Abstract
The relationship between cognitive processing stages and event-related potential components has been extensively researched for single components, but even the simplest task comprises multiple electrophysiological and cognitive components. Here we examined the relationship between behavioral measures and several visual evoked potentials (VEPs) related to global motion onset during a visual motion discrimination task. In addition to reaction time and accuracy, the EZ diffusion model was used to characterize elements of the decision process. Results showed that latencies, but not amplitudes, from three VEP components reliably predicted about 40% of the variance in reaction times for motion discrimination. These included the latency from stimulus motion onset to N2 onset, the latency from N2 onset to N2 peak, and the latency from the N2 peak to the peak of a late positive potential. These latencies were also able to predict the rate of information accumulation during the decision process and the duration of non-decision processes, but not the observer's threshold (boundary) for making a response. This pattern of results is consistent with an interpretation of these three latencies as reflecting a non-specific visual perceptual process, a motion-specific process, and a decision process respectively. The relationship between the earliest interval and drift rate estimated with the EZ model also supports the notion that early perceptual processing might be a constituent part of the decision process itself.
Introduction
Most models of cognitive performance characterize cognition as a set of processes or stages that operate on information. The latency of responses to stimuli has played a prominent role in testing such models, because of the presumption that the time taken to respond is a function of the timing of the stages (see Luce, 1986 for a review). For example, in a strictly serial model, the response latency is simply the sum of the processing times of each stage. In models with parallel processes, the response latency is the sum of processing along the critical path, defined as the sequence of processes that takes the longest to complete (Schweickert, 1980). More complex architectures can involve much more subtle contingencies between processing times and final response latency, but in all cases the presumption is that reaction time is determined by the underlying architecture of cognitive processes.
Event-related potentials (ERP) from the cortical electroencephalogram are usually characterized by a series of prominent deflections between a stimulus and response, which are labeled components of the ERP. Hence it is quite natural to suppose that ERPs from the electroencephalogram might reflect processing stages (Hillyard & Kutas, 1983; Hillyard & Picton, 1987). Indeed, components of the ERP can be modulated by experimental manipulations of the corresponding perceptual-cognitive process (Bahramali Gordon, & Li, 1998; Coles, 1989; Mangun & Hillyard, 1991; Miller, Ulrich, & Rinkenauer, 1999; Näätänen, Gaillard, & Mäntysalo, 1978, Osman & Moore, 1993; Walter et al., 1964). What has more rarely been attempted is to correlate the timing of ERP components directly with behavior, and to attempt to do so for multiple components. If prominent deflections of evoked potentials represent important processing stages, then the latencies of these components should not only be modulated by experimental manipulations that affect behavior, but the latencies themselves should predict the behavior.
Visual motion processing presents a special case of a perceptual decision task with well-known ERP components, the so-called motion-onset visual evoked potential (VEP; Kuba et al., 2007), as well as extensively-researched cognitive operations (Albright & Stoner, 1995; Lu & Sperling, 1995). As such, it provides a powerful opportunity to more comprehensively map multiple stages of perceptual and cognitive processing onto multiple ERP components.
While there are many models of motion perception, most agree on a broad outline where early processing precedes and sets the stage for motion-specific processing, which then feeds forward to cognitive and response processes. A typical example is the three-system model of Lu and Sperling (1995) and its first-order sub-system (van Santen & Sperling, 1984) based on the modified Reichardt detector (Reichardt, 1961). Because first-order motion (luminance-defined) is the focus of the current report, we will focus on this sub-system of the three-system model. The first stage of this model is light adaptation, in which absolute luminance levels are adjusted by local mean luminance to give contrast, which is then fed forward to the rest of the visual system. This is thought to be a retinal function (Blackwell, 1946). Contrast is then subject to gain modulation in the retina, LGN, and V1 (Dean, 1981; Sclar, Maunsell, & Lennie, 1990). First-order motion (motion defined by spatiotemporal changes in luminance) is then detected with Reichardt motion detectors, which themselves involve five stages to compare time-varying signals at adjacent points in space and emit a directionally selective response. The net motion signal is then fed forward, with added noise, to a threshold decision stage.
Motion-evoked VEPs have been extensively studied for several decades. An early positivity around 130 ms, the P1 or P100, is sometimes seen in response to motion onset but does not appear to be specific to motion processing (Bach & Ullrich, 1994; Bach & Ullrich, 1997; Kuba et al., 2007; Kubová et al., 1995). A negativity peaking around 150-200 ms, the motion-evoked N2, is found predominantly in posterior electrode sites (Kuba et al., 2007). The motion-evoked N2 is modulated by motion parameters such as coherence (Patzwahl & Zanker, 2000), and appears to be the main motion-specific ERP (Kuba et al., 2007). For the remainder of this report, the term N2 will be used exclusively to refer to this [occipital] component. The motion-evoked N2 is thought to be generated by the human MT+ complex (hMT+), a likely homologue of monkey areas MT and MST (Ahlfors et al., 1999; Duffy & Wurtz, 1995; Probst et al., 1993). The N2 peak magnitude is reduced by motion adaptation (Hoffmann, Dorn, & Bach, 1999; Müller, Göpfert, & Hartwig, 1986) and saturates at low contrast (Bach & Ullrich, 1994; Bach & Ullrich, 1997). Finally, the P2 deflection, peaking around 240 ms at more anterior sites, is more variable than N2. Its presence and peak amplitude depend on the type of motion stimulation provided, with more complex stimuli being more effective at producing it (Kuba et al., 2007).
The conceptual separation between pre-motion and motion-specific processes is supported by several lines of evidence. Retinal responses do not appear to be sensitive to motion characteristics (Bach & Hoffman, 1999), while motion-related components of the ERP are relatively insensitive to low-level stimulus features such as luminance and contrast (Bach & Ullrich, 1997; Kubova et al., 1995). In patients with Williams-Beuren Syndrome, there is a dissociation between low-level magnocellular deficits such as contrast sensitivity and motion perception performance (Castelo-Branco et al., 2007; Mendes et al., 2005).
If the N2 represents visual motion-specific processing in the human brain, then its onset and peak should logically represent important steps in motion processing. For instance, the onset of N2 could reflect the onset of motion-specific processing, while its peak might represent the end of such processing or the point at which subsequent stages (decision, motor response) begin to dominate. The interval between stimulus onset and N2 onset would then represent pre-motion processing (light adaptation, luminance detection etc.). The interval between onset and peak could likewise represent local motion information extraction and integration of motion signals. In either case, we would expect the interval from stimulus onset to N2 onset and the interval from N2 onset to N2 peak to make independent contributions to the prediction of reaction time in a motion discrimination task.
Consequently, later components of the ERP might represent subsequent stages such as decision-making and response processes. The P2 peak is not universally observed in motion tasks, and when it is present, it appears to be related to the complexity of the motion stimulus (Kuba et al., 2007). Since the decision process is presumably engaged in all motion-processing tasks, even one so simple that the P2 peak is not present, it is unlikely that the P2 is related to basic decision processes. Kuba, Kremláček, and Langrová (1998) observed a positivity at frontal sites Cz and Fz, with a latency around 392 ms, which they hypothesized might be related to post-perceptual cognitive processing. If that hypothesis is correct, then the interval between the N2 peak and this later component would presumably reflect the duration of these processes.
A review of motion models and motion onset VEPs suggests a rough mapping, with early perceptual processes (i.e., light adaptation and gain modulation) reflected by VEP components prior to the N2. Motion-specific processes (i.e., those carried out by the Reichardt detector) would be reflected by the N2 component. Later decision processes would be reflected by a post-N2 component. From this rough mapping, the following specific hypotheses can be derived:
Hypothesis 1. If the period prior to the N2 component reflects pre-motion processes such as light adaptation and gain modulation, then the duration from motion onset to the onset of the N2 should predict reaction time.
Hypothesis 2. If the N2 represents motion-specific processes, then the duration between the onset of the N2 and its peak should predict RT independently of the pre-N2 onset period.
Hypothesis 3: If a decision process subsequent to motion-specific processing exists and is reflected in a later component, the duration between this late potential and N2 peak should predict RT independently of the pre-N2 onset duration and N2 onset-N2 peak duration.
In summary, our suggested mapping between motion models and the ERP points to three epochs defined by ERP components, each of which should make an independent contribution to the prediction of response latency. The present study tests these hypotheses in the context of a two-alternative, global direction discrimination task whose performance has been shown to depend on normal processing by area MT (Pasternak & Merigan, 1994; Rudolph & Pasternak, 1999). The task uses a variant of the well-known moving random dot cinematogram (Newsome and Pare, 1988; Watamaniuk & Sekuler, 1992), with each dot's direction of motion being randomly selected at each time frame from a given range around the mean leftward or rightward direction.
While predicting response latencies is often important in testing hypothesized relationships between brain activity and perceptual-cognitive processes, RT can be quite limited by itself. Recently developed behavioral models that explicitly estimate characteristics of intermediate stages of processing can provide additional insight into the underlying processes. Therefore, we also took advantage of the opportunity to conduct a more exploratory investigation of the relationship between ERP components and one such model, the EZ diffusion model (Wakenmakers, van der Maas, & Grasman, 2007). The EZ diffusion model is a simplification of the diffusion model of Ratcliff (1978). According to the diffusion model, following target onset in a multiple-alternative, forced-choice task, evidence for one of the possible responses accumulates, subject to some degree of random drift (Figure 1). Once this accumulation process passes a boundary for one of the possible responses, that response is initiated. Performance is therefore a function of the rate of information accumulation (drift rate), the distance of the boundary for the correct response from the starting location of the diffusion process (boundary separation), and perceptual and response factors that are combined in the model into so-called non-decision time (NDT). In addition to these three parameters of the model, estimates can be obtained of the variability in drift rate, mean and range of the starting point of the accumulation process relative to the boundary, and the range of NDT. The EZ model assumes that the starting point of the accumulation process relative to the boundary is equal for all decisions (i.e., there is no appreciable bias on the part of the observer), and there is no variability in drift rate, starting point, or NDT from trial to trial (Wakenmakers, van der Maas, & Grasman, 2007). These assumptions allow for explicit solutions of estimators for the model parameters, whereas the full diffusion model requires iterative estimation methods (Ratcliff & Tuerlinkckx, 2002).
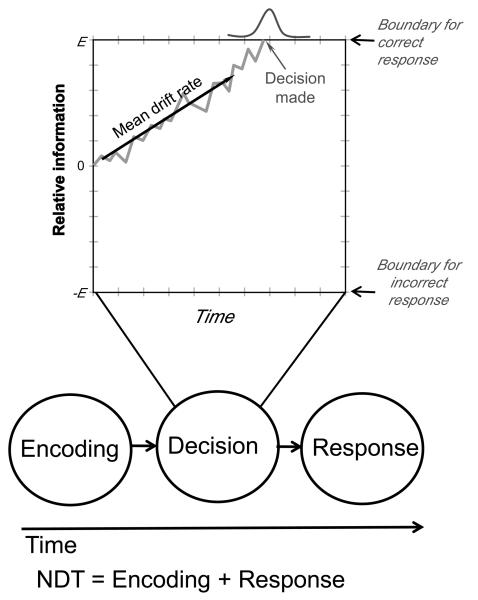
Figure 1.
Schematic illustration of the diffusion model first proposed by Ratcliff (1978). Following appearance of a visual stimulus in a two-alternative forced-choice task, sensory encoding processes take some amount of time. Following sensory encoding, evidence for one of the possible responses (E) accumulates subject to random drift (grey lines). Once this accumulation process passes a threshold for one of the possible responses (correct or incorrect), that response is initiated. Performance is therefore a function of the rate of information accumulation (drift rate), the location of the threshold (boundary) for each possible response (correct and incorrect), and perceptual and responses factors that are combined into the non-decision time (NDT).
Equations for estimating the drift rate, boundary separation and NDT are given in Wakenmakers et al. (2007) and reproduced in the methods below. It can be seen that the drift rate and boundary separation are functions of variability (as indexed by variance) in response latency and accuracy. Increasing variance in response latency at a given accuracy implies a decreasing drift rate, increasing boundary, and decreasing NDT. Increases in accuracy imply primarily an increasing drift rate, with a much smaller influence on increasing boundary separation. The function relating accuracy to NDT has an inverted-U shape, with increases in NDT from very low to moderate accuracy and a slight decrease in NDT from very high to almost perfect accuracy. The mean decision time (MDT) is then a function of drift rate and boundary separation, with NDT simply being the mean response latency minus mean decision time.
If the drift rate, as estimated by the EZ model, reflects the decision stage of a perceptual decision task, then we might expect it to correlate with the hypothesized post-N2 cognitive component, but not earlier components. Earlier components, and the N2 itself, would be expected to correlate with NDT, including as it does, pre-decision perceptual processing and post-decision motor processes.
Methods
Participants
23 visually-, neurologically- and cognitively-intact adults participated in this study, including two of the authors (TM and VK). Ten were male and 13 female. Elderly participants were recruited from the control group of an ongoing study of Alzheimer's disease. Ages ranged from 21-84 years, mean 52 yrs, standard deviation 22.4 yrs. All had normal or corrected-to-normal visual acuity.
All procedures were carried out in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board of the University of Rochester Medical Center. Participants were informed of the procedures and informed consent was obtained.
Materials and Procedure
Participants were seated in a darkened booth in front of 21-in. CRT monitor, positioned 57 cm from the participant, with viewing distance maintained by the use of a chin-rest and forehead-bar combination.
Global motion stimuli were presented on the computer monitor and consisted of white dots subtending 0.125° of visual angle presented within a circular aperture 10° in radius on a uniform black background (see Fig 2). Dot density was approximately 0.6 dots/deg2, and motion speed was 10°/s. Dot motion was controlled by custom software, programmed in C language, compiled with the MinGW compiler (http://www.mingw.org/), on a Windows XP computer.
Figure 2.
Schematic illustration of global motion discrimination task and stimuli. A: On each trial, after a uniformly random inter-trial interval of 1-2 s, a fixation spot appeared for 1 s, followed by an array of stationary random dots. After a uniformly random interval between 1-2 s, the randomly positioned dots began to move to the right or left. B: On coherent motion (DR0) trials, all dot moved in the same direction, while on noise (DR320) trials, the motion of each dot varied randomly at each time frame within a range of 320 degrees about the mean leftward or rightward direction.
The design was a 2 (direction) × 2 (direction range) repeated-measures factorial, with 50 trials in each condition. Later analyses collapsed across direction of motion, so there were 100 trials at each level of direction range. Each trial began when a fixation spot subtending approximately 0.5° of visual angle appeared centrally on the monitor. This was followed 1000 ms later by the onset of a random dot stimulus. After a uniformly distributed random interval lasting between 1-2 s, the dots began to move either to the left or the right, with a specified amount of direction range. After the response or 500 ms, whichever came first, the stimulus disappeared. We used two types of motion stimuli: on half of the trials, all dots moved in the same direction (i.e. coherently, with the range of dot directions = 0°). On the other half of the trials, the direction of each dot was randomized within a range of 320° about the mean direction (always to the right or left). We refer to these conditions as direction range 0° (DR0) and direction range 320° (DR320) respectively. A direction range of 320° was chosen based on past experience that this level of direction noise appreciably reduces global direction discrimination performance in humans (for example, see Huxlin et al. 2009). There were 50 trials for each combination of direction range and direction of motion. The order of trials was randomized within the motion direction discrimination task.
Participants were instructed to report the left/right direction of motion as quickly as possible. If uncertain about the direction of motion in the 320° condition, they were instructed to guess if the dots moved to the left or right. Responses were given using a standard computer mouse by pressing the left button for leftward motion and the right button for rightward motion. To familiarize themselves with task, participants completed 10 practice trials.
Electrophysiological Recording
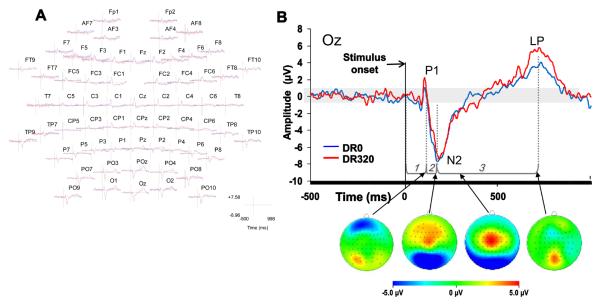
Scalp electroencephalographic (EEG) activity was recorded using Brain Vision equipment (BrainVision, Inc.), with a high-density electrode Acti Cap (64 electrodes) modified according to the International 10-20 System. The recording locations included eight midline sites, with FCz electrode as an on-line average reference and ground at a midline location at AFz (Figure 3A). Low and high pass filter settings were 70 Hz and 0.1 Hz, respectively. The cutoff frequencies for these filters were set at 3 dB down; the roll off was 12 dB per octave at both sides. Impedances were maintained below 10 kΩ for each channel and balanced across all channels within a 5 kΩ range. EEG sampling was set at 500 Hz with 32 bit resolution.
Figure 3.
A. Example of evoked responses to DR0 (red lines) and DR320 (blue lines) motion onsets at different scalp locations. More anterior scalp locations are situated at the top of the figure, Posterior scalp locations are situated at the bottom of the figure. Left and right are veridical. B. Motion-onset VEP from a single, typical participant, collected from the Oz electrode (see A for relative scalp location). Waveform components P1, N2, and LP are labeled. The grey bar illustrates the region within 2 standard deviations of the mean of the baseline epoch. Below the waveform, the horizontal brackets and dotted lines illustrate the three intervals used in the regression analysis: 1 = motion onset to onset of the N2, defined as the point at which the N2 component crosses the threshold of 2 standard deviations below the mean of the baseline epoch, tracking backward from the N2 peak. 2 = the interval between the onset and peak of the N2. 3 = the interval between the peak of the N2 and the peak of the late positive potential LP. Scalp topographies from the same participant are presented below the waveform, corresponding to the P1 peak, N2 peak, P2 peak (which is absent in most participants, including the example) and LP peak.
VEP Data Analysis
VEP analysis was done with EEGLAB 4.51 (Delorme & Makeig, 2004). Scalp topography visualizations (Figure 3) were done with Brain Vision Analyzer software (Brain Products, Inc.). Off-line inspection identified and removed segments of EEG contaminated either by excessive noise, saturation or lack of EEG activity. This resulted in the loss of an average of 3.28% of trials (range 0 – 17%). For eye blink artifacts, we used an independent component analysis (ICA) approach (Makeig et al., 2004). The continuous EEG was pruned by selecting only that data contained within the epochs that would subsequently be used in averaging. ICA (runica algorithm) was performed on the remaining data: the independent component related to eye blinks was identified, and the EEG was reconstructed with the eye blink component removed. Averages were computed for each subject for each electrode and the two stimulus conditions (collapsing across direction of motion). Only trials with correct responses were included in the averages. This resulted in the average loss of 1.4% (range 0-7%) of trials in the DR0 condition, and 2.4% (range 0-13%) of trials in the DR320 condition. Averages were baseline-corrected to the mean of the 500 ms pre-onset time, and extended to 1 s post-stimulus onset. A 45 Hz low-pass filter was applied to the averages.
Averaged responses were used to identify waveform components. We specifically looked for the following standard motion onset VEP components (see Figure 3B for graphical illustration of those components which were actually identified in the data):
The P1 component: defined as the positive peak between 80 and 150 msec after stimulus onset.
The N2 component: defined as the peak negative amplitude in the range of 100 to 250 ms after the motion onset. The onset of the N2 was defined as the point of the leading edge of the N2 waveform where it first exceeded two standard deviations of the mean of the baseline epoch.
The P2 component: defined as the peak positive amplitude in the range of 200 to 300 ms after the motion onset, following the N2 component.
Late Positive (LP) component: defined as a prominent positive deflection following the N2 and P2 components, expected at frontal electrodes.
Data processing and analysis
Analyses were collapsed across left and right, global directions of motion. Trials with response latencies greater than 2.5 standard deviations from a participant's mean, or less than 250 ms, were removed from the analysis. This resulted in the loss of an average of 1.6% of trials (range 0-2.4%). Differences between conditions were assessed with paired-sample t-tests.
Parameters of the EZ-diffusion model were calculated separately for the DR0 and DR320 conditions using the formulas developed by Wagenmakers et al. (2007), using response times and variances to correct trials and accuracy (proportion correct). Drift rate was calculated as
| (1) |
where sign is a function that returns −1 for numbers less than 0 and +1 for numbers greater than 0, PC is percent correct, s is a constant representing variability in drift rate within trials (set to 0.1 following Wakenmakers et al., 2007), VRT is the variance of response latency, and
| (2) |
Given the drift rate, the boundary separation is given by
| (3) |
Mean decision time (MDT) is then
| (4) |
Finally, non-decision time (NDT) is simply
| (5) |
where MRT is the mean reaction time.
The relationship between VEP epochs and behavioral measures was characterized with simultaneous multiple regression. Specifically, response latency and diffusion model parameter estimates were regressed onto N2 onset latency (“1” in Figure 3B), the duration between the onset of the N2 component and the N2 peak (“2” in Figure 3B), and the time between the N2 peak and the late positivity peak LP (“3” in Figure 3B). Participant age was also initially included in the models, but was not a significant predictor of performance. Consequently, the more parsimonious models without age as a predictor are reported below.
Results
Behavioral performance
Means and standard errors for behavioral measures and EZ model parameter estimates are given in Table 1. Participants were significantly faster in the DR0 than DR320 condition (603 vs 696 ms), t(22) = 6.602, p <0.0005, partial η2 = 0.665. Likewise, they were slightly but significantly more accurate in the DR0 than DR320 condition (98.6 vs 97.3%), t(22) = 2.20, p = 0.039, partial η2 = 0.18. Drift rate was significantly greater in the DR0 condition compared to the DR320 condition (0.366 vs 0.303), t(22) = 3.172, p = 0.003, partial η2 = 0.332, indicating that the rate of information accumulation was higher in the DR0 condition. Non-decision time was significantly shorter in the DR0 than in the DR320 condition (401 vs 452 ms), t(22) = −2.954, p = 0.007, partial η2 = 0.284. However, there was no significant effect of direction range on the boundary location, t(22) = −0.243, p = 0.81, partial η2 = 0.003.
Table 1.
Behavioral and diffusion model estimates
| DR0 | DR320 | |||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| RT (s) | 0.603 | 0.027 | 0.696 | 0.033 |
| Accuracy (%) | 0.986 | 0.004 | 0.973 | 0.007 |
| Drift Rate | 0.366 | 0.017 | 0.303 | 0.018 |
| Boundary | 0.145 | 0.007 | 0.146 | 0.006 |
| NDT (s) | 0.401 | 0.02 | 0.452 | 0.025 |
Note. DR0 = motion with a direction range of 0°, DR320 = motion with a direction range of 320°, SE = standard error of the estimate, RT = reaction time, NDT = non-decision time.
Electroencephalography (EEG)
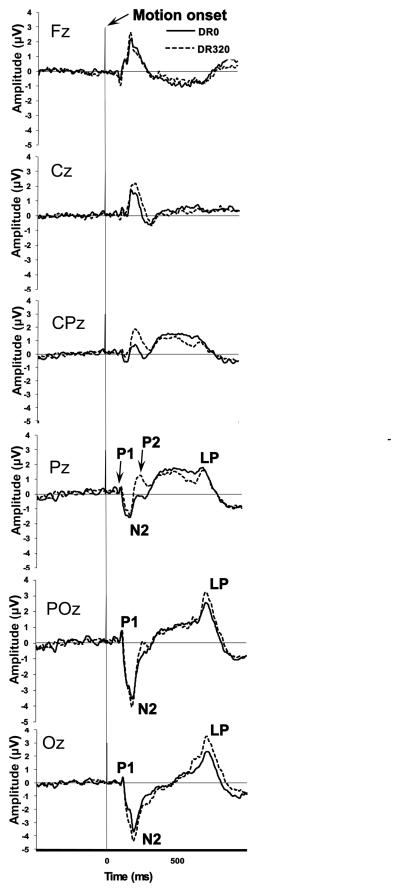
The grand-averaged waveforms for the central electrode locations Oz, POz, Pz, CPz, Cz, and Fz are presented in Figure 4. The two posterior electrode sites (Oz and POz) showed a clear N2 response, but the P1 peak was not present in the grand average, and was present in only some of the waveforms of individual participants. There was also a prominent positive deflection, peaking at around 700 ms. Because such a component was absent or much less prominent at more anterior sites, we interpreted this deflection to be the predicted, post-N2 component “LP”. Both the N2 and LP at Oz and POz had greater amplitudes in the DR320 than the DR0 condition. There was a P2 component reflected in POz, Pz and CPz, which was more prominent in the DR320 condition. These components were followed by a slow, sustained positivity until about the time of the LP. Finally, Cz and Fz showed a clear positive deflection at very near the same latency as the N2 peak, which we interpret as reflecting largely the same source as the N2 component at posterior sites, followed by a negativity which may correspond to the P2 at more posterior sites. At Fz, this component was followed by a prominent, slow, sustained negative potential, which followed a similar time course as the sustained positivities at the more central sites, with a final positive deflection that peaked somewhat later than the LP observed at Oz and POz. The Cz waveform was without prominent deflections following the early deflections.
Figure 4.
Waveforms from central electrodes Oz, POz, Pz, CPz, Cz, and Fz, averaged across all 23 participants. Thick continuous lines represent the DR0 condition, dashed lines the DR320 condition, and the vertical line at 0 ms represents motion stimulus onset. The P1, N2 and LP peaks are indicated where present.
Based on these observations, we focused further analysis on the Oz electrode, which we used to characterize the most prominent components as described below.
Consistent with past studies that separated stimulus onset and motion onset (Kuba et al., 2007), a P1 component was only present in 13 participants (56.5%) in the DR0 condition, and 12 participants (52.2%) in the DR320 condition. The P2 component was likewise not present at the OZ electrode and although present in the grand average, was not reliably detected in individual VEPs at other electrodes. Given their inconsistent occurrence in individual subjects, the P1 and P2 peaks were not analyzed further for the purposes of this study.
The N2 was easily detectable in 22 (96%) participants in the DR0 condition, and all participants in the DR320 condition. The latency of the N2 was not significantly affected by DR, t(21) = 1.03, p = 0.315, partial η2 = 0.048. N2 peak amplitude was significantly greater (i.e., more negative) in the DR320 condition, t(21) = 2.714, p = 0.013, partial η2 = 0.26.
The LP was detectable in 22 participants (92%) in both conditions. LP latency was not significantly affected by DR, t(21) = −1.51, p = 0.147, partial η2 = 0.102, but the peak amplitude of this component was significantly greater in the DR320 condition, t(21) = −3.213, p = 0.004, partial η2 = 0.34.
Interestingly, when correlations between N2 and LP parameters were estimated, the peak latencies of the N2 and LP deflections were not significantly correlated, but their amplitudes were. For the DR0 condition, the N2-LP amplitude correlation was r = −0.825, p <0.0005, while in the DR320 condition, r = −0.74, p <0.0005. Thus, when the N2 was large (more negative), the LP was correspondingly large.
N2 onset latency (the interval between stimulus motion onset and N2 onset), the interval between N2 onset and N2 peak, and the interval between N2 peak and LP peak were also contrasted with paired-sample t-tests as a function of condition. None were significantly different as a function of direction range: for N2 onset latency, t(22) = 0.253, p = 0.803, partial η2 = 0.003, for N2 onset-peak interval, t(20) = 338, p = 0.739, partial η2 = 0.005, and for the interval between N2 and LP peaks, t(21) = 1.597, p = 0.126, partial η2 = 0.113. The same three durations were also used in a regression analysis with behavioral and diffusion model parameter outcomes (see below).
EEG-behavior correlations
VEP measures were entered simultaneously into separate multiple regression models predicting RT, accuracy, and EZ model parameter estimates. Scatter plots illustrating the relationships between electrophysiologically-defined epochs and behavioral measures are presented in Figure 5. Results of the regressions of behavioral measures on these epochs are presented in Table 2. Participants who were missing either the N2 or LP component were excluded from the analysis. Thus, the regression models for the DR0 condition had an N = 20, and for the DR320 condition, an N = 21. Overall, all tolerances were >0.2. Therefore, we conclude that there were no problems of collinearity. Influence was assessed with Cook's distance statistic. All Cook's distances were <1.0 (largest observed = 0.432). Therefore there were no overly influential cases in the analysis.
Figure 5.
Scatter plots showing correlations between reaction time residuals and different VEP epochs. Black diamonds represent the DR0 condition, open circles represent the DR320 condition, black lines represent model fits for the DR0 condition and grey lines represent model fits for the DR320 condition. In order to approximately represent the multiple regression fit (which is a hyperplane), the ordinate plots unstandardized residuals of the regression of RT on the two predictors that are not included in each graph (i.e., the variance not accounted for by those predictors), and fit lines are then based on the regression of these residuals on the predictor indicated on the abscissa. A. RT (residuals after removing variance accounted for by N2 onset – N2 peak and N2 peak – LP intervals) as a function of the interval from motion onset to N2 onset (interval 1 in Figure 3). B. RT residuals as a function of the interval from N2 onset to N2 peak (interval 2 in Figure 3). C. RT residuals as a function of the duration between N2 and LP peaks (interval 3 in Figure 3).
Table 2.
Simultaneous multiple regression models relating durations of VEP epochs to reaction time and diffusion model parameters.
| DR0 | ||||
|---|---|---|---|---|
| RT | Drift Rate | Boundary | NDT | |
| F | 5.915 (0.005) | 5.327 (0.008) | 1.073 (0.384) | 4.556 (0.014) |
| Adjusted R2 | 0.401 | 0.371 | 0.01 | 0.327 |
| SE | 0.101 | 0.065 | 0.033 | 0.081 |
| β N2 Onset | 0.714 (0.003) | −0.577 (0.014) | −0.011 (0.967) | 0.725 (0.004) |
| β N2 Peak-Onset | 0.539 (0.04) | −0.42 (0.11) | 0.416 (0.201) | 0.305 (0.253) |
| β LP - N2 Peak | 0.475 (0.033) | −0.549 (0.018) | 0.427 (0.124) | 0.206 (0.359) |
|
| ||||
| DR320 | ||||
| RT | Drift Rate | Boundary | NDT | |
| F | 5.911(0.005) | 3.18(0.048) | 0.404(0.752) | 3.852(0.026) |
| Adjusted R2 | 0.401 | 0.229 | −0.088 | 0.28 |
| SE | 0.124 | 0.076 | 0.032 | 0.102 |
| β N2 Onset | 1.138 (0.001) | −0.726(0.033) | 0.337(0.381) | 0.867(0.01) |
| β N2 Peak-Onset | 0.804(0.015) | −0.715(0.049) | 0.446(0.285) | 0.388(0.253) |
| β LP - N2 Peak | 0.177(0.356) | −0.545(0.019) | 0.133(0.606) | −0.073(0.727) |
Note. Significant parameters are in bold, p-values are in parentheses. Slopes are standardized. SE = standard error of the estimate, LP = late potential, RT = reaction time, NDT = non-decision time.
In the DR0 condition, each of the epochs contributed significantly to the prediction of RT, and were able to account for about 40% of the variance (p = 0.005). The diffusion model analysis indicated that this was due to their relationships with drift rate and non-decision time, and not with the decision boundary location. The N2 onset latency (p = 0.014) and N2-LP durations (p = 0.018) were significant predictors of estimated drift rate. Both had a negative relationship with drift rate, indicating that longer intervals were associated with slower drift rates. Only N2 onset latency was significantly correlated with NDT (p = 0.004). Its slope was positive, indicating that longer intervals were associated with longer NDTs.
In the DR320 condition, N2 onset latency and N2 onset-to-peak duration were significant predictors of RT, and the model overall accounted for about 40% of the variance in RT (p = 0.005). The drift rate in the DR320 condition was predicted by all three intervals, which accounted for about 23% of the variance (p = 0.048). The slopes for each interval were negative, indicating that longer intervals were associated with slower drift rates. There were again no significant predictors of boundary location. N2 onset latency was the only significant predictor of NDT (p = 0.01), with the overall model accounting for about 28% of the variance (p = 0.026). As for DR0, the slope was positive, indicating that longer intervals were associated with greater NDTs.
Discussion
Based on our understanding of models of motion processing and the motion-onset VEP, we proposed to test three hypotheses to better define the relationship between behavioral performance on a global motion discrimination task and different motion-related epochs of the VEP. First, we predicted that the duration from motion onset to the onset of the N2 should predict reaction time. Second, we hypothesized that the duration between the onset of the N2 and its peak should predict RT independently of the pre-N2 onset period. Finally, we hypothesized that the duration between the N2 peak and LP should predict RT independently of the pre-N2 onset duration or N2 onset-N2 peak duration. All three a priori hypotheses were largely confirmed. Each of the three intervals examined made an independent contribution to the prediction of response latency when motion was totally coherent. However, when motion was noisy, within a range of 320° around the mean direction, only the first two of these epochs were significant predictors of RT. Additionally, we found that these epochs predicted the parameter estimates of a more general model of perceptual decisions, the EZ diffusion model (Wagenmakers et al., 2007). Finally, we found differences in the amplitudes of the N2 and LP peaks as a function of direction range.
Amplitude modulations
The N2 and LP peaks were negatively correlated with each other, and both had greater amplitudes in the DR320 condition. There are many possible reasons why this might be the case, given that the peak amplitude of the ERP is the weighted sum of activity in each current source. The different amplitudes might reflect nothing more than the shorter average duration of the motion stimulus in the DR0 condition because more trials in this condition were terminated by the response before the maximum duration of 500 ms was reached. This might also have caused different relative contributions from generators associated with response preparation, although we would expect such generators to contribute primarily to electrode sites anterior to Oz, such as C3 and C4 (Coles, 1989; Müller-Gethman et al., 2000).
It is possible that the amplitude differences are more meaningful for motion processing, although our experimental design cannot isolate these potential interpretations. Within area MT+/V5, presumed to be the primary generator of the N2 (Ahlfors et al., 1999; Probst et al., 1993), motion opponency may explain greater activity in response to random than coherent motion. Greater numbers of directionally selective neurons are activated by motion in their preferred direction within their receptive fields, countering inhibitory interactions from surrounding neurons. However, such opponency is not seen in striate cortex (Heeger et al., 1999), another likely contributor to N2. Even within monkey MT, separate populations have been identified, one with lateral inhibition and one with lateral excitation, supposed to contribute to local vs. global motion perception respectively (Born & Tootell, 1992).
Neuroimaging studies have arrived at conflicting results regarding the response of MT+ to motion coherence. McKeefry et al. (1997) found some regions, including MT+, that showed a significantly greater change in regional cerebral blood flow in response to incoherently moving dots than to coherently moving dot. Other regions showed greater activity in response to coherent than incoherent motion. Both sets of generators would likely contribute to some degree to the N2. Muckli et al. (2002) found that activity in MT+ and striate cortex increased with increasing angle between two sets of coherently moving dots, corresponding to a change in perception from coherent to transparent motion. Castelo-Blanco et al. (2002) found a similar result with overlapping plaid stimuli, indicating that the existence of multiple motion direction signals in a region of space may increase activity in MT+. Other studies have found larger BOLD signal changes in MT+ with increasing motion coherence (Braddick et al., 2001; Rees et al., 2000). Some magnetoencephalographic (MEG) studies have found greater MT+ response amplitudes to coherent than incoherent motion (Maruyama et al., 2002). Händel et al. (2007), also using MEG, found that one frequency component of striate cortex decreased with increasing motion coherence while a different frequency component of MT+ (or possibly V3A) increased with increasing coherence. Given this complexity of responses, and the presumption that the N2, while reflecting primarily MT+ activity, also likely represents activity from other motion-sensitive regions such as V1 and V3A, the greater amplitude of N2 in response to a wide direction range in this study may represent an increase in activity in any one of these contributing source generators, or some combination of them.
Possible interpretations of individual intervals
The interval from stimulus motion onset to the onset of the N2 component predicted RT in both the DR0 and DR320 conditions. Additionally, it predicted the estimated drift rate and NDT in both conditions, but not the boundary. NDT reflects both sensory processes, presumably occurring (or at least beginning) prior to the decision stage, and motor processes occurring after the decision stage. It seems reasonable to conclude that the N2 onset latency largely reflects sensory processes prior to the decision stage, and thus accounts for its relationship to RT and NDT.
The interpretation of this early epoch as reflecting pre-motion processes is consistent with several lines of evidence, including the lack of motion specificity for earlier motion VEP components (Bach & Ullrich, 1997; Kubová et al., 1995). However, single-cell studies of MT indicate that motion-selective activity can begin as early as 30-40 ms after motion onset (Maunsell, 1987; Raiguel et al., 1999; Schmolesky et al., 1998). Thus motion-specific processing likely begins prior the onset of the N2. Stages are unlikely to be strictly serial (Miller & Hackley, 1992). Later processes likely begin before the completion of earlier processes, continue in parallel with earlier processes, and provide feedback to earlier processes. A better interpretation, then, might be that the pre-N2 epoch reflects a period during which pre-motion processes dominate the posterior ERP signal, while the onset of the N2 component represents the point in time at which pre-motion processing begins to recede and motion-specific processing rapidly ramps up to dominate the posterior ERP signal. However, the lack of strict serial processing does not imply that stages are indistinguishable. The success of the regression models reported here indicates that N2 onset may be behaviorally relevant, independent of the peak latency of the N2. The earliest responses reviewed above represent perhaps the cutting edge of motion-specific processing, but motion detection appears to rely for its input on earlier transformations of the retinal image (Lu and Sperling, 1995; van Santen & Sperling, 1984). We suggest that the onset of N2 represents an approximate but adequate demarcation point between these pre-motion processes and motion-specific processes.
The relationship of this early interval with drift rate would also be puzzling from a strictly serial processing point of view, since the decision stage must logically follow sensory and motion processing. Therefore, correlation of this early interval with the EZ Diffusion Model (Wagenmakers et al., 2007)'s estimate of drift rate is inconsistent with an interpretation of the drift rate parameter as the rate of accumulation of information at a strictly post-perceptual decision stage. If drift rate reflects the rate of accumulation of information by a decision mechanism, likely this accumulation at the decision stage will begin before information is fully encoded at the sensory stage. An even more radical notion from a stages-of-processing point of view is that they are the same process: sensory encoding is the accumulation of information by a decision mechanism. In either case, the diffusion process would begin earlier and be faster when sensory encoding is fast than when sensory encoding is slow.
The interval between the onset and peak of the N2 predicted RT in both the DR0 and DR320 conditions, consistent with the N2 peak's putative role in complex motion processing. However, the fact that this interval was not significantly modulated by the amount of direction range in the stimulus is puzzling. It is possible that a direction range of 320° was not an adequately strong manipulation, as suggested by the relatively high accuracy (mean 97% correct) in the DR320 condition.
The interval between the N2 peak and LP peak was associated with drift rate in both coherence conditions, although in the DR320 condition it was not a significant independent predictor of RT itself. The relationship between this interval and the drift rate of a diffusion process is sensible if the drift rate reflects the accumulation of information by a decisional mechanism. However, the latency of the LP peak suggests that it is associated with the offset of stimulus motion. Because motion was terminated upon a response from the participant, this relationship may be spurious if the LP is in fact an offset-related potential. This would also explain its smaller (and non-significant) relationship to response latency in the DR320 condition. In the DR320 condition, response latencies tended to be around 90 ms longer, and therefore more responses would have occurred after the maximum duration of the motion stimulus. If the LP is actually an offset potential, this would mean that fewer trials were terminated by a response prior to the maximum (500 ms) duration. The offset of motion would no longer be as strongly correlated with response latency, and consequently the contribution of offset to the spurious relationship between LP and RT would be reduced.
The LP at posterior sites
The observation of the LP at electrode locations Oz and POz was unexpected. A late component, interpreted as reflecting decisional and/or motor processes subsequent to motion-specific processing, was observed by Kuba et al. (1998), but at more frontal sites. Indeed, Kuba et al. (1998) observed a similar peak at Cz and Fz around 392 ms, in response to three types of oddball motion stimuli. In the current experiment, neither Cz nor Fz electrodes showed such a peak in grand-averaged waveforms, nor consistently in individual observers. There are several possible reasons for the discrepancy between the LP observed here and that observed by Kuba et al. First, Kuba et al. used motion stimuli with durations of only 200 ms, meaning that the late positive peak that they observed had about the same relationship to the offset of stimulation as the LP in this study. Second, they used an oddball paradigm and had observers count the oddballs. Thus, there were several additional processes engaged by their task that were not engaged in ours; these processes are known to modulate VEP components, including those engaged by differential stimulus probability (Banquet & Contreras-Vidal, 1992; Duncan-Johnson & Donchin, 2007), oddballs (Picton, 1992; Ritter, Vaughan, & Costa, 1968), and counting (Courchesne, Hillyard, & Galambos, 1975). While the late positivity that Kuba and colleagues observed was maximal at frontal sites, it was apparent at Oz as well. Thus, they may have observed a more distributed process than that invoked by the task described here.
VEP epochs and a diffusion process
The EZ diffusion model analysis indicated that faster reactions in the DR0 condition were likely due in part to a greater rate of information accumulation and reduced NDT. The reduced NDT could be a reflection of shorter periods of perceptual processing, shorter motor delays, or both. The significant relationship between NDT and N2 onset, but not later epochs, favors the interpretation that it is due to shorter perceptual processing.
To our knowledge, ours are the first reported correlations between motion-onset VEPs and decisional process characteristics estimated by a diffusion process model. The only previous correlation of a diffusion model analysis with EEG-derived data that we are aware of is that of Philiastides, Ratcliff, and Sajda (2006). While their methods and the visual tasks required of their subjects (object and color discriminations in a simple categorization task) were quite different from those used in the present study, their results exhibit some similarity with those reported here. They used single-trial discriminant analysis of two experimental conditions and found an early (around 170 ms) and late (around 300 ms) component that discriminated conditions better than chance. That is, over a small epoch around these times relative to the stimulus, a linear combination of sequential samples of the electroencephalogram from 60 channels was able to discriminate experimental conditions. A measure of how well the discriminant function categorized trials was correlated with the drift rate estimated by the full diffusion model of Ratcliff (Ratcliff, 1978; Ratcliff & Tuerlinckx, 2002). This finding of a relationship between a post-perceptual component and diffusion rate is consistent with our finding of a relationship between a late epoch and drift rate in a very different paradigm, with a different estimator of drift rate, and a different metric of brain electrical activity.
Limitations
Lateral electrodes, and other components evident in the central electrodes (e.g. P1 and P2 peaks), were examined but not included in the analysis presented here. This is not meant to imply that the only important information (or for that matter, the most important information) about the motion perception task used here is contained in the central electrodes, and primarily Oz. To the contrary, it is likely that lateral electrodes are more sensitive to other source generators (in addition to those noted at central electrode sites) that reflect important processing components contributing to the current task. However, we do believe that this would be a more important consideration in paradigms with lateralized stimulus presentation, where bilateral activation of regions such as hMT+ may be asynchronous and unequal.
Likewise, the adequacy of the assumption of additivity of component processes was not assessed by including the interaction terms in the regression models. To do so would require a much larger sample for adequate statistical power, and the additivity of stages was not the focus of interest. It is possible that the component processes observed here do interact, and that this interaction significantly contributes to final reaction time, but at the current sample size adding just the three 2-way interaction terms would over-parameterize the regression models to a serious degree. Nevertheless, the success of the regression models in accounting for significant variance in RT suggests that this approach results in a useful, albeit incomplete, representation of response latency in a global motion direction discrimination task.
Conclusion
We identified three intervals defined by deflections of the motion VEP that together accounted for 40% of the variance in reaction time in a global motion direction discrimination task. The use of a mathematical model of the decision process (the EZ diffusion model of Wagenmakers et al., 2007) allowed us to better specify which cognitive-perceptual processes were likely related to specific aspects of the VEP. The results were largely consistent with an interpretation of the earliest epoch, from motion onset to N2 onset, as primarily reflecting perceptual processing not specific to motion, followed by motion-specific processing between the onset and peak of the N2, followed by a decision stage between the peak of the N2 and the LP, although the correlation between this latter epoch and a decision process (drift rate) may be spurious. The correlation of the earliest VEP epoch with the estimate of drift rate obtained from the diffusion model is inconsistent with an interpretation of this estimate as representing strictly post-perceptual processing. Rather, this relationship suggests that the process indexed by this parameter reflects early perceptual processing, at least to some degree. Finally, our study shows that the VEP measures obtained from the posterior recording site Oz to motion onset can reliably predict global motion discrimination performance.
Research Highlights.
Multiple epochs in the motion-related visual evoked potential make significant independent contributions to the prediction of response latency.
These epochs can be related to cognitive models of motion perception and perceptual decision-making.
The relationship between these epochs and a diffusion process model indicate that model estimates of information accumulation rate reflect both early and late processes, not only a post-perceptual decision process.
Acknowledgments
TM was funded by NIH Loan Repayment Program Award L30 EY01773 and Training Grant T32EY007125. KRH and TM were supported by grants from the Pfeiffer Foundation, the Schmitt Foundation, NIH Core Grant P30EY0131, and an unrestricted grant to the Department of Ophthalmology from the Research to Prevent Blindness Foundation. VK was supported by Alzheimer's Association Award HAT-07-60437.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors assert that they have no competing interests.
References
- Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotinainen M, Tootell RBH, Aronen HJ, Ilmoniemi RJ. Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. Journal of Neurophysiology. 1999;82:2545–2555. doi: 10.1152/jn.1999.82.5.2545. [DOI] [PubMed] [Google Scholar]
- Albright TD, Stoner GR. Visual motion perception. Proceedings of the National Academy of Sciences. 1995;92:2433–2440. doi: 10.1073/pnas.92.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Hoffman MB. Visual motion detection in man is governed by non-retinal mechanisms. Vision Research. 1999;40:2379–2385. doi: 10.1016/s0042-6989(00)00106-1. [DOI] [PubMed] [Google Scholar]
- Bach M, Ullrich D. Motion adaptation governs the shape of motion-evoked cortical potentials (motion VEP) Vision Research. 1994;34:1541–1547. doi: 10.1016/0042-6989(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Bach M, Ullrich D. Contract dependency of motion-onset and pattern-reversal VEPs: Interaction of stimulus type, recording site and response component. Vision Research. 1997;37:1845–1849. doi: 10.1016/s0042-6989(96)00317-3. [DOI] [PubMed] [Google Scholar]
- Bahramali H, Gordon E, Li WM. Fast and slow reaction time changes reflected in ERP brain function. International Journal of Neuroscience. 1998;93:75–86. doi: 10.3109/00207459808986414. [DOI] [PubMed] [Google Scholar]
- Banquet JP, Contreras-Vidal JL. An integrated neural network-event-related potentials model of temporal and probability context effects on event categorization. Proceedings of the International Joint Conference on Neural Networks. 1992;1:541–546. [Google Scholar]
- Blackwell HR. Contrast thresholds of the human eye. Journal of the Optical Society of America. 1946;36:624–646. doi: 10.1364/josa.36.000624. [DOI] [PubMed] [Google Scholar]
- Born RT, Tootell RBH. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357:497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O'Brien JMD, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30:61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco M, Formisano E, Backes W, Zanella F, Neuenschwander S, Singer W, Goebel R. Activity patterns in human motion-sensitive areas depend on the interpretation of global motion. Proceedings of the National Academy of Sciences. 2002;99:13914–13919. doi: 10.1073/pnas.202049999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo-Branco M, Mendes M, Sebastião, Reis A, Soares M, Saraiva J, Bernardes R, Flores R, Pérez-Jurado L, Silva E. Visual phenotype in Williams-Beuren syndrome challenges magnocellular theories explaining human neurodevelopmental visual cortical disorders. Journal of Clinical Investigation. 2007;117:3720–3729. doi: 10.1172/JCI32556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles MGH. Modern mind-brain reading: Psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Dean AF. The relationship between response amplitude and contrast for the cat striate cortical neurons. Journal of Neurophysiology. 1981;318:413–427. doi: 10.1113/jphysiol.1981.sp013875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. Journal of Neuroscience. 1995;15:5192–5208. doi: 10.1523/JNEUROSCI.15-07-05192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology. 2007;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Handel B, Lutzenberger W, Their P, Haarmeier T. Opposite dependencies on visual motion coherence in human area MT+ and early visual cortex. Cerebral Cortex. 2007;17:1542–1549. doi: 10.1093/cercor/bhl063. [DOI] [PubMed] [Google Scholar]
- Heeger DJ, Boynton GM, Demb JB, Seidemann E, Newsome WT. Motion opponency in visual cortex. Journal of Neuroscience. 1999;19:7162–7174. doi: 10.1523/JNEUROSCI.19-16-07162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annual Review of Psychology. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Picton TW. Electrophysiology of cognition. In: Plum F, editor. Handbook of physiology: Sec. 1. The nervous system: Vol. 5. Higher functions of the brain. Part 2. Waverly Press; Bethesda, MD: 1987. pp. 519–584. [Google Scholar]
- Hoffman M, Dorn TJ, Bach M. Time course of motion adaptation: Motion-onset evoked potentials and subjective estimates. Vision Research. 1999;39:437–444. doi: 10.1016/s0042-6989(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Kuba M, Kremláček J, Kubová Z. Cognitive evoked potentials related to visual perception of motion in human subjects. Physiological Research. 1998;47:265–270. [PubMed] [Google Scholar]
- Kuba M, Kubová Z, Kremláček J, Langrová J. Motion-onset VEPs: Characteristics, methods, and diagnostic use. Vision Research. 2007;47:189–202. doi: 10.1016/j.visres.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Kubová Z, Kuba M, Spekreijsi H, Blakemore C. Contrast dependence of motion-onset and pattern-reversal evoked potentials. Vision Research. 1995;35:197–205. doi: 10.1016/0042-6989(94)00138-c. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Sperling G. The functional architecture of human visual motion perception. Vision Research. 1995;35:2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- Luce RD. Response times: Their role in inferring elementary mental organization. Oxford University Press; New York: 1986. [Google Scholar]
- Makeig S, Debener S, Onton J, Delorme A. Mining event-related brain dynamics. Trends in Cognitive Science. 2004;8:204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. Journal of Experimental Psychology: Human Perception and Performance. 1991;4:1057–1074. doi: 10.1037//0096-1523.17.4.1057. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Kaneoke Y, Watanabe K, Kakigi R. Human cortical responses to coherent and incoherent motion as measured by magnetoencephalography. Neuroscience Research. 2002;44:195–205. doi: 10.1016/s0168-0102(02)00129-3. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR. Physiological evidence for two visual subsystems. In: Vaina LM, editor. Matters of Intelligence. Reidel; Dordrecht, Holland: 1987. [Google Scholar]
- McKeefry DJ, Watson JDG, Frackowiak RSJ, Fong K, Zeki S. The activity in human areas V1/V2, V3, and V5 during the perception of coherent and incoherent motion. NeuroImage. 1997;5:1–12. doi: 10.1006/nimg.1996.0246. [DOI] [PubMed] [Google Scholar]
- Mendes M, Silva F, Simões L, Jorge M, Saraiva J, Castelo-Branco M. Visual magnocellular and structure from motion perceptual deficits in a neurodevelopmental model of dorsal stream function. Cognitive Brain Research. 2005;25:788–798. doi: 10.1016/j.cogbrainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Miller J, Hackley SA. Electrophysiological evidence for temporal overlap among contingent mental processes. Journal of Experimental Psychology: General. 1992;121:195–209. doi: 10.1037//0096-3445.121.2.195. [DOI] [PubMed] [Google Scholar]
- Miller J, Ulrich R, Rinkenauer G. Effects of stimulus intensity on the lateralized readiness potential. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1454–1471. doi: 10.1037//0096-1523.24.3.915. [DOI] [PubMed] [Google Scholar]
- Muckli L, Singer W, Zanella FE, Goebel R. Integration of multiple motion vectors over space: An fMRI study of transparent motion perception. NeuroImage. 2002;16:843–856. doi: 10.1006/nimg.2002.1085. [DOI] [PubMed] [Google Scholar]
- Müller R, Göpfert E, Hartwig M. The effect of movement adaptation on human cortical potentials evoked by pattern movement. Acta Neurobiologia Experimentalis. 1986;46:293–301. [PubMed] [Google Scholar]
- Müller-Gethman H, Rinkenauer G, Stahl J, Ulrich R. Preparation of response force and movement direction: Onset effects on the lateralized readiness potential. Psychophysiology. 2000;37:507–514. [PubMed] [Google Scholar]
- Näätänen R, Gaillard A, Mäntysalo S. Early selective attention reinterpreted. Acta Psychologica. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Paré EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman A, Moore CM. The locus of dual-task interference: Psychological refractory effects on movement-related brain potentials. Journal of Experimental Psychology: Human Perception and Performance. 1993;19:1–21. doi: 10.1037//0096-1523.19.6.1292. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cerebral Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- Patzwahl DR, Zanker JM. Mechanisms of human motion perception: Combining evidence from evoked potentials, behavioural performance and computational modelling. European Journal of Neuroscience. 2000;12:273–282. doi: 10.1046/j.1460-9568.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. Journal of Neuroscience. 2006;26:8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology. 1992;9:456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Probst T, Plendl H, Paulus W, Wist ER, Scherg M. Identification of the visual motion area (area V5) in the human brain by dipole source analysis. Experimental Brain Research. 1993;93:345–351. doi: 10.1007/BF00228404. [DOI] [PubMed] [Google Scholar]
- Raiguel SE, Xiao D-K, Marcar VL, Organ GA. Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. Journal of Neurophysiology. 1999;82:1944–1956. doi: 10.1152/jn.1999.82.4.1944. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Ratcliff R, Tuerlinkckx F. Estimating parameters of the diffusion model: Approaches to dealing with contaminant reaction times and parameter variability. Psychonomic Bulletin and Review. 2002;9:438–481. doi: 10.3758/bf03196302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nature Neuroscience. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Reichardt W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. In: Rosenblith WA, editor. Sensory Communication. Wiley; New York: 1961. [Google Scholar]
- Ritter W, Vaughan HG, Jr., Costa LD. Orienting and habituation to auditory stimuli: A study of short-term changes in averaged evoked responses. Electroencephalography and Clinical Neurophysiology. 1968;25:550–556. doi: 10.1016/0013-4694(68)90234-4. [DOI] [PubMed] [Google Scholar]
- Rudolph K, Pasternak T. Transient and permanent deficits in motion perception after lesions of the cortical areas MT and MST in the macaque monkey. Cerebral Cortex. 1999;9:90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- van Santen JPH, Sperling G. Temporal covariance model of human motion perception. Journal of the Optical Society of America A. 1984;1:451–473. doi: 10.1364/josaa.1.000451. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. Journal of Neurophysiology. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schweickert R. Critical-path scheduling of mental processes in a dual task. Science. 1980;209:704–706. doi: 10.1126/science.7394529. [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Research. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, van der Maas HLJ, Grasman RPPP. An EZ-diffusion model for response time and accuracy. Psychonomic Bulletin and Review. 2007;14:3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: An electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Watamaniuk SN, Sekuler R. Temporal and spatial integration in dynamic random dot stimuli. Vision Research. 1992;32:2341–2347. doi: 10.1016/0042-6989(92)90097-3. [DOI] [PubMed] [Google Scholar]