Abstract
The objective of this investigation was to study the relationship between the symptoms of female gonococcal infections and serum progesterone level and the genotypes of Neisseria gonorrhoeae multi-antigen sequence type (NG-MAST) in Wuhan, China. Eighty-one strains of N. gonorrhoeae were harvested from the vaginal discharge of 975 adult females in Wuhan and were genotyped by using NG-MAST. Serum progesterone (P) and estradiol (E2) levels were measured by radio immunoassay (RIA) in 39 gonorrhea-infected patients with slight symptoms (asymptomatic group) and 42 patients with conspicuous symptoms (symptomatic group). The average levels of serum progesterone in the asymptomatic group were significantly higher than in the symptomatic group (p < 0.05), while no significant difference was found in serum estradiol between the two groups. Of 81 wild-type isolates, 50 NG-MAST sequence types were associated with female infections in Wuhan, and N. gonorrhoeae ST2951, ST735, and ST436 were principally found in asymptomatic patients. ST809 and ST369, however, were mainly detected in asymptomatic female subjects. Gonococcal genetic island (GGI)-positive and GGI-negative strains were found in both the asymptomatic group and the symptomatic group. In females with gonococcal infection, high serum progesterone level is associated with the absence of symptoms, but no association was revealed between genotypes and the presence of symptoms. The GGI bears no relation to the absence of symptoms in the patients.
Introduction
Gonorrhea is the most common sexually transmitted bacterial infection in China. Recent studies indicate that molecular mechanisms of the infection vary with anatomical sites [1]. In male patients and a few female patients, gonorrhea is characterized by purulent discharge (infiltration of polymorphonuclear neutrophils), but in most female patients, the symptoms of genital tract infection, such as pus discharge, abdominal pain, and dyspareunia, tend to be slight or even absent.
With a number of genital infections, sex hormones are associated with the susceptibility to and progression of these conditions. Some studies demonstrated that, with chlamydia infection, susceptibility and inflammatory responses in the female genital tract were regulated by sex hormones [2]. MacDonald et al. also found that hormones could modify the susceptibility of human primary genital epithelial cells to herpes simplex virus in females [3]. Moreover, women, especially pregnant women, at the luteal phase of the menstrual cycle when progesterone levels are highest, are most susceptible to Candida albicans infections [4]. It was also found that, in women, the susceptibility to ascending gonococcal infection changes with menstrual cycle. Our previous studies showed that progesterone delayed the onset of apoptosis in polymorphonuclear leucocytes (PMNs) challenged by Neisseria gonorrhoeae (Ngo) in vitro [5]. Furthermore, progesterone could inhibit the mRNA expression of inflammatory factors such as iNOS, TNF-α, and IL-1β. On the basis of these findings, we postulate that progesterone may be related to the absence of symptoms of gonorrheal infections in females. Progesterone, progesterone-related nitric oxide (NO), and the progesterone production-related enzymes may be implicated in the mechanism.
Besides hormones, the status of the wild-type strains per se is presumably an important factor leading to the presence or absence of the symptoms. The virulence of Ngo varies with their genotypes, and it directly determines the intensity of inflammatory responses of organisms to Ngo. On the other hand, progesterone influences not only the inflammatory response of organisms to Ngo, but also the virulence and other biological properties of Ngo [6, 7]. For example, progesterone may affect the mtrCDE efflux system of some Ngo, thereby, changing their survival ability [8]. This study was designed to examine the relation between the presence of symptoms and the serum progesterone level in patients with gonorrheal infection.
Materials and methods
Isolation of N. gonorrhoeae
N. gonorrhoeae were collected from 975 female patients aged 18 to 42 years who visited the sexually transmitted disease (STD) clinics of Union Hospital and Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, from February 2006 to March 2008. Isolates were inoculated onto non-selective or selective media, and, when necessary, subjected to microcultures. Ngo were identified by employing the oxidase test and Gram-staining, and finally confirmed by using coagglutination (Phadebact or specific monoclonal antibodies) [9, 10]. The symptomatic group was defined if both of the following were observed: (1) easily induced endocervical bleeding or mucopurulent endocervical exudate were present on examination and (2) 30 or more PMN/HPF were present on Gram stain smear of endocervical secretions. The asymptomatic group did not develop obvious symptoms, but cervical hypertrophy and erosion were found in gynecological examination and 10~30 PMN/HPF were present on Gram stain smear of endocervical secretions. In the normal group, less than 10 PMN/HPF were found on Gram stain smear of endocervical secretions [11, 12].
Hormone assay
Blood was drawn from all of the subjects on an empty stomach. The blood was centrifuged at 3,000g for 15 min at 4°C, and the supernatant was harvested and stored at −70°C. Serum estradiol and progesterone were measured by radioimmunoassay (RIA). RIA kits were provided by Shenzhen Larewen Biomedical Engineering Technology Co. Ltd., China. The assay sensitivity was 10 pg/ml for estradiol and was between 0.2~0.6 ng/ml for progesterone. The inter- and intra-assay coefficients of variation were all <10%.
Strain culture and characterization
The bacteria were cultured on Thayer-Martin agar and GC-based chocolate agar, and identified by Gram staining and oxidase test. Pure cultures supplemented with 8% skimmed milk and 10% fetal calf serum were used for long-term storage at −70°C. The strains had not been passaged prior to this study. Auxotyping and serotyping were not done on the strains. For DNA analysis, strains were retrieved from the stock and cultured on GC-based chocolate agar overnight at 36°C in 5% CO2. A single colony was subcultured once before the DNA was prepared. In brief, bacterial suspensions (OD at 540 nm of 1.0~2.0; ~108–109 cfu/mL) were made in 0.17 mol/L PBS (pH 7.3), and the bacteria were pelleted by centrifugation at 2,000g for 5 min, washed once, resuspended in PBS, and boiled for 5 min. The lysate was centrifuged at 2,000g for 5 min, and the supernatant was stored at −20°C. All positive isolates were genotyped by using NG-MAST as described previously [13, 14]. DNA sequencing of por–tbpB was carried out by Shanghai Sangon Biological Engineering Technology & Services Co. Ltd., China. Alignments of trimmed sequences were performed by the use of ClustalX (version 1.8). Gonococcal genetic island (GGI) positive-specific and GGI negative-specific polymerase chain reaction (PCR) amplification was performed on all strains examined. Chromosomal DNA of each strain was amplified using primers 77F (5′-TAACAGCAGACGCTCCATTC-3′) and 86R (5′-CAAGCGCATGGTACATGAAT-3′) (Tm 57°C; extension time 90 s) and primers 73F (5′-AGCCATCAGGGAGGCGGATA-3′) and hlh-ggiR (5′-CAGGCAAACAGCTATTTGAG-3′) (Tm 54°C; extension time 90 s).
Statistical analysis
The Statistical Package for Social Sciences (SPSS v13.0 for windows) was used for the data analysis. Descriptive statistics were performed for each variable. Quantitative results are presented as mean (± SD). the means were compared by using one-way analysis of variance (ANOVA) and the two-sample t-test. Proportions for the two groups were compared by using the χ2 test and the Fisher exact test. A p-value < 0.05 was considered to be statistically significant.
Results
Serum estradiol and progesterone levels
The serum progesterone and estradiol levels were measured in the three groups. Comparison of the symptomatic group and the asymptomatic group showed that serum progesterone levels of the asymptomatic group were significantly higher (p < 0.01) than those of the symptomatic group. However, no significant difference was found in the serum estradiol levels between the symptomatic group and its asymptomatic counterpart (Table 1). We found that the asymptomatic subjects were mostly at the luteal phase, while the subjects in the symptomatic group were mainly at the follicular or ovulatory phase.
Table 1.
Serum progesterone and estradiol levels in the two different groups (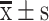 ) (pg/ml)
) (pg/ml)
| Groups | Symptomatic | Asymptomatic |
|---|---|---|
| n | 42 | 39 |
| Progesterone | 0.65 ± 0.32 ng/mL* | 9.04 ± 4.95 ng/mL |
| Estradiol | 87.24 ± 7.06 pg/mL | 119.35 ± 6.45 pg/mL |
Comparison of the symptomatic group and the asymptomatic group, *p < 0.01
Phenotypic and genotypic characterization of isolates
Among the 975 women enrolled in this study, 91 women (9.33%) were diagnosed with gonococcal infection and in ten women, specimens were not collected. A total of 81 isolates were obtained. In 39 women, the symptoms were not apparent, but cervical hypertrophy and erosion were found in gynecological examination (asymptomatic group). Eighty-one isolates showed 41 and 30 divergent partial NG-MAST por and tbpB alleles, respectively, which resulted in the assignment of 50 different sequence types (STs). The genotypic characterization of ST isolates (total 81 strains) is summarized in Table 2. ST2951 was the most prevalent ST found in asymptomatic patients. Among them, 7 of 8 strains of the ST2951 genotype were from the asymptomatic group.
Table 2.
The no-unique Neisseria gonorrhoeae multi-antigen sequence types (NG-MAST) associated with female infections in Wuhan, China
| Groups | Collected time | No-unique ST types (por allele–tbpB allele) | GGI | |||
|---|---|---|---|---|---|---|
| Luteal phase | Follicular phase | Uncertain | Positive | Negative | ||
| Asymptomatic | 27 | 9 | 3 | 2951 (217-679), 735 (242-4), 436 (90-21), 2943 (1226-33), 314 (241-75), 147 (53-21) | 27 | 12 |
| Symptomatic | 16 | 25 | 1 | 809 (543-33), 369 (256-137), 2948 (4-8), 421 (206-156), 641 (90-186) | 29 | 13 |
ST, sequence type
Discussion
Strong evidence demonstrated that sex steroids influence the pattern of inflammatory substances in human. The role of progesterone as a natural immune-suppressant has been known for a long time. The higher incidence of immune-mediated diseases in women has been attributed to progesterone down-regulating proinflammatory cytokines in cross-sectional trials [15–17]. Progesterone may be of particular relevance in female asymptomatic gonococcal infections and need to be further investigated. Hedges et al. conducted an in vivo experiment and found that women with gonococcal cervicitis did not exhibit elevated local levels of IL-1, IL-6, and IL-8 [18]. Their study, however, did not to take the influence of hormones into account. In this study, we demonstrated that, in females with gonococcal infection, the levels of serum progesterone were significantly higher in asymptomatic patients than in symptomatic patients (p < 0.01). In combination with our previous studies, our present results suggest that progesterone may play a role in female asymptomatic infections by down-regulating inflammatory response and delaying the onset of apoptosis. The levels of serum progesterone were higher in the asymptomatic group than in the symptomatic group. The reason for this may be that the serum was collected from asymptomatic subjects mostly at the luteal phase, while in the symptomatic group, the serum was taken mainly at the follicular phase.
Variation within the por and tbpB loci is likely to be selected by the human immune response, and there are large numbers of alleles at both loci [19]. The internal fragments of por and tbpB from 81 isolates were amplified, and both strands were sequenced. There were 41 different por alleles and 30 different tbpB sequences, resulting in 50 different two-locus allelic profiles. Among the 81 isolates, the most common alleles were por-217 and tbpB-33, which were found in 10 and 13 isolates, respectively. Only in one isolate were 30 por alleles and 19 tbpB alleles found. ST2951, ST735, and ST436 were common in asymptomatic patients, but ST809 and ST369 was prevalent in symptomatic patients. This subpopulation of Ngo prevails in females with asymptomatic infections in our series (Wuhan) now. In Sweden, the genetic characterization identified one widely spread ciprofloxacin-resistant N. gonorrhoeae ST147 strain [20]. The ST147 strain successively appeared in America and Africa. It is the most reported genotype of N. gonorrhoeae in the literature at present. We obtained only three isolates of the ST147 strain. After comparison, we know that NG-MAST sequence types might be related to the asymptomatic gonococcal infections, but the connection remains unknown.
The GGI, present in 80% of gonococcal strains, contains two lytic transglycosylase homologs, AtlA and LtgX, which may produce peptidoglycan (PG) fragments [21]. PG fragments can induce IL-8 production and cause the infiltration of PMNs to the sites of infection. N. gonorrhoeae can survive in PMNs and infect other individuals through purulent secretion. It was presumed that Ngo mutants defective in the type IV secretion system may induce less IL-8 production and less PMN infiltration. Table 2 shows that both GGI-positive and GGI-negative strains were found in the asymptomatic group and there were GGI-positive and GGI-negative strains in the symptomatic group, suggesting that GGI has nothing to do with female asymptomatic gonococcal infection.
In conclusion, our results showed that the Ngo GGI genotype is not linked with female gonococcal infections, but progesterone is associated with the outcome of the condition. A better understanding of the mechanisms of female asymptomatic gonococcal infections can help to improve therapeutic and diagnostic results of the disease. The molecular mechanism of the interaction between progesterone and PMNs in gonorrhea warrants further investigation.
Acknowledgments
This project was supported by grants from the National Natural Sciences Foundation of China (no. 30700717) and the Research Fund for the Doctoral Program of Higher Education of China (no. 20070487140).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Zhihong Wu, Li Xu, and Yating Tu contributed equally to this work.
References
- 1.Edwards JL, Apicella MA. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev. 2004;17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushic C, Zhou F, Murdin AD, et al. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207–4216. doi: 10.1128/IAI.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald EM, Savoy A, Gillgrass A, et al. Susceptibility of human female primary genital epithelial cells to herpes simplex virus, type-2 and the effect of TLR3 ligand and sex hormones on infection. Biol Reprod. 2007;77:1049–1059. doi: 10.1095/biolreprod.107.063933. [DOI] [PubMed] [Google Scholar]
- 4.Fidel PL, Jr, Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–657. doi: 10.1128/IAI.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Wu Z, Li J, Chen R, et al. Effect of progesterone on gonococci-induced apoptosis and respiratory burst of human polymorphonuclear leukocytes in vitro. Int J Dermatol. 2009;48(9):1011–1016. doi: 10.1111/j.1365-4632.2009.04177.x. [DOI] [PubMed] [Google Scholar]
- 6.Morse SA, Fitzgerald TJ. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun. 1974;10:1370–1377. doi: 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lysko PG, Morse SA. Effects of steroid hormones on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980;18:281–288. doi: 10.1128/aac.18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerse AE, Sharma ND, Simms AN, et al. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003;71:5576–5582. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paine TC, Fenton KA, Herring A, et al. GRASP: a new national sentinel surveillance initiative for monitoring gonococcal antimicrobial resistance in England and Wales. Sex Transm Infect. 2001;77:398–401. doi: 10.1136/sti.77.6.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenton KA, Hughes G, Herring A, et al. The Gonococcal Resistance to Antimicrobials Surveillance Programme: Protocol 2004 Collection. London: Health Protection Agency Centre for Infections; 2004. [Google Scholar]
- 11.Marrazzo JM, Handsfield HH, Whittington WL. Predicting chlamydial and gonococcal cervical infection: implications for management of cervicitis. Obstet Gynecol. 2002;100:579–584. doi: 10.1016/S0029-7844(02)02140-3. [DOI] [PubMed] [Google Scholar]
- 12.Bozicevic I, Fenton KA, Martin IM, et al. Epidemiological correlates of asymptomatic gonorrhea. Sex Transm Dis. 2006;33:289–295. doi: 10.1097/01.olq.0000194582.44222.c9. [DOI] [PubMed] [Google Scholar]
- 13.Palmer HM, Young H, Martin IM, et al. The epidemiology of ciprofloxacin resistant isolates of Neisseria gonorrhoeae in Scotland 2002: a comparison of phenotypic and genotypic analysis. Sex Transm Infect. 2005;81:403–407. doi: 10.1136/sti.2004.013565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moodley P, Martin IM, Pillay K, et al. Molecular epidemiology of recently emergent ciprofloxacin-resistant Neisseria gonorrhoeae in South Africa. Sex Transm Dis. 2006;33:357–360. doi: 10.1097/01.olq.0000194581.02022.f0. [DOI] [PubMed] [Google Scholar]
- 15.Cutolo M, Sulli A, Capellino S, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus. 2004;13:635–638. doi: 10.1191/0961203304lu1094oa. [DOI] [PubMed] [Google Scholar]
- 16.Kaushic C, Ashkar AA, Reid LA, et al. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks-Asplund EM, Tupper CE, Daun JM, et al. Hormonal modulation of interleukin-6, tumor necrosis factor and associated receptor secretion in postmenopausal women. Cytokine. 2002;19:193–200. doi: 10.1006/cyto.2002.1963. [DOI] [PubMed] [Google Scholar]
- 18.Hedges SR, Mayo MS, Mestecky J, et al. Limited local and systemic antibody responses to Neisseria gonorrhoeae during uncomplicated genital infections. Infect Immun. 1999;67:3937–3946. doi: 10.1128/iai.67.8.3937-3946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin IM, Ison CA, Aanensen DM, et al. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189:1497–1505. doi: 10.1086/383047. [DOI] [PubMed] [Google Scholar]
- 20.Unemo M, Sjöstrand A, Akhras M, et al. Molecular characterization of Neisseria gonorrhoeae identifies transmission and resistance of one ciprofloxacin-resistant strain. APMIS. 2007;115:231–241. doi: 10.1111/j.1600-0463.2007.apm_487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton HL, Domínguez NM, Schwartz KJ, et al. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]


