Abstract
BACKGROUND AND PURPOSE
Acute pancreatitis is an autodigestive process resulting in acute inflammation of the pancreas. Accumulating evidence indicates the essential contribution of cyclooxygenase (COX)-2 and 5-lipoxygenase (5-LOX) to acute pancreatitis. We studied the effects of flavocoxid, a plant-derived dual inhibitor of COX-2 and 5-LOX, in a model of caerulein (CER)-induced acute pancreatitis.
EXPERIMENTAL APPROACH
Rats were given CER (80 µg·kg−1 for each of four injections at hourly intervals) or vehicle (Sham-CER). Animals were then randomized to receive flavocoxid (20 mg·kg−1 i.p.) or vehicle, 30 min after the first CER injection. Two hours after the last CER injection, we evaluated damage to the pancreas by histological methods; serum levels of amylase, lipase, leukotriene (LT)B4 and prostaglandin (PG)E2; pancreatic expression of COX-2 and 5-LOX and tumour necrosis factor-α (TNF-α) gene expression by real-time polymerase chain reaction.
KEY RESULTS
Caerulein induced inflammatory changes in the pancreas and raised values of the other variables measured. In CER-treated animals, but not in those given saline, flavocoxid inhibited COX-2 and 5-LOX expression, reduced serum levels of lipase and amylase and the degree of pancreatic oedema. Treatment with flavocoxid blunted the increased pancreatic TNF-α mRNA expression, serum leukotriene B4 and prostaglandin E2 levels, and protected against histological damage in terms of vacuolization and leukocyte infiltration.
CONCLUSIONS AND IMPLICATIONS
Our results confirm the key role of both COX-2 and 5-LOX in the inflammatory response to acute pancreatitis. Flavocoxid may provide a potential therapeutic approach to the treatment of patients at high risk of developing this life-threatening condition.
Keywords: flavocoxid, acute pancreatitis, COX, 5-LOX, TNF-α
Introduction
Acute pancreatitis (AP) is an inflammatory disease characterized by acute inflammation and necrosis of the pancreatic parenchyma (Bhatia et al., 2005). AP is often associated with remote organ failure, sepsis and high mortality despite the development of new diagnostic and therapeutic procedures. The activation of pancreatic enzymes leads to ‘autodigestion’ of the gland, followed by a massive infiltration of neutrophils and macrophages, leading to a local and systemic inflammatory response. The latter event is responsible for the high morbidity, thus much effort has been made to elucidate the initiation of AP, in order to discover potential therapeutic approaches (Steer, 1998; Lerch and Gorelick, 2000; Bhatia et al., 2005).
In experimental pancreatitis induced by caerulein (CER) (Altavilla et al., 2003a,b; Minutoli et al., 2004), a stable cholecystokinin analogue, the block of secretion is followed by lysosomal degradation of intercellular organelles within autophagic vacuoles in acinar cells. Activation of trypsin from its precursor trypsinogen is believed to be one of the principal events responsible for initiating damage (Lerch and Gorelick, 2000). The initial stage of acute pancreatitis is characterized by interstitial oedema coupled with infiltration of inflammatory cells that generate reactive oxygen species (ROS), which consequently destroy lipid membranes by peroxidation of fatty acids and trigger a variety of inflammatory processes (Lerch and Adler, 1994; Norman, 1998; Bhatia et al., 2000). Recently, it has been proposed that the release of secondary inflammatory mediators including interleukin (IL)-1α, IL-6, tumour necrosis factor-α (TNF-α) and arachidonic acid metabolites contributes to the induction of the systemic inflammatory response and multiple organ failure (Kingsnorth, 1997; Uhl et al., 1998; Kingsnorth and O'Reilly, 2006). Accumulating evidence indicates the essential role of prostanoids in the pathogenesis of acute pancreatitis (Van Ooijen et al., 1988; Vollmar et al., 1989; Zhou et al., 1994). Previous studies have demonstrated that cyclooxygenase (COX)-2 mRNA as well as its serum levels are elevated in the setting of AP, particularly in the early stages of the inflammatory process, when the enzyme acts as a pro-inflammatory agent (Zabel-Langhennig et al., 1999). In CER-induced AP, an over-expression of COX-2, as well as the potentially beneficial role of the inhibition of COX-2 pathway have been demonstrated (Song et al., 2002; Foitzik et al., 2003). Furthermore, other studies reported that mice without a functional COX-2 gene exhibited an attenuated severity of the disease (Ethridge et al., 2002). Despite these experimental results, in the late stages of the disorder, COX-2 may have anti-inflammatory properties, although this still is matter of debate (Colville-Nash and Gilroy, 2001).
Activation of a related pathway, catalysed by 5-lipoxygenase (5-LOX), has been proposed to contribute to the pathogenesis of acute pancreatic inflammation. A significant increase of leukotriene (LT) levels in the pancreas accompanied experimental AP (Ersoz et al., 1999; Oruc et al., 2004), and mice with the 5-LOX gene deleted were protected against experimental pancreatitis (Cuzzocrea et al., 2003). Furthermore, an antagonist of LT receptors, pranlukast, significantly improved pancreas function and decreased cellular inflammatory responses, associated with experimental AP (Hirano, 1997; Folch et al., 1998). These reports confirm the key role of arachidonic acid metabolites in acute pancreatic inflammation.
The classical non-steroidal anti-inflammatory drugs (NSAIDs) were known to inhibit both isoforms of COX and their adverse gastro-intestinal toxicities were attributed to the inhibition of gastroprotective prostaglandins (PGs) produced via the COX-1 pathway. Moreover, inhibition of COX may lead to a shunt of the arachidonic metabolism towards the 5-LOX pathway and treatment with NSAIDs may increase the formation of LTs, which can further induce gastric damage and ulceration (Rainsford, 1987; 1993; Hudson et al., 1993; Laufer, 2001; Oruc et al., 2004). These considerations led to the design of more selective COX-2 inhibitors in order to develop improved anti-inflammatory and analgesic agents with reduced adverse effects compared with the classical NSAIDs. Also, a variety of dual COX/LOX inhibitors with differing structures, have been designed and several compounds are currently undergoing clinical development as anti-inflammatory drugs. Compared with selective inhibitors of the COX or the LOX pathways, dual inhibitors (of COX and LOX) appear to be almost free of gastric toxicity, which is the most common side effect of COX inhibitors. However, the efficacy of a combined inhibition of COX-2 and 5-LOX has not been fully investigated in AP.
Flavocoxid, marketed as an FDA regulated medical food Limbrel®, for the clinical dietary management of the metabolic aspects of osteoarthritis in the United States, is a mixture of the naturally occurring flavonoids, baicalin and catechin, and has been shown to act as a dual inhibitor of COX-2 and 5-LOX (Burnett et al., 2007). Flavocoxid is equivalent to naproxen for efficacy as measured by quality of life endpoints in at least one well-controlled clinical trial (Levy et al., 2009; Morgan et al., 2009). It also appears to exert beneficial effects in many inflammatory disease models. Flavocoxid significantly reduced the expression of both COX-2 and 5-LOX enzymes in rat peritoneal macrophages stimulated by bacterial lipopolysaccharide (Altavilla et al., 2009). Preliminary experimental in vivo data have suggested that flavocoxid decreased, in a dose-dependent manner, oedema in an arachidonic acid-induced mouse ear swelling model and abates the swelling and restored function in mice injected with arachidonic acid in the intra-articular space (Burnett et al., 2007). Moreover, administration of flavocoxid exerted beneficial effects in a murine model of Duchenne muscular dystrophy via inhibition of NFκB and COX/LOX pathways (Messina et al., 2009).
In light of these data, the aim of the present study was to investigate the effects of flavocoxid in an experimental model of CER-induced AP, in order to assess its effects on pancreatic damage, measured biochemically and histologically.
Methods
Animals
All procedures complied with the standards for care and use of animal subjects as stated in the Guide for the care and use of Laboratory animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, Maryland). The protocol was evaluated and accepted by the Ethics Committee of the University of Messina. A total of 28 male Sprague-Dawley rats were used, maintained on 12 h dark/light cycle at 21°C and allowed free access to water and standard rodent diet.
Experimental protocol and dose selection
Acute pancreatitis was induced by repeated administration of CER (CER group, n = 14; 80 µg·kg−1 i.p. for each of four injections at hourly intervals) as previously described (Altavilla et al., 2003b). A control group (n = 14) received four i.p. injections of 0.9% saline at hourly intervals (Sham-CER). CER and Sham-CER animals were randomized to receive flavocoxid (n = 7 animals per group; CER + flavocoxid and Sham-CER + flavocoxid; 20 mg·kg−1 i.p. 30 min after the first injection of CER) or its vehicle (n = 7 animals per group; CER and Sham-CER; 1 mL·kg−1 of NaCl solution). Animals were killed 2 h after the last injection of either CER or its vehicle: blood was drawn for measuring serum amylase and lipase activity and eicosanoid levels; the pancreas was removed for molecular and histological evaluations.
To select the optimal dose of flavocoxid, a short initial experiment was performed using four different doses (5–10–20–40 mg·kg−1) administered 30 min after the first CER injection and serum amylase and lipase activities evaluated as main outcomes. The results showed a significant effect of flavocoxid at the dose of 20 mg·kg−1 with no further increase at the dose of 40 mg·kg−1. On the basis of these data, a dose of 20 mg·kg−1 was chosen for all subsequent experiments.
Isolation of cytoplasmic proteins
Briefly, samples of pancreas were homogenized in 1 mL lysis buffer (25 mM Tris/HCL, pH 7.4, 1.0 mM EGTA, 1.0 mM EDTA, 0.5 phenyl methylsulphonyl fluoride, aprotinin, leupeptin (10 µg·mL−1 each) with a Ultra Turrax (IKA, Staufen, Germany) homogenizer. The homogenate was subjected to centrifugation at 15 000×g for 15 min at 4°C. The supernatant was collected and used for protein determination using the Bio-Rad protein assay kit (Bio-Rad, Richmond, CA, USA).
Determination of cyclooxygenase-2 and 5-lipoxygenase by Western blot analysis
Protein samples (30 µg) were denatured in reducing buffer (62 mM Tris pH 6.8, 10% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.003% bromophenol blue) and separated by electrophoresis on an SDS (12%) polyacrylamide gel. The separated proteins were transferred on to a nitrocellulose membrane using the transfer buffer (39 mM glycine, 48 mM Tris pH 8.3, 20% methanol) at 200 mA for 1 h. The membranes were blocked with 5% non-fat dry milk in TBS-0.1% Tween for 1 h at room temperature, washed three times for 10 min each in TBS-0.1% Tween and incubated with a primary COX-2, or 5-LOX antibody (Abcam, Cambridge, UK) in TBS-0.1% Tween overnight at 4°C. After being washed three times for 10 min each in TBS-0.1% Tween, the membranes were incubated with a second antibody peroxidase-conjugated goat anti-rabbit immunoglobulin G (Pierce, UK) for 1 h at room temperature. After washing, the membranes were analysed by the enhanced chemiluminescence system according to the manufacturer's protocol (Amersham, UK).
The protein signal was quantified by scanning densitometry using a bio-image analysis system (Bio-Profil, Milan, Italy). The results from each experimental group were expressed as relative integrated intensity compared with control normal pancreas, measured with the same batch.
RNA extraction and real-time polymerase chain reaction
Total RNA was isolated using Trizol Reagent (Invitrogen, Milan, Italy) and the procedure was performed according to the protocol provided by the manufacturer. RNA (5 µg) from each sample was reverse transcribed using High Capacity cDNA Archive Kit according to the manufacturer's procedures (Applied Biosystem). cDNA from each sample (5 ng) was amplified by real-time polymerase chain reaction (PCR) with 2X TaqMan universal PCR Mastermix (Applied Biosystem), 20× target primer and probe. β-actin was used as the housekeeping gene. Each sample was analysed in duplicates using SDS 7300 (Applied Biosystem). The results were expressed as an n-fold difference relative to internal controls (relative expression levels).
Serum amylase and lipase content
Serum amylase and lipase activity were determined using commercially available kits (Sigma Chemical, St Louis, MO). The values of serum amylase and lipase activity were expressed as units per liter (U·L−1).
Leukotriene B4 and prostaglandin E2 production
Serum samples stored at −80°C were assayed for leukotriene B4 (LTB4) using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) based on the forward sequential competitive binding technique in which LTB4 present in samples competes with a fixed amount of horseradish peroxidase-labelled LTB4 for sites on a chicken polyclonal antibody. Samples were run in duplicate and the absorbance was read at 450 nm. The intensity of the colour was proportional to the concentration of LTB4 in the sample. Prostaglandin E2 (PGE2) was directly assayed without purification by using the Cayman EIA kit (Cayman, Ann Arbor, MI). Samples were run in duplicate and the absorbance was spectrophotometrically read at 412 nm and was directly proportional to the content of PGE2 in samples.
Histological studies and evaluation of pancreatic oedema
For light microscopy, a piece from the central body of the pancreas was rapidly removed and fixed in 10% buffered formalin. Subsequently, it was embedded in paraffin, cut and stained with hematoxylin and eosin. Assessment of tissue changes was carried out by an experienced pathologist who was unaware of the treatments. Histological grading of oedema and infiltrate of inflammatory cells were based on the following scale: 0, absent; 1, mild; 2, moderate; 3, severe. Grading of vacuolization was based on the approximate fraction of cells involved 0, 0–25%; 1, 25–50%; 2, 50–75%; 3, 75–100%. To measure pancreatic oedema, a piece from the body of the pancreas was rapidly removed, weighed and then blotted dry on filter paper. The extent of pancreatic oedema was calculated from the difference in weight before and after desiccation at 95°C for 24 h. The difference was expressed as a percentage of the tissue wet weight and taken to represent the degree of oedema.
Data analysis
Results are expressed as mean ± SD, except for those from the histological grading which are expressed as the group mode. Statistical evaluation was performed by using one-way analysis of variance followed by Dunnett's post hoc tests and paired Student's t-test with the use of the InPlotPrism software version 3.0 (GraphPad Software, San Diego, USA); P-values < 0.05 were considered significant.
Materials
Flavocoxid (Limbrel®) was a kind gift of Primus Pharmaceuticals, Inc (Scottsdale, Arizona, USA) and is a mixture of baicalein and catechin (4.5:1; see Altavilla et al., 2009). The compound was administered i.p. in 0.9% NaCl solution. CER was obtained from Bachem AG laboratories (Bubendorf, Switzerland). All substances were prepared fresh daily and administered in a volume of 1 mL·kg−1.
Results
Flavocoxid inhibits caerulein-induced cyclooxygenase-2 and 5-lipoxygenase expression
To test the effects of flavocoxid in inhibiting COX-2 and 5-LOX expression, we investigated the expression of these enzymes in pancreas obtained from the several experimental groups 2 h after the last CER injection (Figure 1A and B). No significant changes in COX-2 or 5-LOX expression were found in pancreas of either Sham-CER or Sham-CER + flavocoxid groups. Administration of CER increased the expression of both COX-2 and 5-LOX (P < 0.05, for both assays) in pancreas compared with those in the Sham-CER group. Treatment with flavocoxid almost completely inhibited CER-induced pancreatic COX-2 and 5-LOX (P < 0.01) expression, 2 h after the last CER injection (Figure 1A and B).
Figure 1.
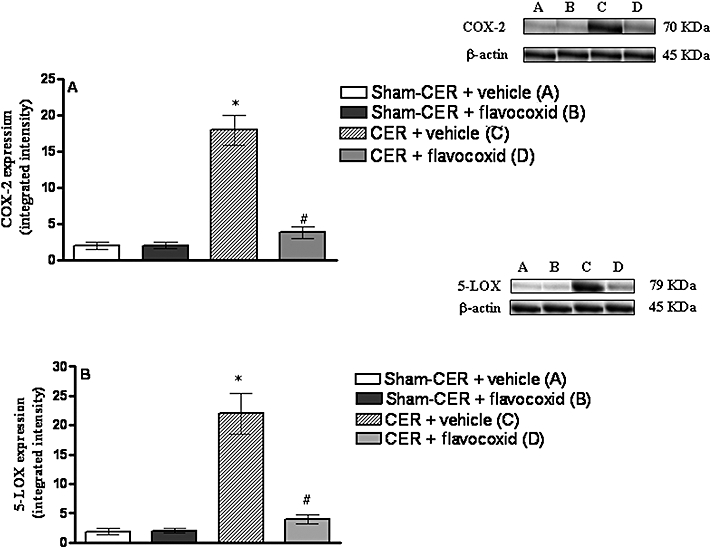
(A) Western blot analysis of COX-2 in the pancreas obtained from the experimental groups, 2 h after the last caerulein injection (CER; 80 µg·kg−1 i.p. for each of four injections at hourly intervals) or with four i.p. injections 0.9% saline at hourly intervals (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid; 20 mg·kg−1 i.p., administered 30 min after the first injection of caerulein) or its vehicle (CER + vehicle; 1 mL·kg−1 of 0.9% NaCl solution). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. (B) Western blot analysis of 5-LOX in the pancreas obtained from the experimental groups, 2 h after the last injection of caerulein (CER) or saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. COX, cyclooxygenase.
Inhibition of cyclooxygenase-2 and 5-lipoxygenase affects serum levels of prostaglandin E2 and leukotriene B4
The activation of COX-2 and 5-LOX was demonstrated indirectly by assessing PGE2 and LTB4, the bioactive products of each enzymatic cascade. As shown in Figure 2, the Sham CER and Sham-CER + flavocoxid groups showed a low constitutive presence of PGE2 and LTB4. A significant increase of both PGE2 and LTB4 (P < 0.05 for both assays) occurred 2 h after the last CER injection. Inhibition of COX-2 and 5-LOX activation by flavocoxid caused a strong reduction in both PGE2 and LTB4 (P < 0.01; Figure 2A and B).
Figure 2.
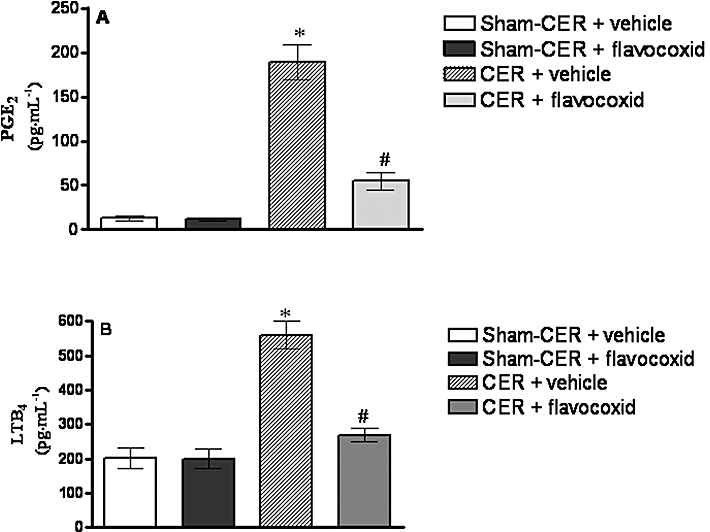
(A) Prostaglandin E2 (PGE2) levels in serum samples obtained from the experimental groups, 2 h after the last injection of caerulein (CER; 80 µg·kg−1 i.p.) or saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. (B) LTB4 levels in serum samples obtained from the experimental groups, 2 h after the last injection of caerulein (CER; 80 µg·kg−1 i.p.) or saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. LTB4, leukotriene B4.
Flavocoxid reduces caerulein-induced tumour necrosis factor-α expression
Tumour necrosis factor-α was investigated as a downstream product of eicosanoid release and the inflammatory cascade: no significant change in TNF-α mRNA was detected in pancreatic samples from the Sham-CER and Sham-CER + flavocoxid groups (Figure 3). AP resulted in a marked increase of TNF-α message (P < 0.05; Figure 3). TNF-α expression was strongly attenuated in pancreatic samples from CER-injected rats treated with flavocoxid (P < 0.01; Figure 3).
Figure 3.
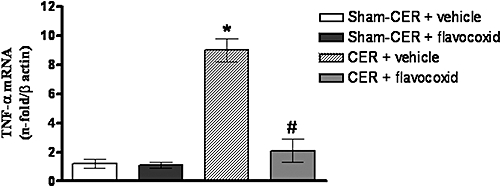
mRNA for TNF-α in pancreatic samples obtained from the experimental groups, 2 h after the last injection of caerulein (CER; 80 µg·kg−1 i.p.) or saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. TNF-α, tumour necrosis factor-α.
Flavocoxid ameliorates the severity of caerulein-induced pancreatitis
Serum amylase and lipase levels were measured as parameters that characterize the severity of secretagogue-induced pancreatitis. Injection of CER dramatically increased serum amylase and lipase (P < 0.05 for both enzymes) levels compared with those in the Sham-CER or Sham-CER + flavocoxid groups (Figure 4A and B). CER-induced pancreatitis resulted also in a significant organ oedema, compared with the Sham-CER group (P < 0.05; Figure 4C). Animals treated with flavocoxid after CER showed a marked reduction in serum amylase or lipase levels (P < 0.01; Figure 4A and B) and decreased pancreatic oedema (Figure 4C).
Figure 4.
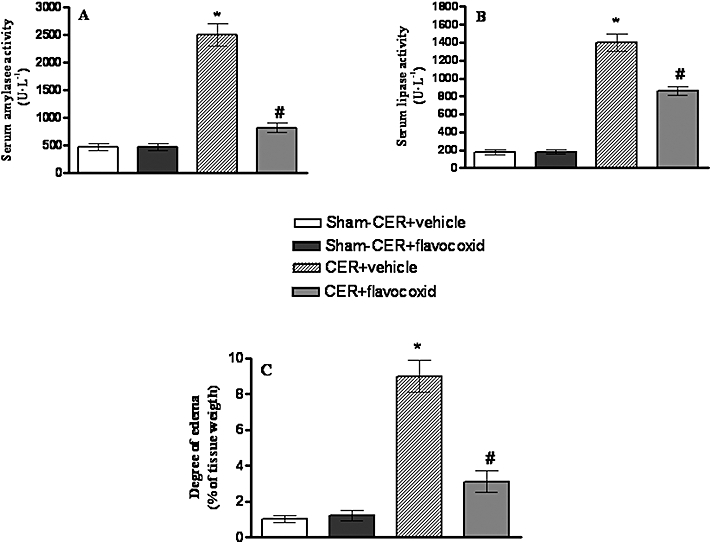
(A) Serum amylase activity in rats injected with caerulein (CER; 80 µg·kg−1 i.p.) or with saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. (B) Serum lipase activity in in rats injected with caerulein (CER; 80 µg·kg−1 i.p) or with saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER. (C) Degree of pancreatic oedema in rats injected with caerulein (CER; 80 µg·kg−1 i.p) or with saline (Sham-CER). Animals were treated with flavocoxid (CER + flavocoxid) or its vehicle (CER + vehicle). Bars represent the mean ± SD of seven animals. #P < 0.01 versus CER + vehicle. *P < 0.05 versus Sham-CER.
Histopathological evaluation of pancreatitis
The pancreas from the Sham-CER animals showed a normal histological pattern with intact lobules and acini, no signs of oedema, intact acinar cells and acinar nuclei in a peripheral placement, as illustrated in Figures 4C and 5A and Table 1. No significant structural differences were observed between Sham-CER and Sham-CER + flavocoxid animals (Table 1, Figure 5A and B). Histopathological evaluation of samples from the CER + vehicle group (Table 1 and Figure 5C) showed massive oedema, infiltration of inflammatory cells and cytoplasmatic vacuolization compared with Sham-CER (P < 0.05). Flavocoxid treatment after CER significantly reduced oedema formation (Figure 4C and Table 1, P < 0.01), inflammatory infiltrate and cytoplasmic vacuolization (Table 1 and Figure 5D, P < 0.01).
Figure 5.
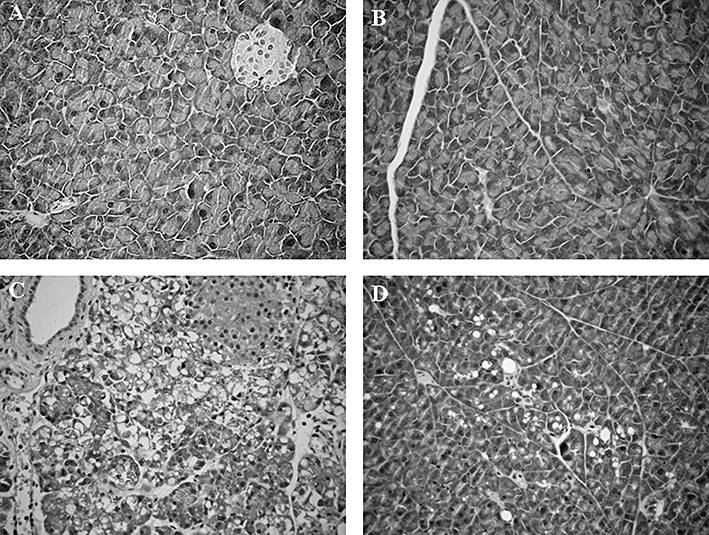
(A) Normal histology of a sample of pancreas obtained from a Sham-CER rat, i.e. saline only injections. Original magnification × 20. (B) Normal histology of a sample of pancreas obtained from a Sham + flavocoxid rat, i.e. flavocoxid after saline. Pancreas lobules and acini are intact, uniform size of acinar cells and peripheral localization of acinar nuclei. Original magnification × 20. (C) Representative sample of pancreas from a rat treated with CER. The pancreas shows a massive oedema, infiltration of inflammatory cells and cytoplasmatic vacuolization. Original magnification × 20. (D) Representative sample of pancreas from a rat treated with flavocoxid after CER. There was a significant reduction in oedema and inflammatory cell infiltrate. Original magnification × 20. CER, caerulein.
Table 1.
Histology of pancreas harvested from control (Sham-CER) and caerulein (CER) injected rats treated with either vehicle or flavocoxid
| Treatment groups | Grade of interstitial oedema | Grade of inflammatory cell infiltrate | Grade of vacuolization |
|---|---|---|---|
| Sham-CER + vehicle | 0 | 0 | 0 |
| Sham-CER + flavocoxid | 0 | 0 | 0 |
| CER + vehicle | 3 | 3 | 3 |
| CER + flavocoxid | 1 | 1 | 1 |
Histological grading of oedema and infiltrate of inflammatory cells were based on the following scale: 0, absent; 1, mild; 2, moderate; 3, severe. Grading of vacuolization was based on the approximate fraction of cells involved: 0, 0–25%; 1, 25–50%; 2, 50–75%; 3, 75–100%. Values shown are the group modes; n = 7; significant differences between modes was assessed by the Student-Fisher t-test.
Discussion
The present paper shows the protective and beneficial effects of a plant-derived dual inhibitor, flavocoxid, in the early stages of pancreatic damage induced by a cholecystokinin analogue. The current management of AP is limited to supportive care and treatment of complications when they develop, thus an effective treatment is still needed. Inhibition of the inflammatory pathway seems to be the most promising approach to prevent the development of the disease. In AP, both COX-2 and 5-LOX are crucial and central mediators in the development and severity of the disease (Vollmar et al., 1989; Song et al., 2002; Cuzzocrea et al., 2003; Foitzik et al., 2003), leading to an increase in LT and PG levels in early and late stages (Ersoz et al., 1999; Foitzik et al., 2003; Oruc et al., 2004). The PGs have a variety of functions in the pancreas, including pro-inflammatory effects and regulation of blood flow. Thus, selective inhibition of COX-2 ameliorated the severity of CER-induced pancreatitis in the rat model (Song et al., 2002; Foitzik et al., 2003). Ethridge et al. (2002) showed that COX-2 gene expression was increased in CER-induced pancreatitis and that the severity of pancreatic necrosis and leukocyte infiltration were significantly decreased by treatment with the selective COX-2 inhibitor NS-398. However, because LTs can also maintain local inflammatory reactions by causing aggregation and degranulation of polymorphonuclear leukocytes, shunting of arachidonic acid to the 5-LOX pathway may account for further organ damage. Therefore, caution is needed in the use of COX-2 inhibitors alone for the treatment of AP in clinical practice. Moreover LTB4 in a CER-induced AP model in rats may be responsible for cell infiltration and oedema in pancreas. In addition, 5-LOX knockout mice were protected against experimental pancreatitis and associated liver and lung injury (Cuzzocrea et al., 2003).
These observations led us to hypothesize a possible beneficial effect of the concomitant and combined blockade of both COX-2 and 5-LOX pathways to reduce organ damage in acute pancreatitis as, to our knowledge, this therapeutic strategy had not been investigated. Flavocoxid is a mixture of the naturally occurring flavonoids, baicalin and catechin, and it has been shown to act as a dual inhibitor of COX and 5-LOX (Burnett et al., 2007). Previous studies reported that baicalin and catechin have a protective effect on the pathogenesis of CER-induced pancreatitis through suppression of inflammatory mediators, such as TNF-α (Xue et al., 2006).
Flavocoxid also reduced activation of both COX-2 and 5-LOX in the pancreas, confirming its mechanism of action, with a consequent significant reduction of PGE2 and LTB4 levels. The reduction of eicosanoid production may account for the reduced oedema and inflammatory infiltrate in CER + flavocoxid animals. As a consequence also TNF-α mRNA was strongly reduced in pancreas obtained from CER + flavocoxid rats. This cytokine (TNF-α) plays a key role in the pathogenesis of acute pancreatitis and related systemic complications: in fact, this cytokine leads to oedema formation and production of other pro-inflammatory mediators that reinforce and amplify the pancreatic injury (Murr et al., 2003; Laveda et al., 2005). Excessive generation of TNF-α, or imbalance between it and other cytokines, induce generation of IL-1, IL-6 and aggravate cell damage. The reduced inflammatory process decreased the release of amylase and lipase enzymes by pancreatic acinar cells demonstrating a protective role of flavocoxid in the autodigestive process. These biochemical and molecular data correlated very well with each histological parameter considered. Pancreas obtained from CER-injected rats showed massive oedema, infiltration of inflammatory cells and presence of cytoplasmatic vacuolization. The administration of flavocoxid markedly reduced the degree of oedema and decreased the histological alterations. Our results clearly show, for the first time, that the concomitant inhibition of COX-2 and 5-LOX by flavocoxid ameliorated the severity of CER-induced pancreatitis, acting directly on the inflammatory response elicited by the secretagogue agent, thus blocking the degenerative process.
In conclusion, flavocoxid, a plant-derived dual inhibitor of COX-2 and 5-LOX, improved the molecular and histopathological features of CER-induced experimental AP in rats. The findings of this study might provide a basis for new experimental and clinical studies investigating the therapeutic role of flavocoxid in this life-threatening condition.
Glossary
Abbreviations
- CER
caerulein
- COX
cyclooxygenase
- 5-LOX
5-lipoxygenase
- LTB4
leukotriene B4
- NSAID
non-steroidal anti-inflammatory drug
- PGE2
prostaglandin E2
- TNF-α
tumour necrosis factor-α
Conflicts of interest
None to declare.
References
- Altavilla D, Famulari C, Passaniti M, Galeano M, Macrì A, Seminara P, et al. Attenuated caerulein-induced pancreatitis in nuclear factor-kappaB-deficient mice. Lab Invest. 2003a;83:1723–1732. doi: 10.1097/01.lab.0000101734.82054.be. [DOI] [PubMed] [Google Scholar]
- Altavilla D, Famulari C, Passaniti M, Campo GM, Macrì A, Seminara P, et al. Lipid peroxidation inhibition reduces NF-kappaB activation and attenuates caerulein-induced pancreatitis. Free Radic Res. 2003b;37:425–435. doi: 10.1080/1071576031000070093. [DOI] [PubMed] [Google Scholar]
- Altavilla D, Squadrito F, Bitto A, Polito F, Burnett BP, Di Stefano V, et al. Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophages. Br J Pharmacol. 2009;157:1410–1418. doi: 10.1111/j.1476-5381.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Wong FL, Cao Y, Lau HY, Huang J, Puneet P, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- Burnett BP, Jia Q, Zhao Y, Levy RM. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J Med Food. 2007;10:442–451. doi: 10.1089/jmf.2006.255. [DOI] [PubMed] [Google Scholar]
- Colville-Nash PR, Gilroy DW. Potential adverse effects of cyclooxygenase-2 inhibition: evidence from animal models of inflammation. BioDrugs. 2001;15:1–9. doi: 10.2165/00063030-200115010-00001. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Rossi A, Serraino I, Di Paola R, Dugo L, Genovese T, et al. 5-lipoxygenase knockout mice exhibit a resistance to acute pancreatitis induced by caerulein. Immunology. 2003;110:120–130. doi: 10.1046/j.1365-2567.2003.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ersoz G, Huseyinov A, Ozutemiz O, Yuce G, Coker I, Batur Y. Leukotrienes in caerulein-induced acute pancreatitis in rats and effect of octreotide. Turk J Gastroenterol. 1999;10:40–47. [Google Scholar]
- Ethridge RT, Chung DH, Slogoff M, Ehlers RA, Hellmich MR, Rajaraman S, et al. Cyclooxygenase-2 gene disruption attenuates the severity of acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 2002;123:1311–1322. doi: 10.1053/gast.2002.35951. [DOI] [PubMed] [Google Scholar]
- Foitzik T, Hotz B, Wittig F, Buhr HJ. Selective inhibition of cyclooxygenase-2 (COX-2) reduces prostaglandin E2 production and attenuates systemic disease sequelae in experimental pancreatitis. Hepatogastroenterology. 2003;50:1159–1162. [PubMed] [Google Scholar]
- Folch E, Closa D, Prats N, Gelpí E, Roselló-Catafau J. Leukotriene generation and neutrophil infiltration after experimental acute pancreatitis. Inflammation. 1998;22:83–93. doi: 10.1023/a:1022399824880. [DOI] [PubMed] [Google Scholar]
- Hirano T. Peptide leukotriene receptor antagonist diminishes pancreatic edema formation in rats with caerulein-induced acute pancreatitis. Scand J Gastroenterol. 1997;32:84–88. doi: 10.3109/00365529709025068. [DOI] [PubMed] [Google Scholar]
- Hudson N, Balsitis M, Everitt S, Hawkey CJ. Enhanced gastric mucosal leukotriene B4 synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993;34:742–747. doi: 10.1136/gut.34.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1–4. doi: 10.1136/gut.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsnorth A, O'Reilly D. Acute pancreatitis. Br Med J. 2006;332:1072–1076. doi: 10.1136/bmj.332.7549.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer S. Discovery and development of ML 3000. Inflammopharmacology. 2001;9:101–112. [Google Scholar]
- Laveda R, Martınez J, Munoz C, Penalva JC, Saez J, Belda G, et al. Different profile of cytokine synthesis according to the severity of acute pancreatitis. World J Gastroenterol. 2005;11:5309–5313. doi: 10.3748/wjg.v11.i34.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549–563. doi: 10.1016/s0025-7125(05)70239-x. [DOI] [PubMed] [Google Scholar]
- Levy RM, Saikovsky R, Shmidt E, Khokhlov A, Burnett BP. Flavocoxid is as effective as naproxen for managing the signs and symptoms of osteoarthritis of the knee in humans: a short-term randomized, double-blind pilot study. Nutr Res. 2009;29:298–304. doi: 10.1016/j.nutres.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Messina S, Bitto A, Aguennouz M, Mazzeo A, Migliorato A, Polito F. Flavocoxid counteracts muscle necrosis and improbe functional properties in mdx mice: a comparison study with methylprednisolone. Exp Neurol. 2009;220:349–358. doi: 10.1016/j.expneurol.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Minutoli L, Altavilla D, Marini H, Passaniti M, Bitto A, Seminara P, et al. Protective effects of SP600125 a new inhibitor of c-jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK1/2) in an experimental model of caerulein-induced pancreatitis. Life Sci. 2004;75:2853–2866. doi: 10.1016/j.lfs.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Baggott JE, Moreland L, Desmond R, Kendrach AC. The safety of flavocoxid, a medical food, in the dietary management of knee osteoarthritis. J Med Food. 2009;12:1143–1148. doi: 10.1089/jmf.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr MM, Yang J, Fier A, Gallagher SF, Carter G, Gower WR, Jr, et al. Regulation of Kupffer cell TNF gene expression during experimental acute pancreatitis: the role of p38-MAPK, ERK1/2, SAPK/JNK, and NF-nB. J Gastrointest Surg. 2003;7:20–25. doi: 10.1016/s1091-255x(02)00053-7. [DOI] [PubMed] [Google Scholar]
- Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- Oruc N, Yukselen V, Ozutemiz AO, Yuce G, Celik HA, Musoglu A, et al. Leukotrien receptor antagonism in experimental acute pancreatitis in rats. Eur J Gastroenterol Hepatol. 2004;16:383–388. doi: 10.1097/00042737-200404000-00003. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. The effects of 5-lipoxygenase inhibitors and leukotriene antagonists on the development of gastric lesions induced by nonsteroidal antiinflammatory drugs in mice. Agents Actions. 1987;21:316–319. doi: 10.1007/BF01966502. [DOI] [PubMed] [Google Scholar]
- Rainsford KD. Leukotrienes in the pathogenesis of NSAID-induced gastric and intestinal mucosal damage. Agents Actions. 1993;39:24–26. doi: 10.1007/BF01972709. [DOI] [PubMed] [Google Scholar]
- Song AM, Bhagat L, Singh VP, Van Acker GG, Steer ML, Saluja AK. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:1166–1174. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- Steer ML. The early intracinar cell events wich occur during acute pancreatitis. Pancreas. 1998;17:31–37. doi: 10.1159/000026152. [DOI] [PubMed] [Google Scholar]
- Uhl W, Schrag HJ, Schmitter N, Aufenanger J, Nevalainen TJ, Büchler MW. Experimental study of a novel phospholipase A2 inhibitor in acute pancreatitis. Br J Surg. 1998;85:618–623. doi: 10.1046/j.1365-2168.1998.00674.x. [DOI] [PubMed] [Google Scholar]
- Van Ooijen B, Kort WJ, Zijlstra FJ, Vincent JE, Wilson JH, Westbroek DL. Prostanoid imbalance in experimental acute pancreatitis in rats. Scand J Gastroenterol. 1988;23:193–198. doi: 10.3109/00365528809103967. [DOI] [PubMed] [Google Scholar]
- Vollmar B, Waldner H, Schmand J, Conzen PF, Goetz AE, Habazettl H. Release of arachidonic acid metabolites during acute pancreatitis in pigs. Scand J Gastroenterol. 1989;24:1253–1264. doi: 10.3109/00365528909090796. [DOI] [PubMed] [Google Scholar]
- Xue D, Zhang W, Zhang Y, Wang H, Zheng B, Shi X. Adjusting effects of baicalin for nuclear factor-kappaB and tumor necrosis factor-alpha on rats with caerulein-induced acute pancreatitis. Mediators Inflamm. 2006;2006:1–6. doi: 10.1155/MI/2006/26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel-Langhennig A, Holler B, Engeland K, Mössner J. Cyclooxygenase-2 transcription is stimulated and amylase secretion is inhibited in pancreatic acinar cells after induction of acute pancreatitis. Biochem Biophys Res Commun. 1999;265:545–549. doi: 10.1006/bbrc.1999.1719. [DOI] [PubMed] [Google Scholar]
- Zhou W, Levine BA, Olson MS. Lipid mediator production in acute and chronic pancreatitis in the rat. J Surg Res. 1994;56:37–44. doi: 10.1006/jsre.1994.1007. [DOI] [PubMed] [Google Scholar]


