Abstract
Little is known about the role of GABAB receptors (GABABRs) in the maintenance of memories associated with using abused substances. We have embarked on a series of studies designed to determine if enhancing the efficacy of GABA-occupied GABABRs with positive allosteric modulators (PAMs) can negate previously established conditioned place preference (CPP) induced by methamphetamine. In the current study, we evaluated the effects of acute administration of GABABR PAMs, GS39783 and CGP7930. We determined that post-conditioning treatments with these PAMs, administered in the home cage, blocked the subsequent expression of methamphetamine-induced CPP. These data indicate that selectively augmenting GABA-occupied GABABR signaling is sufficient to reduce memory maintenance and/or the salience of contextual cues previously associated with methamphetamine.
Keywords: Associative learning, Memory maintenance, Addiction, Rat, Psychostimulant, GABAB receptor, GS39783, CGP7930, Drug-seeking, Reward
1. Introduction
Cues associated with the use of abused substances can activate limbic brain regions [1,2] and elicit drug-craving and drug-seeking [3,4]. This powerful, long lasting associative learning process, and the resulting salience attributed to drug-associated cues, can be studied in laboratory rats using conditioned place preference (CPP) [5–7]. Methamphetamine is a potent psychostimulant that is highly abused throughout the world [8]; thus, the current study evaluated CPP induced by the psychostimulant, methamphetamine (Meth).
There is no FDA-approved pharmacotherapy for psychostimulant addiction. However, literature suggests that the GABAB receptor (GABABR) system is a viable target [9,10]. The GABABR system is down-regulated by repeated psychostimulant administration [11,12]. Accordingly, administration of the GABABR agonist baclofen inhibits the development and expression of CPP induced by Meth [13] as well as the development and expression of amphetamine-induced motor sensitization [14,15]. Baclofen also decreases several aspects of Meth [16], amphetamine [17] and cocaine self-administration [18] in rodents and cocaine-primed reinstatement of cocaine-seeking in baboons [19]. These data demonstrate that baclofen prevents the development of psychostimulant-induced behaviors and subsequent stimulant-seeking. Imaging studies in stimulant-abusing humans indicate that baclofen blunts the limbic activation associated with visual drug cues [9]. Clinical studies also demonstrate the efficacy of baclofen to reduce cocaine craving [20], and decrease cocaine [21] and Meth use [22]. These studies indicate that augmenting GABABR signaling may have beneficial effects on the effects of stimulants. While the use of GABABR agonists as a therapy for addiction has considerable support [10,23], the side effects associated with agonist administration (e.g., sedation and motor impairment) present significant drawbacks [20–22,24,25]. These undesirable effects reflect the fact that direct acting GABABR agonists bind to and activate all GABABRs in the brain regardless of endogenous GABAergic tone. Positive allosteric modulators (PAMs) of the GABABR provide an alternative means to augment GABAergic signaling. GABABR PAMs do not directly activate receptors; instead they increase the efficacy of endogenous GABA [26–29]. Moreover, while GABABRs are located throughout the brain, expression levels differ greatly among brain regions with high expression levels found in regions important for reward and motivated behavior (e.g., ventral tegmental area and thalamus) [30]. These characteristics contribute to the ability of PAMs to maintain temporal and regional specificity compared to GABABR agonists.
Recent reports reveal the ability of GABABR PAMs to reduce the development and expression of reward-mediated behaviors. The PAM CGP7930 reduces cocaine self-administration [18,31] and cocaine- and cue-induced reinstatement of cocaine-seeking [32]. The PAM GS39783 blunts locomotion induced by acute cocaine, blocks the development of cocaine-induced motor sensitization, and normalizes molecular adaptations resulting from repeated cocaine administration [33]. These studies demonstrate the capacity of GABABR PAMs to modulate cocaine-mediated effects; yet, the capacity of PAMs to modify the maintenance of Meth-induced behaviors is unknown.
Administration of GABAergic ligands during the development and expression phases of reward-mediated behaviors provides insight into the role of the GABABR system in these behaviors; however, there are limited clinical applications for these treatment protocols. A more relevant approach is to administer a potential therapy after the brain and behavioral adaptations have taken place (i.e., to target the maintenance of drug-associated memories). Using this treatment strategy, baclofen disrupts the maintenance of previously established amphetamine-induced motor sensitization [14]. We have revealed that a similar strategy with the atypical antidepressant mirtazapine can disrupt the maintenance of Meth-induced CPP [34] as well as Meth-induced motor and cellular sensitization [35]. These studies illustrate the feasibility of post-conditioning treatments to nullify previously established Meth-induced behavioral effects. The current study used this approach to determine if GABABR PAMs would inhibit the expression of Meth-induced CPP when administered after the development of the behavior.
The GABA system undergoes many time- and region-specific adaptations following psychostimulant administration, including changes in GABA turnover and receptor-mediated function. These adaptations are influenced by neuronal phenotype, number of drug exposures, and withdrawal duration, all of which will likely influence the effectiveness of any particular therapeutic intervention. We are interested in determining the influence of withdrawal time (early vs. protracted withdrawal phase) and the treatment duration requirements for GABABR ligands to alter the maintenance of Meth-induced CPP [36]. During early withdrawal (<10 days) the GABABR system is down-regulated; including decreases in receptor expression [11,37], uncoupling of the GABABR from the G-protein [12], and increases in extracellular GABA concentrations [38,39]. Accordingly, in the current study, the GABABR PAMs GS39783, and CGP7930 were administered for 2 days during the early withdrawal phase after Meth conditioning and we hypothesized that augmenting GABABR signaling during this phase of maintenance would reduce the salience of Meth-associated cues, thereby inhibiting the maintenance and subsequent expression of Meth-induced CPP.
2. Experimental procedures
2.1. Animals
Male Sprague–Dawley rats (n = 59, Harlan, Indianapolis, IN) weighing 250–300 g at the start of the study were acclimated to the vivarium for at least 1 week prior to the onset of the experiments. Rats were housed in pairs in a climate-controlled environment on a 12 h light/dark cycle and allowed ad libitum access to food and water. Cage mates were given identical pharmacological treatments. Housing facilities at Rush University are accredited through the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments were carried out in accordance with the conditions set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and with the approval of the local Institutional Animal Care and Use Committee.
2.2. Drugs
Conditioning. (+)Methamphetamine HCl (Sigma–Aldrich, St. Louis, MO) was dissolved in 0.9% sterile saline and administrated as 0.1, 0.3, or 1 mg/ml/kg. The saline vehicle for Meth was administered as 1 ml/kg. Home cage injections. To demonstrate that effects of PAM administration could not be attributed to a single chemical entity, two different PAM ligands were tested, GS39783 and CGP7930 (gifts from Novartis Pharma AG, Basel, Switzerland). The PAM dose was selected based on published observations that when tested as 3, 10, and 30 mg/kg i.p., the 30 mg dose of GS39783 or CGP7930 successfully reduces the break point of cocaine self-administration when administered 10 min prior to the session [31]. Thus, we tested the 30 mg/kg dose in the current study. The PAMs were dissolved in 10% propylene glycol sterile water solution as 30 mg/2 ml. All injections were given intraperitoneally (i.p.).
2.3. Apparatus
The apparatus used to monitor motor activity and place preference consisted of three chambers divided by opaque Plexiglas sliding doors (AccuScan Instruments, Inc., Columbus, OH); two large conditioning chambers (25 cm × 30 cm × 30 cm) separated by a small center chamber (13 cm × 30 cm × 30 cm). Each chamber had distinct visual and tactile cues. One conditioning chamber had vertical stripes on the walls and the other had horizontal wall stripes. Each chamber contained either a textured floor with a smooth plastic rectangle glued to the center of the floor or an alternately textured floor with a six-well overturned paint dish glued to the center of the floor. The two types of floors were randomly assigned to each conditioning chamber. The center chamber contained solid white walls and a smooth slightly raised platform floor. Motor activity and time spent in each chamber was monitored via two sets of photobeams (24 in the horizontal orientation and 12 vertical).
2.4. Conditioned place preference
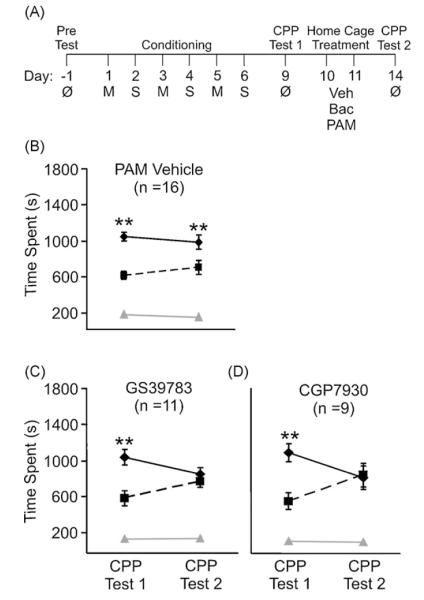
The behavioral testing room was dimly lit (54–108 lx) with white (white noise generator, San Diego Instruments, San Diego, CA) noise continuously present. The rats were transported from the vivarium to the test room (located across the hall, in the same animal facility suite) at least 30 min prior to the start of the experiment. The protocol consisted of the following phases (Fig. 1A): Pre-Test, conditioning, CPP Test 1 to verify the development of CPP, home cage intervening treatments, and CPP Test 2.
Fig. 1.
Meth-induced CPP is inhibited by post-conditioning administration of the PAMs GS39783 and CGP7930. (A) Illustration of treatment protocol. A drug-free Pre-Test was conducted and rats were assigned to receive Meth (1 mg/kg, i.p.) in the initially non-preferred chamber. Conditioning occurred on days 1–6. A drug-free CPP Test was conducted on day 9 to verify the development of CPP (CPP Test 1). Vehicle, baclofen, or a PAM was administered in the home cage on days 10 and 11. A final drug-free CPP Test (CPP Test 2) was conducted on day 14 to determine the capacity of GABAergic ligands to influence the expression of Meth-induced CPP. (B) Rats assigned to the PAM vehicle (10% propylene glycol) group (n = 14) expressed CPP on both Test days (CPP Tests 1 and 2). Rats administered (C) GS39783 (n = 11) or (D) CGP7930 (n = 9) as an intervening treatment did not express a preference for the Meth-paired chamber on CPP Test 2. Post hoc Newman–Keuls test was used to determine between chamber differences (center chamber not included for statistical analysis), **p < 0.01. Solid line, time spent in the Meth-paired chamber; dashed line, time spent in the saline-paired chamber; grey line, time spent in the center chamber. M, methamphetamine (1 mg/kg); S, saline (1 mg/kg); Veh, vehicle (1 ml/kg); Bac, baclofen (2 mg/kg); PAM, positive allosteric modulator: GS39783 (30 mg/kg), CGP7930 (30 mg/kg).
A 30 min Pre-Test was conducted wherein rats were placed into the center chamber and doors were immediately removed allowing free access to the entire CPP box. While the visual and tactile cues were randomized in each conditioning chamber, individual rats spent an average of 64% of total Pre-Test time in one conditioning chamber and 29% in the other chamber with the remaining 7% being spent in the center chamber. For conditioning, rats were administered Meth in the chamber in which they spent the least amount of time during the Pre-Test.
Meth conditioning occurred every other day interposed by saline conditioning days for a total of 6 days. To do so, immediately after each injection the rats were placed into the appropriate chamber for 45 min. 3 days following the last conditioning session (i.e., day 9) rats were given a 30 min drug-free CPP Test. To establish the Meth dose to be used for PAM evaluations, a pilot Meth dose–response was conducted with 0.1, 0.3, and 1 mg/kg (n = 6 in each group). The most robust CPP was obtained with 1 mg/kg. Comparing time spent (s) in the saline-paired vs. time spent in the Meth-paired chamber for each dose resulted in the following: 0.1 mg/kg, 717 ± 189 vs. 949 ± 184; 0.3 mg/kg, 610 ± 107 vs. 1047 ± 120; and for 1 mg/kg, 326 ± 50 vs. 1327 ± 55. A two-way mixed factor ANOVA revealed an effect of chamber (F(1,35) = 27.701, p < 0.0001) and chamber × dose interaction (F(2,35) = 4.723, p = 0.017) but no effect of dose (F(1,35) = 0.001, p = 0.999). A post hoc Newman–Keuls test demonstrated significant CPP (i.e., a significantly greater amount of time spent in Meth-paired compared to the saline-paired chamber) for those rats conditioned with 1 mg/kg Meth. Thus, we selected the 1 mg/kg dose to induce Meth CPP and subsequently evaluated the ability of post-conditioning administration of GABABR ligands to disrupt maintenance of place preference memory. For the current study, the order of Meth- vs. saline-pairings was randomized to ensure that the order of conditioning did not influence behavioral outcomes; Meth conditioning occurred on days 1, 3, and 5 or 2, 4, and 6 and saline conditioning occurred on days 2, 4, and 6 or 1, 3, and 5. As the order or Meth or saline-pairings did not influence CPP outcomes (time spent in the Meth-paired chamber was not different between those that received Meth on days 1, 3, and 5 (time spent = 887 ± 98 s, n = 8) vs. those that received Meth on days 2, 4, and 6 (time spent = 929 ± 95 s, n = 8)) (Student’s t-test, p > 0.05) these data were pooled for analysis. A 30 min drug-free CPP Test was performed on day 9, (termed CPP Test 1) to confirm that the preference developed as described previously. Rats that did not increase time spent in the Meth-paired chamber on CPP Test 1 compared to the same chamber during the Pre-Test by at least 10% (180 s) were excluded from subsequent evaluations of PAM effects (2 rats were excluded based on this criterion). This culling procedure, as previously shown by others [40] helps assure that only those rats that clearly acquired the task were used to determine the potential for GABABR ligands to subsequently reduce the acquired preference. Based on the results generated during CPP Test 1, rats were assigned to one of the following once-daily home cage treatments (days 10 and 11) such that the expression of the preference was approximately equal across vehicle, GS39783, and CGP7930 treatment groups. Selection of the early withdrawal time for PAM treatments was based on prior demonstrations that the GABABR system is down-regulated within the first few days of withdrawal from repeated stimulant exposure [11,12]. We considered that if this adaptation contributed to memory maintenance, then augmenting GABABR signaling during this withdrawal time may disrupt these memories. 3 days after the second, and last, home cage treatment (i.e., day 14) rats were tested for the expression of CPP in a drug-free state (termed CPP Test 2). The presence of PAMs during the CPP Test may influence the expression of CPP, thus to allow the GABABR PAMs to be cleared from the rat prior to CPP testing, a 3-day drug-free period was imposed between the last PAM injection and testing for preference. This approach allowed us to interpret the effects of GABABR ligands in terms of memory maintenance rather than an effect on expression.
2.5. Statistical analysis
CPP was defined as spending significantly more time in the Meth-paired vs. saline-paired chamber. This was accomplished by two-way repeated measures ANOVA (factors were chamber and CPP Test) followed by post hoc Newman–Keuls for between chamber differences for each test. All data are shown as mean ± standard error of the mean (SEM). Statistical outliers were determined as those rats that spent greater than two standard deviations above or below the mean time spent in any chamber.
3. Results
The 6-day conditioning protocol resulted in a significant preference for the Meth-paired chamber. As a group, the 41 rats tested expressed CPP during Test 1 (day 9) (time spent Meth-paired chamber, 1024 ± 41 s; time spent saline-paired chamber, 620 ± 40 s; paired t-test: p < 0.0001). 2 rats did not demonstrate a 10% (180 s) increase in time spent from the Pre-Test to CPP Test 1 thus they were not tested further. This culling procedure helped assure that only those rats that clearly acquired the task were used to determine the potential for GABABR ligands to subsequently reduce the acquired preference (see Section 2). Post-conditioning administration of the vehicle did not impact the ability of drug-free rats to express a preference for the Meth-paired chamber (Fig. 1B; PAM Vehicle (n = 16): chamber, F(1,30) = 22.935, p < 0.0001; Test, F(1,30) = 0.077, p = 0.784; interaction, F(1,30) = 0.980, p = 0.330). This demonstrated that the acquired preference was not diminished by repeated CPP testing or with the intervening home cage injections. In contrast, rats administered GABABR PAMS GS39783 and CGP7930 nullified previously established preference; i.e., the preference for the Meth-paired chamber observed on CPP Test 1 was no longer evident on CPP Test 2 (Fig. 1C and D; GS39783 (n = 11): chamber, F(1,20) = 11.141, p = 0.003; Test, F(1,20) = 0.0002, p = 0.989; interaction, F(1,20) = 6.947, p = 0.016. CGP7930 (n = 9): chamber, F(1,16) = 3.506, p = 0.080; Test, F(1,16) = 0.002, p = 0.965; interaction, F(1,16) = 9.108, p = 0.008). Although the effect of chamber with the rmANOVA was significant only for the GS39783, and not for CGP7930, both groups demonstrated a significant interaction as well as post hoc significance for CPP Test 1 comparing time spent in the saline vs. Meth-paired chamber, which is not apparent during CPP Test 2. Throughout each Test (Pre-Test, CPP Test 1, CPP Test 2) time spent in the middle chamber did not significantly change in any treatment group (Fig. 1B–D). Thus, a dose of GS39783 and CGP7930 which effectively reduces cocaine-induced behaviors including self-administration [16,31], reinstatement of self-administration [32], acute increases in locomotion, development of motor sensitization, and molecular adaptations resulting from repeated administration [33] also inhibits the maintenance of Meth-induced CPP when administered in the home cage.
In addition, we have determined that the inhibitory effect of the PAMs on the maintenance of Meth-induced CPP is not the consequence of altering motor activity on the Test day. During CPP Test 2 (i.e., 3 days after the last home cage PAM injection), total distance travelled is not different between treatment groups (Vehicle, 1581 ± 93; GS39783, 1401 ± 71; CGP7930, 1650 ± 196; one-way ANOVA, p = 0.349).
4. Discussion
This study revealed that two chemically distinct GABABR PAMs, GS39783 and CGP7930, administered to rats in the neutral environment of the home cage, were sufficient to nullify the previously expressed preference for the Meth-paired chamber. At the dose selected, GS39783 and CGP7930 are thought to act only at GABABRs that are occupied by endogenous GABA [27,28]. Therefore, these findings indicate that augmenting the efficacy of occupied GABABRs during early phases of withdrawal is sufficient to disrupt the maintenance of the acquired salience for the Meth-associated context. The ability of a GABABR PAM to nullify robust Meth-induced CPP the 1 mg/kg Meth dose would provide a more compelling argument for the further consideration of the PAMs as potential Meth abuse therapy, thus we evaluated CPP induced by 1 mg/kg Meth and subsequently evaluated the ability of post-conditioning administration of GABABR ligands to disrupt maintenance of place preference memory.
The current study focused on the short-term maintenance of Meth-associated memories, a phase of mnemonic processing that is not widely studied with regard to GABABR influences. In contrast, there is ample literature demonstrating that administration of the GABABR agonist baclofen alters other mnemonic processes including acquisition [41,42], consolidation [43–46], and expression [13,47,48]. Likewise, there are numerous studies demonstrating the efficacy of pharmacological augmentation of GABABR signaling via administration of GS39783, CGP7930, as well as baclofen to modulate the development and expression of behaviors induced by psychostimulants when administered during conditioning, or within 30 min prior to testing [13,15,17–19,31–33,49]. However, to date only Bartoletti et al. [14] have demonstrated efficacy of a GABABR ligand to modify the maintenance of a previously established psychostimulant-induced behavior. These authors report that the maintenance of amphetamine-induced motor sensitization is blunted when 10 administrations of 2 mg/kg baclofen were initiated 36 days after the behavior developed and terminated 20 days prior to the amphetamine challenge. Ongoing studies with a non-selective PAM, fendiline, have indicated that 10 once-daily home cage treatments initiated 4 days after conditioning disrupt the maintenance of Meth-induced CPP that is normally expressed 16 days after the last conditioning session [36]. In sum, our research efforts expanded the literature on direct GABABR agonists, by evaluating GABABR PAM influences on the maintenance of place preference memory induced by Meth.
PAMs act by positively modulating GABA-occupied receptors. The fact that GS39783 and CGP7930 inhibited the maintenance of previously established CPP suggests that during the early withdrawal period, regions that are necessary for the maintenance of Meth-induced CPP are sensitive to disruption by augmenting GABABR signaling which suggests that the sensitive region(s) express GABABRs and have endogenously released GABA. Ongoing studies with Meth-treated rats in our laboratory have not revealed changes in GABABR protein levels or surface expression in several limbic brain regions (unpublished data). Augmenting GABA receptor signaling in areas where GABA is endogenously released in the current study modifying the maintenance of Meth-induced CPP is consistent with the recent demonstration that increasing GABA levels via reducing its metabolism (i.e., with gamma vinyl GABA, an irreversible inhibitor of GABA transaminase), inhibits the expression of Meth-primed reinstatement of CPP (administered 2.5 h prior to the Meth challenge) [50]. Thus, it may be that augmenting GABABR signaling in brain regions where GABA is tonically released is particularly important for disrupting memory maintenance.
Several laboratories report region-specific changes GABABR expression, receptor-mediated function, and GABA turnover during the early days following repeated psychostimulant exposure which suggest a net down-regulation of the GABABR system. GABABRs are reduced throughout the brain of rats after cocaine self-administration [11] and in the ventral tegmental area a decrease in the functional coupling of GABABRs is observed [12]. Changes such as these may contribute to the hyper-excitable state of the brain observed during psychostimulant withdrawal [51]. Additionally increased tone occurs within the first 7 days of withdrawal from repeated psychostimulant withdrawal including increased extracellular GABA levels in the medial prefrontal cortex after repeated cocaine administration [38] and enhanced substantia nigra and striatum K+-evoked GABA release after repeated Meth administration [39] which may be a compensatory response for the down-regulated GABABR expression and/or function. Behavioral observations made in the current study suggest that GABA-occupied GABABRs located in regions critical for the maintenance of Meth-induced CPP are selectively enhanced by PAMs.
In summary, we have identified two GABABR PAMs, GS39783 and CGP7930, which inhibit mnemonic processes necessary to maintain and subsequently express Meth-induced CPP when administered during the early withdrawal phase. This information allows us to infer that GABABR expressing neurons in regions that have endogenous GABAergic tone are critical for the maintenance of the associative memory that forms during Meth-induced CPP. Thus, GABABR PAMs may be a viable therapeutic alternative to direct acting GABABR agonists for the treatment of psychostimulant addiction.
Acknowledgements
Funding for this study was provided by USPHSGs DA015760 to TCN, DA021475 to RMV and TCN, and DA023306 to AAH and TCN. The authors thank Novartis Pharma AG, Basel, Switzerland for the generous gifts of GS39783 and CGP7930. Gratitude is also extended to Dr. John Cryan for his helpful comments on this project.
References
- [1].Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, et al. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–15. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- [4].Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–9. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- [5].Childs E, deWit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–4. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–72. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- [7].Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- [8].United Nations Office on Drugs Crime . World drug report 2009. United Nations Office on Drugs and Crime; Vienna, Austria: 2009. [Google Scholar]
- [9].Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–84. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- [10].Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–27. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Frankowska M, Wydra K, Faron-Gorecka A, Zaniewska M, Kusmider M, Dziedzicka-Wasylewska M, et al. Neuroadaptive changes in the rat brain GABA(B) receptors after withdrawal from cocaine self-administration. Eur J Pharmacol. 2008;599:58–64. doi: 10.1016/j.ejphar.2008.09.018. [DOI] [PubMed] [Google Scholar]
- [12].Kushner SA, Unterwald EM. Chronic cocaine administration decreases the functional coupling of GABA(B) receptors in the rat ventral tegmental area as measured by baclofen-stimulated 35S-GTPgammaS binding. Life Sci. 2001;69:1093–102. doi: 10.1016/s0024-3205(01)01203-6. [DOI] [PubMed] [Google Scholar]
- [13].Li SM, Yin LL, Ren YH, Pan LS, Zheng JW. GABA(B) receptor agonist baclofen attenuates the development and expression of d-methamphetamine-induced place preference in rats. Life Sci. 2001;70:349–56. doi: 10.1016/s0024-3205(01)01397-2. [DOI] [PubMed] [Google Scholar]
- [14].Bartoletti M, Gubellini C, Ricci F, Gaiardi M. The GABAB agonist baclofen blocks the expression of sensitisation to the locomotor stimulant effect of amphetamine. Behav Pharmacol. 2004;15:397–401. doi: 10.1097/00008877-200409000-00014. [DOI] [PubMed] [Google Scholar]
- [15].Bartoletti M, Gubellini C, Ricci F, Gaiardi M. Baclofen blocks the development of sensitization to the locomotor stimulant effect of amphetamine. Behav Pharmacol. 2005;16:553–8. doi: 10.1097/01.fbp.0000179279.98029.e9. [DOI] [PubMed] [Google Scholar]
- [16].Ranaldi R, Poeggel K. Baclofen decreases methamphetamine self-administration in rats. Neuroreport. 2002;13:1107–10. doi: 10.1097/00001756-200207020-00007. [DOI] [PubMed] [Google Scholar]
- [17].Brebner K, Ahn S, Phillips AG. Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology (Berl) 2005;177:409–17. doi: 10.1007/s00213-004-1968-6. [DOI] [PubMed] [Google Scholar]
- [18].Filip M, Frankowska M, Przegalinski E. Effects of GABA(B) receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol. 2007;574:148–57. doi: 10.1016/j.ejphar.2007.07.048. [DOI] [PubMed] [Google Scholar]
- [19].Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABA B receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007;89:206–13. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ling W, Shoptaw S, Majewska D. Baclofen as a cocaine anti-craving medication: a preliminary clinical study. Neuropsychopharmacology. 1998;18:403–4. doi: 10.1016/S0893-133X(97)00128-0. [DOI] [PubMed] [Google Scholar]
- [21].Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, et al. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003;64:1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- [22].Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, et al. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85:177–84. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- [23].Rose ME, Grant JE. Pharmacotherapy for methamphetamine dependence: a review of the pathophysiology of methamphetamine addiction and the the-oretical basis and efficacy of pharmacotherapeutic interventions. Ann Clin Psychiatry. 2008;20:145–55. doi: 10.1080/10401230802177656. [DOI] [PubMed] [Google Scholar]
- [24].Cryan JF, Kelly PH, Chaperon F, Gentsch C, Mombereau C, Lingenhoehl K, et al. Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N,N’-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther. 2004;310:952–63. doi: 10.1124/jpet.104.066753. [DOI] [PubMed] [Google Scholar]
- [25].Jacobson LH, Cryan JF. Differential sensitivity to the motor and hypothermic effects of the GABA B receptor agonist baclofen in various mouse strains. Psychopharmacology (Berl) 2005;179:688–99. doi: 10.1007/s00213-004-2086-1. [DOI] [PubMed] [Google Scholar]
- [26].Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–67. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- [27].Urwyler S, Mosbacher J, Lingenhoehl K, Heid J, Hofstetter K, Froestl W, et al. Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501. Mol Pharmacol. 2001;60:963–71. [PubMed] [Google Scholar]
- [28].Gjoni T, Desrayaud S, Imobersteg S, Urwyler S. The positive allosteric modulator GS39783 enhances GABA(B) receptor-mediated inhibition of cyclic AMP formation in rat striatum in vivo. J Neurochem. 2006;96:1416–22. doi: 10.1111/j.1471-4159.2006.03660.x. [DOI] [PubMed] [Google Scholar]
- [29].Urwyler S, Gjoni T, Koljatic J, Dupuis DS. Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS39783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology. 2005;48:343–53. doi: 10.1016/j.neuropharm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- [30].Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohisto-chemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [31].Smith MA, Yancey DL, Morgan D, Liu Y, Froestl W, Roberts DC. Effects of positive allosteric modulators of the GABAB receptor on cocaine self-administration in rats. Psychopharmacology (Berl) 2004;173:105–11. doi: 10.1007/s00213-003-1706-5. [DOI] [PubMed] [Google Scholar]
- [32].Filip M, Frankowska M. Effects of GABA(B) receptor agents on cocaine priming, discrete contextual cue and food induced relapses. Eur J Pharmacol. 2007;571:166–73. doi: 10.1016/j.ejphar.2007.05.069. [DOI] [PubMed] [Google Scholar]
- [33].Lhuillier L, Mombereau C, Cryan JF, Kaupmann K. GABA(B) receptor-positive modulation decreases selective molecular and behavioral effects of cocaine. Neuropsychopharmacology. 2007;32:388–98. doi: 10.1038/sj.npp.1301102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herrold AA, Shen F, Graham MP, Harper LK, Specio SE, Tedford CE, et al. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009;99:231–9. doi: 10.1016/j.drugalcdep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- [35].McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, et al. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86:55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- [36].Riddle JL, Voigt RM, Herrold AA, Napier TC. Post-conditioning treatment with the GABAB receptor positive allosteric modulator fendiline antagonizes the expression of methamphetamine-induced conditioned placed preference. Soc Neurosci Abstr. 2008;62:20. [Google Scholar]
- [37].Frankowska M, Wydra K, Faron-Gorecka A, Zaniewska M, Kusmider M, Dziedzicka-Wasylewska M, et al. Alterations in gamma-aminobutyric acid(B) receptor binding in the rat brain after reinstatement of cocaine-seeking behavior. Pharmacol Rep. 2008;60:834–43. [PubMed] [Google Scholar]
- [38].Jayaram P, Steketee JD. Effects of cocaine-induced behavioural sensitization on GABA transmission within rat medial prefrontal cortex. Eur J Neurosci. 2005;21:2035–9. doi: 10.1111/j.1460-9568.2005.04000.x. [DOI] [PubMed] [Google Scholar]
- [39].Bustamante D, You ZB, Castel MN, Johansson S, Goiny M, Terenius L, et al. Effect of single and repeated methamphetamine treatment on neurotransmitter release in substantia nigra and neostriatum of the rat. J Neurochem. 2002;83:645–54. doi: 10.1046/j.1471-4159.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- [40].Paolone G, Botreau F, Stewart J. The facilitative effects of d-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–9. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- [41].Nakagawa Y, Ishibashi Y, Yoshii T, Tagashira E. Involvement of cholinergic systems in the deficit of place learning in Morris water maze task induced by baclofen in rats. Brain Res. 1995;683:209–14. doi: 10.1016/0006-8993(95)00302-7. [DOI] [PubMed] [Google Scholar]
- [42].McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–8. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- [43].Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–9. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- [44].Swartzwelder HS, Tilson HA, McLamb RL, Wilson WA. Baclofen disrupts passive avoidance retention in rats. Psychopharmacology (Berl) 1987;92:398–401. doi: 10.1007/BF00210851. [DOI] [PubMed] [Google Scholar]
- [45].Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharmacol. 2002;16:313–9. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]
- [46].Zarrindast MR, Shamsi T, Azarmina P, Rostami P, Shafaghi B. GABAergic system and imipramine-induced impairment of memory retention in rats. Eur Neuropsychopharmacol. 2004;14:59–64. doi: 10.1016/s0924-977x(03)00068-3. [DOI] [PubMed] [Google Scholar]
- [47].Stackman RW, Walsh TJ. Baclofen produces dose-related working memory impairments after intraseptal injection. Behav Neural Biol. 1994;61:181–5. doi: 10.1016/s0163-1047(05)80073-1. [DOI] [PubMed] [Google Scholar]
- [48].Levin ED, Weber E, Icenogle L. Baclofen interactions with nicotine in rats: effects on memory. Pharmacol Biochem Behav. 2004;79:343–8. doi: 10.1016/j.pbb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [49].Fattore L, Spano MS, Cossu G, Scherma M, Fratta W, Fadda P. Baclofen prevents drug-induced reinstatement of extinguished nicotine-seeking behaviour and nicotine place preference in rodents. Eur Neuropsychopharmacol. 2009;19:487–98. doi: 10.1016/j.euroneuro.2009.01.007. [DOI] [PubMed] [Google Scholar]
- [50].DeMarco A, Dalal RM, Pai J, Aquilina SD, Mullapudi U, Hammel C, et al. Racemic gamma vinyl-GABA (R,S-GVG) blocks methamphetamine-triggered reinstatement of conditioned place preference. Synapse. 2009;63:87–94. doi: 10.1002/syn.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hu XT. Cocaine withdrawal and neuro-adaptations in ion channel function. Mol Neurobiol. 2007;35:95–112. doi: 10.1007/BF02700626. [DOI] [PubMed] [Google Scholar]