Abstract
Although some reports have found increasing HLA disparity between donor and recipient to be associated with fewer relapses after allogeneic blood or marrow transplantation (BMT), this potential benefit has been offset by more graft-versus-host disease (GVHD) and nonrelapse mortality. However, the type of GVHD prophylaxis could influence the balance between GVHD toxicity and relapse. We analyzed the impact of greater HLA disparity on outcomes of a specific platform for nonmyeloablative, HLA-haploidentical transplantation. A retrospective analysis was performed on 185 patients with hematologic malignancies enrolled on three similar trials of nonmyeloablative, related donor, haploidentical BMT incorporating high-dose posttransplantation cyclophosphamide for GVHD prophylaxis. No significant association was found between the number of HLA mismatches (HLA-A, -B, -Cw, and -DRB1 combined) and risk of acute grade II–IV GVHD (hazard ratio .89, P = .68 for 3–4 versus fewer antigen mismatches). More mismatching also had no detrimental effect on event-free survival (on multivariate analysis, hazard ratio .60, P = .03 for 3–4 versus fewer antigen mismatches; hazard ratio .55, P = .03 for 3–4 versus fewer allele mismatches). Thus, greater HLA disparity does not appear to worsen overall outcomes after nonmyeloablative haploidentical BMT with high-dose posttransplantation cyclophosphamide.
Keywords: Nonmyeloablative conditioning, allogeneic blood or marrow transplantation, graft-versus-host disease, human leukocyte antigens, cyclophosphamide
INTRODUCTION
Historically, donor-recipient HLA compatibility has been the leading predictor of outcome after allogeneic blood or marrow transplantation (BMT). Increasing degrees of HLA mismatch at either the antigen or allele level have been repeatedly associated with worse overall outcomes in series of myeloablative, related or unrelated donor BMT for hematologic malignancies [1–7]. The reported effect of HLA disparity on relapse risk has been variable [2,3,5]. Although some studies have found a lower relapse risk with increasing degrees of HLA mismatch or specific mismatches, suggesting a graft-versus-tumor effect, this has generally been offset by higher rates of graft-versus-host disease (GVHD), graft failure, and nonrelapse mortality (NRM) [1–7]. Thus, although one or more partially-HLA mismatched (HLA-haploidentical) first-degree relatives can be readily identified in most patients who lack a histocompatible donor, the toxicity of such transplants has prevented many centers from pursuing this approach.
High-dose cyclophosphamide (Cy), when administered in a narrow window after transplantation, depletes alloreactive T-cells from the donor and host and can inhibit both GVHD and graft rejection [8–16]. As a form of drug-induced immunologic tolerance [17], the strategy of giving high-dose Cy after transplantation takes advantage of the heightened cytotoxic sensitivity of proliferating, alloreactive T-cells over non-alloreactive, resting T-cells to being killed by a DNA-damaging agent [12]. High-dose Cy on days 3 and 4 after myeloablative, HLA-matched related or unrelated donor BMT recently has been reported to be effective GVHD prophylaxis without the addition of a calcineurin inhibitor [14]. In early phase trials of nonmyeloablative, HLA-haploidentical BMT, incorporation of posttransplantation Cy, tacrolimus, and mycophenolate mofetil was associated with timely and stable engraftment. Most importantly, this approach carried acceptable rates of acute GVHD, chronic GVHD, and NRM that paralleled those seen with nonmyeloablative HLA-matched transplants [15,16,18]. We postulated that the impact of donor-recipient HLA disparity on GVHD toxicity and overall outcomes could vary depending on the type of post-transplantation immunosuppression. These promising results prompted us to analyze the effects of HLA disparity on outcomes of nonmyeloablative, haploidentical BMT incorporating high-dose posttransplantation Cy.
PATIENTS AND METHODS
Eligibility
We retrospectively analyzed 185 consecutive patients enrolled on three similar clinical trials of nonmyeloablative, related donor, partially HLA-mismatched BMT incorporating high-dose posttransplantation Cy for the prophylaxis of GVHD and graft rejection. Of these patients, 144 were treated at Johns Hopkins University, 13 combined at participating centers (BMT Group of Georgia andHahnemann University Hospital), and 28 at the Fred Hutchinson Cancer Research Center. The protocols received institutional review board approval, and all participants gave signed informed consent. Patients were adults or children with poor-risk or advanced hematologic malignancies who were not candidates for or who had relapsed despite autologous or myeloablative allogeneic BMT. Eligibility criteria included ECOG performance status < 2 or Karnofsky or Lansky-Play score ≥ 60; adequate hepatic function (total bilirubin ≤ 3.0 mg/dL; or absence of clinically significant liver disease); left ventricular ejection fraction ≥ 35%; and adequate pulmonary function (FEV1 and FVC ≥ 40% of predicted and ≥ 60% of predicted if there was prior thoracic or mantle irradiation; or DLCO ≥ 35%). Diagnoses included poor-risk acute leukemia (such as adverse cytogenetics, age > 60, high presenting white blood cell count, mixed lineage leukemia, and delayed response or nonresponse to induction therapy) in first complete remission; acute leukemia in second or subsequent complete remission; myelodysplastic syndrome with or without leukemic transformation; interferon- or imatinib-refractory chronic myelogenous leukemia (CML) in first chronic phase or non-blast crisis CML beyond first chronic phase; other chronic myeloproliferative disorders including chronic myelomonocytic leukemia, myeloid metaplasia, and polycythemia vera; Hodgkin lymphoma; non-Hodgkin lymphoma; chronic lymphocytic leukemia; and multiple myeloma.
Donors were first-degree relatives who were identical at one HLA haplotype and mismatched at one or more loci of the unshared haplotype. Molecular typing was at an allele or allele group level for HLA-A, -B, -Cw, -DRB1, and -DQB1 as previously described [16]. Rare cases were omitted from analyses of allele mismatching because of insufficient resolution but were evaluable for antigen mismatching. Factors in donor prioritization included HLA cross-match compatibility and ABO compatibility.
Treatment
The transplant regimen and outcomes of 67 of the patients have been previously reported [16]. All patients received Cy (14.5 mg/kg IV on days −6 and −5), fludarabine (30 mg/m2 IV on days −6 to −2, adjusted for renal function), total body irradiation (200 cGy on day −1), and T-cell replete bone marrow infusion (day 0). Posttransplantation high-dose Cy (50 mg/kg IV) was administered once on day 3, or once daily on days 3 and 4, with Mesna (80% or 100% dose of Cy, in divided doses). Cy was dosed according to ideal or adjusted body weight, unless actual weight was less than ideal weight. One day later all patients began mycophenolate mofetil (maximum 3 grams orally daily in divided doses, until day 35), tacrolimus, and filgrastim (5 μg/kg/day subcutaneously until neutrophil recovery) [16]. In those receiving one dose of post-transplantation Cy (n = 48), tacrolimus was given until day 50 (n = 10) or day 180 (n = 38) in the absence of GVHD. In those receiving two doses of posttransplantation Cy (n = 137, or 74%), tacrolimus with a target level of 5–15 ng/mL was given until day 180 in the absence of GVHD.
Endpoints
HLA mismatch (assigned by M.S.L.) in the graft-versus-host (GVH) direction was defined as presence of host antigens or alleles not shared by the donor. HLA mismatch in the host-versus-graft (HVG) direction was defined as presence of donor antigens or alleles not shared by the host. All antigen disparities were also counted as allele disparities. GVHD was analyzed according to GVH direction mismatching; graft rejection was analyzed according to HVG direction mismatching; and disease and survival outcomes were analyzed according to either direction or a particular direction as specified.
Disease and survival endpoints are defined below. Acute GVHD was graded according to the Keystone Criteria [19] and was confirmed by biopsy when possible. First-line therapy of acute skin GVHD consisted of methylprednisolone 1–2.5 mg/kg/day IV or prednisone equivalent, while patients with acute visceral GVHD received methylprednisolone in combination with other immunosuppressants such as tacrolimus with or without mycophenolate mofetil. Eligible patients with acute GVHD were treated on protocol BMT CTN 0302 [20]. Chronic GVHD was diagnosed and graded according to both NIH and Seattle standard guidelines [21,22].
Graft failure was defined as persistent lack of donor chimerism (i.e. < 5% donor) by day 30 or day 60. Donor chimerism was determined in blood or bone marrow by restriction fragment length polymorphisms, polymerase chain reaction of variable nucleotide tandem repeats, or X- and Y-chromosome fluorescence in situ hybridization.
Statistical Methods
Event-time distributions for overall survival (OS) and event-free survival (EFS), measured from the day of transplant, were estimated by the Kaplan-Meier method and compared with Cox proportional hazard models. An event was defined as relapse, progression, or death. The simultaneous effect of two or more factors was studied with multivariate proportional hazards models. Cumulative incidences of relapse, NRM, acute grade II–IV GVHD, and chronic GVHD, measured from the day of transplant, were estimated using competing risk analyses [23], and prognostic factors for these endpoints were analyzed using proportional hazards models for competing risks [24]. For competing risk analyses, death without relapse or progression was considered a competing risk for relapse; relapse was a competing risk for NRM; and graft failure, relapse, and death without GVHD were competing risks for GVHD. A case of donor-derived leukemia was also scored as an event and as a competing risk where applicable.
For evaluating differences in group characteristics, a Wilcoxon test was used for continuous variables and a chi-squared test or Fisher’s exact test for cell counts < 5 was used for categorical variables. P-values are two-sided and unadjusted for multiple comparisons. Statistical analysis was performed with R, version 2.6.2 [25].
RESULTS
Patients and Overall Outcomes
Baseline characteristics of the patients and transplants are provided in Table 1, for the group overall (n = 185) and according to the number of HLA mismatches.
Table 1.
Patient Characteristics
| Variable | Entire group (n = 185) | 0–2 antigen mismatches (n = 26) i, j | 3–4 antigen mismatches (n = 159) i, k | P |
|---|---|---|---|---|
| Patient age, y, median (range) | 50 (1 – 71) | 52 (20 – 66) | 49 (1 – 71) | .54 |
| Male gender, n (%) | 117 (63%) | 13 (50%) | 104 (65%) | .20 |
| Diagnosis, n (%) | ||||
| Lymphoid | 106 (57%) | 17 (65%) | 89 (56%) | .51 |
| ALL a | 16 (9%) | – | – | – |
| Hodgkin’s lymphoma | 25 (13.5%) | – | – | – |
| CLL | 14 (8%) | – | – | – |
| Multiple myeloma b | 8 (4%) | – | – | – |
| NHL c | 43 (23%) | – | – | – |
| Myeloid | 78 (42%) | 9 (35%) | 69 (43%) | – |
| AML d | 49 (26%) | – | – | – |
| MDS | 11 (6%) | – | – | – |
| CML e | 12 (6%) | – | – | – |
| CMML | 5 (3%) | – | – | – |
| PV with myelofibrosis | 1 | – | – | – |
| Biphenotypic leukemia | 1 | 0 | 1 | – |
| Prior BMT, n (%) f | 50 (27%) | 10 (38%) | 40 (25%) | .24 |
| Donor age, y, median (range) | 42 (14 – 73) | 45 (23 – 65) | 42 (14 – 73) | .25 |
| Donor relationship, n (%) | ||||
| Mother | 18 (10%) | 0 | 18 (11%) | .08 |
| Father | 16 (9%) | 3 (12%) | 13 (8%) | – |
| Sibling | 97 (52%) | 15 (58%) | 82 (52%) | – |
| Child | 54 (29%) | 8 (31%) | 46 (29%) | – |
| Female donor/male recipient, n (%) | 48 (26%) | 5 (19%) | 43 (27%) | .55 |
| CMV serostatus, patient/donor, n g | ||||
| Negative/negative | 63 (34%) | 11 (44%) | 52 (33%) | – |
| Negative/positive | 34 (18%) | 4 (16%) | 30 (19%) | – |
| Positive/negative | 43 (23%) | 5 (20%) g | 38 (24%) | – |
| Positive/positive | 44 (24%) | 5 (20%) g | 39 (25%) | – |
| CMV mismatch | 77 (42%) g | 9 (36%) g | 68 (43%) | .67 |
| Cell dose infused, median (range) | ||||
| CD34+ cells × 106/kg | 3.9 (1.6 – 12.8) | 3.9 (1.6 – 12.8) | 3.8 (1.7 – 12.6) | .82 |
| CD3+ cells × 107/kg | 3.6 (.5 – 9.8) h | 3.6 (1.8 – 5.9) | 3.7 (.5 – 9.8) h | .68 |
| Two doses of posttransplantation Cy, n (%) | 137 (86%) | 15 (58%) | 122 (77%) | .07 |
| Class I + II mismatches, median (range) i | ||||
| Antigen mismatches | 4 (0 – 4) | – | – | – |
| Allele mismatches | 4 (1 – 4) | – | – | – |
Abbreviations: ALL, acute lymphoblastic leukemia or lymphoblastic lymphoma; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; CMML, chronic myelomonocytic leukemia; PV, polycythemia vera; BMT, blood or marrow transplantation; CMV, cytomegalovirus; Cy, cyclophosphamide.
Fourteen pre-B-cell, 2 T-cell, and 1 T-cell with later features of primitive leukemia.
One with plasma cell leukemia.
Excluding CLL, multiple myeloma, and lymphoblastic lymphoma. Thirteen patients with follicular lymphoma including 1 with previous large B-cell lymphoma; 7 with diffuse large B-cell lymphoma or large B-cell lymphoma including 2 transformations and 1 diffuse large B-cell lymphoma evolved from follicular grade 3 lymphoma; 7 with peripheral T-cell lymphoma; 10 with mantle cell lymphoma; 2 with T-cell prolymphocytic leukemia; and 1 patient each with null-cell type anaplastic large cell lymphoma, marginal zone lymphoma, Waldenstrom’s macroglobulinemia, and hairy cell leukemia.
De novo in 36 cases.
One case of CML had a concurrent diagnosis of MDS.
Forty-eight autologous, 2 allogeneic. Includes 5 patients transplanted for treatment-related MDS/AML arising after previous BMT for lymphoma.
In 1 case donor was CMV negative with patient serostatus unknown.
Unknown in 1 case.
Mismatching in any direction; composite of HLA-A, -B, -Cw, and -DRB1. HLA-DQB1 was typed but mismatching not included in this analysis.
Two donor-recipient pairs had 0 antigen mismatches at HLA-A, -B, -Cw, and -DRB1 but were mismatched at the allele level, with one of these also mismatched for a DQB1 antigen; 8 donor-recipient pairs had 1 antigen mismatch; 16 had 2 antigen mismatches.
57 donor-recipient pairs had 3 antigen mismatches; 102 had 4 antigen mismatches
The median age was 50, similar proportions had lymphoid (57%) and myeloid diseases, and over one-quarter (27%) had received previous BMT. The actuarial EFS at 1 year was 35%, with a median follow-up of 20 months in those without events and 6 months overall (range, < 1 to 71 months). The cumulative incidence of NRM was 6% at day 100 and 15% at 1 year. Graft failure with or without residual bone marrow malignancy occurred in 29 of 177 evaluable patients (16%). Cumulative incidences of acute grade II–IV GVHD and chronic GVHD were 31% and 15%, respectively.
Composite Analysis of Total HLA Mismatches
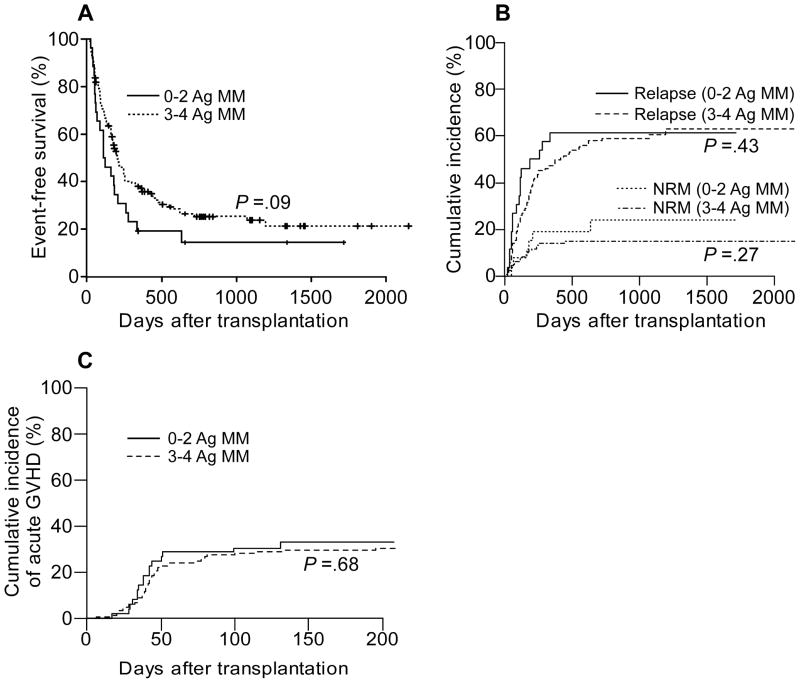
A univariate analysis of total mismatches at HLA-A, -B, -Cw, and -DRB1 in relation to transplant outcomes is presented in Table 2. On univariate analysis, having 3 or 4 total antigen mismatches in any direction was not associated with inferior EFS (HR .67, P = .09), compared with having fewer antigen mismatches (Fig 1a). There was no statistically significant difference found in the cumulative incidence of relapse or of NRM (Fig 1b). Notably, having more antigen mismatches was not associated with a statistically significant difference in the risk of acute grade II–IV GVHD (HR .89, P = .68; Fig 1c). Analyses of chronic GVHD are not presented because it occurred in only 26 patients without competing risks.
Table 2.
Univariate Analysis of HLA Disparity in Relation to Transplant Outcomes
| Variable (reference) | Direction of mismatch | Na | HR for acute GVHD (95% CI) | P for acute GVHD | HR for relapse (95% CI) | P for relapse | HR for NRM (95% CI) | P for NRM | HR for EFS (95% CI) | P for EFS |
|---|---|---|---|---|---|---|---|---|---|---|
| Total antigen MM, 3–4 (vs. 0–2) | Any | 159 (26) | – | – | .79 (.45 – 1.41) | .43 | .61 (.26 – 1.47) | .27 | .67 (.43 – 1.07) | .09 |
| GVH | 136 (49) | .89 (.50 – 1.58) | .68 | .86 (.56 – 1.33) | .50 | .76 (.35 – 1.67) | .50 | .81 (.56 – 1.18) | .27 | |
| HVG | 137 (48) | – | – | .65 (.42 – 1.00) | .05 | 1.06 (.45 – 2.45) | .90 | .69 (.47 – 1.00) | .05 | |
| Total allele MM, 3–4 (vs. 1–2) | Any | 165 (17) | – | – | .58 (.30 – 1.14) | .11 | .86 (.27 – 2.75) | .80 | .58 (.34 – 1.00) | .05 |
| GVH | 152 (30) | 1.03 (.51 – 2.09) | .94 | .70 (.42 – 1.18) | .18 | 1.16 (.41 – 3.33) | .78 | .75 (.48 – 1.19) | .22 | |
| HVG | 153 (29) | – | – | .54 (.32 – .91) | .02 | 1.08 (.38 – 3.08) | .88 | .57 (.37 – .89) | .01 | |
| Class I antigen MM, 2–3 (vs. 0–1) | Any | 166 (19) | – | – | .55 (.30 – .98) | .04 | .98 (.30 – 3.22) | .98 | .54 (.32 – .89) | .02 |
| GVH | 153 (32) | .84 (.43 – 1.61) | .59 | .70 (.44 – 1.11) | .13 | 1.29 (.45 – 3.67) | .63 | .78 (.50 – 1.20) | .26 | |
| HVG | 152 (33) | – | – | .55 (.33 – .91) | .02 | 1.33 (.46 – 3.85) | .59 | .60 (.39 – .92) | .02 | |
| DRB1 antigen MM, 1 (vs. 0) | Any | 153 (32) | – | – | .76 (.47 – 1.22) | .26 | .99 (.38 – 2.59) | .98 | .78 (.51 – 1.21) | .27 |
| GVH | 131 (54) | 1.06 (.60 – 1.88) | .84 | .65 (.43 – .99) | .04 | .87 (.40 – 1.93) | .74 | .62 (.43 – .89) | .009 | |
| HVG | 137 (48) | – | – | .93 (.61 – 1.40) | .71 | .69 (.31 – 1.52) | .35 | .82 (.56 – 1.20) | .31 |
Abbreviations: MM, mismatch; GVH, graft-versus-host; GVHD, graft-versus-host disease (acute grade II–IV); HVG, host-versus-graft; NRM, nonrelapse mortality; EFS, event-free survival.
Number of patient-donor pairs with more mismatches (versus the reference category of fewer mismatches).
Figure 1.
Outcomes according to donor-recipient HLA mismatching. A, Event-free survival and B, cumulative incidences of relapse and nonrelapse mortality according to the number of antigen mismatches in either direction. C, Cumulative incidence of acute grade II–IV GVHD according to the number of antigen mismatches in the GVH direction. Cumulative incidences were estimated by competing risk analysis. Analyses are based upon the number of mismatches at HLA-A, -B, -Cw, and -DRB1 combined. Ag, antigen; MM, mismatch; NRM, nonrelapse mortality.
We also performed a univariate analysis of outcomes according to the total number of antigen mismatches treated as continuous covariates (i.e., 0, 1, 2, 3, or 4 mismatches). For EFS, each additional degree of mismatch in any direction was nondetrimental, with an observed protective effect (HR 0.80, 95% CI .66 – .96, P = .02). Additionally, with this approach we found no statistically significant difference in the cumulative incidence of relapse (HR 0.86, 95% CI .69 – 1.06, P = 0.16) or NRM (HR 0.82, 95% CI .56 – 1.21, P = 0.33), nor was there a statistically significant difference found in the risk of acute grade II–IV GVHD with each additional degree of GVH-direction mismatch (HR 0.87, 95% CI .70 – 1.09, P = 0.24).
Table 3 presents a univariate analysis of other variables that could influence EFS including age, disease type, donor characteristics, and graft characteristics. There was no statistically significant difference in EFS between patients age 50–59 versus < 50 (data not shown), whereas patient age ≥ 60 was associated with an inferior EFS (HR 1.64, P = .01) as was having a female donor for a male recipient (HR 1.47, P = .04). The adverse impact on EFS was not accounted for by a significantly increased risk of acute grade II–IV GVHD (for age ≥ 60, HR 1.18 for acute GVHD, 95% CI .66 – 2.09, P = .57; and for female donor/male recipient, HR .76, 95% CI .42 – 1.37, P = .37). On a multivariate analysis of EFS that included these variables, having 3 or 4 total antigen mismatches, as compared with fewer mismatches, was not detrimental and appeared protective (HR .60, P = .03; Table 4).
Table 3.
Univariate Analysis of Event-Free Survival for Variables Other Than HLA Mismatching
| Variable (reference) | N | HR (95% CI) | P |
|---|---|---|---|
| Patient age ≥ 60 (vs. < 60) | 43 (142) | 1.64 (1.12 – 2.41) | .01 |
| Lymphoid disease (vs. myeloid) | 106 (78) | .81 (.57 – 1.14) | .22 |
| Donor age ≥ 45 (vs. < 45) | 83 (102) | 1.01 (.72 – 1.43) | .94 |
| Female donor/male recipient (vs. other) | 48 (137) | 1.47 (1.02 – 2.14) | .04 |
| Mother as donor (vs. other) | 18 (167) | 1.18 (.69 – 2.03) | .54 |
| CMV mismatch (vs. CMV match) | 77 (107) | .93 (.65 – 1.31) | .65 |
| CD34+ dose ≥ 3.9 × 106/kg (vs. < 3.9 × 106/kg) | 93 (92) | 1.05 (.74 – 1.48) | .78 |
| CD3+ dose ≥ 3.6 × 107/kg (vs. < 3.6 × 107/kg) | 99 (85) | 1.22 (.86 – 1.72) | .26 |
| 2 doses of posttransplantation Cy (vs. 1) | 137 (48) | .98 (.67 – 1.43) | .92 |
Abbreviations: CMV, cytomegalovirus; Cy, cyclophosphamide.
Table 4.
Multivariate Analysis of Event-Free Survival
| Variable (reference) a | N | HR (95% CI) | P |
|---|---|---|---|
| Model 1 | |||
| Total antigen MM, 3–4 (vs. 0–2) | 159 (26) | .60 (.38 – .95) | .03 |
| Patient age ≥ 60 (vs. < 60) | 43 (142) | 1.78 (1.20 – 2.62) | .004 |
| Female donor/male recipient (vs. other) | 48 (137) | 1.60 (1.10 – 2.34) | .01 |
| Model 2 | |||
| Total allele MM, 3–4 (vs. 1–2) | 165 (17) | .55 (.32 – .94) | .03 |
| Patient age ≥ 60 (vs. < 60) | 42 (140) | 1.75 (1.18 – 2.59) | .006 |
| Female donor/male recipient (vs. other) | 47 (135) | 1.58 (1.08 – 2.30) | .02 |
| Model 3 | |||
| Class I antigen MM, 2–3 (vs. 0–1) | 166 (19) | .53 (.32 – .90) | .02 |
| DRB1 antigen MM in GVH direction, 1 (vs. 0) | 131 (54) | .55 (.38 – .80) | .002 |
| Patient age ≥ 60 (vs. < 60) | 43 (142) | 1.96 (1.32 – 2.92) | .001 |
| Female donor/male recipient (vs. other) | 48 (137) | 1.62 (1.11 – 2.36) | .01 |
Abbreviations: MM, mismatch; GVH, graft-versus-host.
HLA mismatches are in either direction unless otherwise specified.
There were no statistically significant differences in patient or transplant characteristics between those having more versus fewer total antigen mismatches (Table 1). In the group with more antigen mismatches, there was a tendency toward more patients receiving a maternal graft or receiving two doses of posttransplantation Cy. Inclusion of these factors in this multivariate model did not change the outcome (data not shown).
Similarly, the presence of a greater number of allele mismatches was not associated with inferior outcomes. Having more allele mismatches was associated with a tendency toward lower relapse risk on univariate analysis (HR .58, P = .11, for 3 or 4 versus fewer mismatches; Table 2), and was not detrimental and appeared protective on multivariate analysis of EFS (HR .55, P = .03; Table 4). However, we found no significantly increased risk of acute grade II–IV GVHD associated with having 3 or 4, as compared with fewer, total allele mismatches on univariate analysis (HR 1.03, P = .94).
Total Mismatches According to Vector
Overall outcomes are also presented according to the direction of the mismatch (Table 2). Such analyses help to address whether the observed effects of HLA disparity are due to imbalances in clinically significant mismatches. For example, having more HVG-direction mismatches may be less clinically significant than GVH-direction mismatches. On univariate analysis, no statistically significant association was found between the total number of antigen or allele mismatches in the GVH direction (3 or 4 versus fewer) and relapse, NRM, or EFS (Table 2). In the HVG direction, more mismatches were associated with a nondetrimental and possibly protective effect on relapse risk and EFS (Table 2). However, because of the tight correlation between GVH- and HVG-direction mismatches at single loci, their relative contributions cannot be dissociated.
The probability of graft failure was examined with respect to the number of antigen mismatches in the HVG direction. A higher incidence of graft failure (recorded as a yes/no) was observed in those having 3 or 4 antigen mismatches in the HVG direction compared with fewer mismatches (130 and 47 evaluable patients per group, respectively). The difference was not statistically significant (odds ratio 1.91, 95% CI .68 – 5.31, P = .22) although analysis is limited by sample size. By treating the number of HVG-direction mismatches as continuous covariates, no statistically significant trend was found with respect to modeling the probability of graft failure (P = .62).
Mismatch Analysis According to HLA Locus
To further investigate whether the observed nondeleterious effects of HLA disparity on EFS reflect skewing of clinically significant variables, overall outcomes were analyzed separately at class I and at HLA-DRB1 (Tables 2 and 4).
The presence of two or more class I antigen mismatches in any direction was associated with a lower risk of relapse on univariate analysis (HR .55, P = .04), and a lower risk for an event on multivariate analysis (HR .53, P = .02). In the GVH direction, on univariate analysis there was no detrimental effect of more class I antigen mismatches on overall outcome (HR .78, P = .26 for EFS) or on the risk of acute grade II–IV GVHD (HR .84, P = .59), and there was a tendency toward lower risk of relapse (HR .70, P = .13). In the HVG direction, more class I antigen mismatching was also associated with a lower risk of relapse (HR .55, P = .02) and a lower risk for an event (HR .60, P = .02) on univariate analysis.
Likewise, HLA-DRB1 antigen mismatching was not associated with inferior outcomes. On univariate analysis (Table 2), DRB1 antigen mismatching in the GVH direction was associated with a lower risk of relapse (HR .65, P = .04), a similar risk of acute grade II–IV GVHD (HR 1.06, P = .84), and an improved EFS (HR .62, P = .009). On multivariate analysis of EFS (Table 4), an HLA-DRB1 antigen mismatch in the GVH direction and two or more class I antigen mismatches in any direction appeared protective.
DISCUSSION
Increasing degrees of HLA mismatch between donor and recipient have been repeatedly associated with greater toxicity and inferior survival after allogeneic BMT [1–7]. However, the present analysis suggests that, with nonmyeloablative, partially-HLA mismatched, T-cell replete BMT that combines high-dose Cy and standard postgrafting immunosuppression, greater HLA disparity does not worsen overall outcome.
With myeloablative, related or unrelated donor BMT, donor-recipient HLA incompatibility at either the serologic or genotypic level has been associated with lower relapse rates in some series [2,3,5,7]. For example, in patients with poor-risk leukemia undergoing myeloablative, related donor BMT, 2- and 3-locus mismatched transplants were associated with a significantly lower relapse than HLA-identical sibling transplants [3]. Likewise, in patients with poor-risk leukemia or myelodysplastic syndrome undergoing myeloablative, related donor BMT, significantly lower relapse was seen with 1 antigen mismatched, versus no antigen mismatched, grafts [5]. Following unrelated donor BMT, specific combinations of allele mismatches have been linked with lower relapse risk and improved overall survival, and may not necessarily be those that lead to severe acute GVHD [4]. Typically, however, the potential antitumor benefit of HLA disparity has been offset by higher rates of GVHD and mortality.
Limitations of this analysis include its retrospective nature and the small number of donor-recipient pairs with fewer than three HLA mismatches (26 or 14% at the antigen level). Analyses of NRM and graft failure in particular may be underpowered, given the relatively low number of occurrences. The small number of patients with fewer mismatches may also limit the power to show minor differences in outcomes based on the degree of HLA disparity. Nevertheless, for the overall outcome of EFS, greater HLA disparity not only appeared nondetrimental, but appeared protective. This could be a chance finding, but makes it unlikely that a worsening of overall outcome would have been missed.
We attempted to analyze potential confounding variables and imbalances in patient characteristics that could skew the data, and this did not change the conclusions regarding EFS in relation to HLA disparity. Although it is possible that variables other than those evaluated influenced the results, this is a limitation of any comparative study. This may explain the unexpected observation that having a female donor for a male recipient was associated with inferior EFS in the absence of a statistically significant increased risk of acute GVHD.
We found no statistically significant association between the incidence of acute grade II or higher GVHD and having more mismatches. Dissociation of an allogeneic graft-versus-tumor effect and GVHD remains an elusive goal [26]. The study is not of the scope to determine whether mismatches at particular loci are associated with differential risk of GVHD or relapse, and it is possible that analyses of acute GVHD are underpowered. Nevertheless, the results raise the possibility that antitumor effects of this particular transplant platform can occur irrespective of clinically significant acute GVHD. Unlike calcineurin inhibitors, which block both T-cell activation and induction of tolerance to alloantigens [27], the strategic delivery of high-dose Cy allows for the effective deletion of proliferating alloreactive cells [12]. It may be that posttransplantation Cy selectively kills cells reactive to abundant alloantigens while minimally affecting cells reactive to tumor-specific antigens, or that Cy is more toxic to cells that cause GVHD than to cells that cause graft-versus-tumor effects [28,29]. Potential effectors of antitumor immunity include HLA class II-reactive CD4+ T-cells, HLA class I-reactive CD8+ T-cells, and natural killer cells recognizing missing self [30–32]. Further studies will be required to discern these mechanisms and to what degree the impact of HLA disparity differs according to disease entity and the vector of the mismatch.
Compared with myeloablative conditioning regimens, reduced intensity conditioning regimens for allogeneic BMT have been associated with lower incidences of NRM [33] and GVHD [34] but higher incidences of graft failure [35] and relapse [36,37]. Although the incidences of NRM and GVHD were acceptably low, the relatively high rates of graft failure and relapse in this study suggest that the transplantation regimen can be optimized further. We are currently exploring strategies to reduce the incidences of graft failure and relapse without increasing transplant-related toxicity. Examples of potential approaches include enhancing the antitumor activity of the conditioning regimen, immunizing donors against tumor-specific antigens, or infusing alloreactive donor natural killer cells after transplantation.
We view these observations as hypothesis generating. Prospective studies will be required to validate the findings. However, this exploratory analysis raises the possibility that, for patients who lack an HLA-matched donor, the choice of a partially mismatched donor may not be dictated solely by the extent of HLA disparity between donor and recipient. For patients who have more than one potential HLA-haploidentical donor, non-HLA characteristics of the graft that affect its antitumor activity may also be considered during donor selection. Examples include selecting grafts according to killer-cell immunoglobulin-like receptors or, for patients with B-cell lymphomas, according to Fc receptor polymorphisms associated with greater responsiveness to rituximab [38].
Acknowledgments
Supported by a National Institutes of Health grant P01 CA015396 to R.J.J., a National Institutes of Health grant K23 CA124465 to Y.L.K., and a Scholar in Clinical Research Award to E.J.F. from the Leukemia and Lymphoma Society of America. Presented in part at the 2008 Annual Meeting of the American Society of Hematology.
References
- 1.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 2.Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 3.Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7:443–452. [PubMed] [Google Scholar]
- 4.Kawase T, Morishima Y, Matsuo K, et al. High-risk HLA allele mismatch combinations responsible for severe acute graft-versus-host disease and implication for its molecular mechanism. Blood. 2007;110:2235–2241. doi: 10.1182/blood-2007-02-072405. [DOI] [PubMed] [Google Scholar]
- 5.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 6.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 7.Morishima Y, Yabe T, Matsuo K, et al. Effects of HLA allele and killer immunoglobulin-like receptor ligand matching on clinical outcome in leukemia patients undergoing transplantation with T-cell-replete marrow from an unrelated donor. Biol Blood Marrow Transplant. 2007;13:315–328. doi: 10.1016/j.bbmt.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Strauss G, Osen W, Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. 2002;128:255–266. doi: 10.1046/j.1365-2249.2002.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eto M, Mayumi H, Tomita Y, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146:1402–1409. [PubMed] [Google Scholar]
- 10.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 11.Luznik L, Jalla S, Engstrom LW, et al. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 12.Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology. 1996;195:129–139. doi: 10.1016/S0171-2985(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 13.Mayumi H, Himeno K, Tanaka K, et al. Drug-induced tolerance to allografts in mice. XII. The relationships between tolerance, chimerism, and graft-versus-host disease. Transplantation. 1987;44:286–290. doi: 10.1097/00007890-198708000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Luznik L, Bolanos-Meade J, Brodsky R, et al. Post-transplantation high dose cyclophosphamide (Cy) is effective single agent for prevention of acute and chronic graft versus host disease after myeloablative HLA matched related and unrelated bone marrow transplantation (BMT) Blood. 2008 abstract 56. [Google Scholar]
- 15.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 16.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–1683. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 18.Burroughs LM, O’Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:1279–1287. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 23.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 25.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 26.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 27.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 28.Anderson BE, McNiff J, Yan J, et al. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–108. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamiryo Y, Eto M, Yamada H, et al. Donor CD4 T cells are critical in allogeneic stem cell transplantation against murine solid tumor. Cancer Res. 2009;69:5151–5158. doi: 10.1158/0008-5472.CAN-08-2517. [DOI] [PubMed] [Google Scholar]
- 31.Hsu KC, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 34.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 35.Le Blanc K, Remberger M, Uzunel M, et al. A comparison of nonmyeloablative and reduced-intensity conditioning for allogeneic stem-cell transplantation. Transplantation. 2004;78:1014–1020. doi: 10.1097/01.tp.0000129809.09718.7e. [DOI] [PubMed] [Google Scholar]
- 36.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez R, Nademanee A, Ruel N, et al. Comparison of reduced-intensity and conventional myeloablative regimens for allogeneic transplantation in non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12:1326–1334. doi: 10.1016/j.bbmt.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 38.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]