Abstract
Mast cells participate in allergies and inflammation by secreting a variety of pro-inflammatory mediators. Curcumin, the active component of turmeric, is a polyphenolic phytochemical with anti-tumor, anti-inflammatory, anti-oxidative, and anti-allergic properties. The effects of curcumin on compound 48/80-induced mast cell activation and passive cutaneous anaphylactoid reactions are unknown. In this report, we investigated the influences of curcumin on the passive cutaneous anaphylactoid response in vivo and compound 48/80-induced mast cell activation in vitro. The mechanism of action was examined by calcium uptake measurements and cAMP assays in mast cells. Curcumin significantly attenuated the mast cell-mediated passive cutaneous anaphylactoid reaction in an animal model. In agreement with this in vivo activity, curcumin suppressed compound 48/80-induced rat peritoneal mast cell (RPMC) degranulation and histamine release from RPMCs. Moreover, compound 48/80-elicited calcium uptake into RPMCs was reduced in a dose-dependent manner by curcumin. Furthermore, curcumin increased the level of intracellular cAMP and significantly inhibited the compound 48/80-induced reduction of cAMP in RPMCs. These results corroborate the finding that curcumin may have anti-allergic activity.
Keywords: Mast cell, Anaphylactoid reaction, Curcumin, Histamine, Calcium
Introduction
Mast cells are the primary effector cells involved in an allergic or immediate hypersensitivity response. Activation of mast cells occurs in response to a challenge by a specific antigen against which the surface immunoglobulin E (IgE) is directed, or by other IgE-directed ligands. Activated mast cells can produce histamine, as well as a wide variety of other inflammatory mediators such as eicosanoids, proteoglycans, proteases and several pro-inflammatory and chemotactic cytokines such as tumor necrosis factor-α, interleukin (IL)-6, IL-4, IL-8, and IL-13 (Kalesnikoff & Galli, 2008). Among them, histamine remains the best-characterized and most potent vasoactive mediator implicated in the acute phase of immediate hypersensitivity (Petersen et al.,1996). Various acute and chronic allergic responses are caused by these mediators. Mast cell degranulation can also be elicited by the basic secretagogues. The most potent secretagogues include the synthetic compound 48/80 and polymers of basic amino acids (Ennis et al.,1980). Compared with the natural process, a high concentration of compound 48/80 induces an almost 90% release of histamine from mast cells. Thus, an appropriate amount of compound 48/80 has been used as a direct and convenient reagent to investigate the mechanisms of allergy and anaphylaxis (Allansmith et al.,1989). The murine mast cell is a good experimental model for the study of compound 48/80-induced histamine release.
Curcumin is a yellow dye in the crude drug "Turmeric" (Curcumae Rhizoma) from the rhizome of Curcuma longa L. (Zingiberaceae). Curcumin is known to have anti-tumor, anti-inflammatory, anti-oxidative, and anti-allergic effects (Cui et al., 2006; Eybl et al., 2006; Kurup & Barrios, 2008). In addition, previous studies demonstrated that curcumin had an inhibitory effect on histamine release from mast cells triggered by IgE, calcium ionophore A23187, or concanavalin A (Suzuki et al., 2005; Lee et al., 2008). However, there are no reports to date concerning the suppressive effect of curcumin on compound 48/80-induced anaphylactoid response.
In this study, we evaluated the effect of curcumin on the passive cutaneous anaphylactoid response and compound 48/80-induced histamine release from mast cells. In addition, the amount of calcium uptake and intracellular cAMP was determined to clarify the mechanism by which curcumin inhibited histamine release from mast cells.
Materials and Methods
Materials
Curcumin, compound 48/80, disodium cromoglycate (DSCG), bovine serum albumin (BSA), and HEPES were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Percoll solution was purchased from Pharmacia (Uppsala, Sweden). For all in vitro experiments, curcumin was dissolved in dimethylsulfoxide (DMSO) and freshly diluted in HEPES-Tyrode buffer (136 mM NaCl, 5 mM KCl, 2 mM CaCl2, 11 mM NaHCO3, 0.6 mM NaH2PO4, 2.75 mM MgCl2, 5.4 mM HEPES, 1.0 mg/mL BSA, 1.0 mg/mL glucose, 0.1 mg/mL heparin, pH 7.4) before use.
Experimental animals
Male Sprague-Dawley rats (8 weeks old, 230~280 g) were purchased from Damool Science (Daejeon, Korea). Animals were housed 3~5 per cages in laminar air-flow cabinets maintained at 22±1℃ and a relative humidity of 55±10% throughout the study. The Animal Research Committee of Chonbuk National University approved the animal study in accordance with the guidelines of the National Institutes of Health (NIH publication #85-23, 1985).
Passive cutaneous anaphylactoid response in rats
Curcumin (10, 50, 100 mg/kg body weight) was orally administered to each rat 1 hour before the injection of compound 48/80, which was injected intradermally (0.25 µg/50 µL) into the dorsal skin. Evans blue solution (1%) was intravenously injected into the penile vein of each rat and 30 minutes after the injection, the rats were sacrificed. Tissue sections around the intradermal injection site were excised and weighed, followed by extraction of extravasated Evans blue dye by incubation of biopsies in 1 mL formamide at 55℃ for 24 hours. Absorbance was measured at 620 nm with a spectrophotometer (Spectra MAX PLUS, Molecular Devices, CA, USA), and tissue Evans blue concentrations quantified by interpolation on a standard curve of dye concentrations in the range of 0.01 to 30 µg/mL.
Preparation of rat peritoneal mast cells (RPMCs)
RPMCs were isolated as previously described (Cochrane & Douglas, 1974). In brief, rats were anesthetized with ether, injected with 10 mL of calcium-free HEPES-Tyrode buffer into the peritoneal cavity, and the abdomen gently massaged for approximately 90 seconds. The peritoneal cavity was opened, and the fluid aspirated using a Pasteur pipette. RPMCs were purified using a Percoll density gradient as described in detail elsewhere (Hachisuka et al.,1988). RPMC preparation was approximately 95% pure as assessed by toluidine blue staining and at least 98% of these cells were viable as assessed by trypan blue exclusion. Purified RPMCs (1×106 cells/mL) were resuspended in HEPES-Tyrode buffer.
RPMC viability assay
To test the viability of RPMCs, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay was performed as previously described (Yoshimura et al., 2004). Briefly, RPMCs (2×105 cells/well) were incubated with various concentrations (10~100 µM) of curcumin at 37℃ for 2 hours. After addition of MTT (100 µg in 100 µL saline), RPMCs were incubated at 37℃ for 1 hour. Crystallized MTT was dissolved and the absorbance measured at 570 nm with a spectrophotometer (Spectra MAX PLUS, Molecular Devices, CA, USA).
Histamine assay
RPMC suspensions (2×105 cells in 200 µL) were pre-incubated with curcumin (10~50 µM) at 37℃ for 5 minutes and then incubated with compound 48/80 (0.25 µg/mL) for 15 minutes. Following centrifugation at 150×g for 10 minutes at 4℃, the amount of histamine in the supernatant was determined by the radioenzymatic method (Harvima et al., 1988). The inhibition percentage of histamine release was calculated using the following formula: % inhibition=[(histamine release without curcumin-histamine release with curcumin)/histamine release without curcumin]×100.
Measurement of 45Ca uptake
The calcium uptake of mast cells was measured according to the method described by Choi et al. (Choi et al., 2006a). Purified RPMCs were resuspended in HEPES-Tyrode buffer containing 45Ca (1.5 mCi/mL; 1 Ci=3.7×1010 becquerels; PerkinElmer Life Sciences, MA, USA), and incubated at 4℃ for 10 minutes. Mast cell suspensions were preincubated with curcumin (10~50 µM) at 37℃ for 5 minutes and then incubated with compound 48/80 (0.25 µg/mL) at 37℃ for 15 minutes. The reaction was stopped by the addition of 1 mM lanthanum chloride. The samples were centrifuged 3 times at 150×g for 10 minutes at 4℃, and then RPMCs were lysed with 10% Triton X-100 and vigorous shaken. Radioactivity of the solution was measured in a scintillation β-counter (Liquid Scintillation Analyzer, A Canberra Company, Australia).
cAMP assay
The cyclic adenosine-3', 5' monophosphate (cAMP) level was measured by the method described by Holmegaard (Holmegaard, 1982). In brief, an RPMC suspension was added to an equivalent volume (200 µL) of prewarmed buffer containing the drug in an Eppendorf tube. The reaction was allowed to proceed for discrete time intervals, terminated by centrifugation at 150×g for 10 minutes at 4℃, and then each sample was added to 250 µL of 50 mM sodium acetate buffer (pH 6.2) under vigorous vortexing, followed by snap freezing in liquid nitrogen. Frozen samples were thawed and vortexed and the debris sedimented by centrifugation at 1,200×g for 10 minutes at 4℃. The cAMP level in the supernatant was determined by radioimmunoassay using a Rianen assay system (PerkinElmer Life Sciences, Boston, MA, USA).
Statistical analysis
The results obtained were expressed as mean±SEM for the number of experiments. Statistical evaluation of the results was performed using one-way ANOVA, followed by Duncan's multiple range tests. Results with P<0.05 were considered statistically significant.
Results
Curcumin attenuates the passive cutaneous anaphylactoid response
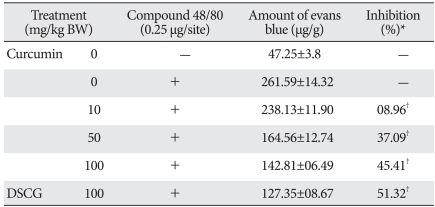
To confirm the anti-anaphylactoid effect of curcumin, we used an in vivo model induced by compound 48/80. It has been previously demonstrated that the intradermal injection of compound 48/80 into the dorsal skin of rats provokes an increase of mast cell-dependent vascular permeability documented by Evans blue extravasation (Choi et al., 2006b). As shown in Table 1, oral administration of curcumin dose-dependently reduced the vascular permeability changes triggered by compound 48/80. Likewise, DSCG (a reference drug) exhibited a significant inhibition at a dose of 100 mg/kg.
Table 1.
Inhibitory effect of curcumin on compound 48/80-induced passive cutaneous anaphylactoid response
Curcumin or DSCG was orally administrated at 1 hour before compound 48/80 injection. Twenty micro liters of compound 48/80 (0.25 µg/site) were intradermally injected into the shaved flank of Sprague-Dawley rats. Each amount of Evans blue represents the mean±S.E.M. of five independent experiments. *Inhibition (%) = [(amount of Evans blue without curcumin - amount of Evans blue with curcumin)/amount of Evans blue without curcumin]×100. †P<0.05, significantly different from the control value.
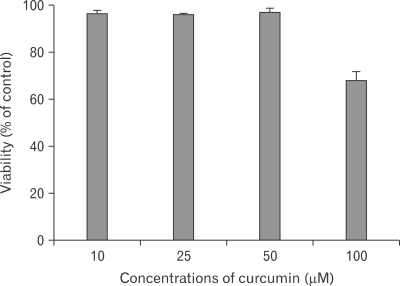
Curcumin has no cytotoxicity on RPMCs at a dose of 10~50 µM
MTT conversion assay was used to determine the viability of RPMCs exposed to curcumin. Viable cells were almost 100% after exposure to 10~50 µM of curcumin for 2 hours. However, at 100 µM, curcumin had cytotoxic effects on RPMCs (Fig. 1). Thus, we performed in vitro experiments at 10~50 µM of curcumin.
Fig. 1.
Effect of curcumin on rat peritoneal mast cell (RPMC) viability. RPMCs were treated with the various concentrations of curcumin for 2 hours. RPMC viability was determined by MTT assay and the percentage of viability was calculated as a ratio of A570 of control cells (treated with HEPES-Tyrode buffered solution). Each data value represents the mean±S.E.M. of five independent experiments.
Curcumin inhibits compound 48/80-induced RPMC degranulation
To investigate how curcumin inhibited the passive cutaneous anaphylactoid response, we evaluated the effect of curcumin on compound 48/80-induced RPMC degranulation (Fig. 2). Normal RPMCs were generally oval in shape and contained many fine granules surrounding a prominent nucleus (Fig. 2A). After stimulation with compound 48/80 for 5 minutes, RPMCs were degranulated (Fig. 2B). Characteristics of mast cell degranulation were cell swelling, cytoplasmic vacuoles, and extruded granules near the cell surface. When RPMCs were incubated with curcumin alone, RPMCs were similar to those seen in (Fig. 2A, C) Pretreatment with curcumin inhibited the degranulation of RPMCs stimulated with compound 48/80, and cell sizes appeared to be somewhat larger than the control (Fig. 2D). The results suggest that curcumin inhibits compound 48/80-induced mast cell degranulation.
Fig. 2.
Inverted light micrographs of rat peritoneal mast cell (RPMC, arrows). (A) The normal RPMC in HEPES-Tyrode buffered solution. (B) The Degranulated RPMC after the addition of compound 48/80. (C) The RPMCs observed within 10 minutes after the addition of curcumin (50 µM) show similar findings as seen in Fig. 2A. (D) The RPMCs pretreated with curcumin observed within 10 minutes after the addition of compound 48/80, show similar findings as seen in Fig. 2C. Bars=10 µm.
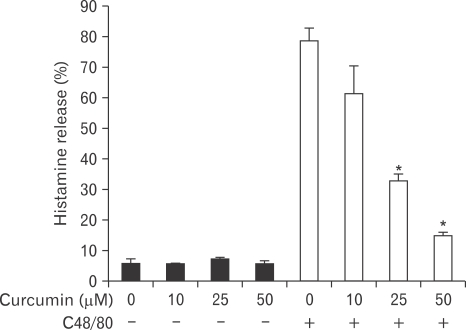
Curcumin inhibits compound 48/80-induced histamine release from RPMCs
To investigate how curcumin inhibited the passive cutaneous anaphylactoid response, we also evaluated the effect of curcumin on compound 48/80-induced mast cell activation. The effect of curcumin on compound 48/80-induced histamine release from RPMCs is shown in Fig. 3. The histamine release from compound 48/80-treated RPMCs was reduced in a dose-dependent manner by curcumin (58 and 80% inhibition at 25 and 50 µM, respectively).
Fig. 3.
Effect of curcumin on compound 48/80-induced histamine release from rat peritoneal mast cell (RPMC). RPMCs were preincubated with curcumin at 37℃ for 5 minutes prior to the incubation with compound 48/80. Curcumin dose-dependently inhibited compound 48/80-induced histamine release. Each data value represents the mean±S.E.M. of five independent experiments. *P<0.001, significantly different from the control value. C48/80: compound 48/80.
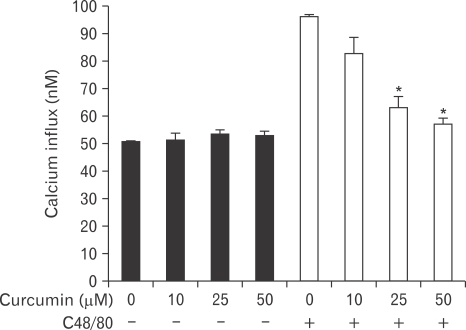
Curcumin reduces compound 48/80-induced calcium uptake into RPMCs
It is well established that an increase in calcium uptake of RPMCs contributes to the release of histamine (Akagi et al., 1994). Treatment with curcumin alone showed no change in calcium uptake. However, calcium uptake was greatly increased by stimulation of RPMCs with compound 48/80. The compound 48/80-induced calcium uptake was inhibited in a concentration-dependent manner by curcumin (Fig. 4).
Fig. 4.
Effect of curcumin on compound 48/80-induced calcium uptake into rat peritoneal mast cell (RPMC). RPMCs were preincubated with curcumin at 37℃ for 5 minutes prior to the incubation with compound 48/80. Curcumin dose-dependently inhibited compound 48/80-induced calcium uptake into RPMCs. Each data value represents the mean±S.E.M. of five independent experiments. *P<0.05, significantly different from the control value. C48/80: compound 48/80.
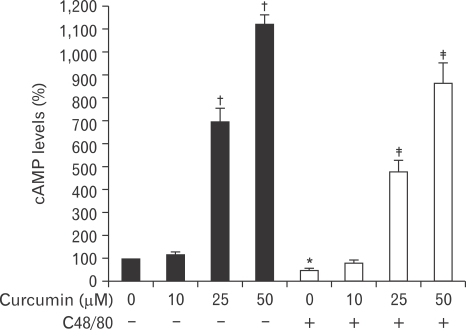
Curcumin increases intracellular cAMP level in RPMCs
The cAMP pathway is believed to be critical for the regulation of mast cell activation. An increase of cAMP is known to precede the inhibition of histamine release from mast cells activated by compound 48/80 (Kaliner & Austen, 1974). To investigate the mechanism of curcumin on the reduction of histamine release from RPMCs stimulated by compound 48/80, we assessed intracellular cAMP levels. Treatment of curcumin alone increased cAMP levels in a dose-dependent manner. Treatment of RPMCs with compound 48/80 showed a significant decrease in cAMP levels as compared to those treated with buffer alone (49.05±3.75% of normal value). However, pretreatment with curcumin blocked the compound 48/80-induced reduction of cAMP in RPMCs (Fig. 5).
Fig. 5.
Effect of curcumin on the compound 48/80-induced decrease of cAMP level in rat peritoneal mast cell (RPMC). Various concentrations of curcumin were added into the RPMCs suspension for 5 minutes, and cAMP levels were measured. Curcumin inhibited the compound 48/80-induced cAMP reduction of RPMCs in a dose-dependent manner. Each data value represents the mean±S.E.M. of five independent experiments. *P<0.05 and †P<0.001, significantly different from the normal value; ‡P<0.001, significantly different from the control value. C48/80: compound 48/80.
Discussion
As part of our studies on bioactive constituents from natural medicines, we previously reported various inhibitors against mast cell degranulation and histamine release induced by compound 48/80 (Choi et al., 2006a; 2006b). In this study, curcumin inhibited compound 48/80-induced systemic anaphylaxis and anti-DNP IgE-mediated PCA. These results indicate that nonspecific and specific mast cell-dependent allergic reactions were significantly inhibited by curcumin. It is well-recognized that compound 48/80 can induce a mast cell-dependent, non-specific anaphylactoid reaction. The mechanism of anaphylactoid response triggered by compound 48/80 is considered to be due to the massive release of vasoactive amines, such as histamine, from mast cells and basophils (Allansmith et al., 1989). As noted, histamine is a typical mediator that causes various pathophysiologic events in acute allergic reactions (Galli, 1993). Thus, it is postulated that curcumin inhibits mast cell-mediated anaphylactoid responses by suppressing histamine release from RPMCs. Compound 48/80 is known to activate mast cell secretory processes by increasing the rate of GTPγS binding to G-proteins (Palomäki & Laitinen, 2006); in turn, the activation of G-proteins can trigger intracellular signaling events such as activation of phospholipase, protein kinase C (PKC) and Ca2+ signaling which ultimately results in the release of histamine from these cells. Inositol 1,4,5-triphosphate then causes the movement of Ca2+ form the endoplasmic reticulum, triggering store-operated Ca2+ entry through specialized Ca2+ release-activated calcium channels (Turner & Kinet, 1999). Degranulation of mast cells is involved in the synergistic activation of PKC and the increase in intracellular calcium concentration. The increase in intracellular Ca2+ induces the movement of granules to the plasma membrane followed by the degranulation of mast cells or basophils and activated formation of inflammatory mediators such as prostaglandins and leukotrienes. Compound 48/80 also stimulates histamine release from RPMCs in both the presence and absence of extracellular calcium. The present study demonstrates that curcumin potently suppressed histamine release probably through the inhibition of the degranulation process following a rise in intracellular Ca2+ levels, in accordance with previous reports (Matsuda et al., 2004; Suzuki et al., 2005; Nugroho et al., 2009).
The cAMP pathway is supposed to be critical to the activation of mast cells. It has been reported that agents that induce the elevation of intracellular cAMP levels can attenuate the stimulated release of mediators from mast cells (Weston & Peachell, 1998). Moreover, several studies have shown an algorithm between cAMP and calcium uptake in RPMCs. In general, increased cAMP inhibits superoxide anion generation via cAMP-dependent protein phosphorylation in RPMCs stimulated by compound 48/80 (Fukuishi et al., 1997). Decreased superoxide anion, as well as cAMP, impedes inositol 1,4,5-triphosphate or GTP-induced calcium release from the endoplasmic reticulum (Yoshii et al., 1988; Akagi et al., 1994). Accordingly, calcium-filling state in the endoplasmic reticulum blocks a calcium influx into RPMCs, which leads to a reduction in the free intracellular calcium content (Hoth & Penner, 1993). Consequently, decreased intracellular calcium prevents histamine release from RPMCs (Yoshii et al., 1988; Akagi et al., 1994). Interestingly, treatment with curcumin significantly increases the cAMP level beyond the basal level. Although the mechanism of curcumin-induced cAMP production has not been elucidated, curcumin may activate adenylate cyclase directly or indirectly, otherwise inhibiting cAMP phosphodiesterase. In addition, curcumin prevents compound 48/80-induced cAMP reduction and calcium uptake of RPMCs in a dose-dependent fashion. According to these observations, the inhibitory mechanism of curcumin on histamine release from compound 48/80-treated RPMCs may be due to an increase of intracellular cAMP. Based upon this information, we speculate that the curcumin-induced increase in cAMP inhibits calcium uptake into RPMCs via the cascade of intracellular events described previously and the subsequent decrease of intracellular calcium content hinders histamine release from RPMCs. In summary, the present data demonstrate that curcumin suppresses both the passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. This is the first study to evaluate the effect of curcumin on the anaphylactoid reaction induced by compound 48/80. Although there are a few differences among the experimental conditions in studies concerning anti-allergic activities of curcumin, our results confirm those of Suzuki and co-workers and Lee and co-workers (Suzuki et al., 2005; Lee et al., 2008). It is well known that reactive species (ROS) are intimately relevant to the induction of inflammation. The ROS generated in macrophages may produce prostaglandins, nitric oxide, and cytokines, leading to the development of inflammation (Haddad et al., 2001). Curcumin was reported to depress the release of ROS from macrophages (Joe & Lokesh, 1994), inhibit the release of histamine from RPMCs (Suzuki et al., 2005), and the release of TNF-α, and IL-4 from RBL-2H3 cells (Matsuda et al., 2004; Lee et al., 2008). These findings indicate that curcumin is closely associated with anti-allergic activities (Kurup & Barrios, 2008). The anti-allergic and anti-oxidative activities of curcumin and related compounds, such as glycosides and bis-demethoxy analogs, have currently been investigated by Suzuki et al. (Suzuki et al., 2005). Their results suggest that most of the compounds develop anti-allergic activities through mechanisms related to both anti-oxidative and non anti-oxidation activities. In conclusions, curcumin may have beneficial effects in the prevention or treatment of mast cell-mediated allergic diseases. The detailed mechanisms of action of curcumin need to be studied further.
Acknowledgements
This work was supported by a Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00014).
References
- 1.Akagi M, Katakuse Y, Fukuishi N, Kan T, Akagi R. Superoxide anion-induced histamine release from rat peritoneal mast cells. Biol Pharm Bull. 1994;17:732–734. doi: 10.1248/bpb.17.732. [DOI] [PubMed] [Google Scholar]
- 2.Allansmith MR, Baird RS, Ross RN, Barney NP, Bloch KJ. Ocular anaphylaxis induced in the rat by topical application of compound 48/80. Dose response and time course study. Acta Ophthalmol Suppl. 1989;192:145–153. doi: 10.1111/j.1755-3768.1989.tb07106.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi YH, Yan GH, Chai OH, et al. Inhibition of anaphylaxis-like reaction and mast cell activation by water extract from the fruiting body of Phellinus linteus. Biol Pharm Bull. 2006a;29:1360–1365. doi: 10.1248/bpb.29.1360. [DOI] [PubMed] [Google Scholar]
- 4.Choi YH, Yan GH, Chai OH, et al. Inhibitory effects of Agaricus blazei on mast cell-mediated anaphylaxis-like reactions. Biol Pharm Bull. 2006b;29:1366–1371. doi: 10.1248/bpb.29.1366. [DOI] [PubMed] [Google Scholar]
- 5.Cochrane DE, Douglas WW. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci USA. 1974;71:408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui SX, Qu XJ, Xie YY, et al. Curcumin inhibits telomerase activity in human cancer cell lines. Int J Mol Med. 2006;18:227–231. [PubMed] [Google Scholar]
- 7.Ennis M, Pearce FL, Weston PM. Some studies on the release of histamine from mast cells stimulated with polylysine. Br J Pharmacol. 1980;70:329–334. doi: 10.1111/j.1476-5381.1980.tb07940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eybl V, Kotyzova D, Koutensky J. Comparative study of natural antioxidants - curcumin, resveratrol and melatonin - in cadmium-induced oxidative damage in mice. Toxicology. 2006;225:150–156. doi: 10.1016/j.tox.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Fukuishi N, Sakaguchi M, Matsuura S, Nakagawa C, Akagi R, Akagi M. The mechanisms of compound 48/80-induced superoxide generation mediated by A-kinase in rat peritoneal mast cells. Biochem Mol Med. 1997;61:107–113. doi: 10.1006/bmme.1997.2594. [DOI] [PubMed] [Google Scholar]
- 10.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 11.Hachisuka H, Nomura H, Sakamoto F, Mori O, Okubo K, Sasai Y. Effect of antianaphylactic agents on substance-P induced histamine release from rat peritoneal mast cells. Arch Dermatol Res. 1988;280:158–162. doi: 10.1007/BF00456847. [DOI] [PubMed] [Google Scholar]
- 12.Haddad JJ, Safieh-Garabedian B, Saade NE, et al. Chemioxyexcitation (delta pO2/ROS)-dependent release of IL-1 beta, IL-6 and TNF-alpha: evidence of cytokines as oxygen-sensitive mediators in the alveolar epithelium. Cytokine. 2001;13:138–147. doi: 10.1006/cyto.2000.0789. [DOI] [PubMed] [Google Scholar]
- 13.Harvima RJ, Harvima IT, Fräki JE. Optimization of histamine radio enzyme assay with purified histamine-N-methyltransferase. Clin Chim Acta. 1988;171:247–256. doi: 10.1016/0009-8981(88)90150-7. [DOI] [PubMed] [Google Scholar]
- 14.Holmegaard SN. Measurement of cyclic AMP in clinical investigations. Acta Endocrinol Suppl (Copenh) 1982;249:1–47. doi: 10.1530/acta.0.1010001-a. [DOI] [PubMed] [Google Scholar]
- 15.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joe B, Lokesh BR. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim Biophys Acta. 1994;1224:255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 17.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaliner M, Austen KF. Cyclic AMP, ATP, and reversed anaphylactic histamine release from rat mast cells. J Immunol. 1974;112:664–674. [PubMed] [Google Scholar]
- 19.Kurup VP, Barrios CS. Immunomodulatory effects of curcumin in allergy. Mol Nutr Food Res. 2008;52:1031–1039. doi: 10.1002/mnfr.200700293. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Kim JW, Ko NY, et al. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J Allergy Clin Immunol. 2008;121:1225–1231. doi: 10.1016/j.jaci.2007.12.1160. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda H, Tewtrakul S, Morikawa T, Nakamura A, Yoshikawa M. Anti-allergic principles from Thai zedoary: structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-23 cells. Bioorg Med Chem. 2004;12:5891–5898. doi: 10.1016/j.bmc.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Nugroho AE, Ikawati Z, Sardjiman, Maeyama K. Effects of benzylidencyclopentanone analogues of curcumine on histamine release from mast cells. Biol Pharm Bull. 2009;32:842–849. doi: 10.1248/bpb.32.842. [DOI] [PubMed] [Google Scholar]
- 23.Palomäki VA, Laitinen JT. The basic secretagogue compound 48/80 activates G proteins indirectly via stimulation of phospholipase D-lysophosphatidic acid receptor axis and 5-HT1A receptors in rat brain sections. Br J Pharmacol. 2006;147:596–606. doi: 10.1038/sj.bjp.0706671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen LJ, Mosbech H, Skov PS. Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: characterization of factors influencing histamine releasability. J Allergy Clin Immunol. 1996;97:672–679. doi: 10.1016/s0091-6749(96)70313-5. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Nakamura T, Iyoki S, et al. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1438–1443. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- 26.Tasaka K, Mio M, Okamoto M. Intracellular calcium release induced by histamine releasers and its inhibition by some antiallergic drugs. Ann Allergy. 1986;56:464–469. [PubMed] [Google Scholar]
- 27.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999;402s(6760 Suppl):B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 28.Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen Pharmacol. 1998;31:715–719. doi: 10.1016/s0306-3623(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 29.Yoshii N, Mio M, Tasaka K. Ca uptake and Ca releasing properties of the endoplasmic reticulum in rat peritoneal mast cells. Immunopharmacology. 1988;16:107–113. doi: 10.1016/0162-3109(88)90019-7. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura T, Hamaguchi E, Usami E, et al. Increased in vitro release of interferon-gamma from ampicillin-stimulated peripheral blood mononuclear cells in Stevens-Johnson syndrome. Biol Pharm Bull. 2004;27:929–931. doi: 10.1248/bpb.27.929. [DOI] [PubMed] [Google Scholar]