Abstract
Experimental autoimmune encephalomyelitis (EAE) is a Th1 and Th17 cell-mediated autoimmune disease of the CNS. IDO and tryptophan metabolites have inhibitory effects on Th1 cells in EAE. For Th17 cells, IDO-mediated tryptophan deprivation and small molecule halofuginone-induced amino acid starvation response were shown to activate general control nonrepressed 2 (GCN2) kinase that directly or indirectly inhibits Th17 cell differentiation. However, it remains unclear whether IDO and tryptophan metabolites impact the Th17 cell response by mechanisms other than the GCN2 kinase pathway. In this article, we show that IDO-deficient mice develop exacerbated EAE with enhanced encephalitogenic Th1 and Th17 cell responses and reduced regulatory T cell (Treg) responses. Administration of the downstream tryptophan metabolite 3-hydroxyanthranilic acid (3-HAA) enhanced the percentage of Tregs, inhibited Th1 and Th17 cells, and ameliorated EAE. We further demonstrate the Th17 cells are less sensitive to direct suppression by 3-HAA than are Th1 cells. 3-HAA treatment in vitro reduced IL-6 production by activated spleen cells and increased expression of TGF-β in dendritic cells (DCs), which correlated with enhanced levels of Tregs, suggesting that 3-HAA-induced Tregs contribute to inhibition of Th17 cells. By using a DC-T cell coculture, we found that 3-HAA-treated DCs expressed higher levels of TGF-β and had properties to induce generation of Tregs from anti-CD3/anti-CD28-stimulated naïve CD4+ T cells. Thus, our data support the hypothesis that IDO induces the generation of Tregs via tryptophan metabolites, such as 3-HAA, which enhances TGF-β expression from DCs and promotes Treg differentiation.
Keywords: EAE, T cells, IDO, cytokines, dendritic cells
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune disease of the CNS (1,2), which has been widely used as an animal model of human multiple sclerosis (3,4).
There are two major types of EAE actively induced by immunization with myelin autoantigens: chronic EAE (C-EAE) and relapsing-remitting EAE, which can be induced by immunizing rodents and other experimental animals with specific myelin Ags (5). C-EAE is characterized by progressive pathogenic lesions in the CNS and ascending paralysis of the nervous system. Unlike C-EAE, relapsing-remitting EAE displays a clear remitting-relapsing profile after the peak of initial clinical onset (6). EAE has been considered a typical Th1 cell-mediated disease (7,8). However, more recent data showed that Th17 cells, a novel lineage of Th cells, play an important role in the pathogenesis of EAE (9–11). Th17 cells produce high levels of IL-17A, which can cause inflammatory cell infiltration, the formation of extensive spinal cord lesions, and clinical dysfunction reflecting CNS damage (10). In immune reactions, Th1- and Th17-mediated responses are negatively regulated at multiple levels by suppressive cytokines (12, 13) and counteracting T cells (e.g., Th1 cells and regulatory T cells [Tregs]). After the initial paralysis, EAE-affected animals tend to exhibit varying periods of spontaneous recovery, suggesting the existence of endogenous mechanisms of immune suppression that prevent prolonged and extensive tissue damage.
Introduction
EAE is an autoimmune disease of the central nervous system (CNS) (1, 2), which has been widely used as an animal model of human multiple sclerosis (MS) (3, 4). There are two major types of EAE actively induced by immunization with myelin autoantigens: chronic EAE (C-EAE) and relapsing-remitting EAE (RR-EAE), which can be induced by immunizing rodents and other experimental animals with specific myelin antigens (5). C-EAE is characterized by progressive pathogenic lesions in the CNS and ascending paralysis of the nervous system. Unlike C-EAE, RR-EAE displays a clear remitting-relapsing profile after peak of initial clinical onset (6). EAE has been considered a typical Th1 cell-mediated disease (7, 8). However, more recent data show that Th17 cells, a novel lineage of Th cells, play an important role in the pathogenesis of EAE (9–11). Th17 cells produce high levels of IL-17A, which can cause inflammatory cell infiltration, the formation of extensive spinal cord lesions, and clinical dysfunction reflecting CNS damage (10). In immune reaction, Th1 and Th17-mediated responses are negatively regulated at multiple levels by suppressive cytokines (12, 13) and counteracting T cells, e.g. Th2 cells and regulatory T cells (Treg cells). After the initial paralysis, EAE-affected animals usually tend to exhibit variable degrees of spontaneous recovery period, suggesting the existence of endogenous mechanisms of immune suppression which prevent prolonged and extensive tissue damage.
An increasing body of data shows that indoleamine 2, 3 dioxygenase (IDO) and IDO-catalyzed tryptophan metabolism play critical roles in the induction of immune tolerance and immune suppression (14). Tryptophan is an amino acid that is essential for cell growth and functioning. The primary metabolism route of tryptophan in vivo is the kynurenine pathway which is essentially controlled by two rate-limiting enzymes: IDO and its isoenzyme, tryptophan 2,3-dioxygenase (TDO) (15). Experimental data in the last decade have shown that IDO, but not TDO, has important immunosuppressive properties involved in immune tolerance and Th1/Th2 regulation. Two major mechanisms are thought to underlie IDO-mediated regulation of immune responses: first, through IDO-mediated tryptophan depletion that directly impacts survival and function of immune cells by inducing cell stress and activating the general control nonrepressed 2 (GCN2) kinase pathwasy (16); and cytotoxic and immune regulatory effects of catabolites derived from the kynurenine metabolism pathway. Accumulating evidence shows that catabolites from the kynurenine-metabolism pathway are important biological mediators in regulating Th1 and Th2 cell function, although Th2 cells are less sensitive to tryptophan metabolites (17).
In a tumor model and CpG (a TLR9 ligand) treatment model, IDO expression was shown to block conversion of Tregs to Th17 cells by activating the GCN2 kinase pathway and suppressing IL-6 production in plasmacytoid denditic cells (pDCs) (18, 19). Moreover, small-molecule halofuginone or selective amino acid depletion-induced amino acid starvation response can directly activate GCN2 kinase and inhibit Th17 cell differentiation, as well as protect mice from Th17-associated EAE (20). It remains unclear whether IDO and tryptophan metabolites also regulate the Th17 cell response by mechanisms other than GCN2 kinase activation.
IDO-tryptophan metabolism and Treg positively regulate one another (21). Tregs can induce IDO expression in dendritic cells (DCs) through interaction between CTLA4 on Treg cells and CD80/CD86 on DCs (22), or through Treg cell-secreted cytokines, e.g. IFN-γ(23). IDO expression and the production of tryptophan metabolites from the kynurenine metabolism pathway can serve as mediators to suppress immune cell responses by direct effects on T cells or by indirect effects through altered APC function (14). In contrast, IDO expression in DCs may induce differentiation of new Tregs from naïve T cells (24). Although the detailed mechanism underlying this process has not been fully elucidated, several experiments showed that coculturing IDO+ DCs or IDO-expressing acute myeloid leukemia cells with naïve T cells, as well as the addition of kynurenine in low tryptophan medium, can convert naïve T cells into CD25+FoxP3+ T cells (25, 26), suggesting that IDO expression can upregulate Treg cell function and tryptophan metabolites may have biological effects that promote the differentiation of Tregs.
Pioneer studies performed with the EAE model showed that IDO and certain products of tryptophan metabolism may play a protective role against immune cell-mediated inflammation of the CNS. IDO is expressed in the CNS and enhancement of its expression correlates with remission of EAE (27, 28). Inhibition of IDO by 1-Methyl-tryptophan (1-MT) exacerbates EAE (27). In the CNS, microglia and macrophages, but not astrocytes, were reported to express IDO upon activation by IFN-γ with synergistic effects of TNF-α (27). These data suggest that IDO expression in the CNS plays a role in initiating a negative feedback loop to self-limit autoimmune inflammation during EAE. Moreover, Platten et al. (29) showed that treatment of adoptively-transferred EAE mice with tryptophan metabolites caused a shift in cellular immune responses from Th1 to Th2, and markedly reduced the severity of autoimmune inflammation in the CNS. To our knowledge, EAE has not been studied in IDO-deficient mice.
In this study, we explored the role of IDO and tryptophan metabolism in encephalitogenic T cell-responses in IDO-deficient mice. We found that IDO deficiency promoted encephalitogenic T cell responses, downregulated Treg responses, and enhanced EAE severity. In contrast, 3-hydroxyanthranilic acid (3-HAA) priming in vitro promoted Treg function and administration of 3-HAA in mice reduced the severity of EAE. We provide evidence that IDO induces Tregs through at least one downstream metabolite from the kynurenine metabolism pathway, 3-HAA. Our data suggest that the IDO-tryptophan metabolite-Treg axis plays a critical role in suppression of T cell-mediated, especially Th17-mediated, encephalitogenic immune responses.
Materials and methods
Mice
C57BL/6 mice and IDO1-deficient mice (IDO−/− mice) on C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME). IDO−/− mice were bred and housed in the Thomas Jefferson University Animal Facility. Female mice, 8 to 10 weeks old, were used in all studies. All experimental procedures were performed in accordance with guidelines of the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Reagents and antibodies
3-HAA was purchased from Sigma-Aldrich (St. Louis, MO). Anti-CD3 (145-2C11) and anti-CD28 (37.51) Abs for T cell stimulation were purchased from BD Biosciences (San Jose, CA). The following Abs for immunostaining were from BD Bioscience: anti-CD4 (RM4–5), anti-CD25 (PC61), anti-IFN-γ (XMG1.2), anti-IL-17 (TC-18H10). Neutralizing Abs against IL-4 and TGF-β, and all recombinant cytokines used for T cell polarization were from R&D Systems (Minneapolis, MN). Anti-FoxP3-PE and Anti-TGF-β-PerCP/Cy5.5 Abs for flow cytometry were purchased from eBioscience (San Diego, CA) and BioLegend (San Diego, CA) respectively.
Induction and treatment of EAE
Mice were immunized subcutaneously (s.c.) on the back with 150 µg of MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) emulsified in Complete Freund’s Adjuvant (CFA) (Difco Lab, Detroit, MI) containing 4 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI). Two hundred ng of pertussis toxin (PTX) (List Biological Lab, Epsom, England) was given i.p. on days 0 and 2 post immunization (p.i). Mice were scored daily for appearance of clinical signs of EAE by a scale from 0 to 5 as described by others (30): 0, no clinical sign; 1, full limp tail; 2, paralysis of one hind limb; 3, paralysis of both hind limbs; 4, paralysis of trunk; 5, death. To test therapeutic effects of 3-HAA in EAE, mice were treated daily with 200 mg/kg of 3-HAA in 500 µl of PBS by i.p. injection. The untreated control group was given the same amount of PBS.
Cell preparation and in vitro-priming
Preparation and in vitro priming of spleen cells were performed as described previously (31). Briefly, spleen lymphocytes were isolated from single cell suspension by centrifugation with Ficoll-Paque™PLUS (Amersham Pharmacia Biotech AB, Piscataway, NJ). Lymphocyte layer was harvested and washed with cold PBS before in vitro stimulation. For in vitro priming, spleen cells were adjusted into 1×106/ml in RPMI 1640 complete medium and stimulated in 24 well plate precoated with 5 µg/ml of anti-CD3 and 2 µg/ml of anti-CD28 antibodies in the presence of 200 µM 3-HAA or vehicle control, supplemented, as needed, with mouse recombinant IL-12 (10 ng/ml) and anti-IL-4 antibody (15 µg/ml) for Th1-polarizing condition, or with TGF-β (2 ng/ml) and mouse recombinant IL-6 (10 ng/ml) for Th17-polarizing condition.
To isolate CNS cells, spinal cords were mechanically dissociated through a 100-µM cell strainer and washed with PBS. Washed cells were fractionated on a 60/30% Percoll gradient by centrifugation at 300 × g for 20 min. Infiltrating mononuclear cells were collected from the interface and washed with PBS for use.
Proliferation assay
T cell proliferation was assayed by 3H-thymidine incorporation as described previously (32). Briefly, triplicate aliquots of 5 × 105 splenocytes in 96 well plate were stimulated with Con A or serial concentration of MOG35–55 peptide in 200 µl of RPMI 1640 complete medium containing 10% fetal calf serum (FCS). After 60 hrs of incubation, cells were pulsed with 1 µCi 3H-thymidine/well for 18 hrs and then were harvested on fiberglass filters. Thymidine incorporation was determined using a Wallac 1450 MicroBeta TriLux scintillation counter (PerkinElmer, Turku, Finland).
Intracellular staining and flow cytometry
Cells isolated from spleens or spinal cords were briefly stimulated with PMA (50 ng/ml), ionomycin (500 ng/ml) (Sigma-Aldrich) and GolgiStop (1 µg/106 cells) (BD PharMingen, San Jose, CA) for 4 hrs at a density of 1 × 106/ml in RPMI 1640 complete medium. Cells were harvested, washed in staining buffer (PBS) containing 1% FCS, 0.02% NaN3 and, in some cases, blocked with anti-CD16/CD32 antibodies. After washes, cells were first stained with fluorescent antibodies to surface markers, followed by intracellular staining according to the manufacturer’s instruction. For intracellular staining, cells were fixed and permeabilized using Fix/Perm® cell permeabilization reagents (BD Bioscience), followed by incubation with fluorescently-labeled antibodies against intracellular cytokine or FoxP3. After the last wash, cells were acquired by using either FACSCalibur, or FACSAria (BD Biosciences). Data were analyzed using FlowJo Software (Ashland, OR).
Cytokine measurement
For cytokine detection, supernatants were collected from culture 48 hrs of stimulation. Levels of IL-4, IL-6, IL-10, IFN-γ and IL-17 in supernatants were determined, as specified, either by ELISA with Duoset cytokine assay reagents (R&D Systems) or by cytokine bead assay (BD Biosciences) per the manufacturer’s instructions.
DC-T cell coculture
To prepare bone marrow stem cell-derived DCs, bone marrow cells were obtained from femurs and tibias of C57BL/6 mice by flushing with cold PBS. Cells were washed in PBS and then were cultured for 6 days in RPMI 1640 complete medium containing 10% FCS, supplemented by 200 U/ml GM-CSF (R&D Systems). Mature DCs were harvested from culture flask by vigorous shaking. Purity of DCs was confirmed by flow cytometry using anti-CD11c antibody. For DC-T cell co-culture, naïve CD4+ T cells were isolated from spleen cells of C57BL/6 mice by cell separation with magnetic anti-mouse CD4 beads according to the manufacturer’s protocol (Miltenyi Biotec). Purified CD4+ T cells (8 × 105/ml) were cultured with DCs (2 × 105 /ml) in anti-CD3/anti-CD28 coated plates containing RPMI 1640 complete medium.
Statistical analysis
For clinical scores of EAE, significance between two groups was examined by using the Two-way ANOVA test. Data are presented as mean ± SE. For cytokine assays, statistical difference between two groups was determined by unpaired, two-tailed Student’s t-test. Results are presented as mean ± SD. In all cases, a value of p < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01 and ***p < 0.001).
Results
IDO deficiency enhances susceptibility to EAE
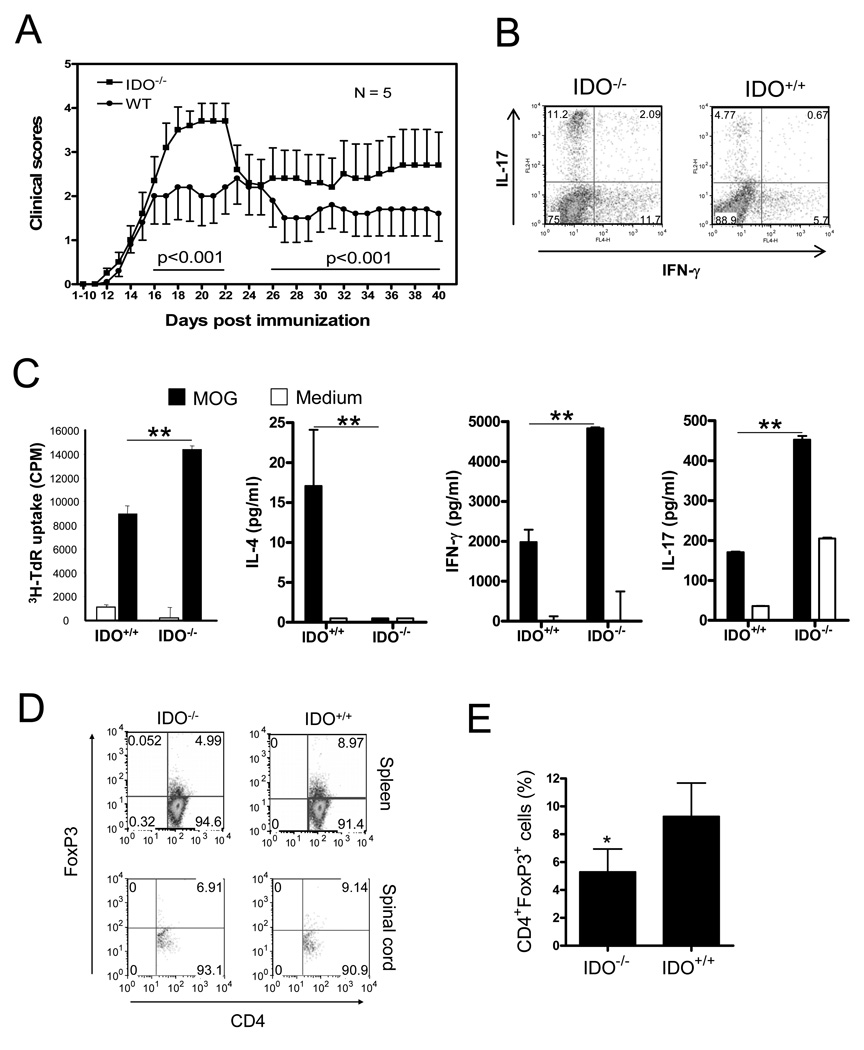
It was documented that blockade of IDO activity by IDO inhibitor 1-MT enhanced the severity of EAE and several other Th1 cell-mediated immune diseases (33). However, the role of IDO in Th17 cell-mediated encephalitogenic responses is not fully understood. Given that 1-MT may also impact immune cells by interacting with TLR (34), we set out to test the role of IDO in suppression of EAE using IDO-deficient mice. We induced active EAE in IDO-deficient and wild type syngeneic mice (control) with MOG35–55 peptide/CFA/PTX and compared EAE development and related T cell responses. The results showed that IDO-deficient mice developed more severe EAE (Figure 1A). To determine whether IDO deficiency impacts Th1 and Th17 responses in EAE mice, we harvested spleen cells from mice at the termination of experiments and analyzed IFN-γ and IL-17-expression in T cells by intracellular cytokine staining. We found that levels of Th1 and Th17 cells in spleens of IDO-deficient mice were increased in comparison with those of wild type mice (Fig. 1B). The enhanced Th1 and Th17 cell response in IDO-deficient mice was also confirmed by increased MOG-induced cytokine production in restimulated spleen cells in vitro. Spleen cells from IDO-deficient mice produced less IL-4 and markedly more IFN-γ and IL-17 (Figure 1C). Interestingly, enhanced Th1 and Th17 cell levels in IDO-deficient mice correlated with decreased levels of CD4+FoxP3+T cells, suggesting that the Treg cell response was reduced in IDO-deficient mice (Fig. 1D, 1E).
Fig. 1. T cell responses and EAE in IDO-deficient mice.
A. IDO-1 knockout and WT C57BL/6 mice were immunized on day 0 with 150 µg MOG peptide in CA. Mice were injected with 200 ng/mouse of PTX on days 0 and 2, respectively. EAE development in mice was assessed by a 0–5 scoring system (mean ± SE). Statistical significance between groups (N=5) was determined by two-way ANOVA. B. Spleen cells were harvested from mice at termination of the experiment and stimulated for 4 hrs in vitro with PMA/inomycin in the presence of Golgi blocker. Cells were stained with anti-CD4-FITC first, followed by intracellular staining with anti-IFN-γ-APC and anti-IL-17-PE. Data were analyzed with Flowjo software. Results are shown as dot plot for IFN-γ versus IL-17 in CD-4 positive gates. C. Spleen cells from tested mice were stimulated in vitro with MOG antigen for 3 d. Lymphocyte proliferation and cytokine production of cells were measured by 3H-TdR incorporation (mean ± SD of triplicate) and cytokine ELISA (mean ± SD of duplicate), respectively. D. Spleen and spinal cord cells of tested mice were stained with anti-CD4-FITC and anti-CD25-APC first, followed by intracellular staining with anti-FoxP3-PE. Data are presented as representative dot plots for CD4 versus FoxP3 in CD4+ gate. E. Percentage of CD4+FoxP3+ cells in spleens from IDO−/− and IDO+/+ mice (mean ± SD, N = 5). One representative of three independent experiments is shown. *p < 0.05.
3-HAA treatment inhibits encephalitogenic T cell responses and ameliorates EAE
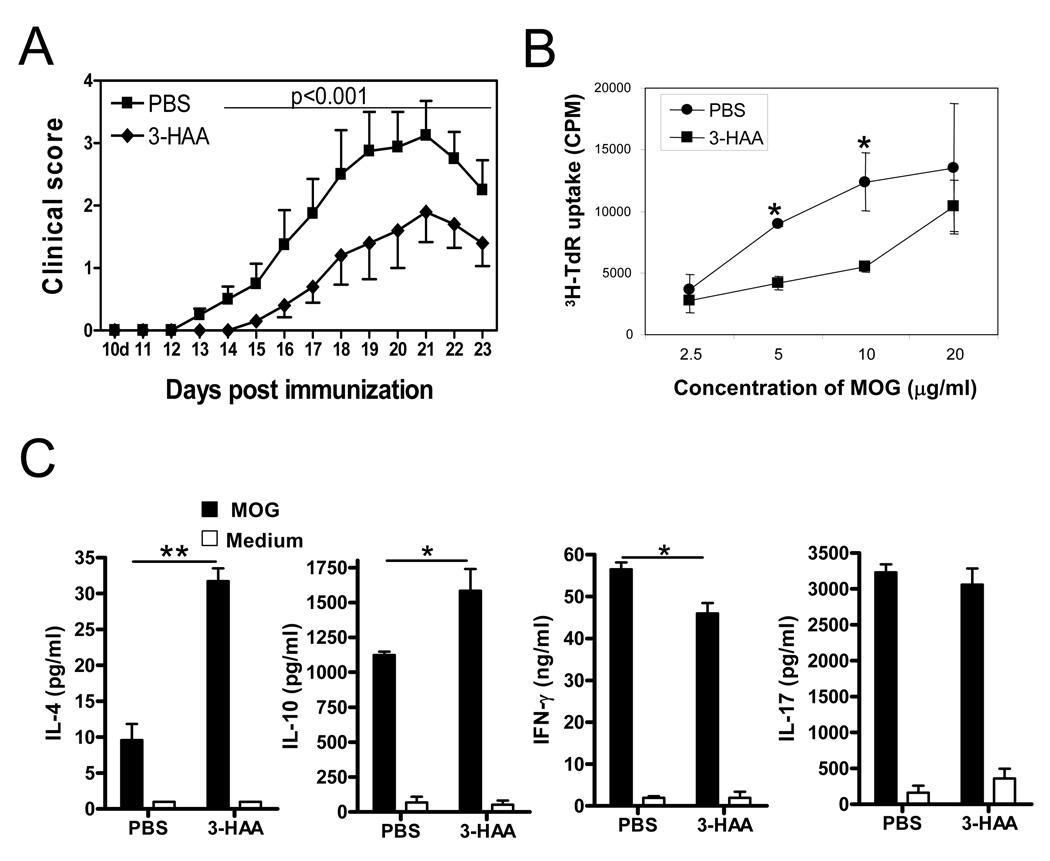
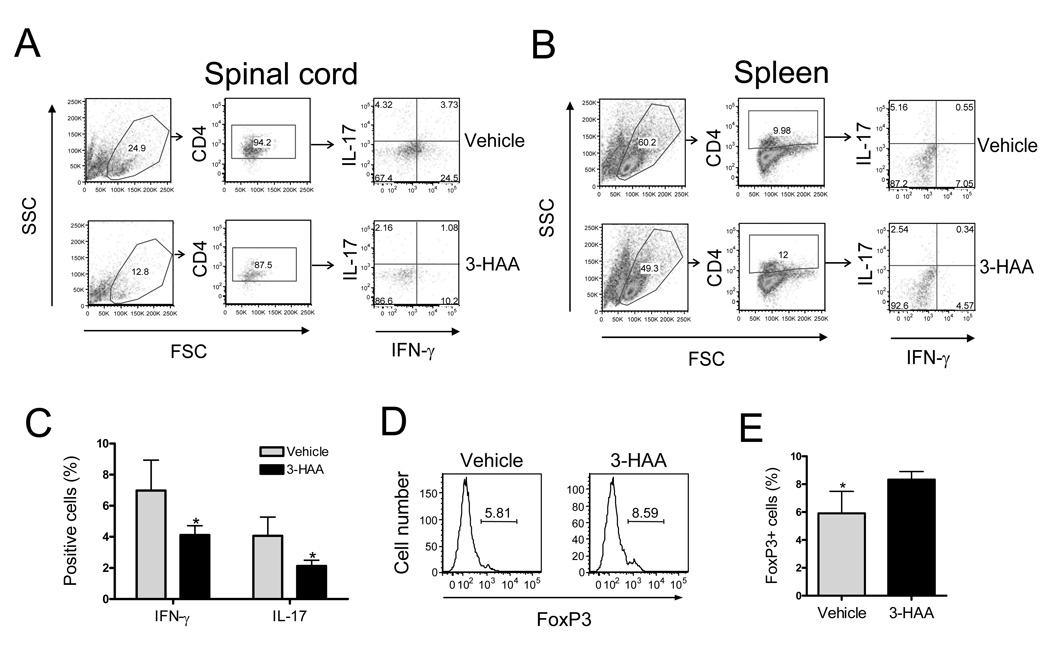
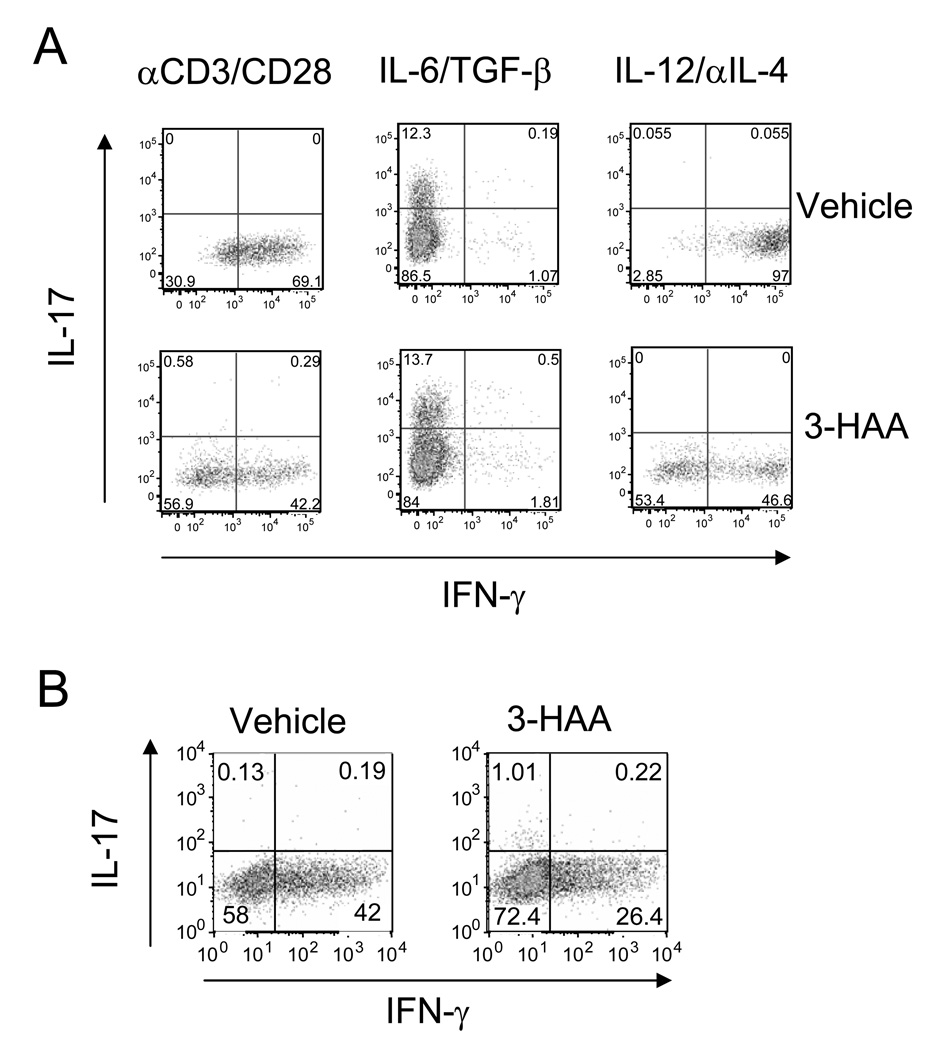
IDO regulates immune responses mainly through two mechanisms: tryptophan depletion-induced cell stress that activates GCN2 kinase activation and the biological effects of tryptophan metabolites (14, 16, 20, 35). Some tryptophan metabolites, including 3-hydroxyanthranillic acid (3-HAA), play roles in suppression of activated Th1 cells. Platten et al. (29) reported that treatment with 3-HAA by i.p. injection or oral administration of N-(3,4-dimethoxycinnamoy) anthranilic acid (3,4-DAA), a synthetic derivative of the tryptophan metabolite anthranilic acid, inhibited Th1 type cytokine responses and reversed paralysis in the adoptively transferred EAE model. However, the effect of 3-HAA on Th17 cells is still unclear. Given that both Th1 and Th17 cell-mediated responses are crucial in the pathogenesis of EAE (36), we analyzed the role of 3-HAA in regulation of T cell responses and EAE pathogenesis in the MOG-induced EAE model. We induced EAE in C57BL/6 mice with MOG35–55 peptide and treated the mice with 3-HAA by i.p. injection starting from day 1 p.i. The results showed that 3-HAA treatment not only delayed clinical onset of EAE but also reduced disease severity (Fig. 2A). In addition, spleen cells from 3-HAA treated mice displayed a lower proliferation, reduced Th1 cytokine production, and increased levels of IL-4 and IL-10 in comparison with those from PBS-treated control mice (Fig. 2B, 2C). However, there was no detectable difference in IL-17 production between the two groups when spleen cells were restimulated for 3 days without continuous 3-HAA treatment in culture (Fig. 2C). Interestingly, levels of IFN-γ and IL-17 expressing cells in spinal cords and spleen cells freshly harvested from 3-HAA-treated mice were decreased in comparison with those from control mice (Fig. 3A-C). In addition, there were fewer CD4+ T cells in spinal cords of 3-HAA-treated mice, suggesting that 3-HAA treatment reduced the numbers of effector Th1 and Th17 cells infiltrating into the CNS.
Figure 2. 3-HAA treatment ameliorates clinical severity of EAE.
C57BL/6 mice were immunized by an EAE-inducing protocol with 150 µg MOG35–55 peptide. Tested mice were injected i.p. with 200 mg/kg of 3-HAA in PBS daily, beginning on day 1 p.i. This route and dose were selected based on the study by Platten et al. (29). Control mice were given the same amount of PBS. One representative of three independent experiments is shown. A. Clinical scores were recorded daily. Results are shown as mean ± SE (n = 5). B. Spleen cells were harvested at the termination of the experiment and were stimulated by MOG35–55 peptide in vitro. Cell proliferation was assessed by 3H-TdR incorporation (mean ± SD of triplicate). p < 0.05. C. Culture supernatants from spleen cells described in B were assayed for cytokines by ELISA. Results are shown as mean ± SD of duplicates. Data are from a representative of three independent experiments. *p < 0.05; **p < 0.01.
Figure 3. 3-HAA treatment inhibits encephalitogenic Th1 and Th17 in vivo.
C57BL/6 mice were immunized by EAE-inducing protocol and treated with 3-HAA as described in Fig. 2. Spinal cord cells and spleen cells were harvested at the end of the experiment and were primed for 4 hrs in vitro with PMA (50 ng/ml) and ionomycin (500 ng/ml) in the presence of GolgiStop (1 µg/106 cells). A and B. IFN-γ and IL-17 expression. Spinal cord cells (A) and spleen cells (B) were stained with anti-CD4-FITC first, followed by intracellular staining with anti-IL-17-PE and anti-IFN-γ-APC. IFN-γ and IL-17-expressing cells were analyzed by flow cytometry. Data are shown as dot plots for spinal cord (pool of each group) and spleen (a representative of each group). C. Percentage of cytokine-expressing cells in spleen (mean ± SD, n = 5). D and E. FoxP3 expression in CD4+ T cells of EAE mice. Spleen cells from 3-HAA-treated and untreated mice were co-stained with anti-CD4-FITC and anti-CD25-allophycocyanin, followed by intracellular staining with anti-FoxP3-PE. Results are shown as representative graphs of CD4+CD25 gates (D) and as the percentage of FoxP3+ cells in CD4+CD25+ gates (mean ± SD, n = 5) (E). One representative of three independent experiments is shown. *p < 0.05.
It is thought that one mechanism of IDO-mediated suppression of the immune response is through upregulation of the Tregs (21, 24). To determine whether Treg levels in 3-HAA-treated mice were increased, we analyzed FoxP3, a marker for Tregs, in spleen cells from 3-HAA- and vehicle-treated mice, respectively. Indeed, CD4+ T cells from spleens of 3-HAA-treated mice expressed more FoxP3 (Fig. 3D, 3E), indicating that Tregs were upregulated in 3-HAA-treated mice and that enhancement of Tregs was associated with decreased encephalitogenic T cell responses and reduced severity of EAE.
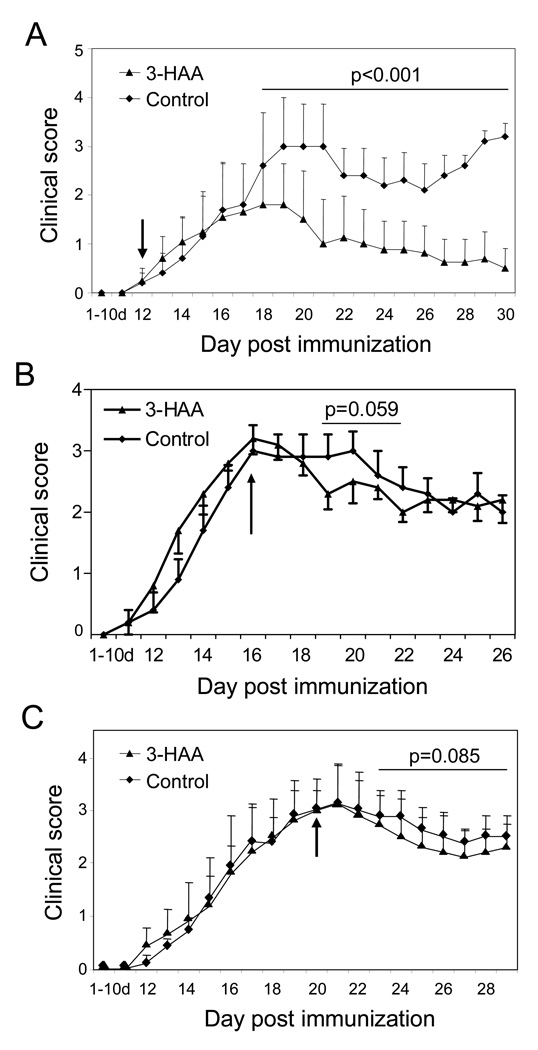
To determine whether 3-HAA functions in the early afferent phase of encephalitogenic immune responses or in the efferent phase of EAE, we treated mice with 3-HAA starting on the day of clinical onset or after they developed severe EAE. Our data showed that 3-HAA treatment of mice starting at the clinical onset of EAE (day 12 p.i.) still decreased the severity of disease (Fig. 4A). When 3-HAA treatment started on day 16 p.i., there was a trend toward decreased disease (Fig. 4B). However, there was no therapeutic effect when 3-HAA treatment was begun at the peak of EAE (day 20 p.i.) (Fig. 4C).
Figure 4. 3-HAA inhibits EAE when treatment begins before or at early disease onset but is not effective when treatment begins at the peak of EAE.
EAE was induced in C57BL/6 mice as described in Fig. 2. Tested mice were injected i.p. with 200 mg/kg of 3-HAA daily, beginning on day 12 (A), day 16 (B), or day 20 (C) p.i. until the end of the experiment. Control mice received the same amount of PBS. Arrows indicate the start date of the treatment. Data are presented as mean ± SE of clinical scores (n = 5). One representative of two independent experiments is shown.
3-HAA inhibits differentiation of Th1 but not Th17 in vitro
We observed that spleen cells from 3-HAA-treated mice produced less IFN-γ in comparison with those from control mice, but IL-17 levels were similar between the two groups. This led us to postulate that 3-HAA may act on Th1 cells and Th17 cells differently. To elucidate the role of 3-HAA in Th1 and Th17 cell differentiation, we stimulated CD4+ naïve T cells with anti-CD3 and anti-CD28 antibodies in vitro, either without polarizing or with Th17- or Th1-polarizing condition in the presence, or absence, of 3-HAA. We found that 3-HAA markedly inhibited Th1 cell differentiation under both Th1-polarizing and no polarizing condition (Fig. 5A). However, no 3-HAA-induced inhibition of Th17 cell differentiation was observed, suggesting that 3-HAA selectively inhibits Th1 cell, but not Th17 cell, differentiation. To exclude the role of antigen presenting cells (APC) in 3-HAA-mediated Th1 cell suppression, we isolated CD4+ T cells from spleens to eliminate APCs and stimulated purified CD4+ T cells with anti-CD3/anti-CD28 in the presence of 3-HAA or vehicle control. The results showed that 3-HAA still suppressed Th1 cell differentiation in the absence of APCs (Fig. 5B). Thus, 3-HAA has a direct effect on suppression of Th1 cell differentiation, but the reduction in Th17 cell responses observed in cells from spleen and spinal cords of 3-HAA-treated mice (Fig. 3A and B) is likely due to indirect inhibition.
Figure 5. 3-HAA inhibits Th1 but not Th 17 differentiation induced by anti-CD3/anti-CD28 stimulation.
Spleen cells from naïve mice (A) and purified naïve CD4+ T cells (B) were stimulated for 3 d on plates precoated with anti-CD3 (5 µg/ml) and anti-CD28 (2 µg/ml) in the presence of 3-HAA (200 µM) or vehicle control. Cells from culture were stained with anti-CD4-FITC, followed by intracellular staining with anti-IL-17-PE and anti-IFN-γ-APC. Intracellular IFN-γ and IL-17 expression was analyzed by flow cytometry. One representative of three independent experiments is shown.
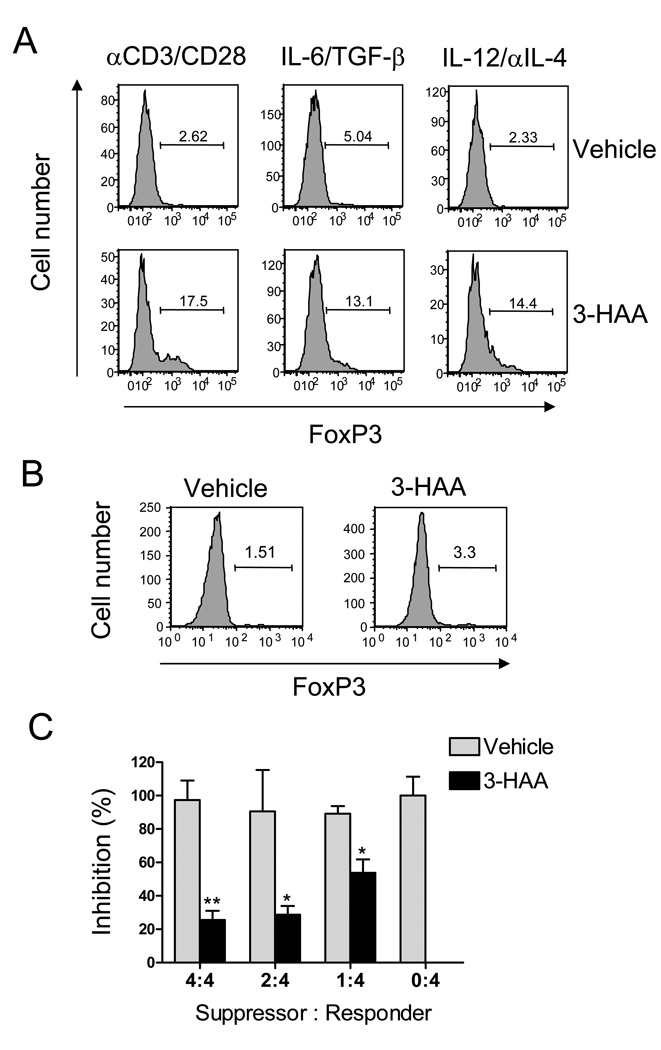
3-HAA treatment promotes anti-CD3/anti-CD28-induced Treg cell development
IDO and Tregs regulate each other positively (14, 22). Tregs induce IDO expression by well-identified mechanisms, e.g. CTLA4-CD80/CD86 interaction (23), but how IDO expression results in generation of Tregs has not been fully elucidated. Given that enhanced T cell-mediated pathogenesis in IDO-deficient mice is correlated with decreased Treg cell response and that levels of Tregs were increased in 3-HAA-treated mice, we postulate that IDO may induce Tregs through, at least in part, certain tryptophan metabolites, e.g. 3-HAA. To test our hypothesis, we stimulated naïve spleen cells with anti-CD3 and anti-CD28 antibodies in vitro, with or without the Th17- or Th1-polarizing condition, in the presence or absence of 3-HAA. Treg development was assessed by analyzing FoxP3 expression in CD4+CD25+ cells with flow cytometry. We found that 3-HAA markedly promoted Treg development under the condition tested (Fig. 6A). To determine the role of APCs in Treg induction by 3-HAA, we stimulated purified naïve CD4+ T cells with anti-CD3/anti-CD28 and treated the cells with 3-HAA or vehicle control. The results showed that 3-HAA treatment had only a minor effect on induction of Tregs in the absence of APCs (Fig. 6B). To determine whether 3-HAA-primed cells have inhibitory function for T cells, we cocultured 3-HAA-primed cells with MOG-TcR transgenic CD4+ T cells in the presence of MOG Ag. The results showed that 3-HAA-primed cells markedly suppressed MOG-stimulated proliferation of MOG-TcR transgenic T cells (Fig. 6C).
Figure 6. 3-HAA promotes anti-CD3/anti-CD28-induced Treg cell differentiation.
A. Spleen cells from naïve C57BL/6 mice (A) and purified naïve CD4+ T cells (B) were stimulated for 3 d on plates pre-coated with anti-CD3 (5 µg/ml) and anti-CD28 (2 µg/ml) in the presence of 3-HAA (200 µM) or vehicle control. Cells from culture were stained with anti-CD4-FITC and anti-CD25-allophycocyanin, followed by intracellular staining with anti-FoxP3-PE. FoxP3 expression is shown as graphs in CD4+CD25+ gate. The numbers represent the percentage of FoxP3+ cells. One representative of three independent experiments is shown. C. Inhibitory effect of 3-HAA-primed cells. Spleen cells were primed for 2 d as described in A and B. The primed cells were harvested from culture and washed with PBS. To determine the inhibitory functions, 2×105 of purified MOG-TcR transgenic CD4+T cells (Responder) were co-cultured in 96 well plate with a different ratio of 3-HAA (or vehicle)-primed cells (Suppressor) for 3 d in the presence of 20 µg/ml of MOG peptide. MOG-induced proliferation of TcR-transgenic T cells was determined by 3H-TdR incorporation. Results are presented as mean inhibitory percentage ± SD of triplicate.
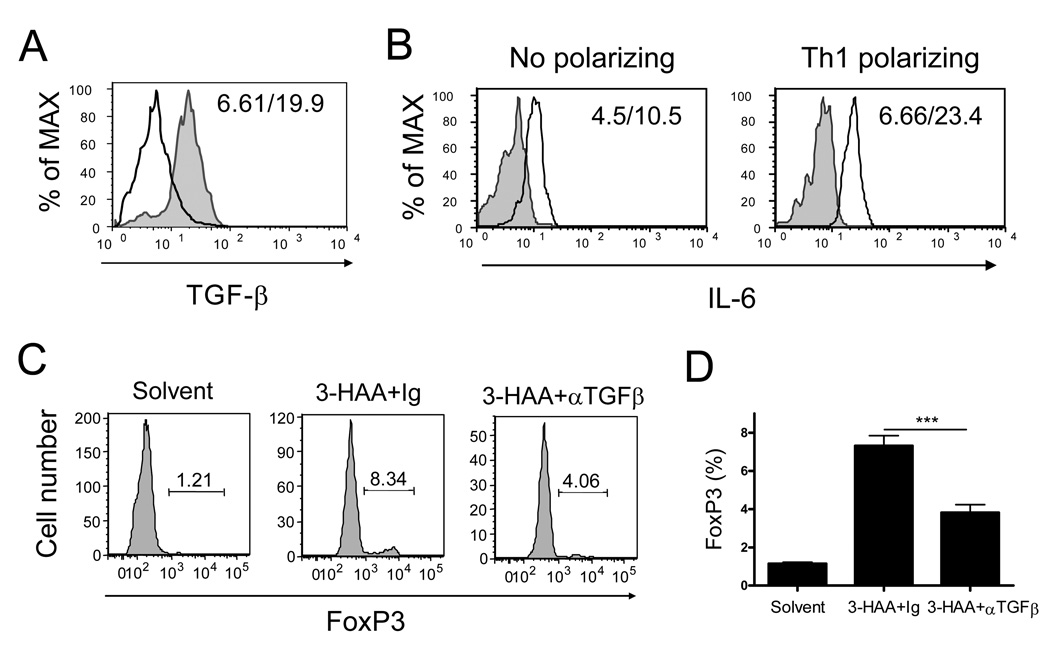
3-HAA-mediated Treg development is correlated with enhanced TGF-β expression and reduced levels of IL-6
To dissect the mechanism underlying 3-HAA-mediated upregulation of Tregs, we treated anti-CD3/anti-CD28-stimulated spleen cells with 3-HAA and analyzed production of TGF-β and IL-6, which are critical in controlling differentiation of activated CD4+ T cells into the Treg or Th17 lineage (13). We found that 3-HAA treatment increased the expression of TGF-β in CD11c+ DCs (Fig. 7A), which correlated with a decreased level of IL-6 in culture supernatants of anti-CD3/anti-CD28-activated spleen cells (Fig. 7B). However, the addition of IL-6 (20 ng/ml) in anti-CD3 and anti-CD28-activated spleen cells without 3-HAA treatment did not affect Treg development or promote Th17 differentiation (data not shown), implying a lack of TGF-β in the culture of activated spleen cells stimulated by anti-CD3/anti-CD28 alone. To confirm the role of TGF-β in 3-HAA –induced Treg differentiation, we added neutralizing anti-TGF-β Ab in culture during 3-HAA priming. The results showed that addition of anti-TGF-β in culture significantly reduced the level of CD4+FoxP3+ T cells (Fig. 7C, 7D). These data suggest that 3-HAA-induced TGF-β expression from DCs plays a pivotal role in the induction of Tregs.
Figure 7. 3-HAA enhances anti-CD3/anti-CD28-induced TGF-β expression and reduces IL-6 production in spleen cells.
A. TGF-β-expression in DCs. Spleen cells from naïve mice were stimulated and primed with either 3-HAA or vehicle control as described in Fig. 5. Cells were harvested from culture on day 2 after stimulation. To determine TGF-β expression, cells were stained with anti-CD11c-FITC, followed by intracellular staining with anti-TGF-β-PerCP/Cy5.5. Data were analyzed by flow cytometry. Data shown are a representative of three experiments. Solid line: vehicle-treated control cells; shaded gray graph: 3-HAA-treated cells. The numbers in each panel represent the mean fluorescence intensity (vehicle/3-HAA). B. IL-6 production in anti-CD3/anti-CD28-activated spleen cells. Spleen cells were stimulated with or without the Th1-polarizing condition, and primed with 3-HAA or vehicle. Supernatants were harvested from culture on day 3 after stimulation. IL-6 levels in culture supernatants were detected by flow cytometry with cytokine bead assay (CBA). Results are shown as graphs reflecting the relative levels of IL-6 in samples. One representative of three experiments is shown. Solid line: vehicle-treated cells; shaded gray graph: 3-HAA-treated cells. The numbers in the panels represent the mean fluorescence intensity (3-HAA/vehicle). C and D. Addition of anti-TGF-b antibody decreased 3-HA-induced FoxP3 expression. Spleen cells were stimulated with anti-CD3/anti-CD28 and primed with 3-HAA (or vehicle) as described in Fig. 6 in the presence of anti-TGF-β antibody (30 µg/ml) or the same amount of Ig control. FoxP3 expression in primed cells was determined as described in Fig. 6. Data are the percentage of FoxP3+ cells in the CD4+CD25+ gate (C) or the mean percentage ± SD of triplicates (D). The results are from a representative of two repeat experiments. ***p < 0.001.
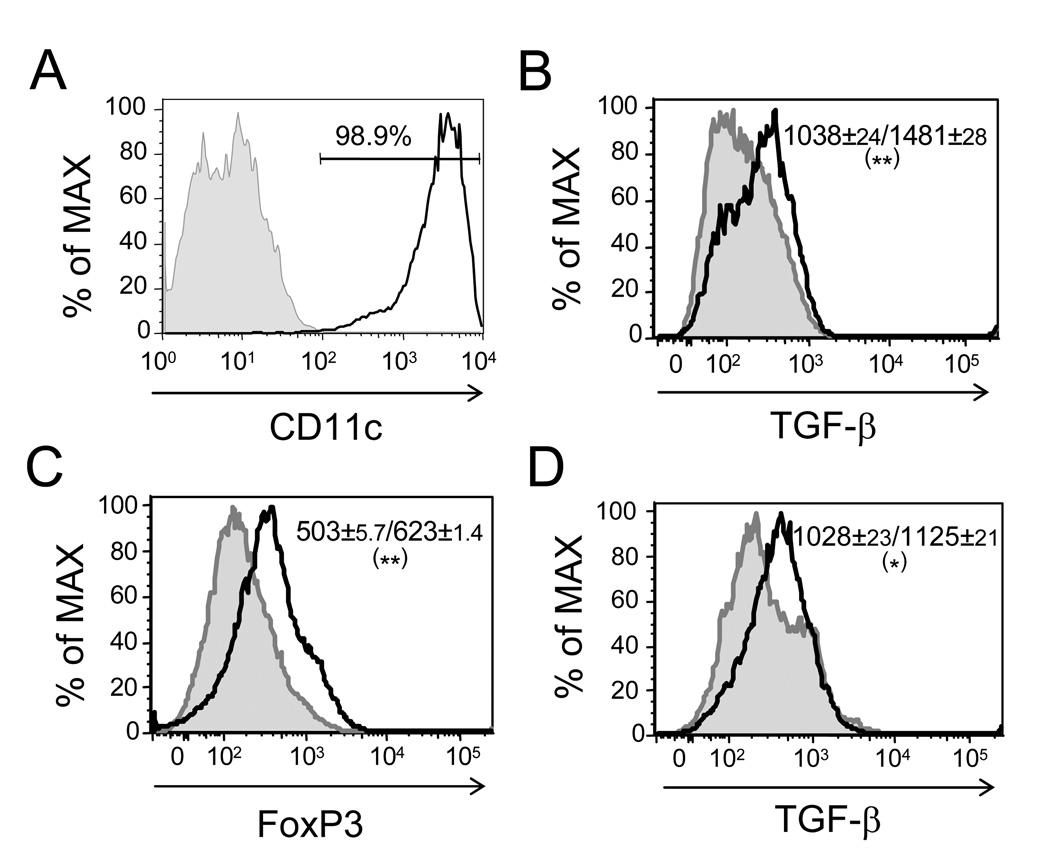
To further confirm the essential role of DCs in expressing TGF-β for induction of Tregs, we prepared bone marrow-derived DCs from C57BL/6 mice (Fig. 8A) and treated DCs with 3-HAA. The results showed that 3-HAA treatment promoted TGF-β expression in DCs in comparison with vehicle-treated DCs (Fig. 8B). We next treated DCs with 3-HAA or vehicle first and then co-cultured the treated DCs with anti-CD4 bead-purified naïve CD4+ T cells in the presence of anti-CD3/anti-CD28 stimulation. Treg development and TGF-β expression of DCs in co-culture were determined by flow-cytometric analysis. We found that 3-HAA-treated DCs enhanced the expression of FoxP3 in T cells compared with vehicle-treated DCs (Fig. 8C), which correlated with an increased expression of TGF-β by DCs (Fig. 8D). These data, together with the results from an anti-TGF-β neutralizing experiment (Fig. 7C, 7D), indicate that DC-expressed TGF-β is essential in 3-HAA-mediated induction of Tregs.
Figure 8. 3-HAA-primed DCs express enhanced levels of TGF-β and are effective in induction of FoxP3 expression in T cells.
A. Bone marrow-derived DCs. DCs were prepared as described in Materials and Methods. Purity of DCs was determined by flow cytometry with anti-CD11c Ab staining. Shaded gray graph: isotype control; solid black line: anti-CD11c Ab. The percentage indicates the purity of DCs. B. Bone-marrow-derived DCs were treated with 200 µM 3-HAA (solid black heavy line) or vehicle control (tinted gray peak) for 48 hrs in culture with GolgiStop (BD Bioscience) for the last 4 h. TGF-β expression in DCs was determined by intracellular staining with anti-TGF-β-PerCP/Cy5.5. Results are data from flow-cytometric analysis. The numbers represent mean fluorescence intensity ± SD of two repeat experiments (vehicle/ 3-HAA). C and D. DCs were treated with either 200 µM 3-HAA or vehicle for 48 h in culture. The treated DCs were washed and then were co-cultured with purified naïve CD4+ T cells for 3 d in the presence of GolgiStop for the last 4 h. C. To analyze FoxP3 expression in T cells, cells were stained with anti-CD4-FITC/anti-CD25-allophycocyanin, followed by intracellular staining with anti-FoxP3-PE. (D) To detect TGF-β expression in DCs, cells were first stained with anti-CD11-FITC, followed by intracellular staining with anti-TGF-β-PerCP/Cy5.5 (D). Results are from flow-cytometric analysis. Thick black line: co-culture with 3-HAA-treated DCs; shaded gray graph: co-culture with vehicle-treated DCs. The numbers indicate mean fluorescence intensity ± SD of two repeat experiments (vehicle-treated DCs/3-HAA-treated DCs). *p < 0.05; **p < 0.01.
Discussion
Previous studies showed that inhibition of IDO activity by systemic administration of IDO inhibitor 1-MT exacerbated clinical scores of EAE (27). Moreover, treatment of EAE mice with tryptophan metabolites or a synthetic derivative of tryptophan metabolite anthranilic acid reduced Th1 cytokine production from encephalitogenic T cells and ameliorated disease severity, suggesting that IDO and tryptophan metabolites play a suppressive role in Th1 cell-mediated immune responses in EAE (29). In comparison with Th1 cells, selective amino acid depletion-induced activation of cytoprotective signaling pathway (GCN2) in T cells was shown to inhibit Th17 cell differentation (20). In addition, IDO-induced activation of GCN2 kinase in pDCs reduces IL-6 production from pDCs and subsequently inhibits Th17 cell differentiation (18, 19). Our studies provide additional evidence for a suppressive role of IDO and tryptophan metabolite 3-HAA in Th1 and Th17 cells in EAE. IDO activity and bystander catabolites from the kynurenine metabolism pathway can impact encephalitogenic T cell responses through direct and indirect mechanisms: by direct suppression of effector T cells through tryptophan depletion-induced GCN2 kinase activation and direct suppressive effects of tryptophan metabolites on effector T cells through their cytotoxic effects or by indirect inhibition of T cells through upregulation of Treg activity by tryptophan metabolites, such as 3-HAA. Both mechanisms may contribute to IDO-mediated inhibition of EAE. However, based on data from our studies, it seems that encephalitogenic Th17 cells are less sensitive to direct suppression by tryptophan metabolites than are Th1 cells. Therefore, the suppressive effect of 3-HAA on Th17 cell-mediated pathogenesis in EAE is largely a result of 3-HAA-induced generation of Tregs.
Treatment with 3-HAA has been shown to reduce Th1 responses in EAE (29) and to decrease Th2-cytokine levels in an asthmatic model (37). Inhibition of encephalitogenic Th1 cells by 3-HAA in our studies is consistent with observations by Platten et al. (29), whereby i.p. injection of 3-HAA or oral administration of 3,4-DAA reduced Th1 cytokine production and relatively enhanced levels of Th2 cytokines. It is not clear whether downregulation of Th1 responses by oral administration of 3,4-DAA shares the same therapeutic mechanism with that of 3-HAA i.p. injection, because we did not compare 3-HAA with 3,4-DAA in our studies. Our data show that, in addition to its suppressive effects on Th1 cells, administration of 3-HAA suppressed Th17 responses by the induction of Tregs. Thus, 3-HAA treatment ameliorates EAE through multiple mechanisms, including inhibition of encephalitogenic Th1 and Th17 responses and upregulation of protective Th2 cell responses. 3-HAA was more effective when treatment was started at the time of antigen expose, or at early clinical onset of EAE, suggesting that 3-HAA inhibited autoantigen-induced T cell activation during the afferent phase of EAE. In contrast, 3-HAA was not effective at inhibiting EAE when its administration began at disease peak. This might have been because existing activated T cells had already migrated into the CNS and caused neural damage. Therefore, early treatment with 3-HAA may improve the clinical outcome of EAE.
It has been well documented that IDO expression and metabolites from the kynurenine metabolism pathway positively regulate the development and function of Tregs (18, 19, 26). Although the mechanism underlying IDO-mediated induction of Tregs has not been fully investigated, tryptophan metabolites are considered to play an important role in Treg cell induction by DCs expressing IDO (24, 38). It was shown that addition of kynurenines to culture is capable of inducing Tregs (26, 39, 40). However, low-tryptophan medium is not effective at inducing Tregs (26), suggesting that tryptophan metabolites (e.g. kynurenines), but not tryptophan depletion in T cells, lead to induction of Tregs. Our data provide additional evidence that IDO can induce Tregs through 3-HAA. Thus, 3-HAA-induced generation of Tregs likely contributes to the inhibition of encephalitogenic Th1 and Th17 cells during EAE. The role of 3-HAA-induced Treg cells is more critical in the inhibition of encephalitogenic Th17 cells because this tryptophan metabolite does not seem to have a direct suppressive effect on Th17 cells.
T cell differentiation is largely controlled by cytokine-mediated polarizing signals (13). TGF-β and IL-6 are two key cytokines that drive CD4+ T cells to differentiate into Tregs or Th17 cells. Successful differentiation of Tregs requires TGF-β while Th17 cell differentiation needs both TGF-β and IL-6 (41, 42). It was shown that pDC-expressed IL-6 plays a critical role in driving Th17 cell differentiation. When IL-6 production is blocked, T cell differentiation is reprogrammed into the Treg lineage (18, 19). Our study showed that IL-6 production was enhanced in anti-CD3/anti-CD28-stimulated spleen cells but no robust Th17 or Treg differentiation was observed, suggesting that anti-CD3/anti-CD28 stimulation alone did not induce enough TGF-β for Treg or Th17 differentiation. This is different from the observation in the tumor model (19), in which tumor cells might provide a source of TGF-β required for Treg or Th17 cell differentiation. In contrast, 3-HAA treatment induced TGF-β expression from DCs and reduced IL-6 production from anti-CD3/anti-CD28-activated cells, which established a favorable microenvironment for Treg, but not Th17 cell, differentiation, indicating that 3-HAA-mediated induction of Tregs and inhibition of IL-6 contribute to the reduced encephalitogenic Th17 response that we observed in 3-HAA-treated mice. In contrast, 3-HAA cannot inhibit Th17 differentiation when IL-6 and TGF-β are added in culture, given that exogenous IL-6 may compensate for the 3-HAA-mediated reduction in IL-6. Our results suggest that 3-HAA-induced TGF-β from DCs is the major contributor for Treg differentiation.
In summary, our data show that IDO-deficiency enhanced encephalitogenic T cell responses, exacerbated EAE, and downregulated Treg function. In contrast, in addition to its direct inhibitory effect on Th1 cells and upregulation of protective Th2 cytokines, tryptophan metabolite 3-HAA inhibited effector T cells (Th1 and Th17) and ameliorated EAE by promoting Treg responses through upregulation of TGF-β produced by DCs. These results suggest that tryptophan metabolite-induced Treg responses constitute an important negative regulatory mechanism in IDO-mediated immune suppression and immune tolerance. Our studies raise the possibility of using tryptophan metabolites to induce Tregs as a therapeutic strategy for immune suppression.
Acknowledgements
We are grateful to Katherine Regan for critical editorial assistance.
This work was supported by grants from the American Heart Association (0830072N) (H.X.) and from the National Institutes of Health (NS040085) (A.M. R.).
Abbreviations
- C-EAE
chronic experimental autoimmune encephalomyelitis
- 3,4-DAA
N-3/4-dimethoxycinnamoy) anthranilic acid
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- GCN2
general control nonrepressed 2
- 3-HAA
3-hydroxyanthranilic acid
- 1-MT
1-methyl-tryptophan
- MOG
myelin oligodendrocyte glycoprotein
- pDC
plasmacytoid dendritic cell
- p.i.
postimmunization
- PTX
pertussis toxin
- TDO
tryptophan 2,3-dioxygenase
- Treg
regulatory T cell
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E. Building different mouse models for human MS. Ann N Y Acad Sci. 2007;1103:11–18. doi: 10.1196/annals.1394.021. [DOI] [PubMed] [Google Scholar]
- 3.Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- 4.Tauber SC, Nau R, Gerber J. Systemic infections in multiple sclerosis and experimental autoimmune encephalomyelitis. Arch Physiol Biochem. 2007;113:124–130. doi: 10.1080/13813450701531227. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamoorthy G, Wekerle H. EAE: an immunologist's magic eye. Eur J Immunol. 2009;39:2031–2035. doi: 10.1002/eji.200939568. [DOI] [PubMed] [Google Scholar]
- 6.Zhang GX, Yu S, Gran B, Li J, Calida D, Ventura E, Chen X, Rostami A. T cell and antibody responses in remitting-relapsing experimental autoimmune encephalomyelitis in (C57BL/6 x SJL) F1 mice. J Neuroimmunol. 2004;148:1–10. doi: 10.1016/j.jneuroim.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM, Chang JT, Segal BM. The critical role of IL-12 and the IL-12Rβ2 subunit in the generation of pathogenic autoreactive Th1 cells. Springer Semin Immunopathol. 1999;21:249–262. doi: 10.1007/BF00812256. [DOI] [PubMed] [Google Scholar]
- 8.Segal BM. Experimental Autoimmune Encephalomyelitis: Cytokines, Effector T Cells, and Antigen-presenting Cells in a Prototypical Th1-mediated Autoimmune Disease. Curr Allergy Asthma Rep. 2003;3:86–93. doi: 10.1007/s11882-003-0017-6. [DOI] [PubMed] [Google Scholar]
- 9.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 10.Aranami T, Yamamura T. Th17 Cells and autoimmune encephalomyelitis (EAE/MS) Allergol Int. 2008;57:115–120. doi: 10.2332/allergolint.R-07-159. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 12.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 15.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–190. doi: 10.1007/978-1-4615-0135-0_21. [DOI] [PubMed] [Google Scholar]
- 18.Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 22.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 23.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 24.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli I, Horenstein AL, Fiore F, Massaia M, Colombo MP, Baccarani M, Lemoli RM. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 26.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 27.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 29.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 31.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu H, Wawrousek EF, Redmond TM, Nickerson JM, Wiggert B, Chan CC, Caspi RR. Transgenic expression of an immunologically privileged retinal antigen extraocularly enhances self tolerance and abrogates susceptibility to autoimmune uveitis. Eur J Immunol. 2000;30:272–278. doi: 10.1002/1521-4141(200001)30:1<272::AID-IMMU272>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for TH1/TH2 regulation. Immunol Lett. 2008;121:1–6. doi: 10.1016/j.imlet.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–2071. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, Lovett-Racke AE. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157–5162. doi: 10.4049/jimmunol.180.8.5157. [DOI] [PubMed] [Google Scholar]
- 39.De Luca A, Montagnoli C, Zelante T, Bonifazi P, Bozza S, Moretti S, D'Angelo C, Vacca C, Boon L, Bistoni F, Puccetti P, Fallarino F, Romani L. Functional yet balanced reactivity to Candida albicans requires TRIF, MyD88, and IDO-dependent inhibition of Rorc. J Immunol. 2007;179:5999–6008. doi: 10.4049/jimmunol.179.9.5999. [DOI] [PubMed] [Google Scholar]
- 40.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 41.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrausch U, Jensen SM, Twitty C, Poehlein CH, Haley DP, Walker EB, Fox BA. Disruption of TGF-β signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J Immunol. 2009;183:3682–3689. doi: 10.4049/jimmunol.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]