Abstract
The transient receptor potential A1 channel (TRPA1) is activated by various compounds, including isothiocyanates, menthol, and cinnamaldehyde. The sensitivities of the rodent and human isoforms of TRPA1 to menthol and the cysteine-attacking compound CMP1 differ, and the molecular determinants for these differences have been identified in the 5th transmembrane region (TM5) for menthol and TM6 for CMP1. We recently reported that caffeine activates mouse TRPA1 (mTRPA1) but suppresses human TRPA1 (hTRPA1). Here we aimed to identify the molecular determinant that is responsible for species-specific differences in the response to caffeine by analyzing the functional properties of various chimeras expressed in Xenopus oocytes. We initially found that the region between amino acids 231 and 287, in the distal N-terminal cytoplasmic region of mTRPA1, is critical. In a mutagenesis study of this region, we subsequently observed that introduction of a Met268Pro point mutation into mTRPA1 changed the effect of caffeine from activation to suppression. Because the region including Met-268 is different from other reported ligand-binding sites and from the EF-hand motif, these results suggest that the caffeine response is mediated by a unique mechanism, and confirm the importance of the distal N-terminal region for regulation of TRPA1 channel activity.
Introduction
Transient receptor potential (TRP) channels are widely expressed in species ranging from nematodes to mammals. The superfamily consists of various members belonging to the TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML subfamilies (1–4). Among these, TRPA1, which was initially named ANKTM1 and contains an ankyrin repeat domain in its N-terminal region, is expressed in subsets of the dorsal root ganglion (DRG) neurons that mediate nociceptive signaling (5,6). As such, TRPA1 is activated by various pungent compounds and environmental irritants, many of which are plant-derived products such as allyl isothiocyanate (AITC) found in wasabi, horseradish, and mustard oils; allicin found in garlic; cinnamaldehyde from cinnamon oil; menthol from peppermint oil; Δ9-tetrahydrocannabinol from marijuana; and sanshool from Sichuan peppers (6–11). Other chemical activators of TRPA1 include formaldehyde, 4-hydroxynonenal (an endogenous aldehyde), methyl p-hydroxybenzoate (an antibacterial compound in cosmetics), H2O2, and Zn2+ (5,12–17). Activation of TRPA1 by these reactive substances is thought to be mediated by their covalent modification of amino acid residues such as Cys (12,18). TRPA1 is also activated by nonreactive substances such as icilin (a synthetic TRPM8 agonist), intracellular Ca2+, alkaline intracellular pH, and nicotine (5,19–22). Activation of TRPA1 by noxious cold temperatures has also been reported (5,7), but it remains controversial whether this activation is a direct effect of the cold (8,19,23). Karashima et al. (24) recently reported evidence that mouse TRPA1 acts as a cold sensor both in vitro and in vivo.
In a previous study (25) we observed, using Ca2+i imaging and electrophysiological recording, that mouse TRPA1 (mTRPA1) in heterologous expression systems and in DRG neurons could be activated by caffeine at millimolar concentrations. We confirmed that the current was carried by mTRPA1 by noting that the response to caffeine was absent in DRG neurons from TRPA1 gene knock-out (KO) mice. Furthermore, we found that wild-type (WT), but not KO mice, were averse to drinking caffeine-containing water in a two-bottle test. Of note, however, we observed that human TRPA1 (hTRPA1) was not activated by caffeine, and instead was suppressed, especially after activation by AITC.
There are other examples of species-specific qualitative differences in TRPA1 responses (26–29). For example, the trichloro(sulfanyl)ethyl benzamides AMG5445 and AMG9090 act as partial agonists of rat TRPA1 (rTRPA1), but act as antagonists to hTRPA1 (26). The thioaminal-containing compound CMP1, which covalently modifies Cys residues, activates rTRPA1 but blocks hTRPA1, and amino acid residues in the 6th transmembrane (TM6) region are critical for that difference (27). Menthol activates mTRPA1 at low concentrations and blocks it at high concentrations, but only activates hTRPA1, and amino acid residues in TM5 are involved in that difference (28).
Caffeine is not a reactive chemical reagent, and it is unknown which region of TRPA1 is critical for activation by caffeine. In this study, we aimed to identify the molecular determinant that is responsible for the species-specific difference between mTRPA1 and hTRPA1. To that end, we carried out chimera studies and observed that it is not a TM region but the N-terminal cytoplasmic region that is responsible. We then used point mutagenesis to identify the critical amino acid residue.
Materials and Methods
Molecular biology
mTRPA1 cDNA was isolated by polymerase chain reaction (PCR), and the DNA sequence was identical to that deposited in the database (25). The hTRPA1 cDNA was provided by Dr. Patapoutian (Scripps Research Institute, La Jolla, CA). All chimeras were constructed using a two-step PCR method with PCR primers at the N- and C-ends and at the junction region. The amplification was done using KOD plus polymerase (TOYOBO, Tokyo, Japan) or ExTaq DNA polymerase (TAKARA, Tokyo, Japan) according to the manufacturer's instructions. All point mutations were introduced into mTRPA1 or hTRPA1 cDNA using a QuikChange kit (Stratagene, La Jolla, CA). All constructs were confirmed by DNA sequencing. cRNA for oocyte injection was transcribed in vitro using a mMESSAGE mMACHINE transcription kit (Ambion, Austin, TX).
Chemicals
For the experiments, we purchased caffeine from Kanto Chemical (Tokyo, Japan), AITC from Tokyo Kasei (Tokyo, Japan), camphor from Sigma (St. Louis, MO), and sucrose from Wako Chemicals (Tokyo, Japan). Stock solutions were made up in recording solution (caffeine) or in DMSO (AITC, camphor) and stored at −20°C. They were diluted in the recording solution just before use.
Electrophysiology
Xenopus oocytes were surgically isolated from frogs anesthetized in water containing 0.15% tricaine. After the final collection, the frogs were humanely killed. Isolated oocytes were treated with collagenase (2 mg/mL, type I; Sigma, St. Louis, MO) for 5 h, after which oocytes at stage V were injected with ∼50 nL of cRNA solution and incubated at 17°C in frog Ringer solution. Experiments with Xenopus conformed to institutional guidelines and were approved by the Animal Experiment Committee of the National Institute for Physiological Sciences.
Membrane currents were recorded from oocytes 1–3 days after cRNA injection under a two-electrode voltage clamp using an OC725C amplifier (Warner Instruments, Hamden, CT) and pCLAMP8 software (Axon Instruments, Union City, CA). The microelectrodes used were drawn from borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) to a resistance of 0.2–0.5 MΩ when filled with 3 M KCl (pH 7.2). The bath solution contained 96 mM NaCl, 2 mM KCl, 3 mM MgCl2, and 5 mM HEPES (pH 7.4), with no added Ca2+. Oocytes were held at −20 mV, and the membrane current was continuously recorded with repeated application of 200-ms ramp pulses from −100 to +100 mV every 5 s. All experiments were carried out at room temperature (23 ± 2°C). To achieve rapid application of agonists, a one-fifth bath volume of 5× concentrated solution was pipetted into the bath. We determined the exchange rate of solutions in the bath of our system by monitoring the change in the Kir3.1/3.2 inward rectifier K+ channel current upon a K+ concentration jump, and the time constant of solution exchange was shown to range from 299 to 533 ms. This speed is sufficient for the analysis of both caffeine effects (see Fig. S5 in the Supporting Material).
Statistical analyses
The data are shown as the means ± SE. Differences between means were analyzed using Student's unpaired t-test. Values of p < 0.01 were considered significant.
Results
Activation of mTRPA1 and suppression of hTRPA1 by caffeine
We examined the effects of caffeine on mTRPA1 and hTRPA1 expressed in Xenopus oocytes by recording the changes in membrane currents under a two-electrode voltage clamp with repeated ramp pulses. mTRPA1 was activated by caffeine, and the EC50-value was ∼5 mM (Fig. 1, A and B). By contrast, hTRPA1 activity was suppressed by caffeine in approximately the same concentration range (Fig. 1, C and D). An accurate estimation of EC50 or IC50 was not practically possible, because the caffeine concentration required to achieve a peak response was too high.
Figure 1.
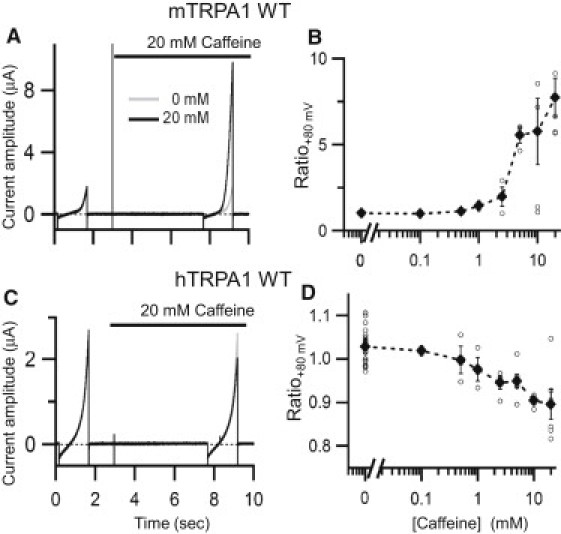
Responses to caffeine by mTRPA1 and hTRPA1 expressed in Xenopus oocytes. (A and C) Representative current traces recorded from Xenopus oocytes expressing mTRPA1 (A) or hTRPA1 (C) under a two-electrode voltage clamp. In each trace, a 1.5-s ramp pulse from −100 to +100 mV was applied twice from a holding potential of −20 mV. Each data set consisted of recordings made without caffeine (first run, gray) and then with caffeine (second run, black). Caffeine was present during the time indicated by the bar. (B and D) The ratios of the current amplitudes recorded at +80 mV from oocytes expressing mTRPA1 (B) or hTRPA1 (D) before and after caffeine application plotted against caffeine concentration. Open circles show data from each oocyte, and the solid diamonds and bars indicate means ± SE. The n-values for each point range from 2 to 6. The n-value for 0 mM caffeine is the sum of all the other points, since data before the application of caffeine were recorded as a negative control in all experiments.
It is well known that caffeine induces Ca2+ release from intracellular Ca2+ stores, which raises the possibility that part of the current elicited by caffeine is not mediated by the TRPA1 channel. To determine how much of the caffeine-induced current was carried by TRPA1, we applied 2 mM camphor, a TRPA1 channel blocker, which almost completely blocked the current (Fig. S1). The percentage of block of the caffeine-induced current was 95.2 ± 2.8% (n = 4). Moreover, no clear responses were observed in oocytes not injected with cRNA (see Fig. 8 C) or in untransfected HEK293 cells (25). Thus, the response to caffeine was confirmed to be mediated almost entirely by the expressed mTRPA1 channel.
Figure 8.
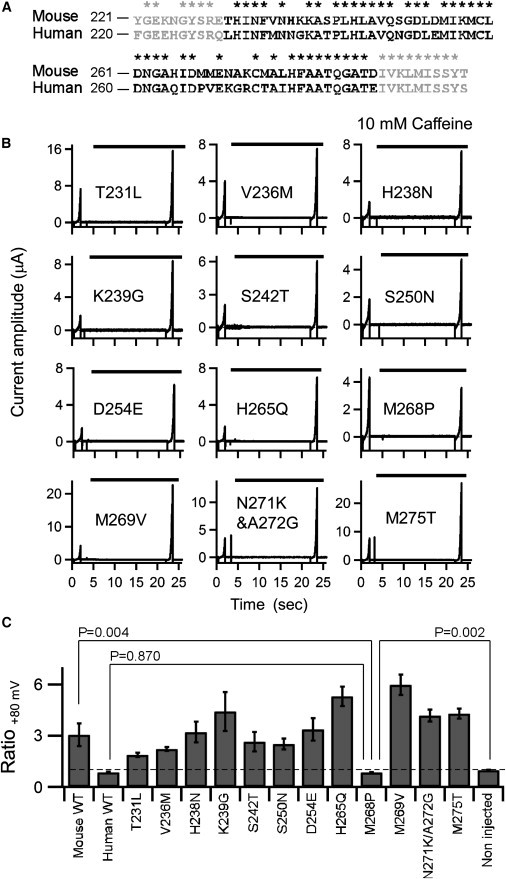
Current recordings and analysis of mTRPA1 point mutants. (A) Alignment of partial amino acid sequences of mTRPA1 and hTRPA1. The identified critical region is highlighted in black (amino acids 231–287 of mTRPA1). Asterisks indicate identical amino acid residues. (B) Representative current recordings from oocytes expressing the indicated mTRPA1 point mutant. Under a two-electrode clamp, 1.5-s ramp pulses from −100 to +100 mV were applied twice from a holding potential of −20 mV. The presence of 10 mM caffeine is indicated by the bar. (C) Ratios of the current amplitudes at +80 mV before and after caffeine application. Plots depict means ± SE. The data for Met268Pro were significantly different from WT mTRPA1, but not from hTRPA1. The data and n-values are shown in Table 2.
Zhang et al. (30) reported that rTRPA1 responds to hyperosmolarity. This raises the possibility that mTRPA1 responds not to the caffeine itself, but to the increase in osmolarity associated with caffeine application. We therefore examined the response to 20 mM sucrose and found that neither mTRPA1 nor hTRPA1 responded to the increase in osmolarity, though they clearly responded to positive control stimuli (Fig. S2 and Table S1 A). Nonetheless, in subsequent experiments we recorded the responses to 5 mM (10 mM in Fig. 7) caffeine to minimize the potential effect of hyperosmolarity.
Figure 7.
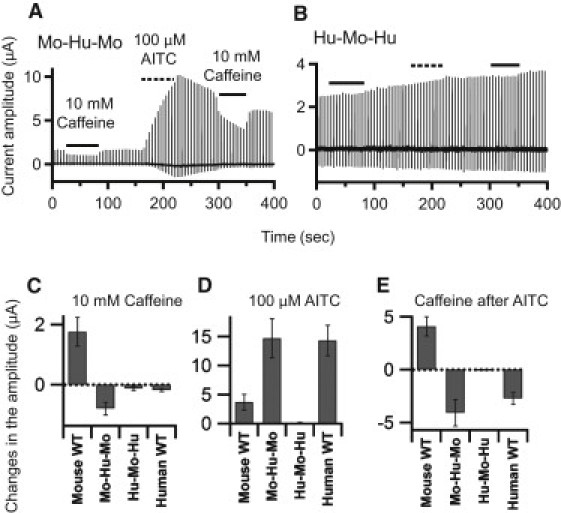
Current recordings and analyses of Mo-Hu-Mo and Hu-Mo-Hu. (A and B) Details of the experiments are the same as in Fig. 3, except that 10 mM caffeine was used. (C–E) Details of the analyses are the same as in Fig. 4, except that 10 mM caffeine was used. Plots depict means ± SE. The data and n-values are shown in Table 1.
Use of chimeras to identify the TRPA1 region critical for the caffeine response
The species-specific difference in the response of TRPA1 to CMP1 and menthol reportedly reflects the fact that they act on amino acids in TM6 and TM5, respectively (27,28). On the other hand, amino acid residues in the N-terminal region are known to be critical for the binding of agonists such as AITC (Cys-415, -422, and -622 of mTRPA1) (12) and Ca2+ (Asp-468 and Asp-479 of hTRPA1 (19), and Leu-474 of hTRPA1 (20)). To identify the critical region for the response to caffeine, we designed the set of chimeric constructs summarized in Fig. 2. In Mo705Hu, the entire N-terminal cytoplasmic region up to amino acid 705 is from mTRPA1, and the remainder of the molecule is from hTRPA1. Hu703Mo has the opposite configuration. Representative recordings from oocytes expressing Mo705Hu and Hu703Mo of the response to caffeine alone and after activation by AITC are shown in Fig. 3, C and D. It is obvious that the Mo705Hu phenotype (Fig. 3 C) is similar to that of mTRPA1 (Fig. 3 A), and that Hu703Mo (Fig. 3 D) is similar to hTRPA1 (Fig. 3 B), which means it is not the TM region but the N-terminal cytoplasmic region that is critical for the difference in the responses to caffeine. We also analyzed the Mo375Hu and Hu374Mo chimeras. Mo375Hu was activated by caffeine as well as by AITC (Fig. 3 E), whereas Hu374Mo was slightly activated by AITC but was inhibited by caffeine (Fig. 3 F). This means that the first half of the N-terminal region is critical for the activation of mTRPA1 by caffeine. It is noteworthy that the critical site for the caffeine response differs from those for AITC and Ca2+, and that it is situated in a more distal part of the N-terminal region. The cumulative data from the experiments represented in Fig. 3 are summarized in Fig. 4 and Table 1. Here the changes in the current amplitude evoked by 5 mM caffeine alone (Fig. 4, A and D), 100 μM AITC alone (Fig. 4, B and E), and 5 mM caffeine after activation by AITC (Fig. 4, C and F) are shown. Note that the data in Fig. 4, A–C, and Fig. 4, D–F, were obtained from different sets of experiments.
Figure 2.
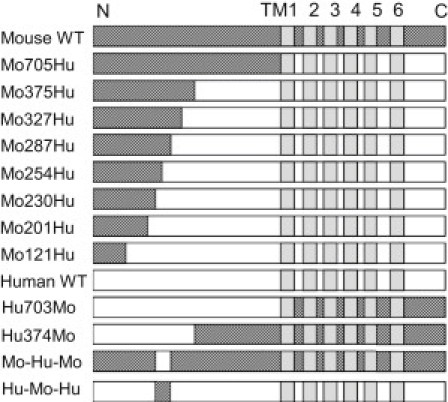
Schematic drawings of the mTRPA1-hTRPA1 chimeras. The components of mTRPA1 and hTRPA1 are shown in dark and white, respectively. The positions of the six TM regions are shown in light gray. In the labels on the left, Mo and Hu stand for mouse and human. Mo705Hu, for example, indicates that the N-terminal 705 residues are from mTRPA1 and the rest are from hTRPA1. Mo-Hu-Mo and Hu-Mo-Hu are chimeras in which only the critical region (231–287 in mTRPA1) was swapped.
Figure 3.
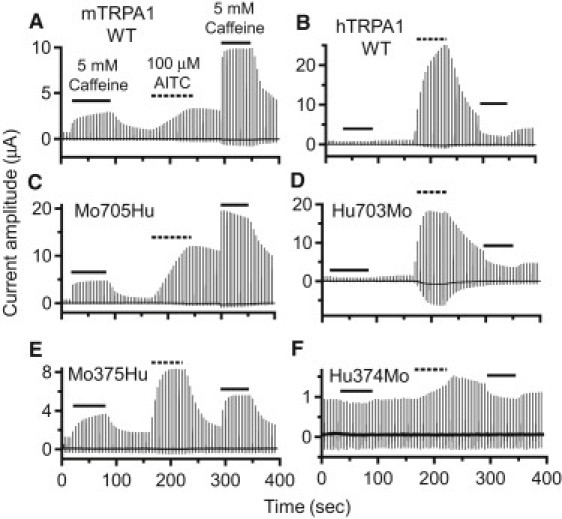
Current recordings of mTRPA1, hTRPA1, Mo705Hu, Hu703Mo, Mo375Hu, and Hu374Mo. Under a two-electrode voltage clamp, 200-ms ramp pulses from −100 to +100 mV were applied every 5 s from a holding potential of −20 mV. Recordings are from oocytes expressing mTRPA1 WT (A), hTRPA1WT (B), Mo705Hu (C), Hu703Mo (D), Mo375Hu (E), or Hu374Mo (F). The presence of 5 mM caffeine or 100 μM AITC is indicated by the bars. Panels A–D and panels E and F are from different batches of oocytes.
Figure 4.
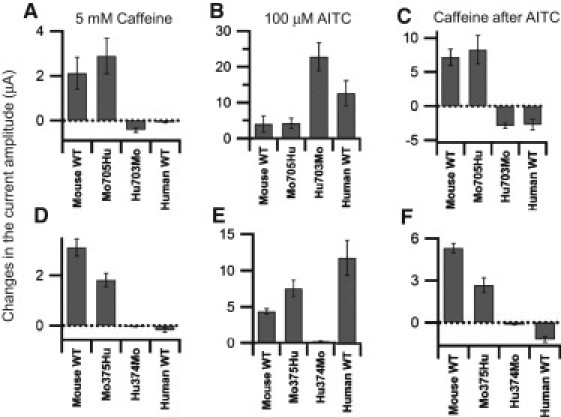
Analysis of the cumulative data from the experiments in Fig. 3. The current amplitude at +100 mV was changed by application of 5 mM caffeine (A, C, D, and F) or by 100 μM AITC (B and E). (A, B, D, and E) Differences in current amplitudes before caffeine application and after the 10th pulse after application are shown. (C and F) Caffeine was applied after the application and subsequent washout of AITC. Differences between current amplitudes before caffeine application and the amplitude of the first pulse after application are shown. Panels A–C and D–F are from different batches of oocytes. Plots depict means ± SE. The data and n-values are shown in Table 1.
Table 1.
Mean ± SE values of the current amplitudes at +100 mV (μA) in the category plots
| Fig. 4, A–C | mTRPA1 WT | Mo705Hu | Hu703Mo | hTRPA1 WT | |
| 5 mM caffeine | 2.12 ± 0.71 | 2.88 ± 0.80 | −0.43 ± 0.10 | −0.08 ± 0.02 | |
| 100 μM AITC | 3.98 ± 2.25 | 4.22 ± 1.45 | 22.77 ± 3.89 | 12.60 ± 3.53 | |
| Caffeine after AITC | 7.15 ± 1.21 | 8.25 ± 2.09 | −2.86 ± 0.37 | −2.71 ± 0.80 | |
| n-value | 4 | 6 | 6 | 4 | |
| Fig. 4, D–F | mTRPA1 WT | Mo375Hu | Hu374Mo | hTRPA1 WT | |
| 5 mM caffeine | 3.11 ± 0.34 | 1.82 ± 0.27 | −0.04 ± 0.02 | −0.18 ± 0.09 | |
| 100 μM AITC | 4.36 ± 0.39 | 7.54 ± 1.13 | 0.22 ± 0.04 | 11.78 ± 2.41 | |
| Caffeine after AITC | 5.31 ± 0.35 | 2.67 ± 0.53 | −0.14 ± 0.01 | −1.21 ± 0.21 | |
| n-value | 5 | 7 | 14 | 5 | |
| Fig. 6, A–C | mTRPA1 WT | Mo327Hu | Mo287Hu | Mo230Hu | hTRPA1 WT |
| 5 mM caffeine | 4.85 ± 0.97 | 0.57 ± 0.12 | 0.26 ± 0.04 | −0.33 ± 0.04 | −0.14 ± 0.05 |
| 100 μM AITC | 9.64 ± 2.76 | 2.73 ± 0.80 | 1.65 ± 0.32 | 14.55 ± 1.72 | 16.78 ± 3.67 |
| Caffeine after AITC | 8.72 ± 1.75 | 2.13 ± 0.42 | 0.96 ± 0.14 | −2.22 ± 0.27 | −3.24 ± 0.77 |
| n-value | 4 | 7 | 7 | 7 | 4 |
| Fig. 6, D–F | mTRPA1 WT | Mo254Hu | Mo201Hu | Mo121Hu | hTRPA1 WT |
| 5 mM caffeine | 2.13 ± 0.39 | 1.25 ± 0.11 | −0.05 ± 0.02 | −0.01 ± 0.01 | −0.15 ± 0.06 |
| 100 μM AITC | 1.56 ± 0.37 | 15.55 ± 0.84 | 12.22 ± 1.67 | 2.98 ± 0.87 | 7.52 ± 2.79 |
| Caffeine after AITC | 5.54 ± 0.79 | 1.23 ± 0.82 | −2.21 ± 0.23 | −0.42 ± 0.14 | −1.22 ± 0.48 |
| n-value | 4 | 6 | 6 | 6 | 4 |
| Fig. 7, C–E | mTPA1 WT | Mo-Hu-Mo | Hu-Mo-Hu | hTRPA1 WT | |
| 10 mM caffeine | 1.76 ± 0.48 | −0.80 ± 0.21 | −0.13 ± 0.07 | −0.19 ± 0.05 | |
| 100 μM AITC | 3.70 ± 1.34 | 14.72 ± 3.36 | 0.17 ± 0.09 | 14.31 ± 2.61 | |
| Caffeine after AITC | 4.09 ± 0.91 | −4.08 ± 1.26 | −0.04 ± 0.02 | −2.73 ± 0.55 | |
| n-value | 14 | 10 | 10 | 12 | |
Further definition of the critical region for the caffeine response
To more narrowly define the region that is critical for the difference in the responses of mTRPA1 and hTRPA1 to caffeine, we constructed additional chimeras (Fig. 2). Because the suppressive effect of caffeine was not very clear in Hu374Mo-expressing oocytes (Figs. 3 F and 4, D and F), and even the activating effect of AITC was very small (Figs. 3 F and 4 E), we considered it likely that there was a problem in the functionality or expression of Hu374Mo, and decided to thereafter concentrate only on the Mo-Hu chimeras shown in Fig. 2.
Both Mo327Hu and Mo287Hu (Fig. 5, A and B) were activated upon application of caffeine, suggesting that the critical region for activation of mTRPA1 is situated in the distal 287 amino acids of the N-terminal region. In oocytes expressing Mo230Hu (Fig. 5 C), caffeine clearly suppressed the current, and the phenotype was similar to that of WT hTRPA1 (Fig. 3 B), indicating that the critical region for activation of mTRPA1 is not present in the distal 230 amino acids. The phenotype of Mo254Hu (Fig. 5 D) was clearly different from either WT mTRPA1 (Fig. 3 A) or Mo230Hu (Fig. 5 C). Upon application of caffeine, the current amplitude increased slightly but then immediately started to decline, reflecting the presence of activation as well as activation-coupled desensitization, inactivation, or block. This was more prominently observed when caffeine was applied after activation by AITC (Fig. 5 D). As expected, the phenotypes of Mo201Hu (Fig. 5 E) and Mo121Hu (Fig. 5 F) were also similar to that of WT hTRPA1 (Fig. 3 B). The cumulative results of the two sets of experiments shown in Fig. 5, A–C and D–F, are summarized in Fig. 6, A–C and D–F, respectively, and in Table 1. The activation of Mo287Hu by caffeine and the suppression of Mo230Hu are obvious (Fig. 6, A and C). Taken together, these results show that the molecular determinant of mTRPA1 activation by caffeine is situated in the N-terminal cytoplasmic region between amino acid residues 231 and 287.
Figure 5.
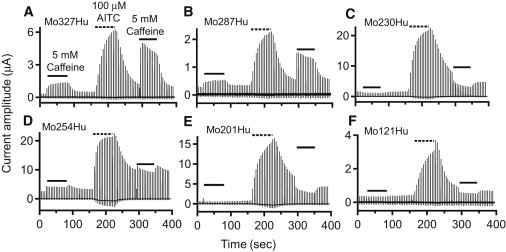
Current recordings for Mo327Hu, Mo287Hu, Mo230Hu, Mo254Hu, Mo201Hu, and Mo121Hu. Details of the experiments are the same as in Fig. 3. Panels A–C and D–F are from different batches of oocytes.
Figure 6.
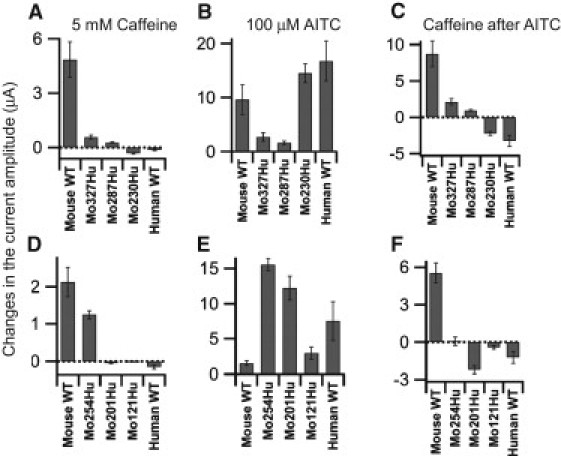
Analysis of the cumulative data from the experiments in Fig. 5. Details of the analyses are the same as in Fig. 4. Panels A–C and D–F are from different batches of oocytes. Plots depict means ± SE. The data and n-values are shown in Table 1.
Effect of swapping only the critical region
To further confirm the importance of the region between amino acid residues 231 and 287, we constructed two chimeras in which only this region is swapped: Mo-Hu-Mo and Hu-Mo-Hu (Fig. 2). The currents recorded from oocytes expressing these two chimeras are shown in Fig. 7, A and B, and the cumulative data are summarized in Fig. 7, C–E, and Table 1. We found that Mo-Hu-Mo was clearly inhibited by caffeine, especially after application of AITC (Fig. 7, A–C, and E), which confirms that without the region between amino acids 231 and 287, mTRPA1 would not be activated by caffeine. By contrast, Hu-Mo-Hu did not show a clear response to caffeine, or even to AITC (Fig. 7, B and D). Thus, Hu-Mo-Hu provided no information about the response to caffeine, which could reflect the molecule's lack of functionality or a lack of surface expression. More complete overall integrity or unity may be necessary for intact hTRPA1 expression and function.
A point mutation that changed the effect of caffeine from activation to suppression
We next used point mutagenesis to identify the critical amino acid residue that accounts for the difference in the responses of mTRPA1 and hTRPA1 to caffeine. Taking the result obtained with Mo-Hu-Mo into consideration (Fig. 7), we decided to introduce human type mutations on the background of mTRPA1. Amino acid residues 231–287 are shown in black in Fig. 8 A, with asterisks indicating the residues conserved between the murine and human channels. We recorded from oocytes expressing the 11 single-point mutants and one double-point mutant shown in Fig. 8 B, and the cumulative data are summarized in Fig. 8 C and Table 2. One can see the effect of caffeine in Fig. 8 B by comparing the currents elicited by ramp pulses applied in the absence and presence of caffeine. Although the extent of the activation varied, all of the mutants except Met268Pro were clearly activated upon caffeine application. Only with Met268Pro was the current amplitude suppressed in a manner similar to that seen with hTRPA1 (Fig. 1 C). Moreover, the caffeine dose-inhibition curve for Met268Pro (Fig. 9) was very similar to that for hTRPA1 (Fig. 1 D). We also confirmed that the response to AITC was retained by Met268Pro (1.12 ± 0.44 (μA), n = 3; Fig. S3). These results demonstrate that we were able to change the phenotype of mTRPA1 to that of hTRPA1 by substituting a Pro for Met-268, which suggests that Met-268 in mTRPA1 (Pro-267 in hTRPA1) may be the critical molecular determinant for the species-specific difference in the caffeine response.
Table 2.
Mean ± SE values of the ratio of the current amplitude at +80 mV (μA) in the presence and absence of caffeine
| Fig. 8C | mTRPA1 WT | hTRPA1 WT | T231L | V236M | H238N hTRPA1 |
| Ratio | 3.05 ± 0.66 | 0.86 ± 0.03 | 1.88 ± 0.13 | 2.22 ± 0.12 | 3.21 ± 0.61 |
| n-value | 17 | 17 | 13 | 15 | 14 |
| K239G | S242T | S250N | D254E | H265Q | |
| Ratio | 4.41 ± 1.14 | 2.64 ± 0.57 | 2.51 ± 0.31 | 3.37 ± 0.66 | 5.30 ± 0.56 |
| n-value | 14 | 14 | 16 | 15 | 17 |
| M268P | M269V | N271K/A272G | M275T | Non-inj | |
| Ratio | 0.85 ± 0.03 | 5.96 ± 0.59 | 4.18 ± 0.34 | 4.30 ± 0.29 | 0.98 ± 0.02 |
| n-value | 14 | 13 | 13 | 13 | 17 |
| Fig. 10B | hTRPA1 WT | mTRPA1 WT | P267M | N237H/ P267M | G238K/P267M |
| Ratio | 0.77 ± 0.04 | 6.07 ± 0.66 | 0.65 ± 0.02 | 0.64 ± 0.02 | 0.70 ± 0.05 |
| n-value | 11 | 11 | 12 | 13 | 11 |
| N237H/G238K/P267M | N239S/ P267M | P267M/ V268M | Non-inj | ||
| Ratio | 0.69 ± 0.03 | 0.76 ± 0.01 | 0.78 ± 0.02 | 0.93 ± 0.02 | |
| n-value | 12 | 12 | 11 | 9 | |
Figure 9.
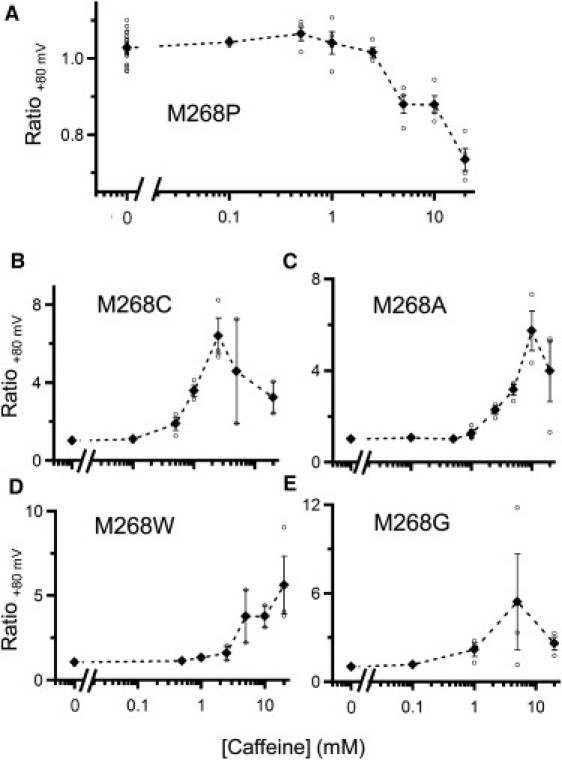
Caffeine dose-response relationships of mTRPA1 Met-268 point mutants. The ratios of the current amplitudes at +80 mV before and after caffeine application are plotted against caffeine concentration. Data for M268P (A), M268C (B), M268A (C), M268W (D), and M268G (E) are shown. Open circles show data from each oocyte, and the solid diamonds and bars indicate means ± SE. The n-values for each point range from 1 to 4 (n = 1 only for the case in which 0.1 mM was applied to Met268Ala). The n-value of 0 mM caffeine is the sum of all other points, since data before caffeine application were recorded as a negative control in all experiments.
To further investigate the importance of Met-268, we also substituted Met-268 of mTRPA1 with Cys, Ala, Trp, or Gly. Cys has an -SH group instead of the -S-CH3 group in Met; Ala and Trp are representative of small and bulky amino acids, respectively; and Gly has a structural flexibility that enables it to kink in a manner similar to that of Pro. In contrast to Met268Pro, however, there was clear activation of the channel by caffeine with all four of these mutants (Fig. 9, B–E). Notably, reductions in the response amplitude at high caffeine concentrations, which were not seen with WT mTRPA1 (Fig. 1 B), were clearly observed with the Cys, Ala, and Gly mutants (Fig. 9, B, C, and E). This suggests that the Cys, Ala, and Gly mutants may have dual features of activation and activation-coupled desensitization, inactivation, or block, and that their phenotypes may reflect aspects of both activation of mTRPA1 and suppression of hTRPA1. We also found that rather bulky amino acid residues, such as Met or Trp, will sustain caffeine-induced activation of mTRPA1, and that introduction of Pro disrupts the activation phenotype of mTRPA1, most likely by introducing a kink into the structure. It would be more appropriate to say that the absence of Pro at 268 of mTRPA1, rather than the presence of Met here, is critical for the activation effect of caffeine.
Finally, we analyzed the effect of introducing mouse type point mutations on a background of hTRPA1 (Fig. 10). The Pro267Met hTRPA1 mutant, which had the substitution opposite to that of Met 268Pro, was not activated by caffeine but was clearly suppressed (Fig. 10, A and B). In addition, four double-point mutants containing the Pro267Met substitution plus an Asn237His, Gly238Lys, Asn249Ser, or Val268Met substitution, and a triple mutant containing Pro267Met, Asn237His, and Gly238Lys substitutions were also suppressed, not activated, by caffeine (Fig. 10 B and Table 2). These phenotypes were all similar to that of WT hTRPA1 (Fig. 1 B). It is thus plausible that the mechanism by which caffeine activates mTRPA1 is more complex than the mechanism by which it inhibits hTRPA1, and requires more structural background of mTRPA1 other than Met-268.
Figure 10.
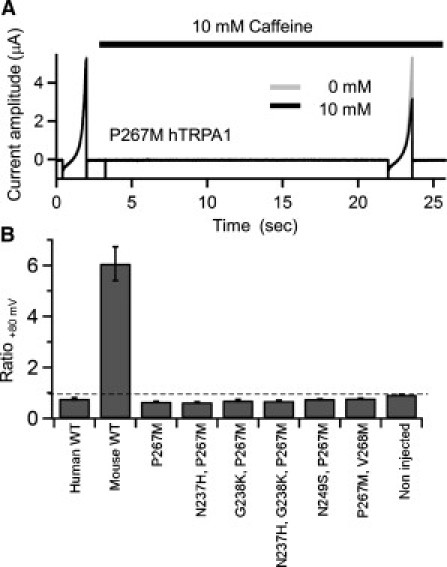
Current recordings and analyses of hTRPA1 point mutants. (A) Details of the experiments are the same as in Fig. 8B. (B) Details of the analyses are the same as in Fig. 8C. Plots depict means ± SE. The data obtained with the mutants did not statistically differ from WT hTRPA1. The data and n-values are shown in Table 2.
Discussion
Comparison of Met-268 with sites determining the response of TRPA1 to other ligands
A number of amino acid residues involved in the response of TRPA1 channels to various ligands have already been identified. It is now accepted that activation of TRPA1 channels by reactive compounds such as AITC is due to covalent modification of Cys residues (12,18). Furthermore, Hinman et al. (18) identified Cys-621, Cys-641, and Cys-665, situated within the ankyrin repeat domain in the N-terminal cytoplasmic region of hTRPA1, as critical factors in that activation, and Macpherson et al. (12) identified Cys-415, Cys-422, and Cys-622 in the same region of mTRPA1 as being critical. It is unlikely, however, that the effect of caffeine reflects such covalent modification, because caffeine is not a reactive substance. We observed that the Cys622Ser mutant, which reportedly does not respond to AITC (12), was clearly activated by caffeine (Table S1 B).
Zurborg et al. (19) reported that cytoplasmic Ca2+ directly activates hTRPA1, and identified the negatively charged Asp-468 and Asp-479 residues in the EF-hand motif in the N-terminal cytoplasmic region as critical sites, and Doerner et al. (20) reported that Leu-474 in the EF-hand motif is critical for Ca2+i sensitivity. On the other hand, in our study the Ca2+i-insensitive mTRPA1 Asp469Ala and Leu475Ala mutants did not show a qualitatively different response to caffeine (Table S1, C and D), which confirms that the effect of caffeine is not mediated by an increase in Ca2+i.
Tyrosine residues situated between TM1 and TM4 have been identified as being critical for activation of TRPV1 by capsaicin or activation of TRPM8 by menthol (31,32). Because all of these Tyr residues are conserved in mTRPA1 and hTRPA1, we were unable to analyze their function using the chimera approach in this study. We therefore carried out point mutation of Tyr residues in TM1–TM4 and found that none of these mutations, which included Tyr-728Ala (TM1), Tyr-788Ala (TM2), Tyr-815Ala (TM3), Tyr-845Ala (TM4), and Tyr-852Ala (TM4), affected the caffeine response of mTRPA1 qualitatively (Table S1, E and F). We also introduced a Tyr-1009Ala or Tyr-1009Leu mutation into the cytoplasmic region, immediately after TM6, which enhanced the responses to both AITC and caffeine (Fig. S4 and Table S1 G), but this change likely reflects an increase in the channel's surface expression.
In this study, we carried out thorough and nonbiased chimera analyses and identified the distal N-terminal cytoplasmic region of mTRPA1 between amino acid residues 231 and 287, which includes Met-268, as being critical for the activation effect of caffeine. However, it remains to be clarified whether Met-268 is the actual caffeine-binding site or is the site of a critical conformational change that occurs after caffeine binds to an adjacent region.
Qualitative species-specific differences in the ligand sensitivity of TRP channels
The qualitative species-specific differences in the ligand sensitivity of some TRP channels and the structural background for that difference have been described previously. One well-known example is TRPV1. Mammalian TRPV1 is activated by capsaicin, but the isoform from chick is completely insensitive (31). With respect to TRPA1, AMG5445, and AMG9090 are potent antagonists of hTRPA1 but are partial agonists of rTRPA1 (26). CMP1 activates rTRPA1 but blocks hTRPA1, and amino acid residues in the TM6 region, Ala-946 and Met-949 in rat, and Ser-943 and Ile-946 in human are critical for that difference (27). Menthol is known to have a bimodal action: it activates mTRPA1 at low concentrations but inhibits the channel at high concentrations (6). Xiao et al. (28) confirmed these results and further showed that hTRPA1 is only activated by menthol, even at high concentrations, and that Drosophila TRPA1 is insensitive to methanol. Moreover, they identified Ser-876 and Thr-877 in the TM5 region as critical sites in determining the sensitivity to menthol (28).
The results summarized above suggest that species-specific activation/suppression of TRPA1 is mainly switched by residues in the TM5 and TM6 regions. These findings might be expected because these TM regions form part of the pore and gate of the channel. However, TM5 and TM6 do not appear to be involved in the species-specific difference in the responses to caffeine, as replacements of the latter half of the protein, including all of the TM regions, in Mo705Hu and Hu703Mo (Fig. 3, C and D) did not change the channel's response to caffeine. Our results suggest the possibility that the region between 231–287 amino acid residues within the ankyrin repeat domain may serve not only as a simple on/off switch, but also as a regulator of bifurcated responses to caffeine.
Possible mechanism for the bifurcated effects of caffeine
It is clear that caffeine binds to both mTRPA1 and hTRPA1, as both channels respond to the compound, though in opposite ways. It is not yet clear whether Met-268 is the binding site for caffeine, but given that the caffeine-induced conformational change that couples with gating differs between mouse and human, it seems reasonable to speculate that the caffeine-binding site is in the three-dimensional proximity of Met-268.
Upon substitution of Met-268 of mTRPA1 with Pro, as is the case with hTRPA1, caffeine no longer activates the channel, but suppresses it instead (Fig. 8). On the other hand, the opposite substitution in hTRPA1 (Pro267Met) and double-point mutations that included Pro267Met did not confer the activation response to caffeine on hTRPA1 (Fig. 10), suggesting that additional mouse type mutations are necessary to achieve activation. Similarly, Chen et al. (27) were able to change the CMP1 response of rTRPA1 (activation) to an hTRPA1-like response (suppression), but they also failed to confer the activation property on hTRPA1 using point mutagenesis.
A reduction in the amplitude of the activation response was observed at higher caffeine concentrations with the mTRPA1 Met268Cys, Met268Ala, and Met268Gly mutants (Fig. 9 B). This is reminiscent of the effect of menthol on mTRPA1 (6,28) and suggests that caffeine binding causes both activation and a type of desensitization/inactivation that is coupled to activation. Perhaps activation of mTRPA1 and suppression of hTRPA1 can be interpreted as parts of the same phenomenon, though with different aspects predominating.
Another possible mechanism for the bifurcated effects of caffeine
A second possibility is that the caffeine-induced suppression of hTRPA1 currents reflects a sort of pore block. It is possible that a sensitivity to caffeine block is shared by both mTRPA1 and hTRPA1 but is masked in mTRPA1, as caffeine also activates it. In the case of the Met268Pro mutant, the blocking effect of caffeine is unmasked by the disappearance of the activation effect. In this scenario, the biphasic caffeine dose-response relationship seen with M268C (Fig. 9 B), for example, might reflect a lower EC50 for activation and higher IC50 for block.
If the current decrease of hTRPA1 by caffeine is due to pore block, a mechanism that is totally unrelated to activation of mTRPA1, it is possible that the kinetics of the effect differ between the two. We therefore analyzed their kinetics (Fig. S5). The current decrease in hTRPA1 by 10 mM caffeine could be fitted with two exponential functions, and their time constants were 0.9 ± 0.1 s and 7.0 ± 0.7 s (n = 10; Fig. S5, A, B, and E). The activation phase of mTRPA1 could be also fitted with two exponential functions with time constants of 1.6 ± 0.3 s and 17.0 ± 3.3 s (n = 10; Fig. S5, C–E). Because the values were in a similar order range, the results did not positively support the notion that the decrease is due to a totally different mechanism, such as pore block. However, the data do not exclude the possibility that the decrease is due to pore block, since the speed of pore block could be rather slow depending on the rate.
To further examine the possibility that the current decrease is due to pore block, we made chimera constructs based on the caffeine-insensitive rat TRPV1, whose latter half after TM5 was replaced with that of hTRPA1 or mTRPA1. We also made similar chimera constructs based on the caffeine-insensitive rat TRPM8. We expected that the chimeras might show a current decrease upon caffeine application if the decrease was due to block at the pore region. However, none of the chimeras were functional and they showed no response to their ligands (data not shown). Therefore, the mechanism for the current decrease of hTRPA1 by caffeine, including the possibility of pore block, remains to be determined.
Physiological significance of species-specific differences in the caffeine response
Met-268 is conserved in mice and rats, whereas Pro-267 is conserved in humans, chimpanzees, and monkeys. The sequence in the region containing Met-268 not well conserved in zebrafish, Drosophila, or mosquitos, which have Gln, Thr, and Thr, respectively, at the corresponding site. It is possible that mice acquired the capability to sense caffeine as a pungent substance to avoid its intake, and that the Met-to-Pro mutation occurred in monkeys to allow intake of caffeine so that they could take advantage of its useful pharmacological effects.
Acknowledgments
We thank Dr. A. Patapoutian (Scripps Research Institute) for the human TRPA1 cDNA, and Ms. Y. Asai for technical support. K. Nagatomo especially thanks Dr. K. Yamada (Hirosaki University) for encouragement and support.
This work was supported by research grants to Y.K. from the Ministry of Education, Science, Sports, Culture and Technology of Japan, and the Japan Society for the Promotion of Science.
Footnotes
Katsuhiro Nagatomo's present address is Department of Physiology, Hirosaki University Graduate School of Medicine, Aomori, Japan.
Supporting Material
References
- 1.Clapham D.E., Julius D., Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 2.Montell C. The TRP superfamily of cation channels. Sci. STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey I.S., Delling M., Clapham D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam K., Montell C. TRP channels. Annu. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Story G.M., Peier A.M., Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 6.Karashima Y., Damann N., Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandell M., Story G.M., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 8.Jordt S.E., Bautista D.M., Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 9.Macpherson L.J., Geierstanger B.H., Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Al-Anzi B., Tracey W.D., Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr. Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Koo J.Y., Jang Y., Oh U. Hydroxy-α-sanshool activates TRPV1 and TRPA1 in sensory neurons. Eur. J. Neurosci. 2007;26:1139–1147. doi: 10.1111/j.1460-9568.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- 12.Macpherson L.J., Dubin A.E., Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 13.McNamara C.R., Mandel-Brehm J., Fanger C.M. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trevisani M., Siemens J., Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita F., Moriyama T., Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br. J. Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson D.A., Gentry C., Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H., Bandell M., Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat. Chem. Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinman A., Chuang H.H., Julius D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zurborg S., Yurgionas B., Heppenstall P.A. Direct activation of the ion channel TRPA1 by Ca2+ Nat. Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 20.Doerner J.F., Gisselmann G., Wetzel C.H. Transient receptor potential channel A1 is directly gated by calcium ions. J. Biol. Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 21.Fujita F., Uchida K., Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J. Clin. Invest. 2008;118:4049–4057. doi: 10.1172/JCI35957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talavera K., Gees M., Voets T. Nicotine activates the chemosensory cation channel TRPA1. Nat. Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 23.Bautista D.M., Jordt S.E., Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Karashima Y., Talavera K., Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagatomo K., Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc. Natl. Acad. Sci. USA. 2008;105:17373–17378. doi: 10.1073/pnas.0809769105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klionsky L., Tamir R., Gavva N.R. Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol. Pain. 2007;3:39. doi: 10.1186/1744-8069-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J., Zhang X.F., Faltynek C.R. Molecular determinants of species-specific activation or blockade of TRPA1 channels. J. Neurosci. 2008;28:5063–5071. doi: 10.1523/JNEUROSCI.0047-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao B., Dubin A.E., Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J. Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J., Kym P.R. TRPA1: the species difference. J. Gen. Physiol. 2009;133:623–625. doi: 10.1085/jgp.200910246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X.F., Chen J., Neelands T.R. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur. J. Neurosci. 2008;27:605–611. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]
- 31.Jordt S.E., Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 32.Bandell M., Dubin A.E., Patapoutian A. High-throughput random mutagenesis screen reveals TRPM8 residues specifically required for activation by menthol. Nat. Neurosci. 2006;9:493–500. doi: 10.1038/nn1665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


