Abstract
Exercise echocardiography has been used for 30 years. It is now considered a consolidated technique for the diagnosis and risk stratification of patients with known or suspected coronary artery disease (CAD). Of the stress echocardiography techniques, it represents the first choice for patients who are able to exercise. Given that the cost-effectiveness and safety of stress echocardiography are higher than those of other imaging techniques, its use is likely to be expanded further. Recent research has also proposed this technique for the evaluation of cardiac pathology beyond CAD. Although the role of new technology is promising, the assessment of cardiac function relies on good quality black and white harmonic images.
Keywords: Exercise echocardiography, Coronary artery disease, Peak imaging
INTRODUCTION
Stress echocardiography is a useful tool for clinical decision-making, given its accuracy in the diagnosis of coronary artery disease (CAD) and its demonstrated prognostic value. Among the different stress echocardiography modalities, exercise is safer and more physiologic. Therefore, according to all current guidelines, exercise stress echocardiography (ESE) must be considered the first choice for patients who are able to exercise[1-3].
New technologies have not yet found a definitive role during stress echocardiography. Doppler tissue imaging and speckle imaging can assess myocardial velocities and deformation. However, neither of them has been demonstrated to be better than visual assessment when the latter is performed by experienced observers. Three-dimensional echocardiography (3-DE) can also be used during exercise since an image of the entire myocardium can be obtained in a few cardiac cycles.
Although ESE has long been used for the diagnosis and risk stratification of patients with known or suspected CAD[4], there has been a change in its targets, as this method has also been used in recent years as a useful tool for evaluation of dyspnea in different clinical situations[5,6].
EXERCISE ECHOCARDIOGRAPHY: 30 YEARS OF DEVELOPMENT
ESE was introduced 30 years ago when the group led by Feigenbaum first reported wall motion abnormalities (WMA) during exercise in patients with CAD[4]. Since then, a significant number of technological landmarks have been developed, including digital imaging, continuous loop quad format display for comparison of rest and exercise images, continuous imaging acquisition, broadband transducer technology, harmonic imaging, and the use of echocardiographic contrast agents for endocardial border delineation. These are major tools currently needed for state-of-the-art performance of ESE. These tools have led to the consolidation of ESE as an accepted and established technique. Other technologies, such as myocardial Doppler imaging, myocardial perfusion, and 3-DE, although newer, have not yet found a definitive role in the ESE scenario.
A number of clinical landmarks merit mention. The first studies focused on the detection and risk stratification of patients with CAD. In the late 1990s, ESE began to be used for the functional assessment of patients with valve disease. Over the last few years ESE has been found to be useful for the evaluation of patients with dyspnea and different clinical scenarios, such as those with suspected diastolic dysfunction, those who are candidates for cardiac resynchronization therapy, and those with hypertrophic cardiomyopathy.
EXERCISE ECHOCARDIOGRAPHY: STATE OF THE ART
Common ESE modalities include treadmill ESE and semi-supine bicycle ESE. The treadmill has several advantages over the bicycle, including achievement of higher O2 consumption and the fact that all patients able to exercise can effectively walk on a treadmill, but back-pedaling or stopping pedaling are frequent in untrained patients when a bicycle is used. Also, muscular pain or discomfort before achievement of submaximal age-predicted heart rate is a common reason for stopping exercise on a bicycle[3]. However, whatever the method we use for ESE, images should be acquired at peak exercise because peak imaging is more sensitive for the diagnosis of CAD[7-9] and because the prognostic value of peak imaging is higher than that of post-exercise imaging[10].
ESE consists simply of the addition of echocardiography to conventional exercise electrocardiography (ECG) testing. Thus, besides the clinical information (symptoms during exercise) and ECG information (ST segment changes), we obtain resting echocardiographic data (resting WMA suggesting scar, valvular or myocardial disease, etc.), and exercise echocardiography data (new WMA indicating ischemia). This is the reason for its superior sensitivity and specificity when compared to conventional exercise ECG testing. As can be inferred from above, its major advantage rests on the evaluation of patients with resting ECG abnormalities, and in the evaluation of those with an inconclusive result on exercise ECG testing. Table 1 shows the up-to-date recommendations for the performance of stress echocardiography on the management of patients with chest pain according to recent European Society of Cardiology guidelines[3]. The recommendation stating that ESE, where available, should be considered as an alternative to exercise ECG testing for diagnostic and prognostic purposes will likely lead to more widespread use of this method of stress testing. In this regard our group has recently demonstrated the prognostic value of ESE in patients with normal exercise ECG testing[11]. We have found that in 1 of every 6 patients with suspected or known CAD who had a completely normal exercise ECG test, ischemia could be detected at peak exercise by echocardiography. These patients were at double the risk of overall mortality and major cardiac events as compared with those without ischemia (5-year mortality rate of 12.1% vs 6.4%; 5-year major cardiac events rate of 10.1% vs 4.2%). Therefore, even in patients who would be considered low risk according to the absence of symptoms or ischemic ECG changes during exercise, ESE allows more accurate risk stratification. Concerns regarding cost-effectiveness are the only reason not to replace exercise ECG by ESE.
Table 1.
Recommendations for the use of exercise stress echocardiography testing in the initial diagnostic assessment of angina1
| Class I | |
| I | Patients with resting ECG abnormalities, LBBB, > 1 mm ST-depression, paced rhythm, or WPW which prevent accurate interpretation of ECG changes during stress |
| II | Patients with a non-conclusive exercise ECG but reasonable exercise tolerance, who do not have a high probability of significant coronary disease and in whom the diagnosis is still in doubt |
| Class IIa | |
| III | Patients with prior revascularization (PCI or CABG) in whom localization of ischemia is important |
| IV | As an alternative to exercise ECG in patients where facilities, costs, and personnel resources allow |
| V | As an alternative to exercise ECG in patients with a low pre-test probability of disease such as women with atypical chest pain |
| VI | To assess functional severity of intermediate lesions on coronary angiography |
| VII | To localize ischemia when planning revascularization options in patients who have already had arteriography |
From: ESC Guidelines on the management of stable angina pectoris: executive summary[3].
Pharmacological stress echocardiography is recommended if the patient is unable to exercise adequately.
ECG: Electrocardiography; WPW: Wolf-Parkinson-White syndrome; LBBB: Left bundle branch block; PCI: Percutaneous coronary intervention; CABG: Coronary artery bypass graft. Class I: Benefits are greater than risks, therefore the procedure should be performed; Class IIa: Benefits are greater than risks, although additional studies are required - it is reasonable to perform the procedure.
HOW TO PERFORM AN EXERCISE ECHOCARDIOGRAM
ECG and blood pressure are monitored during the test as they are during a conventional exercise ECG test. Protocols adjusted to the patient’s clinical characteristics should be used, although the Bruce protocol (change in speed and slope every 3 min) is the most commonly used. We occasionally observe how the patient walks, prior to the exercise testing, to decide the most appropriate protocol for the particular patient.
When a treadmill is used, resting images are acquired on a table-bed, and peak exercise images are acquired with the patient walking or running. When a cycloergometer is used, images are acquired with the patient on the bicycle, at rest and at peak exercise. A set of 3 apical views (long-axis, 4-chamber and 2-chamber) and 2 parasternal views (long- and short-axis) is obtained. In case of non-conclusive peak images (poor quality or doubtful), images during the immediate post-exercise time are also acquired. We have previously shown that peak treadmill imaging is more sensitive than post-exercise imaging for the diagnosis of CAD, and that the quality of peak images in the apical views are similar to those acquired post-exercise[7,8]. In addition, the prognostic value of peak treadmill exercise imaging is higher because, in up to 1 in 4 patients with ischemia, the latter is limited to peak exercise, and the post-exercise study is completely normal[10]. Even in patients with post-exercise ischemia, WMA are markedly greater at peak than at post-exercise. Other authors have also demonstrated higher sensitivity for the detection of CAD with peak rather than post-exercise imaging during bicycle echocardiography[9]. Besides the superior diagnostic and prognostic capabilities of peak exercise imaging, a proper training in this approach has additional advantages: (1) patient scanning during different phases of exercise testing is feasible, and can be performed whenever there is concern about the need for termination of the test, for example in cases of doubtful symptoms or ECG changes; (2) the narrow acquisition time window we have for post-exercise imaging does not exist any more. In contrast, post-exercise imaging acquisition should take no longer than 45 s, otherwise WMA can rapidly recover, particularly in young patients and patients taking β-blockers. To scan the patient both during peak exercise and quickly during post-exercise requires a particular spatial disposition of the treadmill, the table and the echocardiographic machine, each one being close to the others. Figure 1 demonstrates the arrangement of these equipments in our laboratory. The transducer cable length should be adequately long, recognizing that it is not made the same length by all manufacturers; and (3) the post-exercise imaging period can be used to obtain other important information, such as mitral regurgitation (MR), diastolic function and systolic pulmonary artery pressure measurements.
Figure 1.

Exercise echocardiography. When the patient is exhausted or termination criteria appear (symptoms, significative ST changes, decrease or increase in blood pressure, etc.), the observer acquires images by placing the transducer in the cardiac apex, then in the parasternal region. Note the placement of the table, the treadmill and the echocardiography machine for feasible imaging evaluation at peak and post-exercise. The left lateral handlebar of the treadmill has been removed to allow for rapid post-exercise positioning of the patient on the table.
Three technical requirements are mandatory to optimize peak and post-exercise imaging acquisition and also for interpretation: (1) a direct ECG wire connection between the echocardiography machine and the ergometer, which is indispensable for obtaining well registered cardiac cycles; (2) a continuous imaging acquisition system, which allows the acquisition of multiple cardiac cycles during several minutes. After the test, the interpreter only has to choose the loops with the highest quality corresponding to the different views; and (3) a quad-format screen to compare resting and exercise images. Almost all the manufacturers offer equipment with these requirements. The cost-effectiveness of a stress echocardiography laboratory may be optimized if an off-line computer is available to read the studies, as the echocardiography machine may require to be used in the meantime.
IMAGING INTERPRETATION
With stress echocardiography, CAD is considered when there are resting or stress-induced WMA (hypokinesia, akinesia, dyskinesia). When the same WMA are present at rest and during stress, the condition is defined as fixed WMA or scar. When the WMA appear only during stress, the condition is defined as induced WMA or ischemia. When there are WMA at rest that worsen with stress the condition is defined as mixed scar and ischemia. In this latter situation the WMA may develop in the same or in a different territory (ischemia at a distance). A semi-quantitative scale is used to calculate wall motion score by dividing the left ventricle (LV) into 16 or 17 segments[12] and assigning 1 to normal, 2 to hypokinetic, 3 to akinetic, and 4 to dyskinetic segments. The sum of all the scores divided by the number of visualized segments is the wall motion score index. Most laboratories also calculate resting and exercise LV ejection fraction. Figures 2 and 3 show a normal and an abnormal ESE, respectively.
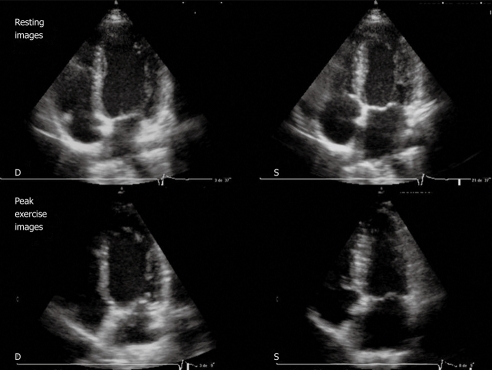
Figure 2.
Resting echocardiography (top) and peak exercise echocardiography (bottom). Four-chamber apical view (diastolic frames on the left, systolic frames on the right) in a patient with normal results. Note the left ventricular (LV) cavity dimensions decrease with exercise and an increase in LV ejection fraction. D: Diastolic; S: Systolic.
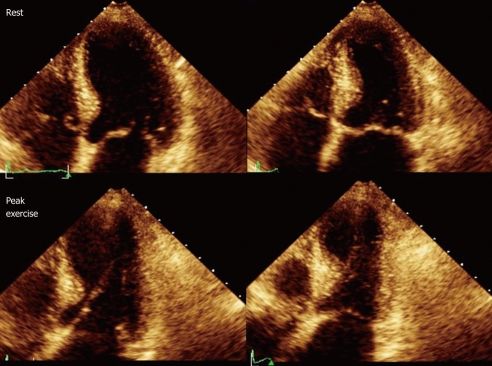
Figure 3.
Resting echocardiography (top) and peak exercise echocardiography (bottom). Four-chamber apical view (diastolic frames on the left, systolic frames on the right) in a patient with significant coronary stenoses in the left anterior descending artery (99%). At rest, wall motion is normal, whereas during exercise a septoapical dyssynergia is observed with the typical 8-shaped left ventricular.
ESE LIMITATIONS
The main limitation of ESE is the presence of a poor acoustic window in some patients. This percentage has dramatically diminished in recent years with the introduction of harmonic imaging. In the remaining 5%-10% of cases with a poor acoustic window, contrast agents can be used to improve myocardial border delineation. However, “excellent” visualization of LV segments is more crucial for pharmacological stress echocardiography (where diagnostic and prognostic information are almost exclusively dependent on the imaging), than for ESE, that provides diagnostic and prognostic information beyond that provided by imaging alone (clinical symptoms, ECG changes, functional capacity). Although current guidelines recommend the use of contrast agents when at least 2 myocardial segments are non-visualized[13], it should be pointed out that the absence of visualization of 2 contiguous apical segments does not have the same significance as the absence of visualization of 2 non-contiguous basal segments.
Secondly, imaging acquisition during ESE is more difficult than during pharmacological stress, due to the greater increase in both heart and respiratory rates with exercise. Pharmacological stress echocardiography requires less skills than ESE[14]. However, subtle WMA during pharmacological stress echocardiography may be equated with more severe ischemic burden during ESE[15]. In our department, most physicians and fellows in training acquire the necessary expertise to perform peak exercise studies on a treadmill with confidence after 100 cases, although this number may vary depending on the trainee’s background (i.e. previous expertise in pharmacological stress echocardiography or post-exercise imaging).
Thirdly, imaging interpretation is made by a semi-quantitative approach, which has led to only moderate agreement between observers in different studies[16,17]. However, this agreement is higher when either significant WMA or extensive CAD, as defined by angiography, is present. Interpretation of WMA may also be more challenging in certain situations such as left bundle branch block, paced rhythm, and atrial fibrillation. Among 8088 patients having a first ESE study in our institution, atrial fibrillation was present in 5.2% of the patients and left bundle branch block or paced rhythm in 7.7%. The diagnostic accuracy of WMA for the detection of CAD may be compromised in patients with these ECG abnormalities, in comparison with the overall population of patients referred for stress echocardiography[18-20]. Minor abnormalities in the septoapical region should not be considered abnormal in patients with left bundle branch block or pacemakers; instead careful assessment of the anterior wall in the 2-chamber view should be performed.
Fourthly, some patients may have limited capacity for exercising and, as 85% of the age-predicted maximal heart rate may not be achieved in up to 25%-30% of the patients, these tests are often considered as non-conclusive. This percentage can be reduced by using atropine during exercise, therefore reducing the need for pharmacological stress[21]. Atropine is particularly useful for patients with reduced resting heart rate as a result of β-blocker therapy, peripheral artery disease or arthropathies.
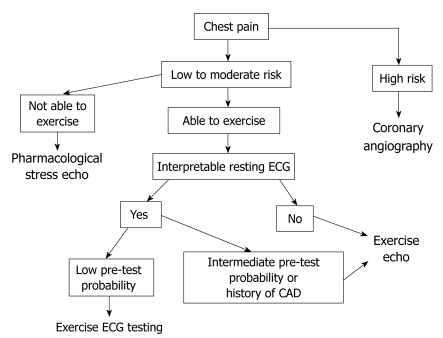
Finally, since in routine clinical practice not all patients can be referred for an ESE study, despite wide availability as in our case, proper selection is mandatory. In our institution, patients with either resting ECG abnormalities, known CAD, or intermediate pre-test probability of CAD are preferentially referred for ESE. Figure 4 shows the algorithm used in our center for patients referred for stress testing.
Figure 4.
Algorithm used in our institution for patients with chest pain. CAD: Coronary artery disease; ECG: Electrocardiography.
COST-EFFECTIVENESS
The cost-effectiveness of ESE has been explored in a few studies. Marwick et al[22] demonstrated that ESE was more cost-effective than exercise ECG. Due to a better risk stratification with the former, downstream costs were particularly low in patients deemed at low risk by ESE, in contrast with patients deemed at low risk by exercise ECG results. The difference in cost was mainly due to the higher rate of catheterization and revascularization procedures performed during follow-up in patients stratified as low risk by exercise ECG. The same group found ESE to be a more cost-effective strategy than exercise ECG for the decision-making process in women with suspected CAD. This approach led to a reduced number of unnecessary coronary angiograms in comparison with a pure exercise ECG only approach or with a selective ESE approach (ESE for patients with non-diagnostic exercise ECG results)[23]. Also, ESE was superior to exercise ECG in risk stratification of patients evaluated in chest pain units, resulting in less diagnostic uncertainty, fewer referrals for further unnecessary investigation, and hence, a significant cost benefit over exercise ECG testing[24].
PROGNOSTIC VALUE OF EXERCISE ECHOCARDIOGRAPHY
Resting LV function and ischemia are important prognostic markers. These indicators can be evaluated together during stress echocardiography. The independent prognostic value of stress-induced WMA, as well as the excellent outcome of patients with negative stress echocardiography (< 1% events/year)[25] have been demonstrated with each stress echocardiography modality. ESE is particularly useful for prognostic assessment because exercise parameters with well-demonstrated prognostic value (such as metabolic equivalents achieved, blood pressure response, or percentage of age-predicted maximal heart rate) may be obtained. ESE has its maximal cost-effectiveness in the assessment of patients with intermediate likelihood of events[22,26]. We have observed that ESE further categorizes patients with intermediate-risk Duke treadmill score into those at higher and lower risk of events. ESE also allows a better stratification of patients with low-risk Duke treadmill score, aiding in the decision whether to perform coronary angiography[26]. Several studies have shown that gender, functional capacity, rate-pressure product, resting LV function, and ischemia are independently associated with cardiac events[26,27], and that ESE has incremental predictive value in patients with different pre-test probabilities of CAD[27]. Patients with WMA involving the territories of the 3 coronary arteries, patients with peak wall motion score index > 1.5, and patients with ischemia at a distance are at higher risk for cardiac events[28].
Furthermore, ESE can show worsening of existing MR or development of new MR during exercise[29-31], due to adverse LV remodeling (Figure 5). Assessment of functional MR is unfeasible with pharmacologic stress testing because both dobutamine and dipyridamole reduce LV preload. Our group has demonstrated that worsening of MR or development of new MR during ESE is associated with a poor outcome. In a recent study on nearly 2000 patients, those with positive ESE and worsening of MR had an event rate of 11% during a follow-up period of 4 years, as compared with 6% in those with a positive echocardiogram and no worsening of MR[30]. In addition, worsening of MR was predictive of death independent of the ESE results.
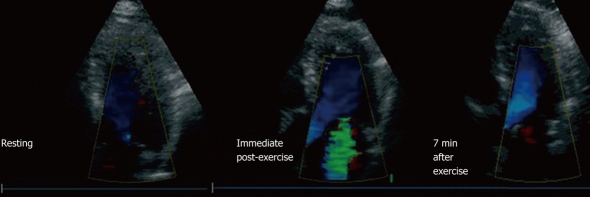
Figure 5.
Example of a patient with mild ventricular dysfunction (resting left ventricle ejection fraction 49%, exercise left ventricle ejection fraction 46%) who developed severe mitral regurgitation (MR) during exercise. This patient had no MR at rest (left), severe MR developed in the immediate post-exercise period (center), which did not completely disappear until 7 min after exercise (right) (From Peteiro et al[32]).
NEW TECHNOLOGY
ESE can be complemented by the addition of new modalities of imaging. As mentioned above, contrast agents may be used to improve endocardial border delineation, improve diagnostic accuracy of ESE[32] and reduce the need for additional diagnostic tests. However, its use on a routine basis may not be cost-effective[33]. Contrast agents can also be used to detect myocardial perfusion defects during stress. As perfusion defects precede WMA, assessment of myocardial perfusion may improve the sensitivity of stress (particularly pharmacologic) echocardiography for detection of ischemia, albeit at the expence of specificity[34]. It should be noted that the assessment of myocardial perfusion during ESE is not without certain technical limitations[35].
Tissue Doppler imaging and speckle imaging can assess myocardial velocities and strain, increase the agreement between observers, and also improve the accuracy of wall motion assessment by less experienced observers, but do not increase the accuracy of the visual assessment when performed by experienced observers[36,37]. Speckle imaging can be effectively used to assess the torsional movement of the myocardium, which is altered during ischemic conditions[38]. However, only one study has assessed LV torsion during stress echocardiography[39]. In this study, apical counterclockwise rotation was increased in patients with ischemia, suggesting that subendocardial ischemia might have led to a compensatory enhancement of the subepicardial counterclockwise rotation. In contrast, in previous experimental studies, more intense ischemia was associated with a reduced counterclockwise apical rotation and LV torsion[40]. Therefore the clinical applications of the assessment of LV torsion remain to be better defined.
Speckle imaging may also be used to assess tardokinesis, which is difficult to observe visually. In a recent ESE study, delayed strain was found to be more sensitive than WMA for the detection of CAD. While WMA usually recovered in less than 2 min, strain delay recovery lasted as long as 10 min[41].
Finally, 3-DE is able to acquire LV full-volume images over a few cardiac cycles. Off-line management of the obtained images (cropping) then allows evaluation of wall motion in any desired image plane at rest and at peak stress[42-44]. Although the imaging acquisition is therefore very quick, a relatively long time is necessary to crop the required images. We have demonstrated similar sensitivity and specificity with exercise 3-DE (Figure 6) and exercise 2-DE, but the feasibility of performing 3-DE was lower[44]. Present limitations are suboptimal imaging quality and low frame rate. Recent developments now allow a full-volume acquisition in only 1 cardiac cycle (i.e. 1 s) with a frame rate of around 20, which is promising for ESE.
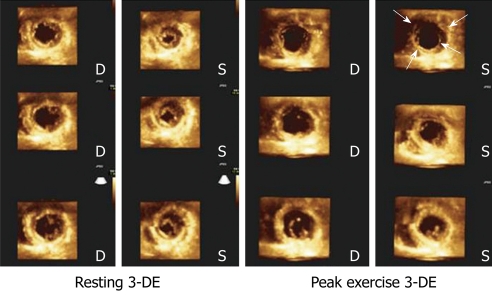
Figure 6.
Cropped views obtained from a left ventricle full-volume during 3-dimensional exercise echocardiography in resting conditions (left panel) and during peak exercise (right panel) in a patient with severe 3-vessel disease. Note exercise-induced akinesia and dilation in the short axis apical views (arrows), as well as hypokinesia and dilation in the short-axis view at the papillary muscles level. 3-DE: Three-dimensional exercise; D: Diastolic; S: Systolic.
To summarize, these features are not available in all ultrasound systems, their advantages over conventional 2-D imaging are not fully clear, and additional expertise is necessary for their interpretation. Although the value of these techniques, when added to traditional assessment, is currently unknown, it can be speculated that speckle tracking imaging has the potential to reduce the need for alternative tests in selected cases.
EXERCISE ECHOCARDIOGRAPHY: FROM THE EVALUATION OF CHEST PAIN TO THE ASSESSMENT OF DYSPNEA
ESE has been a useful tool for evaluation of chest pain in patients with known or suspected CAD for the last 30 years. Nevertheless, its capacity for comprehensive cardiac assessment during exercise has made this technique also valuable for evaluation of dyspnea in patients with a variety of cardiac diseases. ESE has been found to be particularly useful for functional assessment of patients with valve disease or cardiomyopathies, better prediction of response to resynchronization therapy, and identification of diastolic dysfunction as the cause of dyspnea.
Impaired LV diastolic function is associated with an adverse outcome in patients with heart disease. Among the numerous indices of diastolic function, the ratio of early transmitral flow assessed by pulsed Doppler (E) to early diastolic annulus velocity assessed by tissue Doppler (é) is closely related to LV end-diastolic pressure and can be easily measured during ESE. A higher E/é ratio index is associated with the presence of dyspnea, lower functional capacity, exercise-induced LV dysfunction and CAD[5,45]. A change to a pseudonormalized LV inflow pattern during exercise is also associated with the same poor prognostic indicators[45]. These measurements, along with the assessment of pulmonary artery pressure during exercise, may help to clarify causes of dyspnea in patients with different cardiac or noncardiac conditions. The appropriate patients for diastolic exercise testing are likely those with dyspnea of uncertain origin and absence of WMA on ESE. The additional information offered by ESE in patients suspected of having diastolic dysfunction has been recently shown by Holland et al[46]. These investigators performed ESE in 148 patients with the diagnosis of diastolic dysfunction based on resting conventional echo-Doppler measurements[47]. Only 24% of the patients had functional limitation to exercise and only 36% had an increased E/é ratio during exercise. It was concluded that resting measurements may not be adequate for accurate diagnosis of diastolic dysfunction because having diastolic dysfunction in the absence of exercise intolerance and an E/é increase does not seem plausible.
The assessment of dysynchrony during exercise has been found to be a better predictor of response to resynchronization therapy than resting dysynchrony (predictive value 89% vs 70%, P = 0.01)[6]. Also, exercise may change the magnitude of dysynchrony and these changes correlate with the changes in the severity of functional MR during ESE[48,49]. Finally, the appearance of a septal flash (septal movement during the isovolumic contraction period) during stress in patients with left bundle branch block and systolic dysfunction has been correlated with the response to resynchronization therapy, although it has only been studied by dobutamine stress[50].
ESE has also been used in patients with valve disease and discordant symptoms (either symptomatic patients with non-severe valve disease or asymptomatic patients with severe valve disease) helping in the decision making process. Asymptomatic patients with aortic stenosis without inotropic reserve during ESE were found to have worse prognosis during follow-up in one study[51].
In patients with hypertrophic cardiomyopathy, ESE can assess either exercise-induced LV outflow tract obstruction, MR, or LV systolic impairment. We found that patients with hypertrophic cardiomyopathy and lack of increase in LV ejection fraction with exercise have a more adverse outcome[52].
THE COMPETITORS
Several non-invasive techniques can compete with ESE for identification of patients in need of invasive coronary angiography. These include exercise ECG testing, pharmacological stress echocardiography, nuclear myocardial perfusion imaging, coronary computed tomography and cardiac magnetic resonance imaging. Table 2 shows the sensitivity and specificity of these techniques in comparison to stress echocardiography. Although information on the coronary anatomy might be desirable for certain patients, the outcome of CAD patients is more often related to the extent of myocardial ischemia during stress than to the number of diseased vessels assessed by the anatomic methods[57-59]. Therefore, decisions regarding revascularization procedures should not be based solely on the coronary anatomy, but should be based on the evidence that a particular coronary stenosis has an objective functional significance.
Table 2.
Sensitivity and specificity of stress echocardiography and competing methodologies
| Sensitivity (%) | Specificity (%) | |
| Exercise ECG testing[53] | 50 | 90 |
| Stress echocardiography[54] | 80 | 84 |
| Nuclear techniques[54] | 84 | 77 |
| Magnetic resonance[55] | 89 | 87 |
| Coronary computed tomography[56] | 98 | 90 |
ECG: Electrocardiography.
There are further advantages of ESE over these other techniques, including: safety (approximately 1 event/7000 in ESE, 1/700 in pharmacological stress echocardiography)[60,61]; relatively high diagnostic accuracy (sensitivity 80% and specificity 86%)[54], comparable to that of nuclear imaging but higher than that of exercise ECG[53]; solid prognostic data (less that 1% events/year in patients with a negative stress echocardiogram)[22,25,27,62]; and the fact that it is a green technology (compared to nuclear myocardial perfusion imaging and coronary computed tomographic angiography that may expose the patient to radiation levels equivalent to as many as 600 plain chest X-rays)[63]. In addition, the cost of stress echocardiography is lower than that of other noninvasive imaging techniques. According to the American College of Cardiology/American Heart Association guidelines, if 1 is the cost of an exercise ECG test, 2 would be that of an ESE, but 5.7 would be the cost of a nuclear imaging technique[1]. As stress echocardiography is usually performed and interpreted by cardiology staff in a quick fashion after a single procedure, the results are usually readily available. ESE in particular is the only non-invasive modality where an intravenous line is not even required.
According to the consensus statement by experts on stress echocardiography of the European Association of Echocardiography, stress echocardiography should be preferred over stress scintigraphy, because the information provided by both techniques is similar, but the latter poses a significant biological risk not only for the individual but for society[64]. Magnetic resonance might compete in the future with stress echocardiography as it is also a non-radioactive method with relatively higher sensitivity[55]. However, its availability is currently lower and most protocols are based on pharmacological stressors rather than exercise.
CONCLUSION
Because of its low cost, safety, diagnostic and prognostic capabilities, and lack of radiation exposure, ESE should be considered as a first-line technique for patients with suspected or confirmed CAD. It is the first indication for patients with resting ECG abnormalities and for those with inconclusive exercise ECG test results. In addition, ESE may be a useful alternative to conventional exercise ECG testing in centers where facilities and resources allow. The need for exhaustive training should not be a limitation for a technique that adds significantly to the decision-making process for patients with known or suspected CAD.
Footnotes
Peer reviewers: Jamshid Shirani, MD, Director, Cardiology fellowship program, Geisinger Medical Center, 100 North Academy Avenue, Danville, PA 17822-2160, United States; Dr. Thomas Jax, Profil Institut für Stoffwechselforschung, Hellersbergstrasse 9, Neuss 41460, Germany
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
References
- 1.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB Jr, Fihn SD, Fraker TD Jr, Gardin JM, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 2.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 4.Wann LS, Faris JV, Childress RH, Dillon JC, Weyman AE, Feigenbaum H. Exercise cross-sectional echocardiography in ischemic heart disease. Circulation. 1979;60:1300–1308. doi: 10.1161/01.cir.60.6.1300. [DOI] [PubMed] [Google Scholar]
- 5.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Rocchi G, Bertini M, Biffi M, Ziacchi M, Biagini E, Gallelli I, Martignani C, Cervi E, Ferlito M, Rapezzi C, et al. Exercise stress echocardiography is superior to rest echocardiography in predicting left ventricular reverse remodelling and functional improvement after cardiac resynchronization therapy. Eur Heart J. 2009;30:89–97. doi: 10.1093/eurheartj/ehn483. [DOI] [PubMed] [Google Scholar]
- 7.Peteiro J, Fabregas R, Montserrat L, Alvarez N, Castro-Beiras A. Comparison of treadmill exercise echocardiography before and after exercise in the evaluation of patients with known or suspected coronary artery disease. J Am Soc Echocardiogr. 1999;12:1073–1079. doi: 10.1016/s0894-7317(99)70104-5. [DOI] [PubMed] [Google Scholar]
- 8.Peteiro J, Garrido I, Monserrat L, Aldama G, Calviño R, Castro-Beiras A. Comparison of peak and postexercise treadmill echocardiography with the use of continuous harmonic imaging acquisition. J Am Soc Echocardiogr. 2004;17:1044–1049. doi: 10.1016/j.echo.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Park TH, Tayan N, Takeda K, Jeon HK, Quinones MA, Zoghbi WA. Supine bicycle echocardiography improved diagnostic accuracy and physiologic assessment of coronary artery disease with the incorporation of intermediate stages of exercise. J Am Coll Cardiol. 2007;50:1857–1863. doi: 10.1016/j.jacc.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Peteiro J, Bouzas-Mosquera A, Broullón FJ, Garcia-Campos A, Pazos P, Castro-Beiras A. Prognostic value of peak and post-exercise treadmill exercise echocardiography in patients with known or suspected coronary artery disease. Eur Heart J. 2010;31:187–195. doi: 10.1093/eurheartj/ehp427. [DOI] [PubMed] [Google Scholar]
- 11.Bouzas-Mosquera A, Peteiro J, Alvarez-García N, Broullón FJ, Mosquera VX, García-Bueno L, Ferro L, Castro-Beiras A. Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J Am Coll Cardiol. 2009;53:1981–1990. doi: 10.1016/j.jacc.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 12.Bourdillon PD, Broderick TM, Sawada SG, Armstrong WF, Ryan T, Dillon JC, Fineberg NS, Feigenbaum H. Regional wall motion index for infarct and noninfarct regions after reperfusion in acute myocardial infarction: comparison with global wall motion index. J Am Soc Echocardiogr. 1989;2:398–407. doi: 10.1016/s0894-7317(89)80041-0. [DOI] [PubMed] [Google Scholar]
- 13.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Peterson ED, Hendel RC, Stainback RF, Blaivas M, Des Prez RD, Gillam LD, et al. ACCF/ASE/ACEP/AHA/ASNC/SCAI/SCCT/SCMR 2008 appropriateness criteria for stress echocardiography: a report of the American College of Cardiology Foundation Appropriateness Criteria Task Force, American Society of Echocardiography, American College of Emergency Physicians, American Heart Association, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance: endorsed by the Heart Rhythm Society and the Society of Critical Care Medicine. Circulation. 2008;117:1478–1497. doi: 10.1161/CIRCULATIONAHA.107.189097. [DOI] [PubMed] [Google Scholar]
- 14.Picano E. Stress Echocardiography. 4th ed. Berlin: Springer Verlag; 2003. [Google Scholar]
- 15.Rallidis L, Cokkinos P, Tousoulis D, Nihoyannopoulos P. Comparison of dobutamine and treadmill exercise echocardiography in inducing ischemia in patients with coronary artery disease. J Am Coll Cardiol. 1997;30:1660–1668. doi: 10.1016/s0735-1097(97)00376-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann R, Marwick TH, Poldermans D, Lethen H, Ciani R, van der Meer P, Tries HP, Gianfagna P, Fioretti P, Bax JJ, et al. Refinements in stress echocardiographic techniques improve inter-institutional agreement in interpretation of dobutamine stress echocardiograms. Eur Heart J. 2002;23:821–829. doi: 10.1053/euhj.2001.2968. [DOI] [PubMed] [Google Scholar]
- 17.Peteiro J, Alonso AM, Florenciano R, González Juanatey C, de la Morena G, Iglesias I, Moreno M, Rodríguez MA. [Agreement between centers on the interpretation of exercise echocardiography] Rev Esp Cardiol. 2006;59:33–40. [PubMed] [Google Scholar]
- 18.Geleijnse ML, Vigna C, Kasprzak JD, Rambaldi R, Salvatori MP, Elhendy A, Cornel JH, Fioretti PM, Roelandt JR. Usefulness and limitations of dobutamine-atropine stress echocardiography for the diagnosis of coronary artery disease in patients with left bundle branch block. A multicentre study. Eur Heart J. 2000;21:1666–1673. doi: 10.1053/euhj.1999.2008. [DOI] [PubMed] [Google Scholar]
- 19.Peteiro J, Monserrat L, Martinez D, Castro-Beiras A. Accuracy of exercise echocardiography to detect coronary artery disease in left bundle branch block unassociated with either acute or healed myocardial infarction. Am J Cardiol. 2000;85:890–893, A9. doi: 10.1016/s0002-9149(99)00889-9. [DOI] [PubMed] [Google Scholar]
- 20.Hobday TJ, Pellikka PA, Attenhofer Jost CH, Oh JK, Miller FA Jr, Seward JB. Chronotropic response, safety, and accuracy of dobutamine stress echocardiography in patients with atrial fibrillation and known or suspected coronary artery disease. Am J Cardiol. 1998;82:1425–1427, A9. doi: 10.1016/s0002-9149(98)00655-9. [DOI] [PubMed] [Google Scholar]
- 21.Peteiro J, Garrido I, Monserrat L, Aldama G, Salgado J, Castro-Beiras A. Exercise echocardiography with addition of atropine. Am J Cardiol. 2004;94:346–348. doi: 10.1016/j.amjcard.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Marwick TH, Shaw L, Case C, Vasey C, Thomas JD. Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice. Eur Heart J. 2003;24:1153–1163. doi: 10.1016/s0195-668x(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 23.Marwick TH, Anderson T, Williams MJ, Haluska B, Melin JA, Pashkow F, Thomas JD. Exercise echocardiography is an accurate and cost-efficient technique for detection of coronary artery disease in women. J Am Coll Cardiol. 1995;26:335–341. doi: 10.1016/0735-1097(95)80004-z. [DOI] [PubMed] [Google Scholar]
- 24.Jeetley P, Burden L, Stoykova B, Senior R. Clinical and economic impact of stress echocardiography compared with exercise electrocardiography in patients with suspected acute coronary syndrome but negative troponin: a prospective randomized controlled study. Eur Heart J. 2007;28:204–211. doi: 10.1093/eurheartj/ehl444. [DOI] [PubMed] [Google Scholar]
- 25.Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J Am Coll Cardiol. 2007;49:227–237. doi: 10.1016/j.jacc.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 26.Peteiro J, Monserrrat L, Piñeiro M, Calviño R, Vazquez JM, Mariñas J, Castro-Beiras A. Comparison of exercise echocardiography and the Duke treadmill score for risk stratification in patients with known or suspected coronary artery disease and normal resting electrocardiogram. Am Heart J. 2006;151:1324.e1–1324.e10. doi: 10.1016/j.ahj.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Peteiro JC, Monserrat L, Bouzas A, Piñon P, Mariñas J, Bouzas B, Castro-Beiras A. Risk stratification by treadmill exercise echocardiography. J Am Soc Echocardiogr. 2006;19:894–901. doi: 10.1016/j.echo.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Peteiro-Vázquez J, Monserrrat-Iglesias L, Mariñas-Davila J, Garrido-Bravo IP, Bouzas-Caamaño M, Muñiz-García J, Bouzas-Mosquera A, Bouzas-Zubeldia B, Alvarez-García N, Castro-Beiras A. [Prognostic value of treadmill exercise echocardiography] Rev Esp Cardiol. 2005;58:924–933. [PubMed] [Google Scholar]
- 29.Peteiro J, Freire E, Montserrat L, Castro-Beiras A. The effect of exercise on ischemic mitral regurgitation. Chest. 1998;114:1075–1082. doi: 10.1378/chest.114.4.1075. [DOI] [PubMed] [Google Scholar]
- 30.Peteiro J, Monserrrat L, Bouzas A, Piñon P, Mariñas J, Piñeiro M, Castro-Beiras A. Prognostic value of mitral regurgitation assessment during exercise echocardiography in patients with known or suspected coronary artery disease. J Am Soc Echocardiogr. 2006;19:1229–1237. doi: 10.1016/j.echo.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Peteiro J, Monserrat L, Piñón P, Bouzas A, Campos R, Mosquera I, Mariñas J, Bouzas B, Castro-Beiras A. [Value of resting and exercise mitral regurgitation during exercise echocardiography to predict outcome in patients with left ventricular dysfunction] Rev Esp Cardiol. 2007;60:234–243. [PubMed] [Google Scholar]
- 32.Dolan MS, Riad K, El-Shafei A, Puri S, Tamirisa K, Bierig M, St Vrain J, McKinney L, Havens E, Habermehl K, et al. Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am Heart J. 2001;142:908–915. doi: 10.1067/mhj.2001.117608. [DOI] [PubMed] [Google Scholar]
- 33.Moir S, Shaw L, Haluska B, Jenkins C, Marwick TH. Left ventricular opacification for the diagnosis of coronary artery disease with stress echocardiography: an angiographic study of incremental benefit and cost-effectiveness. Am Heart J. 2007;154:510–518. doi: 10.1016/j.ahj.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 34.Rakhit DJ, Becher H, Monaghan M, Nihoyannopoulos P, Senior R. The clinical applications of myocardial contrast echocardiography. Eur J Echocardiogr. 2007;8:S24–S29. doi: 10.1016/j.euje.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Dodla S, Xie F, Smith M, O'Leary E, Porter TR. Real-time perfusion echocardiography during treadmill exercise and dobutamine stress testing. Heart. 2010;96:220–225. doi: 10.1136/hrt.2009.168112. [DOI] [PubMed] [Google Scholar]
- 36.Cain P, Baglin T, Case C, Spicer D, Short L, Marwick TH. Application of tissue Doppler to interpretation of dobutamine echocardiography and comparison with quantitative coronary angiography. Am J Cardiol. 2001;87:525–531. doi: 10.1016/s0002-9149(00)01425-9. [DOI] [PubMed] [Google Scholar]
- 37.Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J. 2007;28:1765–1772. doi: 10.1093/eurheartj/ehm188. [DOI] [PubMed] [Google Scholar]
- 38.Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amundsen BH, Smith HJ, Rosen BD, Lima JA, Torp H, Ihlen H, et al. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 39.Bansal M, Leano RL, Marwick TH. Clinical assessment of left ventricular systolic torsion: effects of myocardial infarction and ischemia. J Am Soc Echocardiogr. 2008;21:887–894. doi: 10.1016/j.echo.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Kroeker CA, Tyberg JV, Beyar R. Effects of ischemia on left ventricular apex rotation. An experimental study in anesthetized dogs. Circulation. 1995;92:3539–3548. doi: 10.1161/01.cir.92.12.3539. [DOI] [PubMed] [Google Scholar]
- 41.Ishii K, Imai M, Suyama T, Maenaka M, Nagai T, Kawanami M, Seino Y. Exercise-induced post-ischemic left ventricular delayed relaxation or diastolic stunning: is it a reliable marker in detecting coronary artery disease? J Am Coll Cardiol. 2009;53:698–705. doi: 10.1016/j.jacc.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad M, Xie T, McCulloch M, Abreo G, Runge M. Real-time three-dimensional dobutamine stress echocardiography in assessment stress echocardiography in assessment of ischemia: comparison with two-dimensional dobutamine stress echocardiography. J Am Coll Cardiol. 2001;37:1303–1309. doi: 10.1016/s0735-1097(01)01159-7. [DOI] [PubMed] [Google Scholar]
- 43.Matsumura Y, Hozumi T, Arai K, Sugioka K, Ujino K, Takemoto Y, Yamagishi H, Yoshiyama M, Yoshikawa J. Non-invasive assessment of myocardial ischaemia using new real-time three-dimensional dobutamine stress echocardiography: comparison with conventional two-dimensional methods. Eur Heart J. 2005;26:1625–1632. doi: 10.1093/eurheartj/ehi194. [DOI] [PubMed] [Google Scholar]
- 44.Peteiro J, Piñon P, Perez R, Monserrat L, Perez D, Castro-Beiras A. Comparison of 2- and 3-dimensional exercise echocardiography for the detection of coronary artery disease. J Am Soc Echocardiogr. 2007;20:959–967. doi: 10.1016/j.echo.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Peteiro J, Pazos P, Bouzas A, Piñon P, Estevez R, Castro-Beiras A. Assessment of diastolic function during exercise echocardiography: annulus mitral velocity or transmitral flow pattern? J Am Soc Echocardiogr. 2008;21:178–184. doi: 10.1016/j.echo.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 46.Holland DJ, Prasad SB, Marwick TH. Contribution of exercise echocardiography to the diagnosis of heart failure with preserved ejection fraction (HFpEF) Heart. 2010;96:1024–1028. doi: 10.1136/hrt.2009.183947. [DOI] [PubMed] [Google Scholar]
- 47.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 48.D'Andrea A, Caso P, Cuomo S, Scarafile R, Salerno G, Limongelli G, Di Salvo G, Severino S, Ascione L, Calabrò P, et al. Effect of dynamic myocardial dyssynchrony on mitral regurgitation during supine bicycle exercise stress echocardiography in patients with idiopathic dilated cardiomyopathy and 'narrow' QRS. Eur Heart J. 2007;28:1004–1011. doi: 10.1093/eurheartj/ehm021. [DOI] [PubMed] [Google Scholar]
- 49.Lafitte S, Bordachar P, Lafitte M, Garrigue S, Reuter S, Reant P, Serri K, Lebouffos V, Berrhouet M, Jais P, et al. Dynamic ventricular dyssynchrony: an exercise-echocardiography study. J Am Coll Cardiol. 2006;47:2253–2259. doi: 10.1016/j.jacc.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 50.Parsai C, Baltabaeva A, Anderson L, Chaparro M, Bijnens B, Sutherland GR. Low-dose dobutamine stress echo to quantify the degree of remodelling after cardiac resynchronization therapy. Eur Heart J. 2009;30:950–958. doi: 10.1093/eurheartj/ehp050. [DOI] [PubMed] [Google Scholar]
- 51.Maréchaux S, Ennezat PV, LeJemtel TH, Polge AS, de Groote P, Asseman P, Nevière R, Le Tourneau T, Deklunder G. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography. 2007;24:955–959. doi: 10.1111/j.1540-8175.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 52.Bouzas Mosquera A, Peteiro J, Fernandez X, Monserrat L, Broullon FJ, Mendez Eirin E, Perez Perez A, Pazos P, Castro Beiras A. Value of exercise echocardiography for predicting outcome in patients with hypertrophic cardiomyopathy (Abstract) Eur Heart J. 2010:In press. [Google Scholar]
- 53.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD Jr, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 54.Schinkel AF, Bax JJ, Geleijnse ML, Boersma E, Elhendy A, Roelandt JR, Poldermans D. Noninvasive evaluation of ischaemic heart disease: myocardial perfusion imaging or stress echocardiography? Eur Heart J. 2003;24:789–800. doi: 10.1016/s0195-668x(02)00634-6. [DOI] [PubMed] [Google Scholar]
- 55.Klem I, Heitner JF, Shah DJ, Sketch MH Jr, Behar V, Weinsaft J, Cawley P, Parker M, Elliott M, Judd RM, et al. Improved detection of coronary artery disease by stress perfusion cardiovascular magnetic resonance with the use of delayed enhancement infarction imaging. J Am Coll Cardiol. 2006;47:1630–1638. doi: 10.1016/j.jacc.2005.10.074. [DOI] [PubMed] [Google Scholar]
- 56.Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J, et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J. 2008;29:531–556. doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- 57.Gohlke H, Samek L, Betz P, Roskamm H. Exercise testing provides additional prognostic information in angiographically defined subgroups of patients with coronary artery disease. Circulation. 1983;68:979–985. doi: 10.1161/01.cir.68.5.979. [DOI] [PubMed] [Google Scholar]
- 58.Bonow RO, Kent KM, Rosing DR, Lan KK, Lakatos E, Borer JS, Bacharach SL, Green MV, Epstein SE. Exercise-induced ischemia in mildly symptomatic patients with coronary-artery disease and preserved left ventricular function. Identification of subgroups at risk of death during medical therapy. N Engl J Med. 1984;311:1339–1345. doi: 10.1056/NEJM198411223112103. [DOI] [PubMed] [Google Scholar]
- 59.Iskandrian AS, Chae SC, Heo J, Stanberry CD, Wasserleben V, Cave V. Independent and incremental prognostic value of exercise single-photon emission computed tomographic (SPECT) thallium imaging in coronary artery disease. J Am Coll Cardiol. 1993;22:665–670. doi: 10.1016/0735-1097(93)90174-y. [DOI] [PubMed] [Google Scholar]
- 60.Varga A, Garcia MA, Picano E. Safety of stress echocardiography (from the International Stress Echo Complication Registry) Am J Cardiol. 2006;98:541–543. doi: 10.1016/j.amjcard.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 61.Picano E, Mathias W Jr, Pingitore A, Bigi R, Previtali M. Safety and tolerability of dobutamine-atropine stress echocardiography: a prospective, multicentre study. Echo Dobutamine International Cooperative Study Group. Lancet. 1994;344:1190–1192. doi: 10.1016/s0140-6736(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 62.Beleslin BD, Ostojic M, Stepanovic J, Djordjevic-Dikic A, Stojkovic S, Nedeljkovic M, Stankovic G, Petrasinovic Z, Gojkovic L, Vasiljevic-Pokrajcic Z. Stress echocardiography in the detection of myocardial ischemia. Head-to-head comparison of exercise, dobutamine, and dipyridamole tests. Circulation. 1994;90:1168–1176. doi: 10.1161/01.cir.90.3.1168. [DOI] [PubMed] [Google Scholar]
- 63.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 64.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL. Stress Echocardiography Expert Consensus Statement--Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30:278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]