Abstract
Six strains of human cytomegalovirus have been sequenced, including two laboratory strains (AD169 and Towne) that have been extensively passaged in fibroblasts and four clinical isolates that have been passaged to a limited extent in the laboratory (Toledo, FIX, PH, and TR). All of the sequenced viral genomes have been cloned as infectious bacterial artificial chromosomes. A total of 252 ORFs with the potential to encode proteins have been identified that are conserved in all four clinical isolates of the virus. Multiple sequence alignments revealed substantial variation in the amino acid sequences encoded by many of the conserved ORFs.
Human cytomegalovirus (HCMV) is a ubiquitous member of the β-herpesvirus family. Although HCMV infection of healthy children and adults is usually asymptomatic, it is a leading cause of birth defects and a major cause of morbidity and mortality in immunocompromised individuals (1, 2).
Until now, AD169 has been the only HCMV genome whose complete sequence has been determined (3). The original annotation of its ≈230,000-bp sequence predicted that AD169 contains 208 ORFs, of which 14 are duplicated within repeated regions. Recently, the annotation has been refined by comparison to the sequence of the chimpanzee cytomegalovirus and by analysis with a gene-finding algorithm (4, 5). AD169 was developed as an attenuated vaccine candidate (6) by serial passage in fibroblasts of a clinical HCMV isolate that was isolated from the adenoids of a child. It has been extensively used as a laboratory strain for molecular studies of HCMV, because its sequence has been available and it replicates more efficiently than clinical isolates in fibroblasts. However, it is different from clinical isolates in terms of genomic structure and biology (7). For example, AD169 lacks a DNA segment containing ≈19 ORFs, which is present in clinical isolates (8), and it fails to replicate in several cell types, such as endothelial cells, which are permissive for replication of clinical isolates (9, 10).
In this article, we report the sequence and annotation of six HCMV strains whose genomes have been cloned as bacterial artificial chromosomes (BACs). We have sequenced BAC clones of AD169 as well as a second laboratory strain, Towne, which was derived from a virus that was isolated from the urine of a congenitally infected infant (11). Like AD169, it was passaged extensively in fibroblasts as a vaccine candidate. Toledo, isolated from the urine of a congenitally infected child (12); FIX (VR1814), isolated from a pregnant woman with a primary HCMV infection (13, 14); PH, isolated from a bone marrow transplant patient (15, 16); and TR, an ocular isolate from an AIDS patient with retinitis (17) are considered clinical isolates. They have been passaged to a more limited extent in fibroblasts, and each replicates in cell types in addition to fibroblasts (Toledo, ref. 18; PH, ref. 16; TR, M.A.J. and J.A.N., unpublished data; and FIX, refs. 13 and 14), although some Toledo variants do not replicate in endothelial cells (19).
We have identified a set of ORFs that are conserved in all four clinical isolates, including 29 putative protein-coding ORFs that have not been previously recognized.
Methods
Virus Clones. The AD169 BAC clone (20) was prepared from a plaque-purified derivative of the AD169 strain of HCMV originally obtained from the American Type Culture Collection (VR-538). Its self-excising BAC insert resides between US28 and US29; no AD169 sequence was deleted. The Towne BAC clone (21) was prepared from a plaque-purified derivative of the Towne strain of HCMV originally obtained from the American Type Culture Collection (VR-977). Its BAC insert replaces the US1–US12 region of the Towne genome. The Toledo BAC clone was prepared from a plaque-purified derivative of a Toledo virus (12) stock received from E. Mocarski (Stanford University) by substituting a derivative of the pMBO131 BAC (22) containing an added puromycin-resistance marker for the US2–US11 region of its genome. The PH BAC clone was prepared from the PH clinical isolate of HCMV (15, 16) and was designed to substitute BAC DNA for the US2–US6 region by using the method of Borst et al. (23). The TR BAC was prepared from the TR clinical isolate, which was originally designated “patient 2” (17). This virus is resistant to ganciclovir and cidofovir and lacks UL97 codons 591–594. The BAC clone was generated by substituting BAC DNA for the US2–US5 region of the TR genome by using the method of Borst et al. (23). The FIX BAC clone (14) was prepared from the VR1814 clinical isolate (13) and was designed to substitute BAC DNA for the US2–US6 region.
DNA Isolation and Sequence Analysis. HCMV BAC DNAs were prepared, subcloned, and sequenced at the Stanford Human Genome Center. BACs were propagated in media containing 15 μg/ml chloramphenicol. A single colony was picked from Luria–Bertani plates, the clone was grown overnight in a 5-ml miniculture, and then it was grown to an OD600 of 1.0 in a 500-ml culture. DNA was isolated by using a Plasmid Maxi Kit (Qiagen, Valencia, CA), sheared by using a Hydroshear Instrument (GeneMachines, San Carlos, CA), size-selected for 3- to 4-kb fragments, and subcloned into pIK96 (http://www_shgc.stanford.edu/Seq/resources/pIK96.html). Randomly selected subclones were sequenced in both directions by using universal primers and BigDye Terminator chemistry (Applied Biosystems) to an average sequence redundancy of 10× across each BAC. After screening to remove subclone and BAC vector sequence, sequences were assembled and edited by using the phred/phrap/consed suite of programs (24–26). After manual inspection of the assembled sequences, finishing was performed by resequencing plasmid subclones and by walking on plasmid subclones or the large insert clone with custom primers. Finishing reactions were performed by using dGTP BigDye Terminator chemistry (Applied Biosystems). Finished sequences contain no gaps and are estimated to contain less than one error per 100,000 bp.
Annotation. macvector 7.2 (Accelrys, San Diego) was used to identify start-to-stop and stop-to-stop ORFs ≥80 aa within the six sequenced BAC clones. blastp (27) was used to search for local alignments between ORFs. Scores with a significance of ≤10–5 were considered matches. clustalw (28) was used for global multiple sequence alignments using the blosum30 scoring matrix with a gap penalty of 3, an open gap penalty of 10, and an extended gap penalty of 0.05.
Results and Discussion
BAC clones of six HCMV isolates were sequenced. As noted above, AD169 and Towne were produced by extensive serial passage in fibroblasts, whereas Toledo, PH, TR, and FIX (VR1814) are clinical isolates that have been passaged to a limited extent in fibroblasts. Each of the BAC clone preparations used for sequencing was tested for its ability to initiate an infection after electroporation into fibroblasts. All DNAs were infectious except for the Towne preparation, which proved to carry a 1,350-bp insertion of bacterial DNA within its essential UL32 ORF. This insertion has been removed from the sequence for our analysis. It is likely that this insert is responsible for the lack of infectivity, but we cannot rule out the possibility that the BAC clone contains another defect.
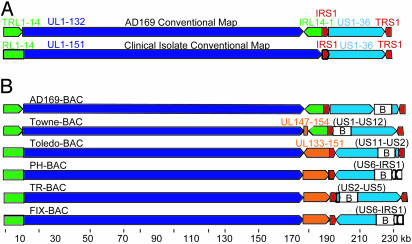
Organization of Sequenced Genomes. The AD169 genome is comprised of unique blocks of ORFs [unique long (UL); unique short (US)] that are bracketed by repeated blocks of ORFs [terminal repeat long (TRL); internal repeat long (IRL); internal repeat short (IRS); terminal repeat short (TRS)]. When a population of DNA is isolated from virions, it is comprised of four genomic isomers in which the UL and US regions are present in both orientations with respect to each other. The conventional map of the AD169 genome (3) displays the ORFs in the order in which they are found in one of the isomers: TRL1-14, UL1-132, IRL14-1, IRS1, US1-36, and TRS1 (Fig. 1A). Each of the BAC clones captured one of the four possible isomers of the viral genome (Fig. 1B).
Fig. 1.
HCMV ORF organization. (A) Conventional ORF maps of the AD169 laboratory strain and clinical isolates. From the left, the AD169 genome contains TRL1-14 (green arrow), UL1-132 (dark blue arrow), IRL14-1 (green arrow), IRS1 (red arrow), US1-36 (light blue arrow), and TRS1 (red arrow). From the left, clinical isolates contain a unique domain including RL1-14 and UL1-151 (green plus dark blue arrow) followed by IRS1 (red arrow), US1-36 (light blue arrow), and TRS1 (red arrow). (B) ORF maps of the BAC clones whose sequences are reported in this article. Sequences were linearized at the position corresponding to nucleotide 1 of the original AD169 sequence. Arrows indicate the relative orientations of the repeated and unique ORF blocks. The RL region is not repeated in the clinical isolates (Toledo, PH, TR, and FIX); there is a single copy of the region (green segment appended to the unique long region). The Towne laboratory strain contains a block of ORFs (UL147–UL154, orange arrow) that is not present in AD169, and the clinical isolates contain a block of ORFs (UL133–UL151, orange arrow) that is not present in the AD169 laboratory strains. The BAC inserts are identified (B), and viral ORFs deleted during BAC insertions are listed in parentheses.
Clinical isolates differ in two major respects from the laboratory strains. First, as originally reported for Toledo (8), clinical isolates contain a DNA segment that is absent from AD169 (UL133–UL151). A similar, but smaller, sequence is missing from Towne. Second, clinical isolates lack an IRL repeat. A deletion plus duplication produced these changes in AD169: a recombination event in the sequences between RL14 and UL148 at one end and within J1 repeats at the other end. A similar rearrangement in Towne resulted from recombination between UL1 and sequences between UL145 and UL146 at one end and J1 repeats at the other end. Clinical isolates do not contain a TRL/IRL repeat bracketing their UL region; rather, their UL region is larger and contains RL1-14 and UL1-151 (Fig. 1 A).
Some of the ORFs missing in laboratory strains reside in an inverted orientation in Toledo as compared with the other clinical isolates, as inferred previously by Southern blot analysis (8). The inversion in Toledo resulted from recombination within sequences located between UL128 and UL129 at one end and UL133–UL148a at the other end.
PH and FIX have lost the IRS1 and US1 ORFs in addition to the ORFs that were intentionally removed during the insertion of the BAC cassette. The loss of these ORFs in the BAC clones was confirmed by PCR analysis. The sequences were present in PH virus that was used to prepare the BAC clone. Apparently, the sequence segments used to target the BAC insert to substitute for the US2–US6 region recombined at an unanticipated site to delete the larger IRS1–US6 ORF block. Both BAC clones generate infectious virus, and no growth defect has been noted for the rescued FIX virus in fibroblasts or endothelial cells (14). This finding is not surprising, because IRS-1 (29) and US1 (30) are not required for replication of AD169 in fibroblasts.
Identification of ORFs. We initially used macvector to prepare a database containing all ORFs present in the six HCMV genomes that encode a polypeptide ≥80-aa long. We used 80 aa as the lower size limit for ORFs to be considered, because >95% of previously recognized AD169 ORFs meet this size criterion (5). This set of ORFs, in turn, was searched by using the blastp algorithm to identify ORFs corresponding to previously recognized HCMV ORFs, which included the original annotated set of AD169 ORFs (3), Toledo- and Towne-specific ORFs (5), a modification to the AD169 UL42–UL43 region (31), AD169 ORFs identified by their correspondence to chimpanzee cytomegalovirus orthologues (4), and AD169 ORFs were predicted by the Bio-Dictionary-based Gene Finder algorithm (5). An ORF in the database with a blastp alignment to a previously recognized ORF at a significance of ≤10–5 was considered a match. Two hundred thirty-three previously identified ORFs (with known sets of spliced ORFs counted as single ORFs) had homologues in all four of the clinical isolates. The annotated ORFs that were deleted in the construction of the BAC clones are included in the set of ORFs considered to be present in all four clinical isolates.
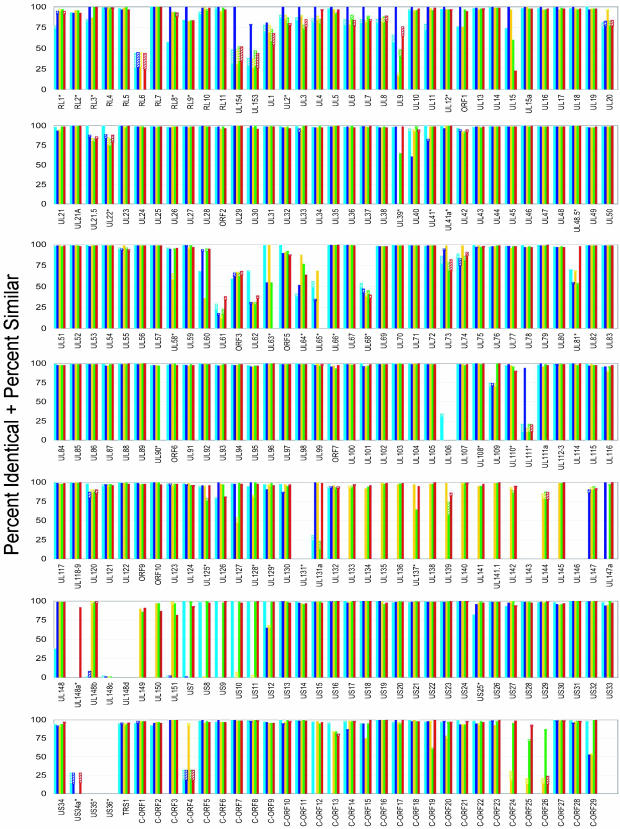
The previously recognized conserved ORFs were analyzed further by performing multiple sequence alignments with clustalw. Each FIX ORF was compared with its homologues in the other HCMV strains, and the percentage identical plus similar amino acids for each ORF-to-ORF comparison were plotted (Fig. 2). ORFs are organized in the Fig. 2 in the order that they are found on the conventional FIX map. The clustalw analysis confirmed that several of the previously recognized ORFs do not have homologues in the genomes of one or more of the four clinical isolates (if there is no homologue for an ORF in FIX, then the plot displays all strains with zero homology). Many of the previously recognized ORFs are highly conserved, and other ORFs exhibit considerable variability among strains. Although there are some clusters of variable ORFs, e.g., UL58–UL68, variable ORFs are spread across the genome. Variability among clinical isolates has been reported for several HCMV ORFs, such as the UL55-coded glycoprotein B (32–35), for which different sequence types have been associated with increased risk of disease in bone marrow transplant patients (36).
Fig. 2.
Variability of homologous ORFs. Global multiple sequence alignments were performed by using clustalw for all ORFs identified by blastp in the six HCMV isolates. For each ORF, the percentage identical (solid bar) plus percentage similar (cross-hatched bar) amino acids are presented for each FIX ORF in comparison to AD169 (light blue), Towne (dark blue), Toledo (yellow), PH (green), and TR (red). In cases where no bars are displayed, the FIX strain did not contain the ORF. ORFs marked with an asterisk do not have an AUG within 80 aa of their stop codon. The ORFs are presented in the order that they are found on the conventional AD169 map; the clinical strain-specific ORFs (UL133–UL151) are presented in the order in which they are found in FIX; the set of newly recognized ORFs (C-ORF1–C-ORF29) are presented after the previously recognized ORFs in the order in which they would occur on the map. Several ORFs, which are combined by splicing, have been analyzed as a single unit: UL21.5 (UL22a), UL36, UL37, UL89, UL111A, UL112-113, UL118-119, UL122, UL123, UL128, and UL131A. Comparisons are not presented for IRS1–US6, which are not present in the FIX BAC. UL154 is related to RL12, and UL153 is similar to RL14.
The comparison of FIX ORFs to their homologues in other strains indicates that many of its ORFs are most related in their amino acid sequence to Towne ORFs (Fig. 2). Furthermore, FIX and Towne contain the UL153 and UL154 ORFs, whereas the other HCMVs contain RL12 and RL14 in this location. UL153 is related to RL14, and UL154 is related to RL12. Consequently, FIX appears to be more closely related to Towne than to the other HCMVs.
We next used blastp to search the database of all HCMV ORFs ≥80 aa for ORFs that were not previously recognized but are present in all four clinical isolates. This search identified 765 conserved ORFs, and these ORFs were subjected to two additional filters. First, we required the presence of an AUG ≥80 codons from a termination signal. This criterion was chosen because >95% of previously annotated HCMV ORFs, which were classified as genuine by using the Bio-Dictionary-based Gene Finder algorithm, start with an AUG (5). The second criterion was designed to address the issue of overlapping ORFs. An ORF on one strand can potentially bias the sequence on the opposing strand, and a high G+C content (HCMV is 57% G+C) can favor the presence of random ORFs because stop codons are A+U-rich (37, 38). This issue has been addressed in the annotation of several β-herpesviruses by limiting the percentage overlap of the smaller ORF to ≤60% (3, 8, 39) or ≤25% (40). Although we required that a putative coding ORF does not completely overlap a known functional ORF, we did not limit the extent of an allowed overlap by percentage. Rather, we limited the length of an allowed overlap to <396 bp, the extent to which UL76 and UL77 overlap. This is the longest overlap among AD169 ORFs that are known to be functional (30, 41). After application of these additional filters, a set of 29 previously unrecognized conserved ORFs remained.
These putative coding ORFs were subjected to clustalw analysis (Fig. 2, C-ORF1–C-ORF29). The four clinical isolates contained homologues for all of the ORFs, and 26 of 29 were found in one or both laboratory strains. Orthologues in the chimpanzee cytomegalovirus genome were found for 14 of 29 ORFs by blastp analysis (Table 1).
Table 1. Previously unrecognized ORFs conserved in the four clinical isolates.
| ORF | Position | Strand | AD169 | Towne | ChCMV |
|---|---|---|---|---|---|
| C-ORF1 | 1911-2219 | + | + | + | - |
| C-ORF2 | 2002-2313 | - | + | + | + |
| C-ORF3 | 3201-3464 | - | - | + | - |
| C-ORF4 | 7721-7993 | + | + | + | - |
| C-ORF5 | 8623-8946 | - | + | + | - |
| C-ORF6 | 23457-23714 | - | + | + | - |
| C-ORF7 | 29049-29441 | + | + | + | + |
| C-ORF8 | 34809-35084 | + | + | + | - |
| C-ORF9 | 35428-35847 | - | + | + | + |
| C-ORF10 | 42908-43321 | + | + | + | + |
| C-ORF11 | 46173-46517 | - | + | + | - |
| C-ORF12 | 53739-54098 | - | + | - | - |
| C-ORF13 | 54532-54792 | + | + | + | + |
| C-ORF14 | 54705-55322 | + | + | + | + |
| C-ORF15 | 120245-120505 | - | + | + | + |
| C-ORF16 | 157089-157358 | - | + | + | - |
| C-ORF17 | 159004-159321 | + | + | + | - |
| C-ORF18 | 161498-161749 | - | + | + | + |
| C-ORF19 | 162157-162567 | - | + | + | - |
| C-ORF20 | 166218-166598 | + | + | + | - |
| C-ORF21 | 167837-168241 | + | + | + | + |
| C-ORF22 | 168302-168568 | + | + | + | + |
| C-ORF23 | 175896-176174 | + | + | + | + |
| C-ORF24 | 184793-185437 | + | - | - | + |
| C-ORF25 | 190194-190643 | - | - | - | - |
| C-ORF26 | 190299-190754 | + | - | - | - |
| C-ORF27 | 204440-204773 | + | + | + | - |
| C-ORF28 | 205251-205532 | + | + | + | + |
| C-ORF29 | 207279-207572 | - | + | + | + |
The ORFs are positioned in the FIX sequence, which has been organized into the order of the conventional map for clinical isolates that is displayed in Fig. 1A. blastp was used to search for homologues to the conserved ORFs in the AD169 and Towne sequences reported here, as well as in the chimpanzee cytomegalovirus (ChCMV) sequence (4).
A map of the FIX genome, with its ORFs organized as they are displayed in the conventional map (3), is presented in Fig. 3. All ORFs on the map are conserved in all four clinical isolates. Consequently, maps for the other clinical strains differ only in the regions altered by insertion of the BAC. Maps of the laboratory strains differ from the FIX map in the regions altered by the BAC inserts, and they lack some of the ORFs conserved in clinical isolates. The original names of the previously recognized ORFs (3–5, 8) are used on the map (Fig. 3, blue arrows). The newly recognized conserved ORFs (C-ORFs) also are indicated on the map (Fig. 3, yellow arrows). It is likely that a portion of the 29 C-ORFs do not code for proteins. Many extensively overlap larger ORFs, and, as discussed above, this overlap might be the basis for their conservation. It will be necessary to assay for expression and function of these ORFs.
Fig. 3.
FIX ORF map. ORFs that are conserved in all four clinical isolates are presented in the order that they reside on the conventional clinical strain map displayed in Fig. 1 A. ORFs with an AUG that were recognized previously in AD169, Towne, or Toledo are oriented with dark blue arrows, previously recognized ORFs without an AUG are marked by light blue arrows, and newly recognized ORFs are shown with yellow arrows. Spliced ORFs are identified by caret symbols, and ORFs deleted by insertion of the BAC element are shown in a box. Two adjacent ORFs are present that were identified as RL6 homologues by blastp. UL141 contains a stop codon, and it is shown as two adjacent ORFs: UL141 and UL141.1.
Davison et al. (4) first noted several regions in AD169 that contain significant gaps between ORFs likely to encode proteins: the RL region, the region between UL57 and UL69, and the region between UL105 and UL112 (Fig. 3). Several of the ORFs in each of these regions are not conserved in all four clinical isolates, most are not conserved in the chimpanzee cytomegalovirus (4), many scored poorly as functional ORFs when tested with the Bio-Dictionary-based Gene Finder algorithm (5), and clustalw analysis demonstrated that many ORFs in these regions are highly variable among HCMV strains (Fig. 2). Consequently, it seems likely that substantial portions of these regions do not serve a protein-coding function. The RL region encodes several abundant early mRNAs (42, 43), but nothing has been reported about their function. The region between UL57 and UL69 contains the origin of lytic DNA replication (44), and its presence might preclude portions of this region from coding proteins. The region between UL105 and UL112 encodes a 5-kb RNA (45) with properties of an intron (C. Kulesza and T.E.S., unpublished data).
Our analysis undoubtedly failed to recognize some functional ORFs. For example, ORFs were discarded if they were not present in all four clinical isolates, and several ORFs were identified in 3 of 4 isolates. ORF content might be somewhat variable among HCMV isolates. Furthermore, numerous ORFs were excluded from consideration because they did not contain an AUG ≥80 aa from a termination codon, even though they were conserved in all clinical isolates. Although most of its ORFs are incorporated into a single exon, the HCMV genome encodes a variety of spliced RNAs (e.g., refs. 46–50). Further computational and experimental analyses are needed to investigate the possibility that additional ORFs, without a start codon, are constituents of spliced transcripts.
Acknowledgments
The sequence determination was funded by the Biological and Environmental Research Program and the U.S. Department of Energy's Office of Science Grant DOE 99ER 62873 (to R.M.M.). The construction of the previously unpublished Toledo, TR, and FIX BACs was supported by National Institutes of Health Grants CA82396 (to T.E.S.) and AI21640 (to J.A.N.) and by Wilhelm Sander-Stiftung Grant 2002.022.1 (to G.H.), and annotation of the sequence was supported by National Institutes of Health Grant CA87661 (to T.E.S.).
Abbreviations: HCMV, human cytomegalovirus; BAC, bacterial artificial chromosome; UL, unique long; US, unique short; TRL, terminal repeat long; TRS, terminal repeat short; IRL, internal repeat long; IRS, internal repeat short; C-ORF, conserved ORF.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AC146851 (Towne), AC146904 (Phoebes), AC146905 (Toledo), AC146906 (TR), AC146907 (FIX), and AC146999 (AD169)].
References
- 1.Pass, R. F. (2001) in Fields Virology, eds. Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott-Raven, Philadelphia), Vol. 2, pp. 2675–2705. [Google Scholar]
- 2.Mocarski, E. S. & Courcelle, C. T. (2001) in Fields Virology, eds. Knipe, D. M., Howley, P. M., Griffin, D. E., Lamb, R. A., Martin, M. A., Roizman, B. & Straus, S. E. (Lippincott-Raven, Philadelphia), Vol. 2, pp. 2629–2673. [Google Scholar]
- 3.Chee, M. S., Bankier, S., Beck, S., Bohni, R., Brown, C. R., Horsnell, T., Hutchison, C. A., III, Kouzarides, T., Martignetti, J. A., Preddie, E., et al. (1990) Curr. Top. Microbiol. Immunol. 154, 125–169. [DOI] [PubMed] [Google Scholar]
- 4.Davison, A. J., Dolan, A., Akter, P., Addison, C., Dargan, D. J., Alcendor, D. J., McGeoch, D. J. & Hayward, G. S. (2003) J. Gen. Virol. 84, 17–28. [DOI] [PubMed] [Google Scholar]
- 5.Murphy, E., Rigoutsos, I., Shibuya, T. & Shenk, T. (2003) Proc. Natl. Acad. Sci. USA 100, 13585–13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elek, S. D. & Stern, H. (1974) Lancet 1, 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Prichard, M. N., Penfold, M. E. T., Duke, G. M., Spaete, R. R. & Kemble, G. W. (2001) Rev. Med. Virol. 11, 191–200. [DOI] [PubMed] [Google Scholar]
- 8.Cha, T. A., Tom, E., Kemble, G. W., Duke, G. M., Mocarski, E. S. & Spaete, R. R. (1996) J. Virol. 70, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacCormac, L. P. & Grundy, J. E. (1999) J. Med. Virol. 57, 298–307. [DOI] [PubMed] [Google Scholar]
- 10.Sinzger, C., Schmidt, K., Knapp, J., Kahl, M., Beck, R., Waldman, J., Hebart, H., Einsele, H. & Jahn, G. (1999) J. Gen. Virol. 80, 2867–2677. [DOI] [PubMed] [Google Scholar]
- 11.Plotkin, S. A., Furukawa, T., Zygraich, N. & Huygelen, C. (1975) Infect. Immun. 12, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinnan, G. V., Delery, M., Rook, A. H., Frederick, W. R., Epstein, J. S., Manischewitz, J. F., Jackson, L., Ramsey, K. M., Mittal, K., Plotkin, S. A., et al. (1984) Ann. Intern. Med. 101, 478–483. [DOI] [PubMed] [Google Scholar]
- 13.Revello, M., Baldanti, F., Percivalle, E., Sarasini, A., De-Giuli, L., Genini, E., Lilleri, D., Labo, N. & Gerna, G. (2001) J. Gen. Virol. 83, 1993–2000. [Google Scholar]
- 14.Hahn, G., Khan, H., Baldanti, F., Koszinowski, U. H., Revello, M. G. & Gerna, G. (2002) J. Virol. 76, 9551–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice, G. P., Schrier, R. D. & Oldstone, M. B. (1984) Proc. Natl. Acad. Sci. USA 81, 6134–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fish, K. N., Depto, A. S., Moses, A. V., Britt, W. & Nelson, J. A. (1995) J. Virol. 69, 3737–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, I. L., Taskintuna, I., Rahhal, F. M., Powell, H. C., Ai, E., Mueller, A. J., Spector, S. A. & Freeman, W. R. (1998) Arch. Ophthalmol. 116, 178–185. [DOI] [PubMed] [Google Scholar]
- 18.Brown, J. M., Kaneshima, H. & Mocarski, E. S. (1995) J. Infect. Dis. 171, 1599–1603. [DOI] [PubMed] [Google Scholar]
- 19.Baldanti, F., Revello, M. G., Percivalle, E., Labo, N. & Gerna, G. (2003) J. Med. Virol. 69, 76–81. [DOI] [PubMed] [Google Scholar]
- 20.Yu, D., Smith, G. A., Enquist, L. W. & Shenk, T. (2002) J. Virol. 76, 2316–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marchini, A., Liu, H. & Zhu, H. (2001) J. Virol. 75, 1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Connor, M., Peifer, M. & Bender, W. (1989) Science 244, 1307–1312. [DOI] [PubMed] [Google Scholar]
- 23.Borst, E. M., Hahn, G., Koszinowski, U. H. & Messerle, M. (1999) J. Virol. 73, 8320–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ewing, B. & Green, P. (1998) Genome Res. 8, 186–194. [PubMed] [Google Scholar]
- 25.Ewing, B., Hillier, L., Wendl, M. C. & Green, P. (1998) Genome Res. 8, 175–185. [DOI] [PubMed] [Google Scholar]
- 26.Gordon, D., Abajian, C. & Green, P. (1998) Genome Res. 8, 195–202. [DOI] [PubMed] [Google Scholar]
- 27.Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990) J. Mol. Biol. 215, 403–410. [DOI] [PubMed] [Google Scholar]
- 28.Chenna, R., Sugawara, H., Koike, T., Lopez, R., Gibson, T. J., Higgins, D. G. & Thompson, J. D. (2003) Nucleic Acids Res. 31, 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blankenship, C. A. & Shenk, T. (2003) J. Virol. 76, 12290–12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu, D., Silva, M. C. & Shenk, T. (2003) Proc. Natl. Acad. Sci. USA 100, 12396–12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mocarski, E. S., Prichard, M. N., Tan, C. S. & Brown, J. M. (1997) Virology 239, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehner, R., Stamminger, T. & Mach, M. (1991) J. Clin. Microbiol. 29, 2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer-Konig, U., Haberland, M., von Laer, D., Haller, O. & Hufert, F. T. (1998) J. Infect. Dis. 177, 1162–1169. [DOI] [PubMed] [Google Scholar]
- 34.Zweygberg-Wirgart, B., Brytting, M., Linde, A., Wahren, B. & Grillner, L. (1998) J. Clin. Microbiol. 36, 3662–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen, L., Geissler, A. & Winters, M. (2003) J. Infect. Dis. 187, 809–819. [DOI] [PubMed] [Google Scholar]
- 36.Torok-Storb, B., Boeckh, M., Hoy, C., Leisenring, W., Myerson, D. & Gooley, T. (1997) Blood 90, 2097–2102. [PubMed] [Google Scholar]
- 37.Silke, J. (1997) Gene 194, 143–155. [DOI] [PubMed] [Google Scholar]
- 38.Cebrat, S., Mackiewicz, P. & Dudek, M. R. (1997) Biosystems 45, 165–176. [DOI] [PubMed] [Google Scholar]
- 39.Rawlinson, W. D., Farrell, H. E. & Barrell, B. G. (1996) J. Virol. 70, 8833–8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gompels, U. A., Nicholas, J., Lawrence, G., Jones, M., Thomson, B. J., Martin, M. E., Efstathiou, S., Craxton, M. & Macaulay, H. A. (1995) Virology 209, 29–51. [DOI] [PubMed] [Google Scholar]
- 41.Wang, S. K., Duh, C. Y. & Chang, T. T. (2000) J. Gen. Virol. 81, 2407–2416. [DOI] [PubMed] [Google Scholar]
- 42.Hutchinson, N. I., Sondermeyer, R. T. & Tocci, M. J. (1986) Virology 155, 160–171. [DOI] [PubMed] [Google Scholar]
- 43.Spector, D. H. (1996) Intervirology 39, 361–377. [DOI] [PubMed] [Google Scholar]
- 44.Masse, M. J., Karlin, S., Schachtel, G. A. & Mocarski, E. S. (1992) Proc. Natl. Acad. Sci. USA 89, 5246–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plachter, B., Traupe, B., Albrecht, J. & Jahn, G. (1988) J. Gen. Virol. 69, 2251–2266. [DOI] [PubMed] [Google Scholar]
- 46.Akrigg, A., Wilkinson, G. W. & Oram, J. D. (1985) Virus Res. 2, 107–121. [DOI] [PubMed] [Google Scholar]
- 47.Stenberg, R. M., Witte, P. R. & Stinski, M. F. (1985) J. Virol. 56, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlinson, W. D. & Barrell, B. G. (1993) Virus Genes 24, 39–48. [DOI] [PubMed] [Google Scholar]
- 49.Tenney, D. J., Santomenna, L. D., Goudie, K. B. & Colberg-Poley, A. M. (1993) Nucleic Acids Res. 21, 2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akter, P., Cunningham, C., McSharry, B. P., Dolan, A., Addison, C., Dargan, D. J., Hassan-Walker, A. F., Emery, V. C., Griffiths, P. D., Wilkinson, G. W. & Davison, A. J. (2003) J. Gen. Virol. 84, 1117–1122. [DOI] [PubMed] [Google Scholar]