Samples may be obtained from either the dosing or the non-dosing lumen. In both cases, the above clearing procedure for a single catheter should be followed. | |
RESULTS
Catheter Clearing Conditions Affect Residual Drug Concentrations
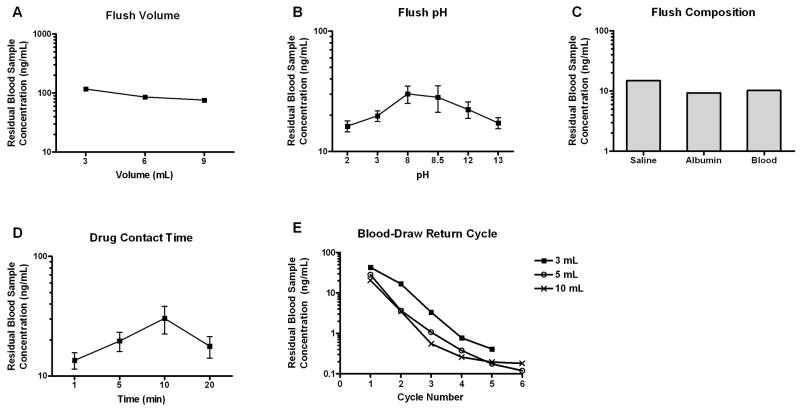
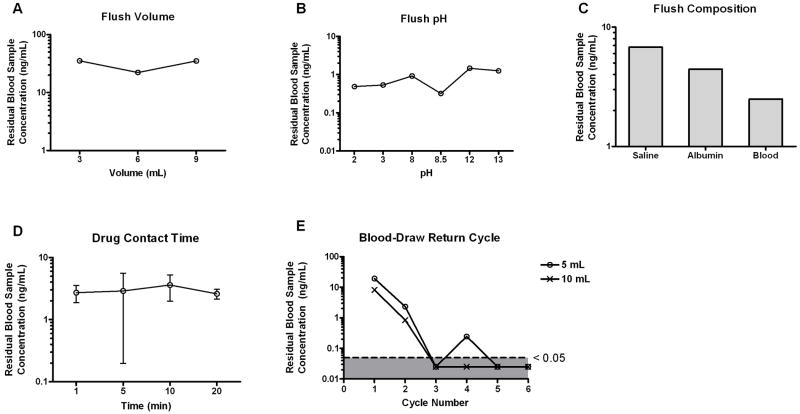
Using the earliest configuration of the in vitro catheter apparatus (Figure 1), we evaluated the effects of various flush conditions on the extent of drug contamination from simultaneous dosing and sampling procedure. Based on the input drug amount and the collection reservoir volume, the initial drug concentrations would be 111 ng/mL and 222 ng/mL for AMD and VCR, respectively. These are clinically achievable concentrations. When the system was subjected to a single flush with 0.9% normal saline, the amount of residual AMD in the blood sample proportionally decreased with increasing flush volume, although high contamination (mean 76 ng/mL, 2 replicates) was still present at the largest tested flush volume (Figure 3A). Residual AMD retention in the catheter was also sensitive to flush pH and flush composition. Extreme acidic and basic conditions promoted removal of adsorbed drug (Figure 3B). One replicate for AMD at pH of 8 was excluded due to its extreme high value of 2330 ng/mL, which most likely was due to analytical assay contamination. Both albumin and whole blood acted as more efficient flushing reagents than normal saline (Figure 3C). In addition, prolonged incubation (10 minutes) of AMD in the catheter lumen resulted in higher residual drug concentration, although exposure time at 20 minutes had similar drug accumulation as that of 5 minutes (Figure 3D). VCR showed lower propensity for catheter adsorption than AMD (Figure 4). The residual drug amount in the blood samples was typically less than 15%, and therefore, not greatly influenced by the tested clearing conditions.
Figure 3.
Effect of clearing conditions on residual AMD concentration. Following the dosing of AMD, the catheter fragment was flushed with (A) varying volumes of normal saline; (B) normal saline with varying pH values; (C) normal saline followed by a second flush of varying compositions; (D) normal saline preceded by varying drug contact time in the catheter lumen; (E) 3 mL 0.9% normal saline flush followed by 3, 5, or 10 mL of blood draw return cycles. Results are plotted as means or means ± SD from 2 or 3 independent experiments, respecively.
Figure 4.
Effect of clearing conditions on residual VCR concentration. Following the dosing of VCR, the catheter fragment was flushed with (A) varying volumes of normal saline; (B) normal saline with varying pH values; (C) normal saline followed by a second flush of varying compositions; (D) normal saline preceded by varying drug contact time in the catheter lumen. (E) 3 mL 0.9% normal saline flush followed by 5 or 10 mL of blood draw return cycles. Results are plotted as means or means ± SD from 2 or 3 independent experiments, respectively.
Taking into account clearing procedures that could be feasibly carried out in the clinical setting, we next examined the effects of blood-draw return cycles along with flush volume. The relationship between number of blood-draw return cycles and residual drug concentration is presented in Figure 3E. The amount of residual AMD decreased in a non-linear fashion with increasing blood-draw return cycle number, and larger flush volume resulted in better drug removal during the initial cycles, which was consistent with our earlier findings. The percent incremental decrease in residual drug concentrations was highest from cycles 1 to 3. By cycles 5 and 6, less than 1% of drug was recovered from the sampling reservoir. Both 5 mL and 10 mL flush volumes were equally efficient in clearing VCR. By cycle 3, residual concentrations were almost all below the limit of detection (Figure 4E).
Drug Clearing Can Be Reproduced in Commercial Catheters
The optimization of number of blood-draw return cycle was performed on commercial catheters to verify the results obtained with catheter fragments. Several types of catheters (single lumen, double lumen and reservoir-style catheters) of varying diameter and length were subjected to four blood-draw return cycles of 5 mL each, followed by a fifth 5 mL pull, then followed by a 2.5 mL sample. In early experiments with few replicates, slightly higher AMD concentrations were observed using the smallest catheters (4 and 5 french) than in larger catheters (6, 6.6, 7 and 9 french and Port-A-Cath®). Drug concentrations were higher when sampled from the “clean” non-dosed lumen comparing to the dosing lumen if the non-dosed lumen was not cleared via the clearing method. This was more evident for VCR where 4- to 17-fold increases in residual drug concentrations were observed from the non-dosed lumen. In final double lumen catheter and single lumen reservoir-style catheter studies in which both dosed and non-dosed lumens were subjected to the clearing procedure, the residual AMD (mean concentration 6.6 french 0.40 ng/mL; 6.6 french Port-A-Cath® 0.42 ng/mL; 9 french double lumen 0.31 ng/mL from dosing lumen and 0.077 ng/mL from non-dosed lumen) and VCR (mean concentration 6.6 french 0.10 ng/mL; 6.6 Port-A-Cath® 0.11 ng/mL) concentrations were all less than 1%.
Central Catheter Drug Contamination Is Measurable In Vivo Following Clearing Procedure
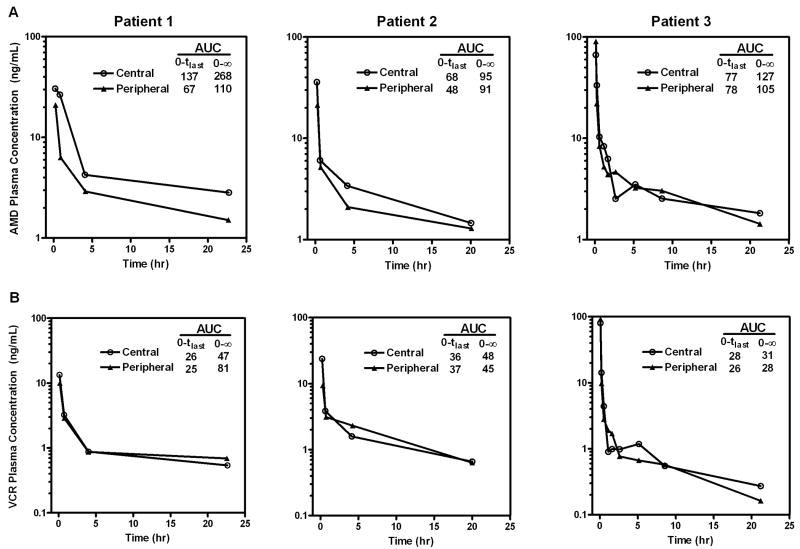
Over the two year enrollment period, a total of 56 patients were invited to participate on the study and five patients consented (9%), of which two patients had zero or one sample drawn from the central venous catheter. Three patients completed the study and a total of 17 paired central and peripheral catheter PK samples were obtained 5 minutes to 24 hours following AMD and VCR IV bolus infusion. The plasma concentration time profiles of both drugs for individual patients are shown in Figure 5 and were similar to historical data in children receiving AMD13 and VCR.14 For AMD, although drug contamination was seen during the entire 24 hour sampling period, the relative deviation from the uncontaminated peripheral catheter sampling method was the greatest during the early sampling time of 5 minutes and 30 minutes post dosing. Minimal central catheter drug contamination was found for VCR. Differences between central and peripherally obtained samples were not statistically significant (P = 0.28 for AMD and P = 0.55 for VCR). Patient 3 showed higher drug concentrations for both drugs obtained from peripheral catheter at the 5 minute time point. Since the rest of the paired samples from this patient followed the expected dosing catheter contamination pattern, it is speculated that this isolated contradictory observation was potentially due to mislabeling or data entry error during the analytical process.
Figure 5.
Plasma concentration time profiles of (A) AMD and (B) VCR in three patients in a pediatric pilot study. Following intravenous administration of AMD and VCR through a central venous line, blood samples were collected from both the dosing catheter and a newly placed peripheral venous catheter. Areas under the curve (AUC, hr•ng/mL) were derived using noncompartmental analysis method.
A Bland-Altman analysis between the central catheter and PIV samples resulted in a bias (standard deviation) of 2.47 (9.14) and 0.70 (4.75) for AMD and VCR, respectively. After removing one pair of outliers where the PIV sample concentration was much higher than the central catheter sample concentration for both drugs, the bias (standard deviation) for AMD was 4.12 (6.34) and for VCR was 1.45 (3.74). These results indicate that samples obtained from the central catheter were, on average, higher than those obtained via the PIV. The bias between the two sampling methods was larger for AMD than VCR, which indicates the clearing procedure may be more efficient in removing VCR than AMD, consistent with our in vitro findings.
The intraclass correlation (ICC) analysis for assessing absolute agreement between the two sampling methods showed moderate correlation (ICC = 0.893) for AMD and strong correlation (ICC = 0.975) for VCR. ICC analysis for assessing observation consistency showed similar results (ICC = 0.894 for AMD; ICC = 0.974 for VCR).
DISCUSSION AND CONCLUSION
Developing a procedure that will allow pharmacokinetic sampling to be performed through the same catheter as drug administration should increase participation by children in important PK trials. Based on a series of in vitro experiments, we have found that five blood-draw return cycles using the patient’s own blood is the most effective method in clearing residual drug from the catheter. Other catheter clearing conditions, such as flush volume, pH, and drug contact time had limited impact on the extent of residual drug binding to the catheter lumen.
AMD exhibited higher tendency for catheter adsorption than VCR. The residual concentration of AMD was 0.16% of the initial infusion concentration after five blood-draw return cycles, whereas an undetectable amount of VCR was observed after only three cycles. Since both drugs were reconstituted with the same vehicle and evaluated using the same catheter configurations, their differences in adsorption is most likely attributed to their physiochemical properties. Smith and coworkers studied the effects of drug molecules on adsorption by polyurethane catheters and found a direct correlation between the drugs’ partition coefficients and catheter uptake in vitro.15
In addition to drug-specific factors, variables such as catheter material and timing of samples will determine whether tested clearing procedures can efficiently remove catheter-bound drugs. Several in vitro studies have demonstrated the presence of drug on the luminal surface of various indwelling catheters, suggesting that the potential for drug adsorption by the catheter is catheter-specific. A series of catheters made of polyvinyl chloride, polyurethane, polyethylene, and silicone were studied for retention of isosorbide dinitrate, demonstrating 4% to 29% drug loss to polyurethane catheters, but less than 1% drug loss to polyethylene catheters.16 We have focused our study on catheters made of silicone to be consistent with our patient population. We were able to achieve almost complete clearance of both AMD and VCR using several common commercial catheter types. The increased luminal surface area of the long catheter may necessitate additional blood-draw return cycles or larger flush volumes than our current clearing procedure. Hence for future PK studies, investigators should clearly define the types of catheters allowed to minimize variable catheter effects on drug adsorption. In the case of double lumen catheters, both catheter configuration and blood flow dynamics may play a role in the inability to sample from a non-dosed lumen without prior drug clearing; downstream collection of upstream (contaminated) blood may contribute to high concentrations in the non-dosed lumen, due to backflow of blood with residual drug on the shared catheter tip.17
Our proof-of-principle study in children demonstrates the feasibility of the proposed catheter clearing approach. Preliminary analysis showed that AMD exposure during the sampling period was higher when blood was drawn from the central catheter relative to the peripheral catheter in two out of the three patients. In the case of VCR, drug exposures were similar between the two sampling catheters. Although residual AMD was present despite the implementation of catheter clearing procedure, high variability from both between-subject (57.2% for population clearance % CV) and residual error (18.5%) can contribute to the heterogeneity of PK observations.13 Modeling and simulation approaches to account for the bias due to sampling method are able to correct the observed data so that accurate PK results can be reported (manuscript in press).18 Furthermore, the partial areas under the curve (AUC) for the first two sampling times (5 and 30 minutes post-dosing) at which time catheter clearing procedure was performed accounted for 1-4% and 8-13% of the total projected AUC, respectively. Precise measurements of earlier time points are not likely to be clinically important for AMD due to their relatively small contributions to total drug exposure and AMD’s long half-life. More importantly, these early time points, while valuable to project maximum exposure, are less likely to influence clearance estimation which depends on later sampling times (during drug elimination).
There were no reported adverse events related to the sampling procedure by study participants although, given the small sample size, this cannot not be generalized to a larger population without further data.
Our study attempted to assess the clearing potential of small-diameter catheters (4 and 5 french) but early in vitro experiments suggested that this was challenging (data not shown). As such we focused on larger catheters (6 french and larger), and cannot comment on the potential to clear catheters in the smallest of children. In addition, the catheters assessed were made of silicone and we did not assess the potential to clear other materials; care should thus be taken when attempting to generalize our methodology to small catheters of non-silicone based materials. In additition, although general knowledge about the partition coefficients of individual drugs might be predictive of their adsorption tendency, characteristics of catheters (e.g. material, configuration) can influence the efficiency of the proposed clearing method. As such, the methodology described in this manuscript should not be applied to other drugs without first assessing its viability in the laboratory.
The methodology described in this paper is not unlike other methods previously described in the academic community.19 However to our knowledge this is the first example of both in vitro and in vivo validation of a waste-return catheter clearing methodology for anti-cancer agents in children.
Our catheter clearing methodology is currently in use in a large national PK/PD study of AMD and VCR being performed in the Children’s Oncology Group (COG). Further prospective validation data from this study is forthcoming. In addition, this procedure has been adopted for a study of the PK of daunorubicin in children with cancer. The implementation of procedures which permit dosing and sampling from the same central catheter should dramatically increase the accrual rate to pediatric PK studies, and advance rational dosing guidance in children with cancer.
Acknowledgments
We thank Dominique Paccaly for his time and effort on the earliest phase of this project. We also thank Cook and Ovation Pharmaceuticals for their support with this project. This work is supported in part by Hope Street Kids and NIH Grant # CA 098543-02S1.
References
- 1.de Jonge ME, Mathot RAA, van Dam SM, et al. Sorption of Thiotepa to Polyurethane Catheter Causes Falsely Elevated Plasma Levels. Ther Drug Monit. 2003;25:261–263. doi: 10.1097/00007691-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Shulman RJ, Ou C, Reed T, et al. Central Venous Catheters Versus Peripheral Veins for Sampling Blood Levels of Commonly Used Drugs. Journal of Parenteral and Enteral Nutrition. 1998;22:234–237. doi: 10.1177/0148607198022004234. [DOI] [PubMed] [Google Scholar]
- 3.Leson C, Bryson S, Giesbrecht E, et al. Therapeutic Monitoring of Cyclosporine Following Pediatric Bone Marrow Transplantation: Problems with Sampling from Silicone Central Venous Lines. Ann Pharmacother. 1989;23:300–303. doi: 10.1177/106002808902300405. [DOI] [PubMed] [Google Scholar]
- 4.Franson TR, Ritch PS, Quebbeman EJ. Aminoglycoside Serum Concentration Sampling via Central Venous Catheters: A Potential Source of clinical Error. Journal of Parenteral and Enteral Nutrition. 1987;11:77–79. doi: 10.1177/014860718701100177. [DOI] [PubMed] [Google Scholar]
- 5.Longley J, Murphy J. Falsely Elevated Digoxin Levels. Another Look. Ther Drug Monit. 1989;11:572–573. [PubMed] [Google Scholar]
- 6.Boodhan S, Maloney AM, Dupuis LL. Extent of Agreement in Gentamicin Concentration Between Serum That Is Drawn Peripherally and From Central Venous Catheters. Pediatrics. 2006;118:1650–1656. doi: 10.1542/peds.2006-0023. [DOI] [PubMed] [Google Scholar]
- 7.Wanwimolruk S, Murphy JE. Effect of Monitoring Drug Concentrations Through Lines Used to Administer the Drugs: An In Vitro Study. Ther Drug Monit. 1991;13:443–447. doi: 10.1097/00007691-199109000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Umstead G. Tobramycin Levels from Hickman Catheters. Drug Intell Clin Pharm. 1985;19:477–478. doi: 10.1177/106002808501900614. [DOI] [PubMed] [Google Scholar]
- 9.Ponto LLB, Graham MM, Richmond JC, et al. Contamination Levels in Blood Samples Drawn from the Injection Intravenous Line. Molecular Imaging and Biology. 2002;4:410–414. doi: 10.1016/s1536-1632(02)00121-x. [DOI] [PubMed] [Google Scholar]
- 10.Frei E. The Clinical Use of Actinomycin. Cancer Chemotherapy Reports. 1974;58:49–54. [PubMed] [Google Scholar]
- 11.Rahmani R, Zhou XJ. Pharmacokinetics and Metabolism of Vinca Alkaloids. Cancer Surv. 1993;17:269–81. [PubMed] [Google Scholar]
- 12.Lee JI, Skolnik JM, Barrett JS, et al. A Sensitive and Selective Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Quantification of Actinomycin-D and Vincristine in Children with Cancer. J Mass Spectrom. 2007;42:761–770. doi: 10.1002/jms.1211. [DOI] [PubMed] [Google Scholar]
- 13.Mondick JT, Gibiansky L, Gastonguay MR, et al. Population Pharmacokinetic Investigation of Actinomycin-D in Children and Young Adults. J Clin Pharmacol. 2008;48:35–42. doi: 10.1177/0091270007310383. [DOI] [PubMed] [Google Scholar]
- 14.Groninger E, Meeuwsen-de Boer T, Koopmans P, et al. Pharmacokinetics of Vincristine Monotherapy in Childhood Acute Lymphoblastic Leukemia. Pediatr Res. 2002;52:113–118. doi: 10.1203/00006450-200207000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Smith JC, Davies MC, Melia CD, et al. Uptake of Drugs by Catheters: the Influence of the Drug Molecule on Sorption by Polyurethane Catheters. Biomaterials. 1996;17:1469–1472. doi: 10.1016/0142-9612(96)89770-5. [DOI] [PubMed] [Google Scholar]
- 16.De Muynck C, Vandenbossche G, Colardyn F, et al. Sorption of Isosorbide Dinitrate to Central Venous Catheters. J Pharm Pharmacol. 1993;45:139–141. doi: 10.1111/j.2042-7158.1993.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 17.Huitema ADR, Holtkamp M, Tibben MM, et al. Sampling Technique From Central Venous Catheters Proves Critical for Pharmacokinetic Studies. Ther Drug Monit. 1999;21:102–104. doi: 10.1097/00007691-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Zhang AY, Skolnik JM, Barrett JS. Evaluating the Extent of Chemotherapeutic Contamination from Central Venous Catheters in Children with Cancer and Providing Guidance for Accurate Reporting of PK Parameters. PAGE. 2010;19 Abstr 1789. [Google Scholar]
- 19.Registered Nurses’ Association of Ontario. see http://www.rnao.org/Page.asp?PageID=924&ContentID=796) or Pediatric Vascular Access Clinicians Network (see http://www.avainfo.org/website/article.asp?id=167759.