Abstract
BACKGROUND AND PURPOSE
We evaluated the role(s) of monoamine oxidase (MAO)-mediated H2O2 generation on 5-hydroxytryptamine (5-HT)-induced tension development of isolated basilar artery of spontaneously hypertensive rats (SHR) and normotensive Wistar-Kyoto (WKY) rats.
EXPERIMENTAL APPROACH
Basilar artery (endothelium-denuded) was isolated for tension measurement and Western blots. Enzymically dissociated single myocytes from basilar arteries were used for patch-clamp electrophysiological and confocal microscopic studies.
KEY RESULTS
Under resting tension, 5-HT elicited a concentration-dependent tension development with a greater sensitivity (with unchanged maximum tension development) in SHR compared with WKY (EC50: 28.4 ± 4.1 nM vs. 98.2 ± 9.4 nM). The exaggerated component of 5-HT-induced tension development in SHR was eradicated by polyethylene glycol-catalase, clorgyline and citalopram whereas exogenously applied H2O2 enhanced the 5-HT-elicited tension development in WKY. A greater protein expression of MAO-A was detected in basilar arteries from SHR than in those from WKY. In single myocytes and the entire basilar artery, 5-HT generated (clorgyline-sensitive) a greater amount of H2O2 in SHR compared with WKY. Whole-cell iberiotoxin-sensitive Ca2+-activated K+ (BKCa) amplitude measured in myocytes of SHR was approximately threefold greater than that in WKY (at +60 mV: 7.61 ± 0.89 pA·pF−1 vs. 2.61 ± 0.66 pA·pF−1). In SHR myocytes, 5-HT caused a greater inhibition (clorgyline-, polyethylene glycol-catalase- and reduced glutathione-sensitive) of BKCa amplitude than in those from WKY.
CONCLUSIONS AND IMPLICATIONS
5-HT caused an increased generation of mitochondrial H2O2 via MAO-A-mediated 5-HT metabolism, which caused a greater inhibition of BKCa gating in basilar artery myocytes, leading to exaggerated basilar artery tension development in SHR.
Keywords: monoamine oxidases, 5-hydroxytryptamine, mitochondrial reactive oxygen species, basilar artery, spontaneously hypertensive rats
Introduction
5-Hydroxytryptamine (5-HT), a potent vasoactive amine, involved in the regulation of cerebral circulation, and it is implicated in the aetiology of cerebral disorders such as migraine, vasospasm following acute subarachnoid haemorrhage and ischaemic brain diseases (Bonvento et al., 1991). One of the endogenous sources of 5-HT is the circulating platelet and during aggregation, a large amount of 5-HT is released from the aggregated platelets into the plasma and causes aberrant vascular responses (Voldby et al., 1982; Vanhoutte, 1983). 5-HT causes vasorelaxation (mainly via endothelial cells) and vasoconstriction (via vascular smooth muscle cells). At sites of atherosclerosis where endothelial damage has occurred, 5-HT released from aggregated platelets has direct contractile effects on the blood vessels and consequently decreases blood flow with serious outcomes.
The magnitude of the contractile response of the basilar artery in spontaneously hypertensive rats (SHR) a commonly used animal model for human essential hypertension, and stroke-prone SHR (SHRSP) in response to 5-HT is greater (but not to other vasoactive agents – U46619, endothelin-1, neuropeptide Y and angiotensin II) compared with normotensive Wistar-Kyoto (WKY) rats (Nishimura, 1996). It is mainly related to an attenuated NO-mediated relaxation (basal release of NO and/or 5-HT-induced NO release from endothelium) in SHR (Schoeffter and Hoyer, 1990; Schmuck et al., 1996; Ullmer et al., 1996). However, there was no differential contractile responses to 5-HT (topical application via the cranial window) of WKY and SHRSP (Paterno et al., 1997).
In vascular smooth muscle cells, vascular tone is coupled to membrane potential (Trapani et al., 1981; Haeusler, 1983) that is determined by potassium (K+) conductance (Nelson et al., 1990; Nelson and Quayle, 1995). Increased Ca2+-activated K+ channel (KCa) current amplitude was observed in arterial smooth muscle from rats with genetic, renal and salt-induced hypertension (Rusch et al., 1992; England et al., 1993; Liu et al., 1994; 1995; Rusch and Runnells, 1994; Martens and Gelband, 1996). The arterioles of SHR constricted twofold to fourfold more intensely in response to iberiotoxin, a highly selective blocker of the large-conductance Ca2+-activated K+ channels (BKCa; ion channel nomenclature follows Alexander et al., 2009) (Liu et al., 1998). In cerebrovascular smooth muscle cells from SHR, there is a higher density (4.7-fold) of iberiotoxin-sensitive BKCa channels at physiological membrane potentials [plus a fourfold increase in BKCa channel α-subunit (a pore-forming subunit)], than in these cells from WKY. Iberiotoxin has a greater inhibitory effect on BKCa channel amplitude of the cerebral arteriole smooth muscle cells of SHR compared with WKY rats. Enhanced BKCa current amplitude plus an increased α-subunit of BKCa channels were observed in the aorta of SHR compared with WKY rats (Liu et al., 1997; 1998;). It has been suggested that the KCa current amplitude is positively correlated to the blood pressure level of animals, and the enhanced KCa channels act as a physiological brake to limit blood pressure elevation (Rusch and Runnells, 1994; Paterno et al., 1997). Thus, agents that suppress the opening of vascular KCa channels of hypertensive animals abolish this beneficial compensatory mechanism and a greater increase in vascular tone is anticipated.
Monoamine oxidase (MAO)-containing nerve fibres is present in the major cerebral arteries including the basilar artery of rats (Shigematsu et al., 1989) and humans (Kalaria and Harik, 1987). The MAOs, flavin-containing enzymes catalysing the oxidative deamination of endogenous monoamines (5-HT, dopamine, adrenaline and noradrenaline), are located in the outer mitochondrial membrane and exhibited in virtually all tissues of mammals. In humans, there are two types of MAO: MAO-A and MAO-B (based on genetic criteria, substrate specificity and inhibition by various synthetic compounds) (Youdim and Finberg, 1991). The physiological significance of MAO in the regulation of cardiovascular activities, reported so far, were mostly derived from the effects of MAO inhibitors used with administered catecholamines in different organs. However, less attention has been paid to the products of MAO activities, such as H2O2, one of the endogenous reactive oxygen species (ROS). MAO-dependent increases in ROS production appeared to be relevant in 5-HT-induced myocardial injury caused by post-ischaemic reperfusion (Bianchi et al., 2005a) and myocyte hypertrophy in vitro (Bianchi et al., 2005b). However, the role(s) of MAO in mediating the exaggerated, 5-HT-elicited, contractile responses of basilar artery of hypertensive animals is unknown.
Therefore, in this study, we tested the hypothesis that 5-HT caused a greater tension development of isolated basilar artery through the mediation of mitochondrial ROS, such as H2O2, generated via MAO-A, in SHR, than in normotensive WKY rats. To eliminate the possible contribution of NO to our results, we used arteries denuded of endothelium after isolation.
Methods
Animals
All animal care and experimental procedures conformed to guidelines for the use of laboratory animals and were approved by the Animal Research Ethics Committee of CUHK (Ref. #: 03/001/ERG). Every effort was made to limit animal suffering and the number of animals used in these experiments. SHR and normotensive WKY rats (22–26 weeks old, male) were used in this study.
Isometric tension development
Isolated basilar artery rings (length: 1 mm, endothelium denuded) were mounted under the optimum tension of 3 ± 0.3 mN in a 5 mL small vessel wire myograph containing physiological salt solution with the composition (mM) of NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11 and CaCl2 1.8, gassed with 16% O2/6% CO2 balanced with N2, pO2∼100 mmHg (the physiological pO2 level), in order to minimize the generation of ROS under the non-physiological conditions with the more commonly used gas mixture (carbogen): 95% O2/5% CO2 (pO2 > 600 mmHg) (Farrow et al., 2008). The endothelium was carefully removed by rubbing the intima of the artery with a human hair for ∼5 min, and endothelium removal was confirmed by the failure of acetylcholine (10 µM)-induced relaxation as reported (Seto et al., 2006). Four basilar artery rings were isolated from individual artery and experiments were performed on the same day. One concentration–response curve of 5-HT (with and without a particular blocker/inhibitor) was constructed in each arterial preparation of individual rats. In order to reduce the number of animals used, controls (inhibitor-free) and drug (blocker/inhibitor)-treated experiments were randomly conducted according to the incomplete block design/protocols (Hinkelmann and Kempthorne, 2005). In this regard, only one ‘control curve’ (i.e. 5-HT-induced contraction without the presence of inhibitor/blocker) of each strain was employed in this study for comparison and data analysis.
Isolation of rat cerebral vascular smooth muscle cells
Single basilar artery smooth muscle cells were enzymatically dissociated as reported (Wu et al., 2005). Cells isolated were used within 8 h after isolation.
Patch-clamp electrophysiology
Conventional whole-cell native iberiotoxin-sensitive BKCa channel gating before, during and after 5-HT (and other drugs) challenge were recorded at room temperature (∼22°C). Whole-cell, membrane-rupture recording of the macroscopic iberiotoxin-sensitive BKCa channels gating of single artery myocytes were recorded, as described by our group previously (Seto et al., 2007). External physiological solutions for recording the BKCa channel amplitude contained (in mM): NaCl 130, KCl 5, MgCl2 1.2, CaCl2 1.5, glucose 10 and HEPES 10 (pH 7.4 with NaOH). Internal pipette solution containing ∼100 nM free [Ca2+] (estimated using the computer programme: Maxchelator, Stanford University, Stanford, CA, USA) had the following composition (in mM): NaCl 10, KCl 110, MgCl2 5, CaCl2 2, EGTA 10, K2ATP 5 and HEPES 10 (pH 7.2 with KOH). To measure the rate of onset of block and recovery from the block in response to drug challenge, the BKCa current was elicited with a test potential to +60 mV (500 ms duration) from a holding potential of −60 mV and stimulated at 0.0333 Hz.
Confocal microscopy
To estimate ROS levels, myocytes were incubated with mitochondrial H2O2-sensitive fluorescent probe: Reduced MitoTracker Red (Philipp et al., 2006). Myocytes were incubated (37°C) with Reduced MitoTracker Red [5 µM in 0.05% dimethyl sulphoxide (DMSO)] for 1 h. After washing, the myocyte was imaged at 15 s intervals under a confocal microscope with a 60× objective (numerical aperture 1.45) (Eclipse CL Plus, Nikon, Japan) and the fluorescence emission (579–599 nm) from MitoTracker Red was acquired. Images were analysed using EZ-C1 3.5 programme (Nikon, Japan).
Chemiluminescence measurement of H2O2
No H2O2 was detected (with or without 5-HT) using one basilar artery when determined by chemiluminescence (Beckman LS-6000, Brea, CA, USA) (Gao et al., 2009). Twenty basilar arteries (cut longitudinally and endothelium denuded) of SHR and WKY were pooled together. Scintillation counting was performed 4–5 times after adding the basilar arteries (20 rats each group) to obtain a stable reading (baseline) before adding 5-HT (1 µM). Data were expressed as counts per minute (cpm) of 20 isolated basilar arteries (length: 8 mm each)·h−1.
Western blots analysis
Basilar arteries were homogenized in the presence of protease inhibitors to obtain extracts of proteins. Different selective antibodies: anti-MAO-A (1:1000), anti-MAO-B (1:1000), anti-5-HT transporter (5-HTT; 1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-mouse HRP-conjugated IgG, 1:1000 and anti-rabbit HRP-conjugated IgG, 1:1000 (Bio-Rad Laboratories, Hercules, CA, USA) were used to detect the presence of protein of interest. The protein expression of MAO-A (61 kDa), MAO-B (60 kDa) and 5-HTT (70 kDa) was detected by Western blot analysis to generate chemiluminescent signals in the presence of the ImmueStar Reagent (Bio-Rad Laboratories). Intensity of individual protein (MAO-A, MAO-B and 5-HTT) bands was measured and quantified (at the corresponding molecular weight of each protein) using the Scion Image analysis programme (Scion Image Ltd., Frederick, MD, USA).
Statistical analysis
Data are expressed as mean ± SEM; n refers to number of basilar arterial ring preparations used in each experiment. Concentration of 5-HT causing 50% of the maximal contraction response (EC50) observed was estimated using Prism (GraphPad Software, USA). Statistical comparisons were performed using one-way and two-way analysis of variance (anova) or Student's t-test, where appropriate.
Materials
All drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. Citalopram hydrobromide and tomoxetine hydrochloride were obtained from Tocris Biosciences (Bristol, UK). All drugs were dissolved at the highest concentrations in either Nano-pure water [5-HT, 5-hydroxytryptophol (5-HTOL), 5-hydroxyindole-3-acetic acid (5-HIAA), clorgyline, pargyline, citalopram, tomoxetine, polyethylene glycol (PEG)-catalase, polyethylene glycol-superoxide dismutase (PEG-SOD), iberiotoxin, Nω-nitro-L-arginine methyl ester (L-NAME) and reduced glutathione (GSH)] or DMSO (indomethacin, apocynin and allopurinol), and diluted directly in external/internal recording solutions (in electrophysiological studies) and physiological salt solution (tension change measurements). Reduced MitoTracker Red was purchased from Invitrogen (Carlsbad, CA, USA), and iberiotoxin was obtained from Alomone Laboratories. (Jerusalem, Israel).
Results
Isometric tension development
5-HT elicited a concentration-dependent tension development of basilar arteries of normotensive WKY and SHR with similar maximum tension (∼10 µM) (Figure 1A), with a significant leftward shift of the concentration–response curve for 5-HT (EC50: WKY, 98.2 ± 9.4 nM; SHR, 28.4 ± 4.1 nM) (P < 0.01) in SHR when compared with that of WKY (Figure 1A). 5-HIAA and 5-HTOL (<30 µM) did not alter the tension of arterial rings from either strain of rat (Figure 1A).
Figure 1.

Concentration–response curves for the in vitro effects of 5-hydroxytryptamine (5-HT)-induced tension development of isolated basilar artery (endothelium-denuded) of spontaneously hypertensive rats (SHR) and Wistar-Kyoto (WKY) rats in the absence or the presence of different agents/treatments. Results are expressed as mean ± SEM (n = 6–8). 5-HIAA, 5-hydroxyindole-3-acetic acid; 5-HTOL, 5-hydroxytryptophol; PEG-catalase, polyethylene glycol-catalase.
Inhibition of MAO, 5-HTT and catecholamine uptake
Clorgyline (1 µM, a MAO-A inhibitor) did not alter the concentration–response curve of 5-HT [EC50: 104.8 ± 6.7 nM (with clorgyline) vs. 98.2 ± 9.4 nM (control) (P > 0.05)] of WKY rats (Figure 1B). Interestingly, clorgyline caused a significant rightward shift (with no change in maximum contraction) of the concentration–response curve for 5-HT of basilar arterial rings from SHR (EC50: 92.3 ± 5.5 nM (with clorgyline) vs. 28.4 ± 4.1 nM (control) (P < 0.01)] (Figure 1C), and the curve (with clorgyline) overlapped with that observed in WKY rats (control) (Figure 1C). Pargyline (10 µM, a MAO-B inhibitor) did not modify the 5-HT-induced tension development in WKY rats [EC50: 96.1 ± 7.0 nM (with pargyline) vs. 98.2 ± 9.4 nM (control) (P > 0.05)] and SHR [EC50: 33.5 ± 5.3 nM (with pargyline) vs. 28.4 ± 4.1 nM (control) (P > 0.05)]. Citalopram (0.1 µM, a potent 5-HTT inhibitor) attenuated 5-HT-induced tension development (a rightward shift of the curve with no change in maximum tension) of SHR [EC50: 93.7 ± 10.3 nM (with citalopram) vs. 28.4 ± 4.1 nM (control) (P < 0.01)] whereas a trend of rightward shift in WKY rats was observed [EC50: 110.5 ± 8.8 nM (with citalopram) vs. 98.2 ± 9.4 nM (control) (P > 0.05)] (Figure 1E). Tomoxetine (10 nM, a potent, selective noradrenaline re-uptake inhibitor) did not modify 5-HT-induced tension development of WKY rats [EC50: 103.7 ± 5.9 nM (with tomoxetine) vs. 98.2 ± 9.4 nM (control) (P > 0.05)] and SHR [EC50: 33.8 ± 9.2 nM (with tomoxetine) vs. 28.4 ± 4.1 nM (control) (P > 0.05)].
Effects of PEG-catalase, H2O2 and PEG-superoxide dismutase
In WKY, PEG-catalase (100 U mL−1, a cell-permeable enzyme that catalyses conversion of H2O2 to H2O and O2) did not modify 5-HT-induced tension development [EC50: 103.4 ± 6.2 nM (with PEG-catalase) vs. 98.2 ± 9.4 nM (control) (P > 0.05)]. In SHR, the enhanced 5-HT-induced tension development was normalized by PEG-catalase (100 U·mL−1) [EC50: 101.9 ± 9.0 nM (with PEG-catalase) vs. 28.4 ± 4.1 nM (control) (P < 0.01)] (Figure 1F). In WKY, H2O2 (100 µM, 30 min) enhanced (PEG-catalase-sensitive) the 5-HT-induced tension development [EC50: 25.7 ± 10.0 nM (with H2O2); 92.3 ± 7.7 nM (H2O2 plus PEG-catalase); 98.2 ± 9.4 nM (control)] which was similar to that observed in SHR. PEG-SOD (a cell-permeable enzyme that catalyses the dismutation of superoxide into O2 and H2O2) (30 U·mL−1) did not modify 5-HT-elicited tension development of both strains of rat [EC50: WKY, 102.4 ± 7.3 nM (with PEG-SOD) vs. 98.2 ± 9.4 nM (control) (P > 0.05); SHR, 32.6 ± 5.6 nM (with PEG-SOD) vs. 28.4 ± 4.1 nM (control) (P > 0.05) Figure 1D].
Estimation of H2O2 generation
A higher basal level of H2O2 was detected in basilar arteries (a pool of 20 arteries) of SHR as compared with that of WKY rats. 5-HT (1 µM) elicited a stronger increase in H2O2 in SHR compared with WKY rats. Clorgyline (1 µM), PEG-catalase (100 U·mL−1) and citalopram (0.1 µM), but not pargyline (10 µM) and PEG-SOD (30 U·mL−1), abolished the 5-HT-induced H2O2 generation (Figure 2).
Figure 2.
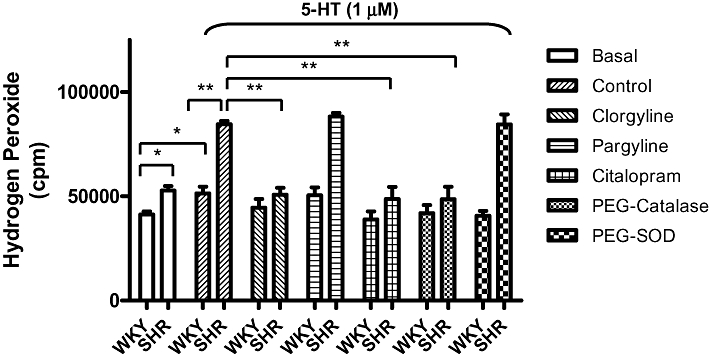
Assay, by chemiluminescence, of H2O2 generation (shown as cpm) in isolated basilar arteries (endothelium denuded) in response to 5-HT (1 µM) in the absence or presence of different agents (clorgyline, pargyline, citalopram, PEG-catalase and PEG-SOD). Results are expressed as mean ± SEM. *P < 0.05; **P < 0.01. 5-HT, 5-hydroxytryptamine; cpm, counts per minute; PEG-catalase, polyethylene glycol-catalase; PEG-SOD, polyethylene glycol-superoxide dismutase; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Protein expression of MAO-A, MAO-B and 5-HTT
The protein level of MAO-A in SHR was ∼50% higher than that of WKY rats (P < 0.01) (Figure 3). However, the level of MAO-B and 5-HTT in WKY and SHR were not different (data not shown).
Figure 3.
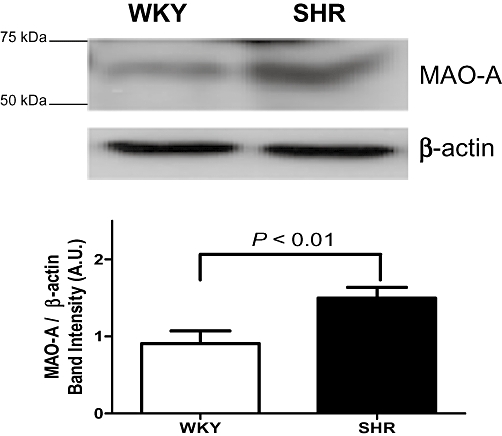
Western immunoblots analysis revealed monoamine oxidase-A (MAO-A, 61 kDa) expression of basilar artery (endothelium-denuded) of spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats. β-Actin was measured as a loading control. Results are normalized to β-actin expression and are expressed as mean (arbitrary units, AU) ± SEM of four independent experiments (P < 0.01, SHR vs. WKY).
5-HT on BKCa channel gating
In single myocytes isolated from basilar arteries, the iberiotoxin-sensitive BKCa current amplitude (measured at +60 mV) in SHR was greater (approximately threefold) than that of WKY (Figure 4) [cell capacitance: 15.2 ± 1.1 pF (SHR) vs. 16.0 ± 0.9 pF (WKY)] (P > 0.05). 5-HT (1 µM) markedly suppressed the BKCa amplitude in SHR (54 ± 6% inhibition) whereas only a relatively small inhibition (12 ± 2%) was observed in WKY (Figure 4). The inhibitory effects of 5-HT on BKCa amplitude could not be reversed after washing (Figure 4). PEG-catalase (100 U·mL−1), clorgyline (1 µM), citalopram (0.1 µM) and GSH (a physiological reductant) (5 mM, included in pipette solution) prevented the inhibitory effects of 5-HT on BKCa channels (Figure 4). Exogenous H2O2 (100 µM) (concentration at which it modified tension development of basilar artery and/or BKCa channels gatings) (Yang et al., 1999; Dong et al., 2008) inhibited BKCa amplitude of SHR (62 ± 8% inhibition; n = 7) and WKY (15 ± 6% inhibition; n = 6) with no recovery after washout (Figure 4). The rate of onset of the steady-state inhibition of BKCa amplitude by exogenous H2O2 (∼8 min) was faster than that observed with 5-HT (∼15 min) (Figure 4). 5-HIAA and 5-HTOL did not have significant effects on BKCa amplitude (data not shown).
Figure 4.
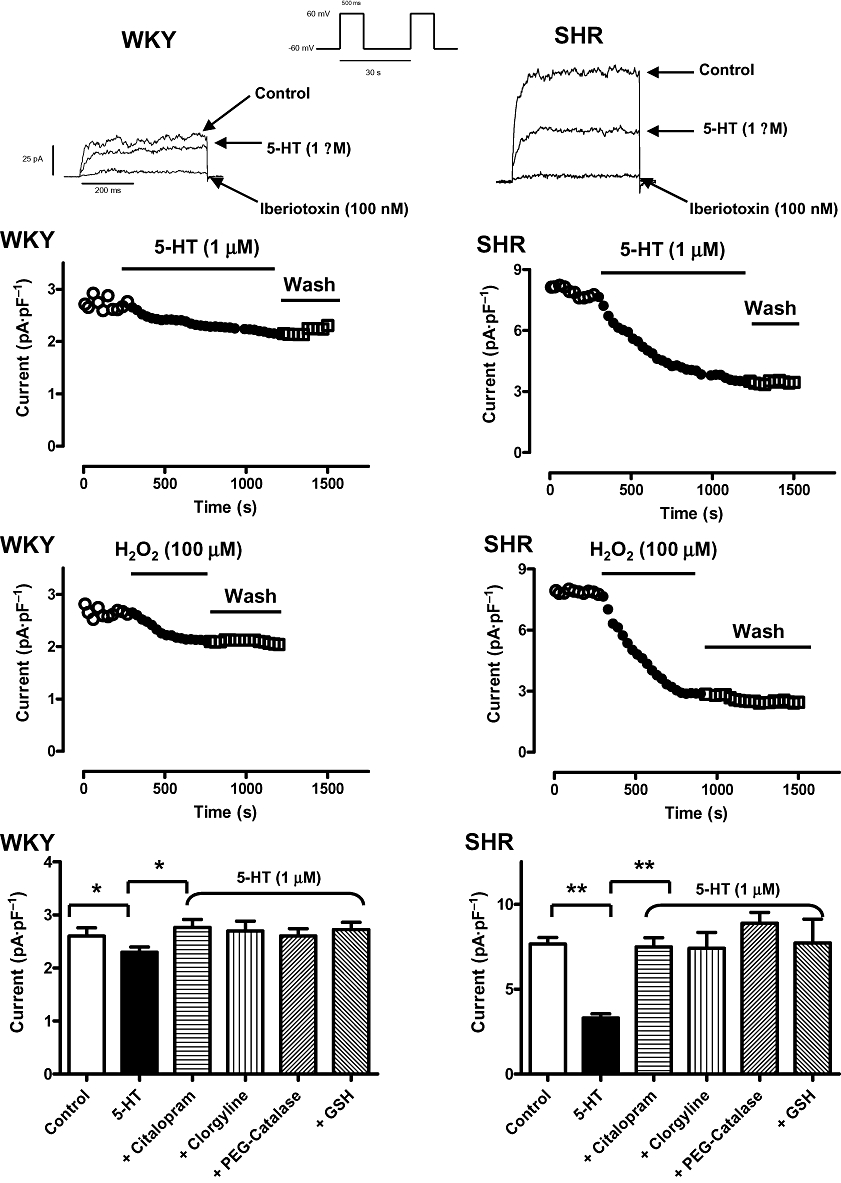
Time course of the inhibitory effects of 5-HT (1 µM, top panel) and H2O2 (100 µM, middle panel) on iberiotoxin-sensitive, large-conductance Ca2+-activated K+ (BKCa) amplitude of single basilar artery myocytes of SHR and WKY rats. The BKCa current was elicited using a train-pulse protocol with a test potential of +60 mV (500 ms pulse duration) from a holding potential of −60 mV at 0.03 Hz. (Bottom panel): summary of the macroscopic BKCa current amplitude recorded [peak BKCa current (pA·pF−1) recorded at +60 mV from a holding potential of −60 mV for 500 ms duration at 0.0333 Hz] in response to 5-HT (1 µM) challenge in the absence or presence of different agents [citalopram, clorgyline, PEG-catalase and reduced glutathione (GSH)]. Mean ± SEM are indicated by columns and vertical bars respectively (*P < 0.05 and **P < 0.01). 5-HT, 5-hydroxytryptamine; PEG-catalase, polyethylene glycol-catalase; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Effects of 5-HT on mitochondrial ROS generation
5-HT (1 µM) caused an increase in mitochondrial ROS generation at ∼5 min after 5-HT administration, and the peak amplitude of signal occurred at ∼15 min. A relatively greater magnitude was detected in SHR when compared with that of WKY (Figure 5). ROS generation was inhibited by clorgyline (1 µM) and citalopram (0.1 µM) but not by pargyline (10 µM) (Figure 5). Indomethacin [10 µM, a cyclooxygenase (COX) inhibitor], L-NAME (100 µM, a nitric oxide synthase (NOS) inhibitor), allopurinol (10 µM, a xanthine oxidase inhibitor) and apocynin [100 µM, an inhibitor of reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases] did not alter the 5-HT-elicited ROS generation (data not shown).
Figure 5.
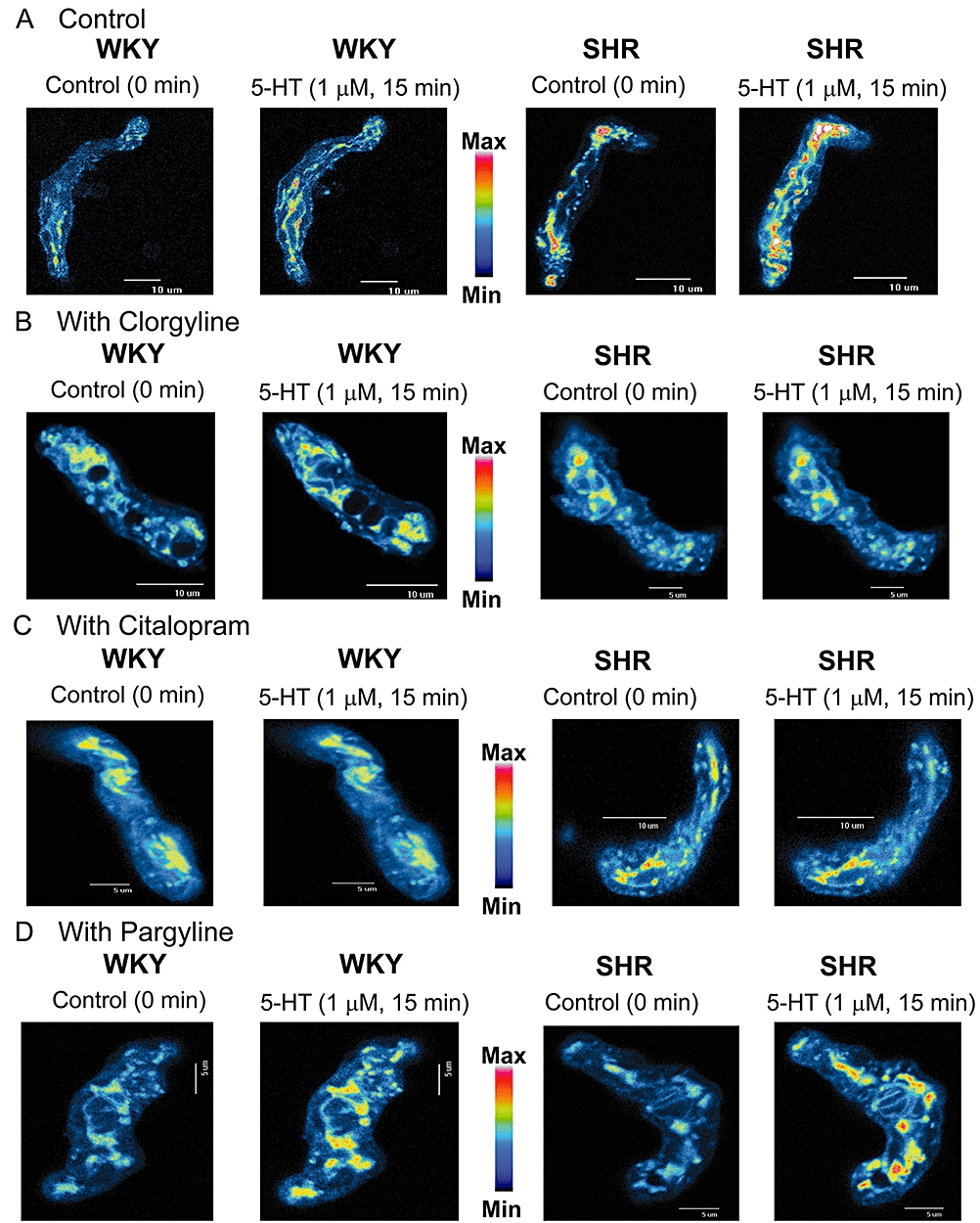
Effects of 5-HT (1 µM) on mitochondrial H2O2 generation estimated using reduced MitoTracker Red. Insets are representative images of single basilar artery myocytes of SHR and WKY rats (at least 20 cells in each condition) in response to 5-HT in the absence (control) (A) or the presence of clorgyline (1 µM) (B), citalopram (0.1 µM) (C) and pargyline (10 µM) (D). 5-HT, 5-hydroxytryptamine; SHR, spontaneously hypertensive rats; WKY, Wistar-Kyoto rats.
Discussion
Consistent with previous studies (Yokota et al., 1994; Nishimura and Suzuki, 1995; Salomone et al., 1997; Budzyn et al., 2008), 5-HT-induced contraction of isolated basilar artery (endothelium-denuded) was greater in SHR than that in normotensive WKY rats. In isolated aorta (Budzyn et al., 2008), the enhanced 5-HT-elicited contraction in SHR was due to an increase in vascular superoxide (O2−) and the destruction of NO. In our study, the exaggerated contraction observed in SHR could not be related to endothelium/NO as the endothelium was mechanically removed.
Exogenous H2O2 enhanced 5-HT-induced contractions (PEG-catalase-sensitive/PEG-SOD-insensitive) in basilar arterial rings from WKY rats, which overlapped with those observed in SHR. In SHR, PEG-catalase reversed the exaggerated 5-HT-induced contraction. Our results therefore suggested that the exaggerated contraction in SHR was mediated by H2O2 (and probably not O2−).
Arterial smooth muscle cells can take up 5-HT via the 5-HT transporter (5-HTT), and MAO is able to metabolize intracellular 5-HT (Small et al., 1977; Brust et al., 2000). In DOCA-salt and LNNA-induced hypertensive rats (Ni et al., 2006), and in rats with pulmonary hypertension (Eddahibi et al., 2001a,b;), up-regulation of 5-HTT expression/function was observed. Similar to findings in the aorta of SHR and WKY rats (Ni et al., 2006), our results failed to reveal a difference in the 5-HTT expression in basilar arteries between SHR and WKY rats. Citalopram (a potent 5-HTT blocker), but not tomoxetine (a potent noradrenaline uptake inhibitor), abolished the ‘enhanced component’ of 5-HT-elicited contraction in SHR suggesting that the uptake of 5-HT via 5-HTT plays an essential role in mediating the exaggerated contraction.
Both isoforms of MAO, MAO-A and MAO-B, have been demonstrated in cerebral microvessels in humans (Kalaria and Harik, 1987; Youdim and Finberg, 1991). As reported earlier, an augmented expression of MAO-A, but not of MAO-B, was detected in cerebral arteries of SHR (Lai and Spector, 1978; Guffroy and Strolin Benedetti, 1984). Given the fact that 5-HT is metabolized by MAOs, and H2O2 is generated (Sagin et al., 2004), it is tempting to suggest that the enhanced MAO-A expression increased 5-HT metabolism and a higher H2O2 level was generated in SHR. Clorgyline (a MAO-A inhibitor) (Ulus et al., 2000) [but not pargyline (a MAO-B inhibitor) (Nishimura, 1996), indomethacin, L-NAME, allopurinol and apocynin] reversed the enhanced contraction in SHR. These results therefore confirm the obligatory role of MAO-A in the enhanced contraction mediated by 5-HT in SHR.
Inside the cell, 5-HT is metabolized by MAO and, in addition to H2O2, 5-HIAA and 5-HTOL are formed (Sagin et al., 2004). Consistent with a previous study performed on isolated mesenteric artery of DOCA-induced hypertensive rats (Thompson and Webb, 1987), neither 5-HIAA nor 5-HTOL (≤30 µM) altered the basal tension development of the basilar artery of both strains of rat, arguing against the participation of 5-HT metabolites (i.e. 5-HIAA and 5-HTOL) in mediating the vascular effects of 5-HT observed.
In the cerebral circulation and other vascular beds, H2O2 is generally believed to be a vasodilator (Faraci, 2006; Modrick et al., 2009). However, in canine basilar artery smooth muscle cells (Yang et al., 1999), H2O2 caused an increase in [Ca2+]i plus contraction of basilar artery myocytes whereas relaxation of pig coronary artery smooth muscle cells was observed (Hayabuchi et al., 1998). Mitochondrial-derived H2O2 inhibits relaxation of bovine coronary arterial smooth muscle in response to hypoxia (Gao et al., 2009). Using a specific fluorescent dye (reduced MitoTracker Red that is oxidized to a fluorescent form after exposure to ROS) (Oldenburg et al., 2003) for monitoring mitochondrial H2O2 generation, we demonstrated, for the first time, that H2O2 was indeed generated (in a PEG-catalase-/citalopram-sensitive and PEG-superoxide dismutase-insensitive fashion) upon the addition of 5-HT, with a relatively greater magnitude of H2O2 generation detected in SHR. Generation of H2O2 in isolated basilar artery of both strains of rat was confirmed using chemiluminescence methods that further strengthens our conclusions on the participation of mitochondrial H2O2 in mediating the exaggerated vascular contractile effects of 5-HT observed in SHR. In addition, the generation of H2O2 by 5-HT was abolished by clorgyline strongly supporting an obligatory role of MAO-A in mitochondrial H2O2 generation after 5-HT challenge.
In KCa channels, a large number of cysteine residues are located within an extensive, presumably cytoplasmic, domain unique to these channels making them likely to be affected by redox modulation (Zhang et al., 2006). In addition, several channel properties are changed when KCa channels are moved from the relatively reduced cellular environment to the more oxidized environments (e.g. ROS/oxidative stress). So far, our results demonstrate the generation of H2O2 during 5-HT metabolism via mitochondrial MAO-A. We therefore decided to assess the modulatory role(s) of H2O2 thus generated on BKCa channel gating. As previously reported (Liu et al., 1998), the basal BKCa amplitude recorded in myocytes from SHR basilar arteries, was greater (approximately threefold) compared with that in myocytes from WKY. 5-HT and H2O2 elicited a greater magnitude of inhibition of BKCa amplitude in basilar artery myocytes of SHR than that observed in WKY. In addition, the rate of onset of steady-state maximum inhibition of BKCa channel caused by exogenous H2O2 (added into the external recording solution) was faster (∼8 min) than that observed with 5-HT (∼15 min). It is probably related to the time required for the generation of H2O2 via MAO-A (upon 5-HT addition) whereas exogenous applied H2O2 (it is freely diffusible) has ‘direct/immediate effects’ on BKCa channel gatings. A greater amplitude of basal BKCa currents in hypertensive states is thought to serve as a physiological brake in controlling blood pressure from rising too high (Liu et al., 1998; Kamouchi et al., 2002). Inhibition of the ‘enhanced’ BKCa channel gating, as by TEA and iberiotoxin, thus resulted in a greater magnitude of vascular contraction. Indeed, our results consistently demonstrate that 5-HT (1 µM) caused a greater degree of inhibition of BKCa amplitude of single basilar artery myocytes of SHR compared with those from WKY rats. It is probably correlated with a higher sensitivity (i.e. a lower EC50 value) of isolated basilar artery of SHR compared with WKY rats in response to 5-HT. In addition, modulation of 5-HT-induced inhibition of BKCa channels by GSH (a physiological reductant, delivered into the cytosol via the patch pipette) clearly illustrated that oxidant-mediated changes are responsible for 5-HT-elicited inhibition of BKCa amplitude. A persistent inhibition of BKCa channel gating by 5-HT was observed after extensive washout and may suggest that there was a long-lasting/permanent change of amino acids residues (e.g. methionine, tryptophan and cysteine) (Soto et al., 2002) of BKCa channels by the H2O2 generated. Hence, our results clearly illustrated that there is a greater amount of mitochondrial H2O2 release upon 5-HT challenge in basilar artery myocytes of SHR, which is associated with a greater protein expression of MAO-A (and thus more 5-HT is metabolized). Interestingly, many MAO inhibitors have anti-hypertensive properties (MacCauley, 1980) that may be related to the inhibition of ROS formation as ROS generated inhibits vascular K+ channels (as demonstrated in our study). It is important to point out that O2− stimulated KCa channels gating of rat basilar artery, leading to relaxation of the artery (Conde et al., 1999) whereas in our study, inhibition of BKCa channels by 5-HT/H2O2 was consistently observed. In porcine renal artery myocytes (Brakemeier et al., 2003), exogenous H2O2 inhibited BKCa channels whereas in porcine coronary artery myocytes (Thengchaisri and Kuo, 2003) H2O2 activated BKCa channels. In our study, effects of 5-HT were not modified by PEG-SOD, and exogenously added H2O2 enhanced 5-HT-evoked contraction of basilar artery from WKY rats. Collectively, our results strongly suggest that H2O2 (but not O2−) is probably the ROS generated (via MAO-A) upon the addition of 5-HT to basilar artery myocytes.
In conclusion, we have provided convincing evidence that an enhanced MAO-A protein expression in the cerebral arteries is closely associated with/responsible for the exaggerated in vitro tension development, induced by 5-HT, of the isolated basilar artery of SHR. More importantly, in isolated cerebral artery myocytes from SHR and WKY rats, we have demonstrated, for the first time, an association between the generation of mitochondrial H2O2 (via metabolism of 5-HT by mitochondrial MAO-A) and the inhibition of BKCa channel gating caused by 5-HT and the H2O2 released (Figure 6).
Figure 6.
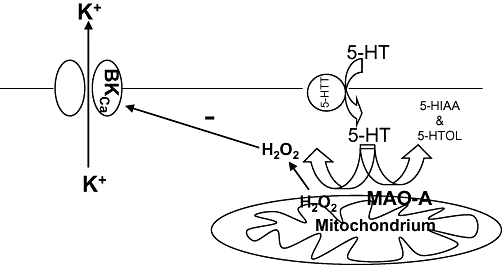
Proposed mechanisms underlying the exaggerated 5-HT-induced basilar artery contraction of spontaneously hypertensive rats (SHR). −, inhibition; 5-HT, 5-hydroxytryptamine; 5-HTT, 5-hydroxytryptamine transporter; 5-HIAA, 5-hydroxyindole-3-acetic acid; 5-HTOL, 5-hydroxytryptophol; BKCa, large-conductance Ca2+-activated K+ channels; H2O2, hydrogen peroxide; K+, potassium ions; MAO-A, monoamine oxidase-A.
Acknowledgments
We are grateful to Li Ka Shing Institute of Health Sciences and Institute of Vascular Medicine (Faculty of Medicine, The Chinese University of Hong Kong) for financial supports (to YW Kwan). This project is financially supported by UGC Earmarked Grants of Hong Kong (Ref. #: 4107/01M; 4166/02M, project code: 2140565) and Direct Grants for Research (The Chinese University of Hong Kong) (Reference no. 2401149; Project code/ID: 2041231; 2401296). Ms CCW Poon, Mr SW Seto, Ms ALS Au, Ms Q Zhang and Mr WYW Lee are recipients of postgraduate studentship of the Department of Pharmacology/School of Biomedical Sciences (The Chinese University of Hong Kong, Hong Kong). Provision of the Student Campus Work Scheme by the Chou's Foundation Fund and the Student Campus Work Scheme (Shaw College, The Chinese University of Hong Kong) is appreciated. Proofreading of the manuscript by Dr Ho Yeung Lam is acknowledged.
Glossary
Abbreviations
- 5-HIAA
5-hydroxyindole-3-acetic acid
- 5-HTOL
5-hydroxytryptophol
- 5-HTT
5-hydroxytryptamine transporter
- BKCa channels
native iberiotoxin-sensitive large-conductance Ca2+-activated K+ channels
- COX
cyclooxygenase
- DMSO
dimethyl sulphoxide
- GSH
reduced glutathione
- L-NAME
Nω-nitro-L-arginine methyl ester
- MAO-A
monoamine oxidase-A
- MAO-B
monoamine oxidase-B
- NADPH oxidases
reduced nicotinamide-adenine dinucleotide phosphate oxidases
- PEG-catalase
polyethylene glycol-catalase
- PEG-SOD
polyethylene glycol-superoxide dismutase
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- WKY
Wistar-Kyoto rats
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005a;112:3297–3305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Pimentel DR, Murphy MP, Colucci WS, Parini A. A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J. 2005b;19:641–643. doi: 10.1096/fj.04-2518fje. [DOI] [PubMed] [Google Scholar]
- Bonvento G, MacKenzie ET, Edvinsson L. Serotonergic innervation of the cerebral vasculature: relevance to migraine and ischaemia. Brain Res Brain Res Rev. 1991;16:257–263. doi: 10.1016/0165-0173(91)90009-w. [DOI] [PubMed] [Google Scholar]
- Brakemeier S, Eichler I, Knorr A, Fassheber T, Köhler R, Hoyer J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int. 2003;64:199–207. doi: 10.1046/j.1523-1755.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Brust P, Friedrich A, Krizbai IA, Bergmann R, Roux F, Ganapathy V, et al. Functional expression of the serotonin transporter in immortalized rat brain microvessel endothelial cells. J Neurochem. 2000;74:1241–1248. doi: 10.1046/j.1471-4159.2000.741241.x. [DOI] [PubMed] [Google Scholar]
- Budzyn K, Ravi RM, Miller AA, Sobey CG. Mechanisms of augmented vasoconstriction induced by 5-hydroxytryptamine in aortic rings from spontaneously hypertensive rats. Br J Pharmacol. 2008;155:210–216. doi: 10.1038/bjp.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde MV, Marin J, Balfagon G. Superoxide anion and K+ channels mediate electrical stimulation-induced relaxation in the rat basilar artery. Eur J Pharmacol. 1999;372:179–186. doi: 10.1016/s0014-2999(99)00215-0. [DOI] [PubMed] [Google Scholar]
- Dong DL, Yue P, Yang BF, Wang WH. Hydrogen peroxide stimulates the Ca2+-activated big-conductance K channels (BK) through cGMP signaling pathway in cultured human endothelial cells. Cell Physiol Biochem. 2008;22:119–126. doi: 10.1159/000149789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S, Frisdal E, Levame M, Hamon M, Raffestin B. Dexfenfluramine-associated changes in 5-hydroxytryptamine transporter expression and development of hypoxic pulmonary hypertension in rats. J Pharmacol Exp Ther. 2001a;297:148–154. [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, et al. Serotonin transporter over-expression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001b;108:1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England SK, Wooldridge TA, Stekiel WJ, Rusch NJ. Enhanced single-channel K+ current in arterial membranes from genetically hypertensive rats. Am J Physiol. 1993;264:H1337–H1345. doi: 10.1152/ajpheart.1993.264.5.H1337. [DOI] [PubMed] [Google Scholar]
- Faraci FM. Reactive oxygen species: influence on cerebral vascular tone. J Appl Physiol. 2006;100:739–743. doi: 10.1152/japplphysiol.01044.2005. [DOI] [PubMed] [Google Scholar]
- Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol. 2008;295:L979–L987. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhao X, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of ERK MAP kinase. Am J Physiol. 2009;297:H2262–H2269. doi: 10.1152/ajpheart.00817.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffroy C, Strolin Benedetti M. Monoamine oxidase and semicarbazide-sensitive amine oxidase in spontaneously hypertensive and in normotensive control rats. Life Sci. 1984;34:535–545. doi: 10.1016/0024-3205(84)90486-7. [DOI] [PubMed] [Google Scholar]
- Haeusler G. Contraction, membrane potential and calcium fluxes in rabbit pulmonary arterial muscle. Fed Proc. 1983;42:263–268. [PubMed] [Google Scholar]
- Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Kempthorne O. Design and analysis of experiments. In: Hinkelmann K, Kempthorne O, editors. Advanced Experimental Design. II. New York: John Wiley & Sons, Inc.; 2005. pp. 1–70. [Google Scholar]
- Kalaria RN, Harik SI. Blood-brain barrier monoamine oxidase: enzyme characterization in cerebral microvessels and other tissues from six mammalian species, including human. J Neurochem. 1987;49:856–864. doi: 10.1111/j.1471-4159.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Kitazono T, Nagao T, Fujishima M, Ibayashi S. Role of Ca2+-activated K+ channels in the regulation of basilar arterial tone in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2002;29:575–581. doi: 10.1046/j.1440-1681.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- Lai FM, Spector S. Monoamine oxidase activity in tissues of spontaneously hypertensive rats. Experientia. 1978;34:1060. doi: 10.1007/BF01915347. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones AW, Sturek M. Increased barium influx and potassium current in stroke-prone spontaneously hypertensive rats. Hypertension. 1994;23:1091–1095. doi: 10.1161/01.hyp.23.6.1091. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones AW, Sturek M. Ca2+-dependent K+ current in arterial smooth muscle cells from aldosterone-salt hypertensive rats. Am J Physiol. 1995;269:H1246–H1257. doi: 10.1152/ajpheart.1995.269.4.H1246. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-activated K+ channels in the cerebral microcirculation of genetically hypertensive rats. Evidence for their protection against cerebral vasospasm. Circ Res. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- MacCauley RB. Monoamine oxidase and the pharmacology of the monoamine oxidase inhibitors. In: Palmer GC, editor. Neuropharmacology of Central Nervous System and Behavioural Disorders. New York: Academic; 1980. pp. 94–107. [Google Scholar]
- Martens JR, Gelband CH. Alterations in rat interlobular artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res. 1996;79:295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Lynch CM, Dayal S, Lentz SR, Faraci FM. Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 2009;29:1130–1137. doi: 10.1038/jcbfm.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial muscle tone. Am J Physiol. 1990;259:C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Ni W, Lookingland K, Watts SW. Arterial 5-hydroxytryptamine transporter function is impaired in deoxycorticosterone acetate and Nω-nitro-L-arginine but not spontaneously hypertensive rats. Hypertension. 2006;48:134–140. doi: 10.1161/01.HYP.0000225754.15146.dd. [DOI] [PubMed] [Google Scholar]
- Nishimura Y. Characterization of 5-hydroxytryptamine receptors mediating contractions in basilar arteries from stroke-prone spontaneously hypertensive rats. Br J Pharmacol. 1996;117:1325–1333. doi: 10.1111/j.1476-5381.1996.tb16732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Suzuki A. Enhanced contractile responses mediated by different 5-HT receptor subtypes in basilar arteries, superior mesenteric arteries and thoracic aortas from stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:99–101. doi: 10.1111/j.1440-1681.1995.tb02986.x. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Critz SD, Cohen MV, Downey JM. Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. J Mol Cell Cardiol. 2003;35:653–660. doi: 10.1016/s0022-2828(03)00083-x. [DOI] [PubMed] [Google Scholar]
- Paterno R, Heistad DD, Faraci FM. Functional activity of Ca2+-depednent K+ channels is increased in basilar artery during chronic hypertension. Am J Physiol. 1997;272:H1287–H1291. doi: 10.1152/ajpheart.1997.272.3.H1287. [DOI] [PubMed] [Google Scholar]
- Philipp S, Cui L, Ludolph B, Kelm M, Schulz R, Cohen MV, et al. Desferoxamine and ethyl-3,4-dihydroxybenzoate protect myocardium by activating NOS and generating mitochondrial ROS. Am J Physiol. 2006;290:H450–H457. doi: 10.1152/ajpheart.00472.2005. [DOI] [PubMed] [Google Scholar]
- Rusch NJ, Runnells AM. Remission of high blood pressure reveres arterial potassium channels alterations. Hypertension. 1994;23:941–945. doi: 10.1161/01.hyp.23.6.941. [DOI] [PubMed] [Google Scholar]
- Rusch NJ, De Lucena RG, Wooldridge TA, England SK, Cowley AW. A Ca2+-dependent K+ currents is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992;19:301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- Sagin FG, Sozmen EY, Ersoz B, Mentes G. Link between monoamine oxidase and nitric oxide. Neurotoxicology. 2004;25:91–99. doi: 10.1016/S0161-813X(03)00089-5. [DOI] [PubMed] [Google Scholar]
- Salomone S, Morel N, Godfraind T. Role of nitric oxide in the contractile response to 5-hydroxytryptamine of the basilar artery from Wistar Kyoto and stroke-prone rats. Br J Pharmacol. 1997;121:1051–1058. doi: 10.1038/sj.bjp.0701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmuck K, Ullmer C, Kalkman HO, Probst A, Lubbert H. Activation of 5-HT2B receptors: an early step in the generation of migraine headache. Eur J Neurosci. 1996;8:959–967. doi: 10.1111/j.1460-9568.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Schoeffter P, Hoyer D. 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J Pharmacol Exp Ther. 1990;252:387–395. [PubMed] [Google Scholar]
- Seto SW, Kwan YW, Ngai SM. Modulatory effect of interleukin-1β on rat isolated basilar artery contraction. Eur J Pharmacol. 2006;531:238–245. doi: 10.1016/j.ejphar.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Seto SW, Au AL, Lam TY, Chim SS, Lee SM, Wan S, et al. Modulation by simvastatin of iberiotoxin-sensitive, Ca2+-activated K+ channels of porcine coronary artery smooth muscle cells. Br J Pharmacol. 2007;151:987–997. doi: 10.1038/sj.bjp.0707327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu K, Akiguchi I, Oka N, Kamo H, Matsubayashi K, Kameyama M, et al. Monoamine oxidase-containing nerve fibers in the major cerebral arteries of rats. Brain Res. 1989;497:21–29. doi: 10.1016/0006-8993(89)90965-7. [DOI] [PubMed] [Google Scholar]
- Small R, Macarak E, Fisher AB. Production of 5-hydroxyindoleacetic acid from serotonin by cultured endothelial cells. J Cell Physiol. 1977;90:225–231. doi: 10.1002/jcp.1040900208. [DOI] [PubMed] [Google Scholar]
- Soto MA, González C, Lissi E, Vergara C, Latorre R. Ca2+-activated K+ channel inhibition by reactive oxygen species. Am J Physiol. 2002;282:C461–C471. doi: 10.1152/ajpcell.00167.2001. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol. 2003;285:H2255–H2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- Thompson LP, Webb RC. Vascular responsiveness to serotonin metabolites in mineralocorticoid hypertension. Hypertension. 1987;9:277–281. doi: 10.1161/01.hyp.9.3.277. [DOI] [PubMed] [Google Scholar]
- Trapani A, Matsuki N, Abel PW, Hermsmeyer K. Norepinephrine produces tension through electromechanical coupling in rabbit ear artery. Eur J Pharmacol. 1981;72:87–91. doi: 10.1016/0014-2999(81)90301-0. [DOI] [PubMed] [Google Scholar]
- Ullmer C, Boddeke HGWM, Schmuck K, Lubbert H. 5-HT2B receptor-mediated calcium release from ryanodine-sensitive intracellular stores in human pulmonary artery endothelial cells. Br J Pharmacol. 1996;117:1081–1088. doi: 10.1111/j.1476-5381.1996.tb16700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulus IH, Maher TJ, Wurtman RJ. Characterization of phentermine and related compounds as monoamine oxidase (MAO) inhibitors. Biochem Pharmacol. 2000;59:1611–1621. doi: 10.1016/s0006-2952(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. 5-Hydroxytryptamine and vascular disease. Fed Proc. 1983;42:233–237. [PubMed] [Google Scholar]
- Voldby B, Engbaek F, Enevoldsen EM. CSF serotonin concentration and cerebral arterial spasm in patients with ruptured intracranial aneurysm. Stroke. 1982;13:184–189. doi: 10.1161/01.str.13.2.184. [DOI] [PubMed] [Google Scholar]
- Wu BN, Tu HF, Welsh DG, Chen IJ. KMUP-1 activates BKCa channels in basilar artery myocytes via cyclic nucleotide-dependent protein kinases. Br J Pharmacol. 2005;146:862–871. doi: 10.1038/sj.bjp.0706387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZW, Zheng T, Wang J, Zhang A, Altura BT, Altura BM. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signalling pathways. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:646–653. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]
- Yokota Y, Imaizumi Y, Asano M, Matsuda T, Watanabe M. Endothelium-derived relaxing factor released by 5-HT: distinct from nitric oxide in basilar arteries of normotensive and hypertensive rats. Br J Pharmacol. 1994;113:324–330. doi: 10.1111/j.1476-5381.1994.tb16212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Finberg JP. New directions in monoamine oxidase A and B selective inhibitors and substrates. Biochem Pharmacol. 1991;41:155–162. doi: 10.1016/0006-2952(91)90471-g. [DOI] [PubMed] [Google Scholar]
- Zhang G, Xu R, Heinemann SH, Hoshi T. Cysteine oxidation and rundown of large-conductance Ca2+-dependent K+ channels. Biochem Biophys Res Commun. 2006;342:1389–1395. doi: 10.1016/j.bbrc.2006.02.079. [DOI] [PubMed] [Google Scholar]


