Abstract
This article reviews the current status of cardiovascular disease (CVD) on the international scale. Presently viewed as an epidemic that has migrated from westernized societies to developing countries, several important issues are elaborated upon. They include the basis for the increasing prevalence of CVD and the associated societal implications. The challenges related to lack of resources and infrastructure support may also impede successful implementation of proven strategies to reduce CVD. In addition to traditional risk factors such as cigarette smoking, hypertension, obesity, hyperlipidemia, diabetes mellitus and insulin resistance, many developing countries must also contend with other risk biomarkers. Included in this grouping are human immunodeficiency virus/acquired immunodeficiency syndrome and other infectious/inflammatory processes as well as nutritional and vitamin deficiencies that make preventive measures more difficult to prioritize. Taken together, greater partnering between local governments, affiliated hospitals and international societies is needed to enhance and facilitate efforts aimed at optimizing standard of care measures in developing countries in order to reduce cardiovascular risk.
Keywords: Heart disease, Developing world, Epidemic, Risk factors, Urbanization, Prevention
CARDIOVASCULAR DISEASE - A GLOBAL PROBLEM
Cardiovascular disease (CVD) has typically been viewed as an affliction of wealthy, industrialized societies. In fact, during the past century minimal if any effort aimed at cardiovascular (CV) prevention has been allocated to developing countries. This in part reflected the higher prevalence of infectious diseases that provided the rationale for not investing time and resources toward chronic diseases. However there is an emerging body of data suggesting that this policy may not only be erroneous but also dangerous. Based upon statistics by the World Health Organization, approximately 80% of the 17 million CV deaths worldwide in 2003, occurred in developing countries[1].
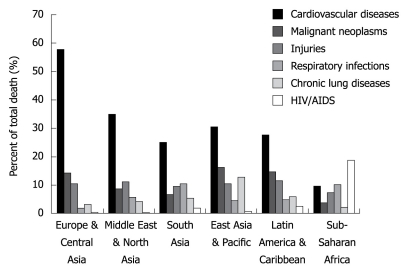
As shown in Figure 1, CVD represents the number one cause of death in all regions except for sub-Saharan Africa; however when the analysis extends beyond adults aged 30 years and older, CVD is number one cause of death in all regions[2].
Figure 1.
Cause of death by percentage in each region[2].
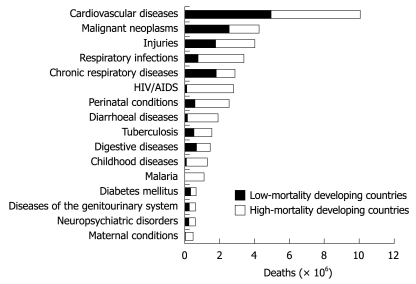
These findings in Figure 2 raise a number of questions: (1) Why is CVD so prevalent in developing countries? (2) What are the implications of this prevalence? (3) Why has there not been as strong a focus on controlling this epidemic? and (4) What can be done to control this continuing epidemic?
Figure 2.
Deaths attributable to 16 leading causes in developing countries, 2001[3].
EPIDEMIOLOGICAL AND NUTRITION TRANSITION
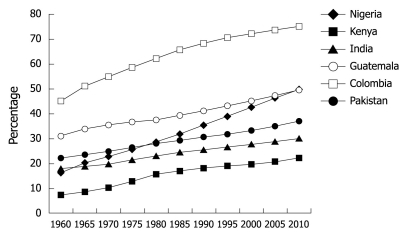
It is widely believed that there are 4 stages of epidemiological transition, ranging from famine and pestilence (stage 1) to degenerative diseases (stage 4). In terms of overall health, each country falls somewhere along this spectrum. Sub-Saharan Africa is the main region that falls under the first stage, while stage 4 regions are the more industrialized nations. Emerging outcomes data now suggest a trend towards stage 4 even for less industrialized countries. While communicable diseases such as human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), tuberculosis, and malaria continue to be associated with high mortality rates, especially in developing countries, considerable progress has been made during the past decades to reduce the burden of disease resulting from these conditions. Consequently, a more favorable prognosis has been achieved in affected infants who are now more likely to survive into adulthood. The ensuing survival rates in turn increase the exposure to risk factors such as cigarette smoking that alters the shift toward enhanced CV risk. Another factor to be considered is the “early malnutrition wars” and their casualties. For example, Amuna et al[4] have hypothesized that exposure of fetuses to early malnutrition led to adaptation to a “thrifty phenotype”. However, when exposed to a more affluent environment and greater caloric means, these “super efficient” specimens are less able to metabolize the nutrients (i.e. fats) that they are exposed to. With increased energy intake at the expense of expenditure, increased fat storage in adipose tissue, skeletal muscle, heart and liver may lead to metabolic dysregulation resulting in inflammation, insulin resistance (IR), metabolic syndrome and increased risk of CVD. An alternative explanation is that there has been a significant increase in the rate of urbanization in most developing countries (Figure 3 below). The result of urbanization is a more frequent exposure to CV related risk factors.
Figure 3.
Trends of urban populations in developing countries[5].
Once in these urban areas, diet and lifestyle changes adopted include high caloric food intake combined with a sedentary lifestyle. With this combination, there has been an appreciable jump in the prevalence of CV disease risk factors. Some of the forces that drive people towards high caloric foods include time constraints (forced to eat on the go), strong advertising and availability[6]. Similar forces come into play when considering the adoption of sedentary lifestyles.
RISK FACTORS
A decades worth of studies have identified risk factors for CVD, the more prevalent ones being hypertension, smoking, obesity (due to poor diet and/or lack of physical activity), hyperlipidemia and genetic predisposition. If there is truly an increase in the prevalence of CVD, one should naturally expect that there has been an increase in one or more of these risk factors. The data supporting this will be explored further.
Smoking
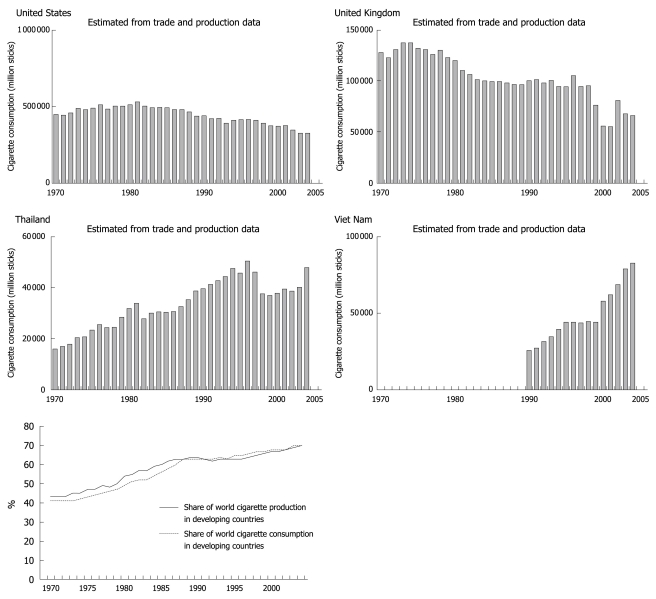
Tobacco use has been established as a risk factor for CVD since the 1940s. This includes both active and passive use[7]. Due to aggressive anti-smoking campaigns, tobacco use has declined significantly in the United States over the past 40 years[8]. Unfortunately, the trend has been the reverse in developing countries (Figure 4).
Figure 4.
Cigarette consumption in select countries-comparing industrialized to non-industrialized countries[8,9].
The reasons for this increase in tobacco use include but are not limited to, urbanization and aggressive marketing by tobacco companies, especially in light of strict regulations in developed countries forcing these companies to shift their business practices elsewhere. Of particular concern is the fact that young women who are typically less likely to smoke are viewed as an untapped market and are heavily targeted[10].
Hypertension
It is difficult to assess the degree to which there has been an increase in the prevalence of hypertension in most developing countries because there are few studies. However there are studies demonstrating the high levels of prevalence today. To support the argument that it is associated with urbanization, numerous studies have demonstrated the difference in prevalence between urban and rural areas. For instance, in Mozambique, the prevalence of hypertension amongst adults is about 33%, while the odds ratio (OR) of hypertension being more frequent in urban areas compared to rural was 2 [95% confidence intervals (CI): 1.2-3][11]. Fezeu et al[12] identified an increase in the blood pressure of adults in Cameroon over a 10-year period (Table 1).
Table 1.
Difference in systolic blood pressure (SBP) over 10-year period in Cameroon
| Increase in SBP | Women | Men | P |
| Rural setting (mmHg) | 18.2 | 18.8 | < 0.001 |
| Urban setting (mmHg) | 8.2 | 6.5 | < 0.001 |
Hypertension is also highly prevalent in Latin America-prevalence rates ranging from 8.6% to as high as 29% of the adult population based on the city[13]. Pre-hypertension has also been assessed in a number of studies, with prevalence rates ranging from 30% in Jamaican adults[14] to as high as 34% in Taiwanese adults[15].
Obesity
Obesity is an intriguing and paradoxical CV risk biomarker in developing countries. This is because in developing countries such as Sub-Saharan Africa, epidemics of famine that culminate in malnutrition are believed to predominate. However, urbanization of some regions has in fact induced higher obesity rates. Amongst Cameroonian adults (older than 15), 21.6% of men are overweight. The number is higher amongst women at 28.6%. The prevalence of obesity is high as well - 6.5% in men, 19.5% in women[16]. Abubakari et al[17] found an approximate 10% rate of obesity in West Africa. Furthermore, in Cameroon, women were more likely to be obese (OR: 3.16, 95% CI: 2.51-3.98). To further support the theory that urbanization plays a significant role in West Africa, prevalence rates of obesity were compared in urban and rural settings. Not surprisingly, urban residents were 2.7 fold more likely to be overweight[18]. Although, it may be difficult in some developing countries to establish changes in the prevalence of obesity due to a lack of baseline comparison data, some studies have been able to demonstrate such increases. For example, in South Asian women, significant increases in obesity rates were identified between 1996 and 2006, as described in Table 2.
Table 2.
Change in obesity prevalence over a 10-year period in Asian countries[17] (%)
| Country | 1996 | 2006 |
| Bangladesh | 2.7 | 8.9 |
| Nepal | 1.6 | 10.1 |
| India | 10.6 | 14.8 |
Lipids
Hyperlipidemia has long been associated with the development of CVD in industrialized nations[19]. There has been an increase in the prevalence of hyperlipidemia in developing countries that can partly be attributed to urbanization - some reports have demonstrated a higher prevalence in urban vs rural areas[20]. In rural China, greater than 30% of adults older than 35 years of age have total cholesterol levels greater than 200 mg/dL. Elevated LDL (greater than 130 mg/dL) ranged from 13.8% of adult males to 17.2% of adult females[21]. Amongst Latin American populations, the prevalence of hypercholesterolemia varied from 6%-20% depending on the city[13]. For comparison, the prevalence of elevated total cholesterol (> 240 mg/dL) in US adults from 2003-2006 was 16.3%[22].
Diabetes
This is another CV risk factor that has become more prevalent in developing countries in recent years. A 2008 review found an increase in the prevalence of diabetes in Nigeria and Ghana. From 1963 to 1998, the prevalence rose from 0.2% to 6.3% of the adult Ghanaian population while amongst Nigerians, the prevalence rose from 1.65% to 6.8% from 1985 to 2000[23]. Not only has there been a rise in the prevalence of diabetes in developing countries, but further increases have been projected in selected regions (i.e. Latin America)[24]. A number of studies have also described a significant prevalence in parts of Asia as well[25,26].
IR
Current data indicate a worldwide surge in the prevalence of diabetes, with particularly worrisome rates in the developing world. As concerning as these new data are, even more concerning is the prevalence of IR, particular among young people. IR is another risk factor that is linked to the progression of CVD, both as an independent risk factor[27] as well as through its association with other risk factors such as obesity and hypertension. The link between IR and CVD is thought to occur through two processes known to be associated with CVD, namely hyperuricemia[28] and inflammation[29]. With this said, is IR a problem in developing countries? Amongst Bolivian children, the prevalence of IR was 39.4%[30]. In another study of obese Chinese children, 77% of the study participants were insulin resistant[31]. The fact that levels this high are being seen in children is more concerning when one considers that these numbers, without early intervention, are only going to rise as they grow into adulthood.
NON-TRADITIONAL RISK FACTORS
Management of CVD in developing countries presents a unique challenge. Because the majority of the limited available resources are devoted to communicable diseases, convincing evidence would need to be provided to justify resource diversion for CV prevention. Among the considerations would be whether the top causes of mortality within developing countries, including respiratory infections, HIV/AIDS, malnutrition and emotional stress, feature prominently in CV risk assessment.
HIV/AIDS
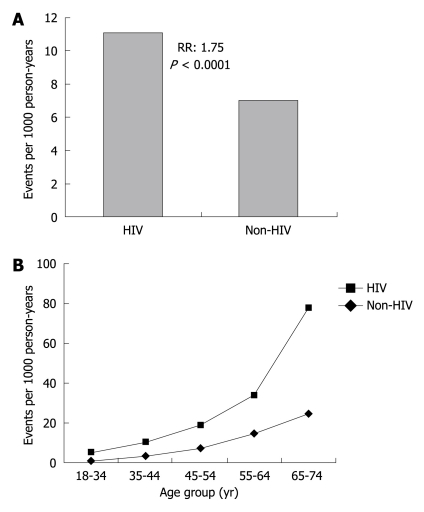
There are an estimated 33 million people living with HIV worldwide and 67% of affected subjects reside in Sub-Saharan Africa[32]. Observational data have linked HIV to coronary artery disease (CAD) (Figure 5).
Figure 5.
Association between coronary events and human immunodeficiency virus (HIV)[33]. A: Myocardial events amongst patients grouped by HIV-infection status; B: Myocardial events grouped by age. Light line represents HIV positive patients, dark line represents patients without HIV.
Endothelial dysfunction is a critical early stage in the development of atherosclerosis. In addition to traditional CV risk factors[34], there is also evidence to implicate HIV in promoting endothelial cell dysfunction. This may occur through several postulated mechanisms. They include the HIV directly activating pro-inflammatory cytokines and procoagulant proteins as well as increasing endothelial cell adhesiveness and apoptosis resulting in endothelial cell damage[35]. Another mechanism may be through the use of highly active antiretroviral therapy. For example, abacavir and didanosine have been associated with increased risk of myocardial infarction (MI) (OR: 1.89, 95% CI: 1.47-2.45 and OR: 1.49, 95% CI: 1.14-1.95) within 6 mo of use[36].
Other infections as risk factors
Due to poor sanitation, close living quarters and low socio-economic status, there remains a high prevalence of infectious pathogens and in part explains the higher prevalence of mortality due to respiratory infections in developing countries. Might any of these pathogens also be linked to elevated CV risk? The influenza virus is a common pathogen worldwide and has been shown to be prevalent amongst children in developing countries[37,38]. This becomes significant when one considers that a history of being infected with influenza A or B (determined by the presence of IgG antibodies) is associated with an increased risk of myocardial infection[39]. Another pathogen that has been associated with the development of CV disease is Chlamydia pneumonia[40]. Other pathogens that have been linked to CAD include Mycoplasma pneumonia and Helicobacter pylori. Nevertheless, more research into the direct causality link between pathogens and development of CVD is warranted.
Vitamin D, B12 and folate
Vitamin D deficiency has been observed in limited studies in populations of people in developing countries[41-43]. Yet despite the fact that some developing countries are in tropical regions (with a lot of sun exposure), a high prevalence of Vitamin D deficiency exists; low vitamin D levels have been associated with increased coronary calcification[44]. Hyperhomocysteinemia and vitamin B12 deficiency have also been linked to development of CVD[45]. B12 is usually obtained from meat products, which in most developing countries may be relatively sparse. Folate is a co-factor in the homocysteine metabolism pathway and it has been proposed that its deficiency can also result in disease in a manner analogous to B12 deficiency. As adequate folic acid levels may be difficult to attain, most developed countries have policies in place that mandate fortification of certain foods with folate. Unfortunately, these policies are absent in developing countries, resulting in high prevalence of folate deficiency.
Psychosocial factors
Amongst Congolese children, greater than 50% meet the symptom criteria for Post Traumatic Stress Disorder (PTSD)[46], while in responders of an Afghanistan survey greater than 20% met criteria for PTSD[47]. Depression has been observed in about a quarter of Brazilian women infected with the HIV[48]. These examples demonstrate the prevalence of psychosocial stressors within the developing world and become more problematic when one considers the association between psychosocial disease and CVD. Specifically, the INTERHEART study[49] examined the association between “stress factors” (stress at work and at home, financial situation and major life events) and acute MI. The OR between cases (people with MI) and controls, depending on the stressor, ranged from 1.38 to 2.17. In addition, depression has been described as a risk factor for CAD[50] and while confounders such as smoking may promote this process, there are direct pathophysiological considerations such as platelet dysfunction and systemic inflammation that may accentuate CV risk.
IMPLICATIONS OF INCREASED PREVALENCE OF CVD RISK FACTORS/CVD
Although data support increased CV risk in developing countries, what are the potential strategies and implications that should be focused upon? Unfortunately, few developing countries are sufficiently equipped to handle medical emergencies such as acute MI and stroke due to lack of resources and trained personnel. Another effect of a CV epidemic is loss of manpower due to earlier onset of disease (by an average of 10 years). In fact, in developing countries (i.e. India) more than 50% of CV deaths occurred before 70 years of age as compared to less than 25% of CV deaths in developed countries[51].
There are a variety of reasons why healthcare policies are geared towards infectious diseases. First, the pediatric population is at high risk and is accompanied by a strong visceral response to treat urgently. In contrast, CV disease is viewed as a chronic process that can be controlled at a personal level.
CONTROLLING CV DISEASE IN DEVELOPING COUNTRIES
Despite the increased prevalence of CV risk factors in developed countries, the first half of this decade saw a peak and decline in CV related mortality[52], related in part to risk factor modification. Nonetheless, developing countries present an uncommon scenario as policies that have worked in developed countries may not be applicable. Thus, the ideal approach may be a dual one - identifying proven strategies in developed countries and then modifying them as necessary for the individual country being targeted.
Prevention at the population level
The most obvious risk factor that should be targeted at this level is smoking. One approach might be to increase taxation of tobacco products and use the extra revenue gained to fund such programs. Another population based prevention strategy is developing national food guidelines/recommendations. Most countries have distinctive cuisines and developing guidelines that incorporate locally grown and healthy products are more likely to be accepted by the general populace. This is of particular importance considering the globalization of Western world fast food chains. The higher prevalence of CV risk factors in urban vs rural areas should be considered when it comes to implementing national health guidelines because it presents an opportunity to implement change before risk factors become more prevalent in rural areas. For example, should fortification (as done in Westernized regions) with folate be incorporated into food products?
Primary prevention
Screening people who may be at high risk for CV disease may forestall the progression to advanced disease. In developing countries where the prevalence of psychosocial stressors is high, routine screening for PTSD and depression ought to be considered. In some countries, there is a shortage of physicians in general (including specialists), hence the primary care approach could be modified for use by non-physicians as primary care providers. Bischoff et al[53] has suggested that nurse based care would be an appropriate approach in Cameroon - this could be extended to other parts of Sub-Saharan Africa as well. Upon identifying those at risk, the next issue becomes how to manage them. How do we deliver cost effective therapies to those in need? Standardized recommendations need to be developed for each country. For instance, should we recommend a standard 3-mo trial of diet and exercise after identifying risk factors in a patient, and due to the cost of medications, should primary care providers be more aggressive with this approach if necessary?
Secondary prevention
Post MI and post stroke care should be placed on standard of care regimens based upon clinical outcome data when feasible. However, due to the high cost of medications, unique combinations of appropriate medicines that balance cost and quality adjusted life years need to be developed for each region. For instance giving just aspirin and β-blocker while sub-standard may be more cost effective than aspirin, β-blocker, angiotensin converting enzyme-inhibitor and statin (standard post MI regimen)[2]. Another factor that needs to be addressed when it comes to secondary prevention is the availability of emergency care resources. A model that could be implemented is a partnership between local hospitals and hospitals in developed countries, whereby physicians from developed countries travel to developing countries to teach local personnel how to use donated equipment.
Overall, there is clearly a need for more research in the field of CV disease in developing countries. The data regarding traditional and, to a lesser degree, non-traditional risk factors however is limited. Research should be geared towards identifying the unique factors that are at play in the developing world. This is especially important because most developing countries are still reeling from communicable disease epidemics and would only be further crippled by a CV epidemic.
Footnotes
Peer reviewers: Serafino Fazio, Associate Professor of Internal Medicine, Department of Internal Medicine, Cardiovascular and Immunologic Sciences, University Federico II, Via S. Pansini 5, 80131 Naples, Italy; Jun R Chiong, MD, MPH, FACC, FCCP, Associate Professor of Medicine, Director, Advanced Heart Failure Program, Loma Linda University, 11234 Anderson Street, Suite 4404, Loma Linda, CA 92354, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
References
- 1.World Health Organization. Cardiovascular disease: prevention and control. Available from: http://www.who.int/dietphysicalactivity/publications/facts/cvd/en/
- 2.Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–3553. doi: 10.1161/CIRCULATIONAHA.105.591792. [DOI] [PubMed] [Google Scholar]
- 3. Available from: http://www.who.int/entity/whr/2003/en/Chapter6-en.pdf.
- 4.Amuna P, Zotor FB. Epidemiological and nutrition transition in developing countries: impact on human health and development. Proc Nutr Soc. 2008;67:82–90. doi: 10.1017/S0029665108006058. [DOI] [PubMed] [Google Scholar]
- 5. Available from: http://esa.un.org/unup/index.asp.
- 6. Available from: http://www.fao.org/docrep/007/y5736e/y5736e00.HTM.
- 7.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ. 1997;315:973–980. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Available from: http://www.who.int/tobacco/mpower/mpower_report_country_profiles_2008.pdf.
- 9. Available from: http://www.who.int/entity/tobacco/mpower/mpower_report_tobacco_crisis_2008.pdf.
- 10.Mackay J, Amos A. Women and tobacco. Respirology. 2003;8:123–130. doi: 10.1046/j.1440-1843.2003.00464.x. [DOI] [PubMed] [Google Scholar]
- 11.Damasceno A, Azevedo A, Silva-Matos C, Prista A, Diogo D, Lunet N. Hypertension prevalence, awareness, treatment, and control in mozambique: urban/rural gap during epidemiological transition. Hypertension. 2009;54:77–83. doi: 10.1161/HYPERTENSIONAHA.109.132423. [DOI] [PubMed] [Google Scholar]
- 12.Fezeu L, Kengne AP, Balkau B, Awah PK, Mbanya JC. Ten-year's change in blood pressure levels and prevalence of hypertension in urban and rural Cameroon. J Epidemiol Community Health. 2009:Epub ahead of print. doi: 10.1136/jech.2008.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schargrodsky H, Hernández-Hernández R, Champagne BM, Silva H, Vinueza R, Silva Ayçaguer LC, Touboul PJ, Boissonnet CP, Escobedo J, Pellegrini F, et al. CARMELA: assessment of cardiovascular risk in seven Latin American cities. Am J Med. 2008;121:58–65. doi: 10.1016/j.amjmed.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson TS, Younger NO, Tulloch-Reid MK, Wright MB, Ward EM, Ashley DE, Wilks RJ. Prevalence of prehypertension and its relationship to risk factors for cardiovascular disease in Jamaica: analysis from a cross-sectional survey. BMC Cardiovasc Disord. 2008;8:20. doi: 10.1186/1471-2261-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai PS, Ke TL, Huang CJ, Tsai JC, Chen PL, Wang SY, Shyu YK. Prevalence and determinants of prehypertension status in the Taiwanese general population. J Hypertens. 2005;23:1355–1360. doi: 10.1097/01.hjh.0000173517.68234.c3. [DOI] [PubMed] [Google Scholar]
- 16.Kamadjeu RM, Edwards R, Atanga JS, Kiawi EC, Unwin N, Mbanya JC. Anthropometry measures and prevalence of obesity in the urban adult population of Cameroon: an update from the Cameroon Burden of Diabetes Baseline Survey. BMC Public Health. 2006;6:228. doi: 10.1186/1471-2458-6-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abubakari AR, Lauder W, Agyemang C, Jones M, Kirk A, Bhopal RS. Prevalence and time trends in obesity among adult West African populations: a meta-analysis. Obes Rev. 2008;9:297–311. doi: 10.1111/j.1467-789X.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 18.Balarajan Y, Villamor E. Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–2144. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- 19.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, Tyroler HA. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 20.Glew RH, Conn CA, Vanderjagt TA, Calvin CD, Obadofin MO, Crossey M, Vanderjagt DJ. Risk factors for cardiovascular disease and diet of urban and rural dwellers in northern Nigeria. J Health Popul Nutr. 2004;22:357–369. [PubMed] [Google Scholar]
- 21.Zhang X, Sun Z, Zheng L, Li J, Liu S, Xu C, Li J, Zhao F, Hu D, Sun Y. Prevalence of dyslipidemia and associated factors among the hypertensive rural chinese population. Arch Med Res. 2007;38:432–439. doi: 10.1016/j.arcmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 22. Available from: http://www.cdc.gov/nchs/data/hus/hus08.pdf.
- 23.Abubakari AR, Bhopal RS. Systematic review on the prevalence of diabetes, overweight/obesity and physical inactivity in Ghanaians and Nigerians. Public Health. 2008;122:173–182. doi: 10.1016/j.puhe.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Aschner P. Diabetes trends in Latin America. Diabetes Metab Res Rev. 2002;18 Suppl 3:S27–S31. doi: 10.1002/dmrr.280. [DOI] [PubMed] [Google Scholar]
- 25.Aekplakorn W, Abbott-Klafter J, Premgamone A, Dhanamun B, Chaikittiporn C, Chongsuvivatwong V, Suwanprapisa T, Chaipornsupaisan W, Tiptaradol S, Lim SS. Prevalence and management of diabetes and associated risk factors by regions of Thailand: Third National Health Examination Survey 2004. Diabetes Care. 2007;30:2007–2012. doi: 10.2337/dc06-2319. [DOI] [PubMed] [Google Scholar]
- 26.Shera AS, Jawad F, Maqsood A. Prevalence of diabetes in Pakistan. Diabetes Res Clin Pract. 2007;76:219–222. doi: 10.1016/j.diabres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab. 2001;86:713–718. doi: 10.1210/jcem.86.2.7202. [DOI] [PubMed] [Google Scholar]
- 28.Yoo TW, Sung KC, Shin HS, Kim BJ, Kim BS, Kang JH, Lee MH, Park JR, Kim H, Rhee EJ, et al. Relationship between serum uric acid concentration and insulin resistance and metabolic syndrome. Circ J. 2005;69:928–933. doi: 10.1253/circj.69.928. [DOI] [PubMed] [Google Scholar]
- 29.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caceres M, Teran CG, Rodriguez S, Medina M. Prevalence of insulin resistance and its association with metabolic syndrome criteria among Bolivian children and adolescents with obesity. BMC Pediatr. 2008;8:31. doi: 10.1186/1471-2431-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung RY, Tong PC, Yu CW, Lau PW, Mok GT, Yam MC, Lam PK, Chan JC. High prevalence of insulin resistance and metabolic syndrome in overweight/obese preadolescent Hong Kong Chinese children aged 9-12 years. Diabetes Care. 2003;26:250–251. doi: 10.2337/diacare.26.1.250. [DOI] [PubMed] [Google Scholar]
- 32. Available from: http://data.unaids.org/pub/GlobalReport/2008/jc1510_2008_global_report_pp29_62_en.pdf.
- 33.Lo J, Grinspoon S. Cardiovascular disease in HIV-infected patients: does HIV infection in and of itself increase cardiovascular risk? Curr Opin HIV AIDS. 2008;3:207–213. doi: 10.1097/COH.0b013e3282fb7ba6. [DOI] [PubMed] [Google Scholar]
- 34.Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- 35.Chi D, Henry J, Kelley J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium. 2000;7:223–242. doi: 10.3109/10623320009072210. [DOI] [PubMed] [Google Scholar]
- 36.Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 37.Gordon A, Ortega O, Kuan G, Reingold A, Saborio S, Balmaseda A, Harris E. Prevalence and seasonality of influenza-like illness in children, Nicaragua, 2005-2007. Emerg Infect Dis. 2009;15:408–414. doi: 10.3201/eid1503.080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai HP, Kuo PH, Liu CC, Wang JR. Respiratory viral infections among pediatric inpatients and outpatients in Taiwan from 1997 to 1999. J Clin Microbiol. 2001;39:111–118. doi: 10.1128/JCM.39.1.111-118.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan XR, Li X, Xin XM, Jiang LX, Cui LY, Wang LF, Li HY. Influenza virus infection and risk of acute myocardial infarction. Inflammation. 2008;31:266–272. doi: 10.1007/s10753-008-9074-2. [DOI] [PubMed] [Google Scholar]
- 40.Jha HC, Vardhan H, Gupta R, Varma R, Prasad J, Mittal A. Higher incidence of persistent chronic infection of Chlamydia pneumoniae among coronary artery disease patients in India is a cause of concern. BMC Infect Dis. 2007;7:48. doi: 10.1186/1471-2334-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters BS, dos Santos LC, Fisberg M, Wood RJ, Martini LA. Prevalence of vitamin D insufficiency in Brazilian adolescents. Ann Nutr Metab. 2009;54:15–21. doi: 10.1159/000199454. [DOI] [PubMed] [Google Scholar]
- 42.Harinarayan CV, Ramalakshmi T, Prasad UV, Sudhakar D, Srinivasarao PV, Sarma KV, Kumar EG. High prevalence of low dietary calcium, high phytate consumption, and vitamin D deficiency in healthy south Indians. Am J Clin Nutr. 2007;85:1062–1067. doi: 10.1093/ajcn/85.4.1062. [DOI] [PubMed] [Google Scholar]
- 43.Sadat-Ali M, AlElq A, Al-Turki H, Al-Mulhim F, Al-Ali A. Vitamin D levels in healthy men in eastern Saudi Arabia. Ann Saudi Med. 2009;29:378–382. doi: 10.4103/0256-4947.55168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 45.Sadeghian S, Fallahi F, Salarifar M, Davoodi G, Mahmoodian M, Fallah N, Darvish S, Karimi A. Homocysteine, vitamin B12 and folate levels in premature coronary artery disease. BMC Cardiovasc Disord. 2006;6:38. doi: 10.1186/1471-2261-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mels C, Derluyn I, Broekaert E, Rosseel Y. Screening for traumatic exposure and posttraumatic stress symptoms in adolescents in the war-affected eastern Democratic Republic of Congo. Arch Pediatr Adolesc Med. 2009;163:525–530. doi: 10.1001/archpediatrics.2009.56. [DOI] [PubMed] [Google Scholar]
- 47.Scholte WF, Olff M, Ventevogel P, de Vries GJ, Jansveld E, Cardozo BL, Crawford CA. Mental health symptoms following war and repression in eastern Afghanistan. JAMA. 2004;292:585–593. doi: 10.1001/jama.292.5.585. [DOI] [PubMed] [Google Scholar]
- 48.Mello VA, Segurado AA, Malbergier A. Depression in women living with HIV: clinical and psychosocial correlates. Arch Womens Ment Health. 2009:Epub ahead of print. doi: 10.1007/s00737-009-0094-1. [DOI] [PubMed] [Google Scholar]
- 49.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 50.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50:2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 51.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 52.Nemetz PN, Roger VL, Ransom JE, Bailey KR, Edwards WD, Leibson CL. Recent trends in the prevalence of coronary disease: a population-based autopsy study of nonnatural deaths. Arch Intern Med. 2008;168:264–270. doi: 10.1001/archinternmed.2007.79. [DOI] [PubMed] [Google Scholar]
- 53.Bischoff A, Ekoe T, Perone N, Slama S, Loutan L. Chronic disease management in Sub-Saharan Africa: whose business is it? Int J Environ Res Public Health. 2009;6:2258–2270. doi: 10.3390/ijerph6082258. [DOI] [PMC free article] [PubMed] [Google Scholar]