Abstract
Zirconium-89 (t1/2 = 3.27 days) is a positron emitting radionuclide which displays excellent potential for use in the design and synthesis of radioimmunoconjugates for immunoPET. In these studies we report the preparation of 89Zr-DFO-J591, a novel 89Zr -labeled monoclonal antibody (mAb) construct for targeted immunoPET imaging and quantification of prostate-specific membrane antigen (PSMA) expression in vivo.
Methods
The in vivo behavior of [89Zr]Zr-chloride, [89Zr]Zr-oxalate and [89Zr]Zr-DFO was investigated by using PET imaging. High level computational studies using density functional theory (DFT) calculations have been used to investigate the electronic structure of [89Zr]Zr-DFO and probe the nature of the complex in aqueous conditions. J591 was functionalized with the hexadentate, tris-hydroxamate ligand desferrioxamine B (DFO) and radiolabeled with [89Zr]Zr-oxalate at room temperature. ImmunoPET imaging experiments in male, athymic nu/nu mice bearing sub-cutaneous LNCaP (PSMA positive) or PC-3 (PSMA negative) tumors were conducted. The change in 89Zr-DFO-J591 tissue uptake in response to high- and low-specific-activity formulations in the two tumor models was measured by using acute biodistribution studies and immunoPET.
Results
Basic characterization of three important reagents, [89Zr]Zr-chloride and [89Zr]Zr-oxalate, as well as the complex, [89Zr]Zr-DFO, demonstrated that the nature of the 89Zr species has a dramatic effect on the biodistribution and pharmacokinetics. DFT calculations provide a rationale for the observed high in vivo stability of 89Zr-DFO-labeled mAbs and suggest that in aqueous conditions, [89Zr]Zr-DFO forms a thermodynamically stable, 8-coordinate complex by coordination of two water molecules. 89Zr-DFO-J591 was produced in high radiochemical yield (>77%) with radiochemical purity >99% and a specific-activity of 181.7±1.1 MBq/mg (4.91±0.03 mCi/mg). In vitro assays demonstrated that 89Zr-DFO-J591 had an initial immunoreactive fraction of 0.95±0.03 and remains active for up to 7 days. In vivo biodistribution experiments revealed high uptake of 89Zr-DFO-J591 in LNCaP tumors after 24, 48, 96 and 144 h (34.4±3.2 %ID/g; 38.0±6.2 %ID/g; 40.4±4.8 %ID/g; and 45.8±3.2 %ID/g, respectively). Specificity for PSMA expression was confirmed by biodistribution studies in PC-3 (PSMA negative) tumor models and by using low specific activity competitive inhibition studies. ImmunoPET studies also demonstrated that 89Zr-DFO-J591 provides excellent image contrast with tumor-to-muscle ratios >20 for delineation of LNCaP tumors between 48 – 144 h post-administration.
Conclusion
These experimental and computational studies demonstrate that 89Zr-DFO-labeled mAbs show exceptional promise as radiotracers for immunoPET imaging of human cancers. 89Zr-DFO-J591 displays tumor-to-background tissue contrast in immunoPET and can be used to delineate and quantify PSMA-positive prostate tumors in vivo.
Keywords: ImmunoPET, zirconium-89, prostate-specific membrane antigen (PSMA), J591, monoclonal antibodies, density functional theory
Introduction
The National Cancer Institute (NCI) estimates that in the United States during 2009, approximately 192,000 cases of prostate cancer (PC) will have been diagnosed with a projected mortality rate of over 27,000 men (>14%). Despite the high incidence of PC, standard diagnostic imaging techniques used for the detection and monitoring of therapy remain inadequate. For example, early stage, hormonally sensitive tumors on treatment, and non-castrate PCs often appear negative in positron emission tomography (PET) scans using either the metabolic radiotracer, [18F]-FDG or the hormone-based radiopharmaceutical, 16β-[18F]-dihydrotestosterone ([18F]-FDHT) for imaging overexpression of androgen receptors.(1) Therefore, there is an urgent requirement to develop new tools for non-invasive delineation and staging of PC in vivo.
Prostate-specific membrane antigen (PSMA) is a 100 kDa, type II transmembrane glycoprotein and is one of the best characterized oncogenic markers/targets.(2, 3) PSMA expression has been detected in a limited range of normal tissues including benign prostatic epithelium, renal proximal tubule, small bowel and brain (a subset of astrocytes). However, these normal sites express PSMA at levels 2–3 orders of magnitude lower than that observed in more than 95% of clinical PC specimens.(4, 5) In addition, as these normal tissue PSMA sites are highly polarized to the apical/luminal aspect of the benign prostatic glands, renal tubules and small bowel, basement membrane and epithelial tight junctions they form substantial barriers to circulating mAbs. PSMA expression by astrocytes is similarly sequestered behind the blood-brain barrier. As a result, antibodies to PSMA are functionally tumor-specific, whereas small molecule PSMA ligands excreted via the renal tubular lumen are not.
PSMA expression levels have been shown exhibit a positive correlation with increased tumor aggression, metastatic spread and the development of castrate-resistance or resistance to hormone-based therapies. PSMA expression has also been reported in the neovasculature of most solid tumors.(6) The failure of [18F]-FDG-PET for detecting early and treated PC, and the acquired resistance of many advanced PCs to androgen-based agents has been the driving force behind recent efforts towards developing novel chemo- and radioimmunoconjugate based drugs and imaging agents. In particular, in 1996 the United States Food and Drug Administration (US-FDA) approved the use of [111In]-ProstaScint ([111In]-Capromab pendetide or [111In]-7E11), a murine-mAb specific for an intracellular epitope of PSMA, for single-photon emission computed tomography (SPECT) imaging of PC soft tissue metastases. However, [111In]-ProstaScint for clinical diagnosis is sub-optimal because of low sensitivity for viable tumor sites (62% for lymph node metastases; 50% for prostate bed recurrence) which is probably because the number of available targets (presented in dead/dying tissue) is limited. Furthermore, [111In]-ProstaScint does not bind to viable PC sites in bone (the most common site of metastatic disease), and in contrast to PET, SPECT imaging remains only semi-quantitative in the clinical setting. Despite these limitations, [111In]-ProstaScint has its supporters, and is still recommended as being useful for specific clinical situations by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines.(5) The NCCN recommends the use of [111In]-ProstaScint before salvage therapy after radiotherapy or prostatectomy. This fact is testament to the relative lack of better imaging methods for detection of metastatic prostate cancer, especially in soft-tissue.
In 1997, Liu et al. produced 4 mAbs (IgG1: J415, J533 and J591; and IgG3: E99) specific for binding to two distinct epitopes (PSMAext1 and PSMAext2) on the extracellular domain of PSMA.(7, 8) Subsequent in vitro and in vivo studies by Smith-Jones et al.(9, 10) and McDevitt et al.(11) identified J591 as the most promising candidate for developing diagnostic and therapeutic immunoconjugates targeting extracellular PSMA in viable tissue. Since these initial studies, J591 has been humanized and a range of preclinical and clinical studies using J591 radiolabeled with 90Y, 177Lu or 131I for β-therapy, 213Bi and 225Ac for α-therapy, and 111In for SPECT imaging have been reported.(9–20)
Positron Emission Tomography has advantages over SPECT imaging in terms of sensitivity and contrast resolution, especially for tissues at a depth within the body, and these improved imaging characteristics are particularly important for radioimmuno-imaging. The work presented here describes the production and preclinical evaluation of 89Zr-radiolabeled J591 for targeted immunoPET imaging of PSMA-positive tumors in vivo. The new radiopharmaceutical, 89Zr-DFO-J591, has been characterized by a range of stability and cellular association assays in vitro. The nature of the electronic structure of [89Zr]Zr-DFO complex has been explored by using high level density functional theory (DFT) calculations and the computational results provide a rationale for the high experimentally observed in vitro and in vivo stability of the [89Zr]Zr-DFO-labeled radioimmunoconjugates. The ability of 89Zr-DFO-J591 to target PSMA expressing tissue has also been examined by using acute biodistribution studies and immunoPET imaging in vivo. The results demonstrate that 89Zr-DFO-J591 has potential to be used in the clinic as a radiotracer for both improved localization and staging of PSMA-positive tumors.
Materials and Methods
All chemicals, unless otherwise stated, were purchase from SigmaAldrich (St. Louis, MO) and were used as received. Water (>18.2 MΩ·cm at 25 °C, Milli-Q, Millipore, Billerica, MA) was purified by passing through a 10 cm column of chelex resin (Bio-Rad Laboratories, Hercules, CA) at a flow rate <1.0 mL/min. All instruments were calibrated and maintained in accordance with previously reported routine quality-control procedures.(21) Radioactivity measurements were made by using a Capintec CRC-15R Dose Calibrator (Capintec, Ramsey, NJ) with a calibration factor of 465 for 89Zr. For accurate quantification of radioactivities, experimental samples were counted for 1 min. on a calibrated Perkin Elmer (Waltham, MA) Automatic Wizard2 Gamma Counter by using a dynamic energy window of 800–1000 keV for 89Zr (909 keV emission). 89Zr-radiolabeling reactions were monitored by using silica-gel impregnated glass-fibre instant thin-layer chromatography (ITLC-SG) paper (Pall Corp., East Hills, NY) and analyzed on a radio-TLC plate reader (Bioscan System 200 Imaging Scanner coupled to a Bioscan Autochanger 1000 (Bioscan Inc., Washington, DC, using Win-Scan Radio-TLC software version 2.2). Solvent systems included diethylene triamine pentaacetic acid in water (DTPA, 50 mM, pH7) and phosphate buffered saline (PBS). Human prostate cancer cell lines LNCaP and PC-3 were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were grown by serial passage.
Density functional theory calculations
All calculations were conducted using density functional theory (DFT) as implemented in the Gaussian03 suite of ab initio quantum chemistry programs.(22) Full computational details and Cartesian coordinates of the optimized structures are presented in the supporting information. Energetic values are reported in S.I. units of kJ mol−1.
Antibody conjugation and radiolabeling
The IgG1 monoclonal antibody J591 was conjugated to the tris-hydroxamate, hexadentate chelate, desferrioxamine B (DFO, Calbiochem, Spring Valley, CA) by using a 6-step procedure modified from that described by Verel et al.(23) Full details are provided in the supporting information.
Zirconium-89 was produced via the 89Y(p,n)89Zr transmutation reaction on an EBCO TR19/9 variable beam energy cyclotron (Ebco Industries Inc., Richmond, British Columbia, Canada) in accordance with previously reported methods.(23, 24) The [89Zr]Zr-oxalate was isolated in high radionuclidic and radiochemical purity (RCP) >99.9%, with an effective specific-activity of 195–497 MBq/µg, (5.28–13.43 mCi/µg).(24)
89Zr-DFO-J591 was prepared by the complexation of [89Zr]Zr-oxalate with DFO-J591. Typical radiolabeling reactions were conducted in accordance with the following procedure. Briefly, [89Zr]Zr-oxalate (153.2 MBq, [4.14 mCi]) in 1.0 M oxalic acid (170 µL) was adjusted to pH7.7–8.1 with 1.0 M Na2CO3(aq.). CAUTION: Acid neutralization releases CO2(g) and care should be taken to ensure that no radioactivity escapes the microcentrifuge vial. After CO2(g) evolution ceased, DFO-J591 (400 µL, 2.1 mg/mL [0.84 mg of mAb], in 0.9% sterile saline) was added and the reaction was mixed gently by aspirating with a pipette. The reaction was incubated at room temperature for between 1–2 h and complexation progress was monitored with respect to time by ITLC (DTPA, 50 mM, pH7). After 1 h, crude radiolabeling yields and RCP was >95%. 89Zr-DFO-J591 was purified by using either size-exclusion chromatography (Sephadex G-25 M, PD-10 column, >30 kDa, GE Healthcare; dead-volume = 2.5 mL, eluted with 200 µL fractions of 0.9% sterile saline) or spin-column centrifugation (4 mL total volume, >30 kDa, Amicon Ultra-4, Millipore, Billerica, MA; washed with 4×3 mL, 0.9% sterile saline). The radiochemical purity (RCP) of the final 89Zr-DFO-J591 (>77% radiochemical yield; formulation: pH5.5–6.0; <500 µL; 0.9% sterile saline) was measured by both radio-ITLC and analytical size-exclusion chromatography (loading <0.74 MBq [20 µCi], ca. 5–10 µL aliquots) and was found to be >99% in all preparations. In the ITLC experiment 89Zr-DFO-J591 and [89Zr]Zr-DFO remain at the baseline (Rf = 0.0), whereas 89Zr4+(aq.) ions and [89Zr]Zr-DTPA elute with the solvent front (Rf = 1.0).
Chelate number
The number of accessible DFO chelates conjugated to J591 was measured by radiometric isotopic dilution assays following a method similar to that described by Anderson et al.(25) From a stock solution, aliquots of [89Zr]Zr-oxalate (10 µL, 660 kBq [18 µCi], pH7.7–8.1 [pH adjusted using 1.0 M Na2CO3]) were added to 10 solutions containing 1:2 serial dilutions of non-radioactive ZrCl4(aq.) (50 µL fractions; 250–0.5 pmol, pH7.7–8.1). The mixture was vortexed for 30 s before adding aliquots of DFO-J591 (5 µL, 2.1 mg/mL, [10.5 µg of mAb, 0.07 nmol], in 0.9% saline). The reactions were incubated at room temperature for 2 h before quenching with DTPA (20 µL, 50 mM, pH7). Control experiments confirmed that 89Zr complexation to DFO-J591 was complete within <2 h. The extent of complexation was assessed by developing ITLC strips (DTPA, 50 mM) and counting the activity at the baseline and solvent front. The fraction of 89Zr-radiolabeled mAb (Ab) was plotted versus the amount of non-radioactive ZrCl4 added. The number of chelates was calculated by using linear regression analysis to calculate the concentration of ZrCl4 at which only 50% of the DFO-J591 was labeled, multiplying by a factor of 2, and then dividing by the moles of mAb present in the reaction.
Immunoreactivity
The immunoreactive fraction of 89Zr-DFO-J591 was determined by using specific radioactive cellular-binding assays following modified procedures derived from Lindmo et al.(26) Briefly, LNCaP or PC-3 cells were suspended in micro-centrifuge tubes at concentrations of 5.0, 4.0, 3.0, 2.5, 2.0, 1.5, and 0.5 ×106 cells/mL in 500 µL PBS (pH7.4). Aliquots (50 µL, <0.37 kBq, [<0.01 µCi]) of 89Zr-DFO-J591 in 1% bovine serum albumin (BSA) were added to each tube (n = 3; final volume: 550 µL) and the samples were incubated on an orbital mixer for 60 min. at room temperature. Cells were then pelleted by centrifugation (600G for 2 min.), resuspended and washed twice with ice-cold PBS before removing the supernatant and counting the 89Zr-radioactivity associated with the cell pellet. The count data were background corrected and compared with the total number of counts in control samples. Non-specific binding was assessed by using the same methods but with PC-3 (PSMA-negative) cells in place of LNCaP cells. Immunoreactive fractions were determined by linear regression analysis of a plot of (total/bound) activity versus (1/[normalized number of cells]), and calculated as 1/y-intercept.
Stability studies
The stability of 89Zr-DFO-J591 with respect to change in radiochemical purity, loss of radioactivity from the mAb and/or change in immunoreactivity was investigated in vitro by incubation in solutions of 0.9% saline, and 1% BSA for 7 days at 37°C. The radiochemical purity was determined by radio-ITLC and γ-counting, and the immunoreactive fraction was measured by using the LNCaP cellular-binding assay (vide supra).
Xenograft models
All animal experiments were conducted in compliance with Institutional Animal Care and Use Committee (IACUC) guidelines. Male athymic nu/nu mice (NCRNU-M, 20–22 g, 6–8 weeks old) were obtained from Taconic Farms Inc. (Hudson, NY), and were allowed to acclimatize at the MSKCC vivarium for 1 week prior to implanting tumors. Mice were provided with food and water ad libitum. LNCaP tumors were induced on the left shoulder by sub-cutaneous (s.c.) injection of 4.0×106 cells in a 200 µL cell suspension of a 1:1 v/v mixture of media with reconstituted basement membrane (BD Matrigel™, Collaborative Biomedical Products Inc., Bedford, MA). Similarly, PC-3 tumors were induced on the right shoulder by s.c. injection of 5.0×106 cells. Palpable LNCaP tumors (50–250 mm3) developed after a period of 24–30 days. PC-3 tumors (70–90 mm3) grew more rapidly with more uniform size distribution and were suitable for use for in vivo studies after 10–14 days. The tumor volume (V / mm3) was estimated by external vernier caliper measurements of the longest axis, a / mm, and the axis perpendicular to the longest axis, b / mm. The tumors were assumed to be spheroidal and the volume was calculated in accordance with Equation 1.(27)
| (1) |
Acute biodistribution studies
Acute in vivo biodistribution studies were conducted to evaluate the uptake of 89Zr-DFO-J591 in mice bearing s.c. LNCaP (50–250 mm3) or PC-3 (70–90 mm3) tumors. Mice were randomized before the study and were warmed gently with a heat lamp 5 min. before administering 89Zr-DFO-J591 (0.55–0.74 MBq, [15–20 µCi], 3–4 µg of mAb, in 200 µL 0.9% sterile saline for injection) via intravenous (i.v.) tail-vein injection (t=0 h). Animals (n = 3–5, per group) were euthanized by CO2(g) asphyxiation at 24, 48, 96 and 144 h post-injection and 12 organs (including the tumor) were removed, rinsed in water, dried in air for 5 min., weighed and counted on a gamma-counter for accumulation of 89Zr-radioactivity. The mass of 89Zr-DFO-J591 formulation injected into each animal was measured and used to determine the total number of counts (counts per minute, [c.p.m.]) by comparison to a standard syringe of known activity and mass. Count data were background- and decay-corrected and the percentage injected dose per gram (%ID/g) for each tissue sample was calculated by normalization to the total amount of activity injected.
Competitive inhibition studies were also performed in vivo to investigate the specificity of 89Zr-DFO-J591 for PSMA. Non-radiolabeled J591 (5 mg/kg solution in 0.9% sterile saline, 0.3 mg/mouse) was added to the 89Zr-DFO-J591 formulation to reduce the specific-activity (60-fold decrease: 3.04 MBq/mg [0.082 mCi/mg]) and biodistribution studies were performed at 48 h post-i.v. administration (n=4).
Small-animal immunoPET imaging
PET imaging experiments were conducted on a microPET Focus 120 scanner (Concorde Microsystems).(28) Mice were administered 89Zr-DFO-J591 formulations (10.9–11.3 MBq, [295–305 µCi], 60–62 µg of mAb, in 200 µL 0.9% sterile saline for injection) via i.v. tail-vein injection. Approximately 5 minutes prior to recording PET images, mice were anesthetized by inhalation of 1% isoflurane (Baxter Healthcare, Deerfield, IL)/oxygen gas mixture and placed on the scanner bed. PET images were recorded at various time-points between 3–144 h post-injection. List-mode data were acquired for between 10 and 30 min. using a γ-ray energy window of 350–750 keV, and a coincidence timing window of 6 ns. For all static images, scan time was adjusted to ensure a minimum of 20 million coincident events were recorded. Data were sorted into 2-dimensional histograms by Fourier re-binning, and transverse images were reconstructed by filtered back-projection (FBP) into a 128×128×63 (0.72×0.72×1.3 mm) matrix. The reconstructed spatial resolution for 89Zr was 1.9 mm full-width half maximum (FWHM) at the center of the field-of-view (FOV). The image data were normalized to correct for non-uniformity of response of the PET, dead-time count losses, positron branching ratio, and physical decay to the time of injection but no attenuation, scatter, or partial-volume averaging correction was applied. An empirically determined system calibration factor (in units of [mCi/mL]/[cps/voxel]) for mice was used to convert voxel count rates to activity concentrations. The resulting image data were then normalized to the administered activity to parameterize images in terms of %ID/g. Manually drawn 2-dimensional regions-of-interest (ROIs) or 3-dimensional volumes-of-interest (VOIs) were used to determined the maximum and mean %ID/g (decay corrected to the time of injection) in various tissues.(29) Images were analyzed by using ASIPro VM™ software (Concorde Microsystems).
Full details of PET imaging of [89Zr]Zr-chloride, [89Zr]Zr-oxalate ([89Zr(C2O4)4]4−) and [89Zr]-Zr-DFO are presented in the supporting information.
Statistical analysis
Data were analysed by using the unpaired, two-tailed Student’s t-test. Differences at the 95% confidence level (P<0.05) were considered to be statistically significant.
Results and discussion
Basic characterization of 89Zr-species in vivo
Prior to commencing full studies on 89Zr-radiolabeled mAbs, it is important to understand the in vivo behavior of various 89Zr-labeled species including the starting reagents, and potential impurities or metabolites. Therefore, we examined the biodistribution of [89Zr]Zr-chloride and [89Zr]Zr-oxalate, and the complex, [89Zr]Zr-DFO by using PET imaging (Figure 1). Maximum intensity projection (MIP) images of [89Zr]Zr-chloride and [89Zr]Zr-oxalate (300 µCi, 200 µL 0.9% sterile saline) were recorded at 24 h post-i.v. tail-vein injection in male, athymic, nu/nu mice.(24) [89Zr]Zr-chloride was found to be sequestered in the liver with little excretion (Figure S1). In contrast, administration of [89Zr]Zr-oxalate (most likely present as the thermodynamically stable species, [89Zr(C2O4)4]4−) showed high accumulation of 89Zr-radioactivity in bones, joints and potentially cartilage (Figure S2). Previous dynamic PET imaging studies on [89Zr]Zr-DFO demonstrated that this complex is excreted rapidly within 20 min. via a renal pathway with a measured biological half-life (t1/2.biol) of 305±6 s (Figures S3 and S4).
Figure 1.
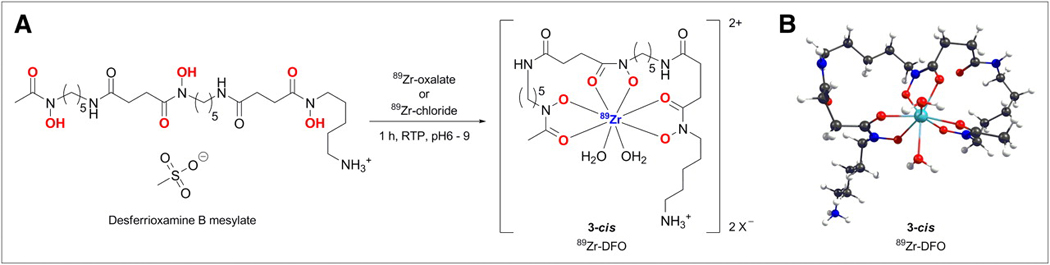
(A) Complexation reaction between [89Zr(C2O4)4]4− and DFO. (B) DFT-optimized structure of 8-coordinate complex [89Zr(HDFO)-cis-(H2O)2]2+ (3-cis).
Density functional theory calculations
The complexation reaction between [89Zr]Zr-chloride or [89Zr]Zr-oxalate with the hexadentate, trihydroxamate chelate, desferrioxamine B (DFO) is shown in Scheme 1. In contrast to the more familiar coordination chemistry of radionuclides such as 64Cu, 68Ga, 111In and 86/90Y, 89Zr displays a number of distinct differences. In particular, the 4+ oxidation state imparts a strong preference for 89Zr4+ ions to bind to highly electronegative (class a) hard donor atoms including oxygen, nitrogen and fluoride. Zirconium complexes have a high propensity towards hydrolysis in aqueous solution. In addition, the relatively large ionic radius of 89Zr4+ allows the first coordination sphere to accommodate up to 8-donor atoms. In this respect, the ionic nature of most Zr4+ complexes and the higher coordination numbers means that the chemistry more closely resembles that of radio-lanthanides and radio-actinides such as 177Lu and 225Ac.
As a consequence of the complicated aqueous-phase chemistry, the nature of the most 89Zr4+(aq.) species, including the [89Zr]Zr-DFO complex is uncertain. Unfortunately, all attempts at crystallizing [89Zr]Zr-DFO have been unsuccessful. Therefore, we conducted high level density functional theory (DFT) calculations to probe the structure, bonding and chemical/thermodynamic stability of [89Zr]Zr-DFO with respect to expansion of the coordination sphere from 6- to 7- or 8-coordinate with the addition of one or two water molecules.
Structures of the octahedral complex [89Zr(HDFO)]2+ (1), the 7-coordinate complexes with mono-H2O coordination in the axial and equatorial sites [89Zr(HDFO)-ax-(H2O)]2+ (2-ax) and [89Zr(HDFO)-eq-(H2O)]2+ (2-eq), and the 8-coordinate bis-H2O complex [89Zr(HDFO)-cis-(H2O)2]2+ (3-cis) were fully optimized using DFT (Cartesian coordinates are given in the supporting information: Tables S1 – S5). Selected structural parameters for complexes 1 – 3 are presented in Table 1 and their relative energies and optimized structures are shown in the reaction coordinate diagram (Figures 2 and S5). The calculations reveal that expansion of the coordination sphere to either 7- or 8-coordinate by the addition of one or two water molecules is thermodynamically favorable. Interestingly, in the 7-coordinate species the axial and equatorial coordination sites, 2-ax and 2-eq, are energetically inequivalent. Axial coordination (2-ax) is thermodynamically more favorable than equatorial coordination (2-eq) by around −41 kJ mol−1. The DFT calculations also suggest that the 8-coordinate complex with cis-coordination geometry with respect to the orientation of the H2O molecules (3-cis) is 95 kJ mol−1 more stable that the parent octahedral complex (1). Furthermore, complex 3-cis is 14 kJ mol−1 more stable than the sum of the thermodynamic stabilization of achieved by complexes 2-ax and 2-eq (sum = −81 kJ mol−1). This additional stability of complex 3-cis arises due to structural relaxation from the cooperativity of two coordinated H2O ligands which allows the r(Zr-OH2(ax)) to decrease from 2.362 Å in complex 2-ax to 2.33 Å in complex 3-cis (Table 1). Natural bond order (NBO) charge analysis (Table S6) also supports the conclusion that thermodynamic stabilization of complex 3-cis arises from increased electrostatic interaction between the Zr4+ ion and axial-H2O ligand (ligand-to-metal charge transfer). NB: It should be noted that we expect the coordinated H2O ligands to be kinetically labile and species 1 – 3 are likely in rapid equilibrium at physiological temperatures.
TABLE 1.
Biodistribution Data of 89Zr-DFO-J591, Administered Intravenously to Mice Bearing Subcutaneous LNCaP Tumors
| Organ | 24 h (n = 4) |
48 h (n = 5) |
96 h (n = 5) |
144 h (n = 4) |
Block (300 µg of mAb) at 48 h (n = 4) |
|---|---|---|---|---|---|
| Blood | 21.8 ± 2.8 | 4.4 ± 1.9 | 1.4 ± 0.8 | 2.6 ± 1.5 | 10.7 ± 0.4 |
| Tumor | 34.4 ± 3.2 | 38.0 ± 6.2 | 40.4 ± 4.8 | 45.8 ± 3.2 | 10.3 ± 0.8 |
| Heart | 7.4 ± 2.2 | 4.0 ± 1.3 | 1.7 ± 0.6 | 1.4 ± 0.5 | 3.8 ± 0.7 |
| Lung | 11.7 ± 1.9 | 5.7 ± 3.1 | 2.2 ± 0.9 | 2.5 ± 0.9 | 5.7 ± 0.3 |
| Liver | 11.7 ± 1.5 | 17.7 ± 1.6 | 17.2 ± 2.7 | 11.2 ± 1.6 | 5.1 ± 0.4 |
| Spleen | 8.8 ± 4.3 | 21.1 ± 0.3 | 24.6 ± 1.8 | 4.6 ± 2.4 | 3.1 ± 0.7 |
| Kidney | 10.1 ± 1.0 | 7.5 ± 1.5 | 5.1 ± 0.5 | 5.3 ± 0.5 | 5.1 ± 0.2 |
| Muscle | 1.1 ± 0.1 | 0.6 ± 0.3 | 0.4 ± 0.4 | 0.2 ± 0.2 | 0.8 ± 0.2 |
| Bone | 4.0 ± 0.8 | 8.2 ± 1.2 | 8.7 ± 1.5 | 7.4 ± 1.3 | 2.4 ± 0.3 |
| Tumor/blood | 1.6 ± 0.2 | 8.7 ± 4.1 | 29.7 ± 17.1 | 18.0 ± 10.5 | 1.0 ± 0.1 |
| Tumor/heart | 4.7 ± 1.5 | 9.6 ± 3.5 | 23.4 ± 9.0 | 31.9 ± 10.7 | 2.7 ± 0.5 |
| Tumor/lung | 2.9 ± 0.5 | 6.7 ± 3.7 | 18.4 ± 7.7 | 18.5 ± 6.8 | 1.8 ± 0.2 |
| Tumor/liver | 2.9 ± 0.5 | 2.1 ± 0.4 | 2.3 ± 0.5 | 4.1 ± 0.7 | 2.0 ± 0.2 |
| Tumor/spleen | 3.9 ± 1.9 | 1.8 ± 0.3 | 1.6 ± 0.2 | 9.9 ± 5.2 | 3.3 ± 0.8 |
| Tumor/kidney | 3.4 ± 0.5 | 5.1 ± 1.3 | 7.9 ± 1.2 | 8.6 ± 1.1 | 2.0 ± 0.2 |
| Tumor/muscle | 32.4 ± 4.6 | 59.2 ± 28.8 | 95.9 ± 95.3 | 306.4 ± 432.2 | 13.3 ± 3.1 |
| Tumor/bone | 8.7 ± 1.9 | 4.7 ± 1.0 | 4.6 ± 1.0 | 6.2 ± 1.2 | 4.3 ± 0.6 |
Complete biodistribution data are presented in Supplemental Table 7. Data are expressed as mean % ID/g ± SD. Errors for tumor-to-tissue ratios are calculated as geometric mean of SD. LNCaP tumors: 3–4 µg mAb; PSMA-positive, 50–250 mm3.
Figure 2.
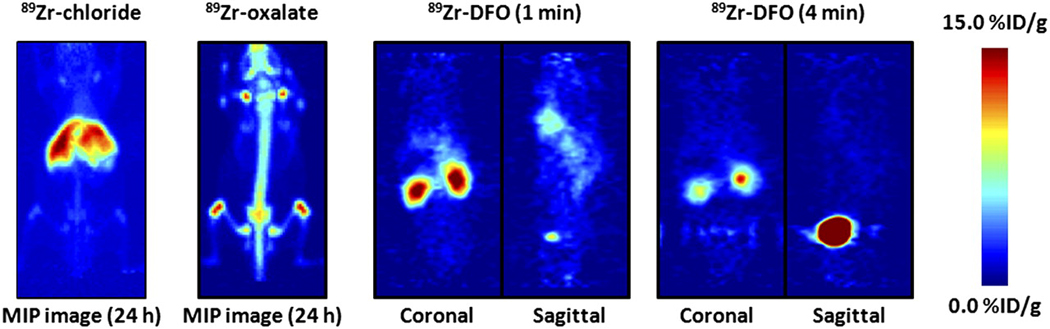
PET images showing maximum intensity projection of 89Zr-chloride and 89Zr-oxalate at 24 h after intravenous administration and dynamic PET images of 89Zr-DFO at 1 and 4 min after injection. For maximum-intensity-projection images, upper and lower intensity thresholds were set at 100% and 0%, respectively. Further details are presented in Supplemental Figures 3–6. MIP = maximum intensity projection.
Previous studies have shown that although DTPA can be used for chelation and radiolabeling of mAbs with 89Zr4+ ions, demetalation occurs in vivo, and although less than optimal, DFO remains the chelate of choice.(24, 30, 31) Experimental studies on 89Zr-DFO-mAbs have reported high in vivo stability with respect to demetalation/ligand dissociation, and relatively low levels of radiotracer accumulation in background tissue.(32–37) These DFT studies suggest that the origin of the observed in vivo stability of 89Zr-DFO-mAbs is a combination of the inherently high thermodynamic and kinetic stability of the [89Zr]Zr-DFO complex due to very strong electrostatic interactions, coupled with the enhancement in thermodynamic stability induced by expansion of the first coordination sphere and geometry relaxation to give an 8-coordinate species. Indeed, in the case of [89Zr]Zr-DFO, and in contrast to the more familiar Cu, Ga, In, and Y complexes, these studies indicate that the presence of water or, for example, coordinating anions such as chloride, actually increases the thermodynamic stability of the Zr complex.
Radiochemistry
J591 was functionalized with DFO by using bioconjugation methods modified from the pioneering work of Verel et al.(23) The conjugation and purification chemistry was found to proceed in moderate-to-high yield (65±5%) with high chemical purity (>95%). Radiolabeling of DFO-J591 with [89Zr]Zr-oxalate was achieved at room temperature in slightly alkaline solutions (pH7.7–8.1) with crude radiochemical yields (>95%, n=6). Facile purification of 89Zr-DFO-J591 from small-molecule radiolabeled impurities was achieved by using either size-exclusion chromatography or spin-column centrifugation. The final radiochemical yield of the purified 89Zr-DFO-J591 was >77% and the product was formulated in 0.9% sterile saline with RCP >99% (n=6) and a specific-activity of 181.7±1.1 MBq/mg (4.91±0.03 mCi/mg) of mAb (Figures S6 and S7). The specific-activity obtained in these studies compares favorably with the previously reported specific-activities of other 89Zr-radiolabeled mAbs.(32–39) Isotopic dilution assays revealed an average of 3.9±0.3 accessible chelates per mAb.
89Zr-DFO-J591 immunoreactivity and stability studies in vitro
The immunoreactive fraction of the 89Zr-DFO-J591 formulations was measured by specific in vitro cellular association assays using LNCaP (PSMA-positive) cells prior to each in vivo experiment (Figure S8).(26) In studies using [213Bi]-labeled J591, McDevitt et al. reported that the LNCaP cell line has an estimated 180,000 PSMA molecules per cell.(11) However, in other studies using 111In and 131I-labeled mAbs (including J415, J533, J591 and 7E11), Smith-Jones et al. found higher PSMA copy numbers of between 600,000 – 800,000 sites/cell for viable LNCaP cells.(9) The average immunoreactive fraction of 89Zr-DFO-J591 was found to be 0.95±0.03 (n=4). Control experiments (n=4) using the PC-3 (PSMA-negative) cell line showed no binding which further demonstrated the specificity of 89Zr-DFO-J591 for PSMA expressing cells.
Incubation of 89Zr-DFO-J591 in either 0.9% saline or 1% BSA for 7 days at 37°C revealed <2% decrease in RCP (via demetalation) with an observed ~17% decrease in the immunoreactive fraction for the 1% BSA experiment (0.78±0.03, Figure S9). Therefore, in the absence of specific proteolysis or reductive/oxidative metabolism, 89Zr-DFO-J591 is expected to remain intact and immunoreactive in vivo on a time-scale suitable for immunoPET imaging.
Biodistribution studies
The ability of 89Zr-DFO-J591 to target an extracellular epitope of the PSMA type II transmembrane glycoprotein receptor in vivo was initially assessed by conducting acute biodistribution studies in LNCaP tumor-bearing mice at 24, 48, 96 and 144 h post-i.v. administration (Tables 2 and S7, and Figure 3). The data reveal that high LNCaP tumor uptake was observed 24 h post-injection (34.4±3.2 %ID/g) with a steady increase through 48 h (38.0±6.2 %ID/g) and 96 h (40.4±4.8 %ID/g), and reaching 45.8±3.2 %ID/g at 144 h (P=0.01 between LNCaP uptake at 24 and 144 h). This high accumulation of 89Zr-DFO-J591 is consistent with extraction of the activity from the blood pool (24 h: 21.8±2.8 %ID/g, 48 h: 4.4±1.9 %ID/g and 96 h: 1.4±0.8), rapid internalization of the J591-PSMA complex followed by sequestration of the 89Zr-radioactivity inside the cell. In contrast, 89Zr-DFO-J591 uptake in the PC-3 (PSMA negative) tumors at 48 h (15.6±2.1 %ID/g, P=0.0025) and 96 h (24.0±2.6 %ID/g, P=0.0017) showed statistically significant decrease in 89Zr-accumulation compared with uptake in PSMA-positive tumors. In mice bearing PC-3 tumors, the 89Zr-DFO-J591 activity in the blood remained 4-fold higher at 48 h (19.0±1.1 %ID/g, P=0.001) and 10-fold at 96 h (13.0±1.8 %ID/g, P<0.05) compared with the corresponding LNCaP mice (48 h: 4.4±1.9 %ID/g and 96 h: 1.4±0.8 %ID/g).
TABLE 2.
Biodistribution Data of 89Zr-DFO-J591, Administered Intravenously to Mice Bearing Subcutaneous PC-3 Tumors (3–4 µg of mAb)
| Organ | 48 h (n = 4) | 96 h (n = 3) |
|---|---|---|
| Blood | 19.0 ± 1.1 | 13.0 ± 1.8 |
| Tumor | 15.6 ± 2.1 | 24.0 ± 2.6 |
| Heart | 6.8 ± 0.1 | 4.3 ± 0.9 |
| Lung | 12.6 ± 1.9 | 7.0 ± 2.3 |
| Liver | 12.4 ± 0.9 | 11.0 ± 1.6 |
| Spleen | 10.2 ± 2.0 | 7.2 ± 0.7 |
| Kidney | 10.5 ± 0.9 | 6.9 ± 1.6 |
| Muscle | 1.5 ± 0.4 | 0.9 ± 0.2 |
| Bone | 4.3 ± 0.6 | 5.1 ± 0.5 |
| Tumor/blood | 0.8 ± 0.1 | 1.8 ± 0.3 |
| Tumor/heart | 2.3 ± 0.3 | 5.6 ± 1.4 |
| Tumor/lung | 1.2 ± 0.3 | 3.4 ± 1.2 |
| Tumor/liver | 1.3 ± 0.2 | 2.2 ± 0.4 |
| Tumor/spleen | 1.5 ± 0.4 | 3.4 ± 0.5 |
| Tumor/kidney | 1.5 ± 0.2 | 3.5 ± 0.9 |
| Tumor/muscle | 10.4 ± 3.3 | 25.4 ± 5.8 |
| Tumor/bone | 3.6 ± 0.7 | 4.7 ± 0.7 |
Complete biodistribution data are presented in Supplemental Table 7. Data are expressed as mean %ID/g ± SD. Errors for tumor-to-tissue ratios are calculated as geometric mean of SD. PC-3 tumors: PSMA-negative, 70–90 mm3.
Figure 3.
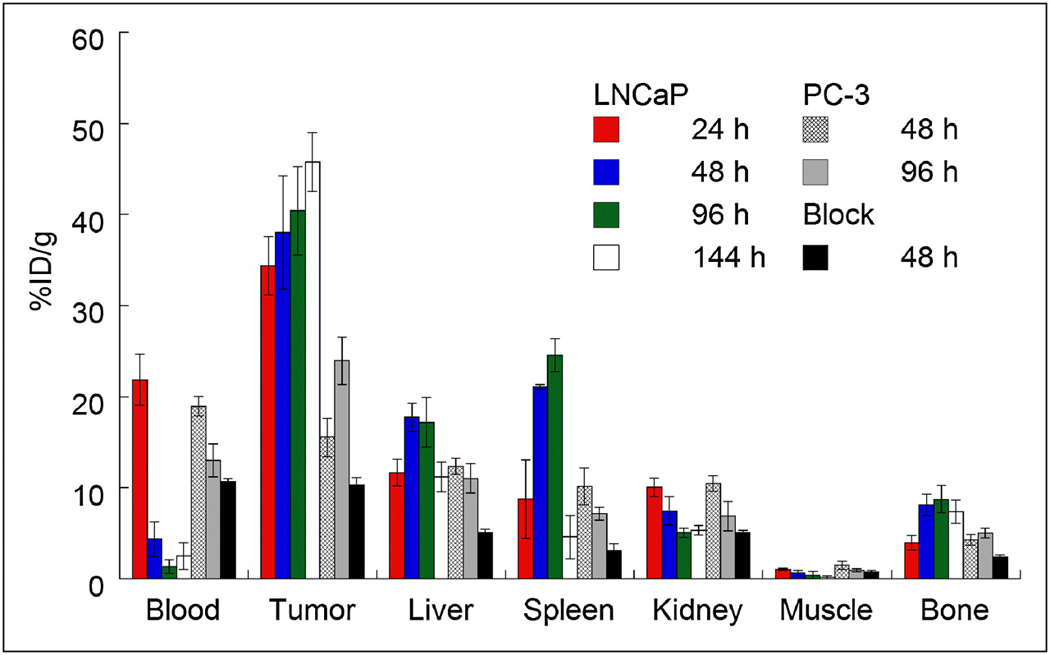
Bar chart showing selected tissue biodistribution data (%ID/g) for uptake of either high- (181.7 ± 1.1 MBq/mg [4.91 ± 0.03 mCi/mg]; 3–4 µg of mAb per mouse) or low-specific-activity (60-fold decrease, 3.04 MBq/mg [0.082 mCi/mg]; 300 µg of mAb per mouse) formulations of 89Zr-DFO-J591 (0.55–0.74 MBq [15–20 µCi], in 200 µL of sterile saline for injection) in male athymic nu/nu mice bearing subcutaneous LNCaP (PSMA-positive) or PC-3 (PSMA-negative) tumors.
Competitive inhibition (“blocking”) studies using low specific-activity formulations (60-fold decrease: 3.04 MBq/mg, [0.082 mCi/mg]) revealed only 10.3±0.8 %ID/g tumor uptake at 48 h post-injection; an approximate 4-fold decrease (P<0.002) versus high specific-activity formulations (Table 2 and Figure 3). Furthermore, in the low specific-activity experiments, 89Zr-DFO-J591 activity in the blood remained high (2–3 fold higher at 48 h: 10.7±0.4 %ID/g, P=0.026) but 89Zr-accumulation in the liver showed a statistically significant decrease from 17.7±1.6 %ID/g to 5.1±0.4 %ID/g (P<0.004). This decreased liver uptake on increasing the mass of administered mAb is fully consistent with the reported decreased liver toxicity observed in Phase I dose escalation trials. The competitive inhibition experiments concur with the in vitro data (vide supra) and further demonstrate the specificity of 89Zr-DFO-J591 for the PSMA antigen in vivo.
Interestingly, in the LNCaP tumor-bearing mice 89Zr uptake in the bone was relatively high and increased between 24 to 96 h (24 h: 4.0±0.8 %ID/g, 48 h: 8.2±1.2 %ID/g and 96 h: 8.7±1.5), before decreasing slightly to 7.4±1.3 %ID/g at 144 h. In contrast, bone accumulation of 89Zr activity in the PC-3 tumor-bearing mice was reduced in magnitude by approximately 45% at 48 and 96 h (4.3±0.6 %ID/g and 5.1±0.5 %ID/g, respectively). The degree of bone uptake is consistent with previously reported studies using various other 89Zr-labeled mAbs including 89Zr-DFO-trastuzumab (37, 39) for imaging HER2/neu expression and 89Zr-DFO-bevacizumab for imaging vascular endothelial growth factor (VEGF).(40) The nature of the radioactive species accumulating in the bone remains uncertain but it is plausible that slow intratumoral metabolism and subsequent recirculation of 89Zr-labeled metabolites may occur. Full metabolic studies are beyond the scope of the current study and will be the subject of further investigations. However, it is worth noting that in a recent clinical trial investigating the radiation dosimetry of 89Zr-DFO-U36 (a chimeric monoclonal antibody directed against CD44v6) in 20 patients with head and neck squamous cell carcinoma (HNSCC), the liver was identified as the dose limiting organ.(34, 38)
ImmunoPET imaging with 89Zr-DFO-J591
Temporal immunoPET images of 89Zr-DFO-J591 (10.9–11.3 MBq, [295–305 µCi], 60–62 µg of mAb, in 200 µL 0.9% sterile saline) recorded in LNCaP and PC-3 tumor-bearing mice between 3 to 144 h are presented in Figure 4. Time-activity curves (TACs) generated from the immunoPET images showing the mean %ID/g radiotracer uptake in various tissues including the heart/blood pool, liver and muscle in mice bearing LNCaP (n=3) or PC-3 (n=3) are given in Figure 5. Radiotracer uptake in LNCaP tumors was observed in <24 h post-injection of 89Zr-DFO-J591 and high tumor-to-muscle (T/M) ratios (calculated by using the mean %ID/g values derived from volume-of-interest [VOI]) analysis of the immunoPET images) were observed. At 48 h post-injection the immunoPET measured mean and maximum %ID/g for radiotracer uptake in LNCaP tumor-bearing mice were found to be 21.9±0.6 and 38.2±4.9 %ID/g, respectively, with a mean T/M ratio of 15.85 (Table S8). By 120 and 144 h, the mean T/M ratio in LNCaP tumors increased to 22.49 and 25.89, respectively. In contrast, low 89Zr-DFO-J591 accumulation in PSMA-negative PC-3 tumors (mean T/M ratios of 3.48, 4.36 and 4.19 at 48, 120 and 144 h, respectively) was found to occur. Uptake in these PSMA-negative tumors is in accordance with the “enhanced permeation and retention” (EPR) mechanism (Table S9 and Figures S10 – S12).
Figure 4.
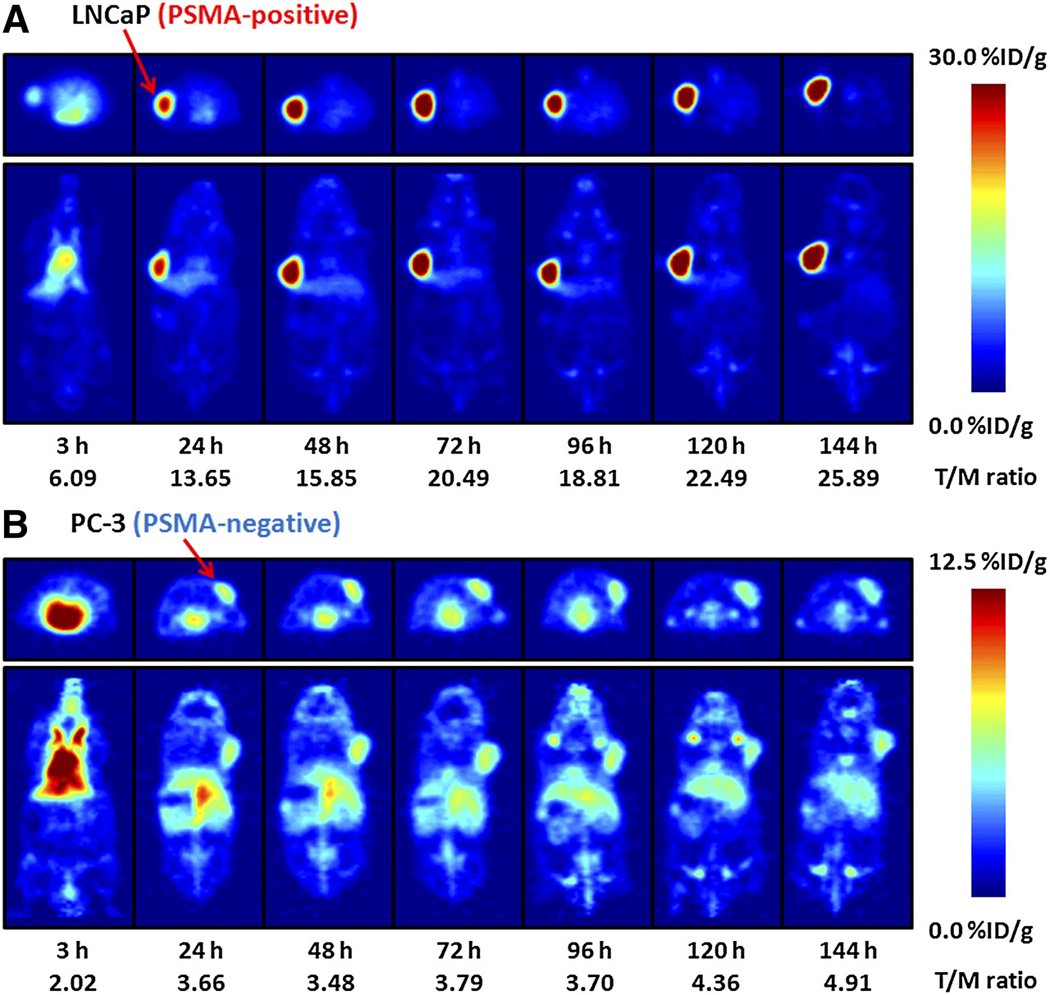
Temporal immunoPET images of 89Zr-DFO-J591 (10.9–11.3 MBq [295–305 µCi], 60–62 µg of mAb, in 200 µL of sterile saline) recorded in LNCaP tumor–bearing (PSMA-positive, left shoulder) (A) and PC-3 tumor–bearing (PSMA-negative, right shoulder) (B) mice between 3 and 144 h after injection. Transverse and coronal planar images intersect center of tumors, and mean tumor-to-muscle ratios derived from volume-of-interest analysis of immunoPET images are given. Upper thresholds of immunoPET have been adjusted for visual clarity, as indicated by scale bars.
Figure 5.
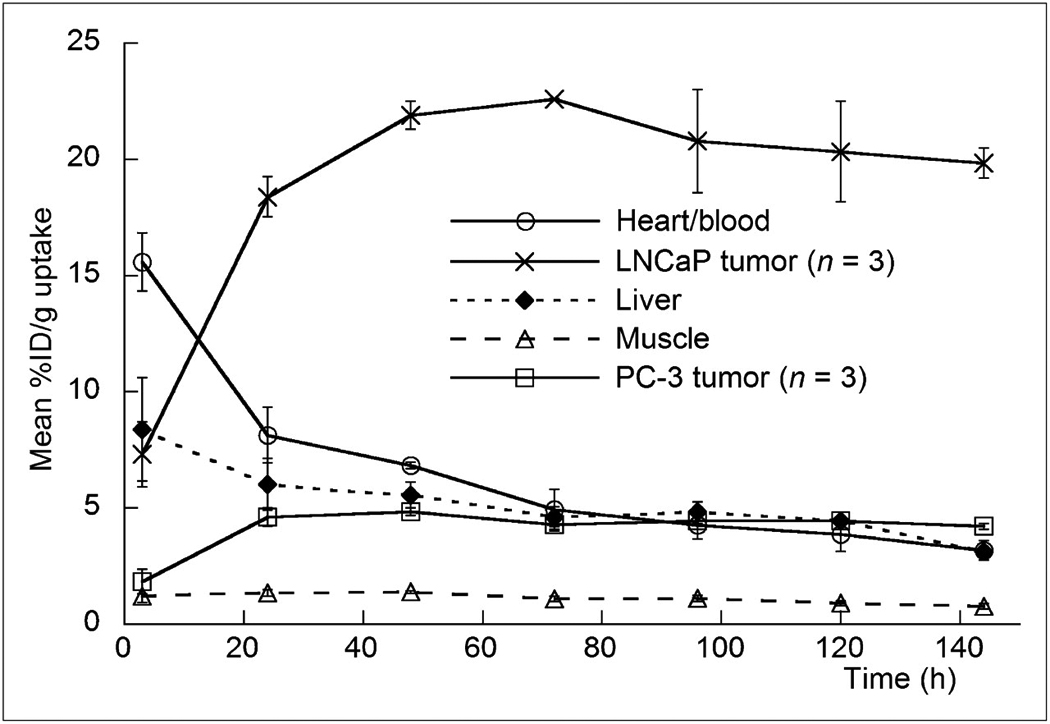
Time–activity curves derived by volume-of-interest analysis of immunoPET images showing mean %ID/g tissue uptake vs. time/h for 89Zr-DFO-J591 radiotracer accumulation in mice bearing subcutaneous LNCaP (PSMA-positive) or PC-3 (PSMA-negative) tumors. Complete time–activity curve data for 89Zr-DFO-J591 immunoPET imaging is given in supplemental materials (Supplemental Tables 9 and 10; Supplemental Figs. 11–13).
These data demonstrate that 89Zr-DFO-J591 immunoPET imaging provides very high tumor-to-background tissue (T/B) ratios and that this high uptake is specific for the presence of PSMA expression in tissue. Overall, the novel radiotracer 89Zr-DFO-J591 represents a promising candidate for translation to the clinic as an immunoPET agent for non-invasive delineation of PSMA-positive primary and metastatic prostate cancers in vivo.
Summary and Conclusions
Basic characterization of the in vivo behavior of several important 89Zr-labeled species, including the starting reagents, [89Zr]Zr-chloride and [89Zr]Zr-oxalate, and the key complex [89Zr]Zr-DFO are reported. Using PET imaging, the nature of the aqueous phase 89Zr species was shown to have a dramatic effect on the in vivo biodistribution with [89Zr]Zr-chloride and [89Zr]Zr-oxalate sequestering for over 24 h in the liver and bones, respectively. In contrast, [89Zr]Zr-DFO is first pass excreted through the kidneys and accumulates in the bladder with a biological half-life of 305±6 s.
The nature of the [89Zr]Zr-DFO complex and its thermodynamic stability with respect to expansion of the coordination environment from 6- to 7- or 8-coordinate through the addition of one or two water ligands has been investigated using high level DFT calculations. The computational studies suggest that formation of an 8-coordinate complex with two labile water ligands is thermodynamically favorable and in this respect, aqueous environments are expected to actively increase the inherent thermodynamic stability of 89Zr-DFO-conjugated radiotracers.
89Zr-DFO-J591 has been prepared with high radiochemical purity (>99%) and specific-activity (of 181.7±1.1 MBq/mg). In vitro stability studies demonstrated that functionalization of J591 with 3.9±0.3 accessible DFO chelates per mAb, and subsequent radiolabeling does not compromise the immunoreactivity, and radiolabeled immunoconjugate remains active for up to 7 days at 37°C. Biodistribution and immunoPET experiments indicated that 89Zr-DFO-J591 shows high potential as a radiotracer for specific, non-invasive delineation of PSMA positive prostate cancers in vivo. Work towards the clinical translation of 89Zr-DFO-J591 and other 89Zr-labeled mAbs is underway.
Supplementary Material
Acknowledgments
We thank Drs. NagaVaraKishore Pillarsetty and Pat Zanzonico for informative discussions, Valerie M. Longo for assistance with the biodistribution experiments, Thomas Ku for advice with in vitro experiments and Bradley Beattie for assistance with the PET imaging. We thank Prof. Jennifer C. Green (Department of Chemistry, University of Oxford, United Kingdom) for access to computational facilities. We also thank the staff of the Radiochemistry/Cyclotron Core at MSKCC.
Grant Support: Funded in part by the Geoffrey Beene Cancer Research Center of Memorial Sloan-Kettering Cancer Center (JSL), the Office of Science (BER) - U. S. Department of Energy (Award DE-SC0002456; JSL), the Ludwig Center for Cancer Immunotherapy of the Sloan-Kettering Institute (SML, PSJ) and the Starr Cancer Consortium (SML, JSL and PSJ). Technical services provided by the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH grants R24 CA83084 and P30 CA08748.
Footnotes
Disclosures: Dr. Bander is the inventor on patents that are owned by Cornell Research Foundation ("CRF") for the J591 antibody described in this manuscript. Dr. Bander is a paid consultant to BZL Biologics, the company to which the patents were licensed by CRF for further research and development.
References
- 1.Apolo AB, Pandit-Taskar N, Morris MJ. Novel tracers and their development for the imaging of metastatic prostate cancer. J Nucl Med. 2008;49(12):2031–2041. doi: 10.2967/jnumed.108.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olson WC, Heston WDW, Rajasekaran AK. Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Reviews on Recent Clinical Trials. 2007;2:182–190. doi: 10.2174/157488707781662724. [DOI] [PubMed] [Google Scholar]
- 3.Jhanwar YS, Divgi C. Current status of therapy of solid tumors. J Nucl Med. 2005;46(1_suppl):141S–150S. [PubMed] [Google Scholar]
- 4.Sokoloff RL, Norton KC, Gasior CL, Marker KM, Grauer LS. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. The Prostate. 2000;43(2):150–157. doi: 10.1002/(sici)1097-0045(20000501)43:2<150::aid-pros10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther. 2008;8(2):175–181. doi: 10.1586/14737140.8.2.175. [DOI] [PubMed] [Google Scholar]
- 6.Morris MJ, Pandit-Taskar N, Divgi CR, et al. Phase I evaluation of J591 as a vascular targeting agent in progressive solid tumors. Clin Cancer Res. 2007;13(9):2707–2713. doi: 10.1158/1078-0432.CCR-06-2935. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Moy P, Kim S, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor endothelium. Cancer Res. 1997;57:3629–3634. [PubMed] [Google Scholar]
- 8.Liu H, Rajasekaran AK, Moy P, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1997;58:4055–4060. [PubMed] [Google Scholar]
- 9.Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al. In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res. 2000;6(18):5237–5243. [PubMed] [Google Scholar]
- 10.Smith-Jones PM, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003;44(4):610–617. [PubMed] [Google Scholar]
- 11.McDevitt MR, Barendswaard E, Ma D, et al. An {{alpha}}-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60(21):6095–6100. [PubMed] [Google Scholar]
- 12.Vallabahajosula S, Smith-Jones PM, Navarro V, Goldsmith SJ, Bander NH. Radioimmunotherapy of prostate cancer in human xenografts using monoclonal antibodies specific to prostate specific membrane antigen (PSMA): studies in nude mice. The Prostate. 2004;58:145–155. doi: 10.1002/pros.10281. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhajosula S, Kuji I, Hamacher KA, et al. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J Nucl Med. 2005;46(4):634–641. [PubMed] [Google Scholar]
- 14.Bander NH, Trabulsi EJ, Kostakoglu L, et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urology. 2003;170:1717–1721. doi: 10.1097/01.ju.0000091655.77601.0c. [DOI] [PubMed] [Google Scholar]
- 15.Vallabhajosula S, Goldsmith SJ, Hamacher KA, et al. Prediction of myelotoxicity based on bone marrow radiation-absorbed dose: radioimmunotherapy studies using 90Y- and 177Lu-labeled J591 antibodies specific for prostate-specific membrane antigen. J Nucl Med. 2005;46(5):850–858. [PubMed] [Google Scholar]
- 16.Vallabhajosula S, Goldsmith SJ, Kostakoglu L, Milowsky M, Nanus DM, Bander NH. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin Cancer Res. 2005;11(19Suppl):7195s–7200s. doi: 10.1158/1078-0432.CCR-1004-0023. [DOI] [PubMed] [Google Scholar]
- 17.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177Lu-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncology. 2005;23(21):4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 18.Milowsky MI, Nanus DM, Kostakoglu L, et al. Vascular targeted therapy with anti-prostate-specific membrane antigen monoclonal antibody J591 in advenced solid tumors. J Clin Oncology. 2007;25(5):540–547. doi: 10.1200/JCO.2006.07.8097. [DOI] [PubMed] [Google Scholar]
- 19.Pandit-Taskar N, O'Donoghue JA, Morris MJ, et al. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J Nucl Med. 2008;49(7):1066–1074. doi: 10.2967/jnumed.107.049502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294(5546):1537–1540. doi: 10.1126/science.1064126. [DOI] [PubMed] [Google Scholar]
- 21.Zanzonico P. Routine quality control of clinical nuclear medicine instrumentation: a brief review. J Nucl Med. 2009;49(7):1114–1131. doi: 10.2967/jnumed.107.050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaussian 03 RC. Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Wallingford CT: Gaussian, Inc.; 2004. [Google Scholar]
- 23.Verel I, Visser GWM, Boellaard R, Stigter-van Walsum M, Snow GB, van Dongen GAMS. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J Nucl Med. 2003;44:1271–1281. [PubMed] [Google Scholar]
- 24.Holland JP, Sheh Y, Lewis JS. Standardized methods for the production of high specific-activity zirconium-89. Nucl Med Biol. 2009;36(7):729–739. doi: 10.1016/j.nucmedbio.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson CJ, Schwarz SW, Connett JM, et al. Preparation, biodistribution and dosimetry of copper-64-labeled anti-colorectal carcinoma monoclonal antibody fragments 1A3-F(ab')2. J Nucl Med. 1995;36(5):850–858. [PubMed] [Google Scholar]
- 26.Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunological Methods. 1984;72(1):77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 27.Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: Comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–796. [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JS, Lee JS, Im KC, et al. Performance measurement of the microPET Focus 120 scanner. J Nucl Med. 2007;48(9):1527–1535. doi: 10.2967/jnumed.107.040550. [DOI] [PubMed] [Google Scholar]
- 29.Tseng J-C, Zanzonico PB, Levin B, Finn R, Larson SM, Meruelo D. Tumor-specific in vivo transfection with HSV-1 thymidine kinase gene using a sindbis viral vector as a basis for prodrug ganciclovir activation and PET. J Nucl Med. 2006;47:1136–1143. [PubMed] [Google Scholar]
- 30.Meijs WE, Herscheid JDM, Haisma HJ, Pinedo HM. Evaluation of desferal as a bifunctional chelating agent for labeling antibodies with Zr-89. Appl Radiat Isot. 1992;43(12):1443–1447. doi: 10.1016/0883-2889(92)90170-j. [DOI] [PubMed] [Google Scholar]
- 31.Meijs WE, Haisma HJ, Klok RP, et al. Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J Nucl Med. 1997;38:112–118. [PubMed] [Google Scholar]
- 32.Verel I, Visser GWM, Boellaard R, et al. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in Xenograft-bearing nude mice. J Nucl Med. 2003;44:1663–1670. [PubMed] [Google Scholar]
- 33.Perk LR, Visser OJ, Stigter-van Walsum M, et al. Preparation and evaluation of 89Zr-Zevalin for monitoring of 90Y-Zevalin biodistribution with positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33(11):1337–1345. doi: 10.1007/s00259-006-0160-0. [DOI] [PubMed] [Google Scholar]
- 34.Börjesson PKE, Jauw YWS, Boellaard R, et al. Performance of immuno-Positron Emission Tomography with Zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin Cancer Res. 2006;12(7):2133–2140. doi: 10.1158/1078-0432.CCR-05-2137. [DOI] [PubMed] [Google Scholar]
- 35.Perk LR, Stigter-van Walsum M, Visser GWM, et al. Quantitative PET imaging of Met-expressing human cancer xenografts with 89Zr-labelled monoclonal antibody DN30. Eur J Nucl Med Mol Imaging. 2008;35(10):1857–1867. doi: 10.1007/s00259-008-0774-5. [DOI] [PubMed] [Google Scholar]
- 36.Aerts HJWL, Dubois L, Perk L, et al. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J Nucl Med. 2009;50(1):123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 37.Dijkers ECF, Kosterink JGW, Rademaker AP, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. 2009;50(6):974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 38.Borjesson PKE, Jauw YWS, de Bree R, et al. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J Nucl Med. 2009;50(11):1828–1836. doi: 10.2967/jnumed.109.065862. [DOI] [PubMed] [Google Scholar]
- 39.Holland JP, Caldas-Lopes E, Divilov V, et al. Measuring the pharmacokinetic effects of a novel Hsp90 inhibitor on HER2/neu expression in mice using 89Zr-DFO-trastuzumab. PLoS ONE. 2010;5(1):e8859. doi: 10.1371/journal.pone.0008859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagengast WB, de Vries EG, Hospers GA, et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med. 2007;48(8):1313–1319. doi: 10.2967/jnumed.107.041301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


